Abstract
Background:
Mobility impairment is a common disability in MS and negatively impacts patients’ lives.
Objective:
Evaluate the effect of prolonged-release (PR) fampridine (extended-release dalfampridine in the United States) on self-assessed walking disability, dynamic/static balance and safety in patients with MS.
Methods:
MOBILE was a randomised, double-blind, exploratory, placebo-controlled trial. Patients with progressive/relapsing-remitting MS and Expanded Disability Status Scale score of 4.0–7.0 were treated with PR-fampridine or placebo twice daily for 24 weeks. Efficacy endpoints included change from baseline in the 12-item MS Walking Scale (MSWS-12), Timed Up and Go (TUG) test and Berg Balance Scale (BBS).
Results:
132 patients were randomised at 24 sites in six countries. PR-fampridine therapy resulted in greater median improvements from baseline in MSWS-12 score, TUG speed and BBS total score versus placebo over 24 weeks. A higher proportion of patients receiving PR-fampridine versus placebo experienced significant improvements at MSWS-12 improvement thresholds ⩾7 (p = 0.0275), ⩾8 (p = 0.0153) and ⩾9 points (p = 0.0088) and TUG speed thresholds ⩾10% (p = 0.0021) and ⩾15% (p = 0.0262). PR-fampridine was well tolerated.
Conclusions:
PR-fampridine therapy resulted in early and sustained improvements in broad measures of walking and balance over six months.
Keywords: Fampridine, balance, walking, randomised clinical trial, multiple sclerosis
Introduction
Walking impairment is common in MS,1,2 even early in disease,3 and maintaining mobility is a high priority among patients with MS.4,5 Prolonged-release (PR) fampridine tablets (known as sustained/modified-release fampridine in some countries and extended-release dalfampridine in the United States) are approved for improving walking in MS based on results from two pivotal trials6,7 that demonstrated consistent improvements in walking speed on the Timed 25-Foot Walk8 and on patients’ self-assessment of ambulatory disability on the 12-item MS Walking Scale (MSWS-12).9 Walking is a complex activity influenced by many variables, including impairments in balance,10–12 which are frequent among patients with MS13 and correlate with slower walking speed.10 Furthermore, balance impairment is a strong predictor for perceived difficulty in mobility in MS.14 This highlights the need for studies that assess effects of PR-fampridine on balance.
PR-fampridine improved standing balance in a small (n = 8) 14-week study;15 however, randomised, placebo-controlled studies assessing the effect of PR-fampridine on balance have not previously been performed. The Timed Up and Go (TUG) test16,17 and Berg Balance Scale (BBS)17,18 measure mobility/balance and have demonstrated high reliability in patients with MS.17,18 The objective of MOBILE was to explore the effect of PR-fampridine on endpoints related to patients’ self-assessed walking disability and dynamic/static balance, assessed using the BBS and TUG, as well as to subjective impression of well-being and patients’ impression of change in walking.
Methods
Standard protocol approvals, registrations and patient consents
This trial was conducted in compliance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, the European Union Clinical Trials Directive and local regulatory requirements. Approval for the trial protocol and all amendments was obtained from local ethics committees. Written informed consent was obtained from each patient before any evaluations were conducted for eligibility. The trial is registered on ClinicalTrials.gov (identifier NCT01597297) and the European Union Clinical Trials Register (EudraCT number 2012-000368-90).
Trial design
MOBILE was a randomised, double-blind, exploratory, multi-centre, placebo-controlled trial in patients with MS (electronic Supplementary Materials, Figure 1). Patients were screened for eligibility during a 14-day screening period. Eligible patients were randomised (1:1) to receive PR-fampridine 10 mg tablets or matching placebo twice daily every 12 hours for 24 weeks. Scheduled visits took place at screening, day 1 and weeks 2, 4, 8, 12, 16, 20 and 24. A post-dosing follow-up visit was conducted two weeks after the end of treatment. Randomisation was determined by a centralised system on day 1 of the trial and treatment assignments were made through an Interactive Web Response System. All patients and trial staff, including the principal investigator, were blinded to patient treatment assignments. Blinding was achieved by using a matched placebo. Treatment kits were prepared centrally and labelled with unique numbers, which were used to maintain the blind when drug supplies were dispensed.
Patients and trial treatments
Eligible patients were male or female, aged 18–70 years with an Expanded Disability Status Scale score of 4.0–7.0 and diagnosis of primary-progressive MS, secondary-progressive MS, progressive-relapsing MS or relapsing-remitting MS per revised McDonald criteria19,20 of ⩾3 months’ duration. Most stable concomitant therapies for treatment of MS were permitted. Key exclusion criteria included: treatment with 4-aminopyridine or 3,4-diaminopyridine in any formulation ⩽30 days before screening; known allergy to pyridine-containing substances; any history of seizure, epilepsy or other convulsive disorder; renal impairment (creatinine clearance <80 ml/min); onset of MS exacerbation ⩽60 days before screening; and a body mass index ⩾40 kg/m2.
Outcome measures and clinical assessments
Outcome measures were assessed at screening, day 1 and weeks 2, 4, 8, 12, 16, 20 and 24 (on-treatment period) and at the week 26 follow-up visit (except where noted otherwise). Because this was an exploratory study, there were no pre-specified primary/secondary endpoints and no formal statistical hypothesis testing was planned. Walking ability was assessed using the MSWS-129 and the seven-item Patient Global Impression of Change (PGIC) scale.21 The MSWS-12 is a 12-item questionnaire that asks patients to rate limitations of their mobility owing to MS during the preceding two weeks on a five-point scale (from 1 = not at all to 5 = extremely). Total score ranges from 1–60 and was transformed to a scale of 0–100; reduced score indicates improvement in walking. The PGIC is a global assessment of the patient’s impression of how the study drug affected their overall walking during the preceding seven days. The PGIC was assessed at weeks 2, 4, 8, 12, 16, 20 and 24, and scored on a seven-point scale (from 1 = very much worse to 7 = very much improved).
Mobility and dynamic balance were assessed using the TUG test,16,17 which measures the time/speed it takes for a patient to stand up from a seated position, walk three metres out, turn around and walk back and return to the seated position. Increased speed on the TUG indicates improvement in mobility and dynamic balance. Static and dynamic balance were measured using the BBS,17,18 which is comprised of 14 balance-related tasks scored from 0 (unable to perform) to 4 (able to perform independently). The BBS is the sum of scores across these tasks and ranges from 0 (poor balance) to 56 (good balance). Positive change on the BBS indicates improvement in balance. The TUG and BBS, which have demonstrated high reliability in patients with MS,17,18 were chosen to further assess the impact of PR-fampridine treatment on mobility, specifically on static and dynamic balance. Balance has not been previously evaluated with PR-fampridine treatment in a randomised, placebo-controlled trial but may impact patients’ walking ability and mobility.
The impact of MS on the patient and quality of life (QoL) were assessed using the 29-item Multiple Sclerosis Impact Scale (MSIS-29) physical subscale (PHYS)22 and the EuroQoL-5 Dimensions 5-level (EQ-5D-5L).23 The self-administered MSIS-29 questionnaire contains a 20-item PHYS and a nine-item psychological subscale. For a particular visit, the MSIS-29 PHYS score was calculated by summing the 20 items and transforming the score to a scale with a range of 0 (no impact of MS) to 100 (extreme impact of MS). Negative change on the MSIS-29 PHYS indicates improvement in physical health. The EQ-5D-5L is a generic instrument comprised of five questions and a visual analogue scale (VAS). A utility score is derived from the five questions addressing mobility, self-care, usual activities, pain/discomfort and anxiety/depression, with responses/scores ranging from 1 (no problem) to 5 (severe problem). The VAS score ranges from 0 (worst imagined health state) to 100 (best imagined health state). The EQ-5D was assessed at day 1 and weeks 4, 8, 12, 16, 20 and 24, and a positive change in both the utility and VAS scores indicates an improvement in health state.
Safety and tolerability were assessed by monitoring adverse events (AEs), serious AEs (SAEs) and concomitant medications and by performing physical examination, vital sign measurements and 12-lead electrocardiograms.
Statistical analyses
A sample size of 120 patients (60 patients per group) was chosen based on bootstrapping that sampled data from a previous study6 to create 1000 datasets of 60 patients each, and repeated for sample sizes of 70 and 80 patients. For each sample, the proportion of patients with a six-point change or more in MSWS-1224 score was calculated, and the percentage of samples that fell within an interval of the actual percentage in the study was calculated to explore whether increasing the sample size increased the precision. Using the sample size of 60 patients for both treatment groups, 84% of samples were within 8% of the true proportion and increasing the size did not appreciably increase precision.
The intent-to-treat population consisted of all patients who were randomised, received at least one dose of treatment and had at least one post-baseline assessment for a given parameter. Efficacy analyses were based on an intent-to-treat analysis with missing data imputed by the last observation carried forward method when at least one post-baseline value was available. Baseline values were not carried forward and were defined as the mean over the screening and baseline visits. Mean changes in each efficacy endpoint, except for the PGIC, were calculated over 24 weeks using the last observation carried forward values to calculate the average. Because the PGIC was designed to assess change since the previous visit, week 2 was the only time point that was valid for between-group comparisons of treatment effect.
This was an exploratory study, and descriptive statistics were used to summarise endpoints. No formal statistical hypothesis testing was planned. Based on the distribution of changes from baseline across patients, all by-visit analyses were summarised using median change from baseline on the outcome. Median changes were presented with corresponding non-parametric 95% confidence intervals.
Mean changes from baseline on the MSWS-12 were categorised using the following thresholds: a less than one-point improvement/no change/worsening and then increasing thresholds of improvement from ⩾1 point to ⩾10 points in one-point increments. These categories were summarised as the number/percentage of patients meeting each threshold. Mean percentage changes from baseline in TUG speed were categorised using the following thresholds: ⩽0% (worsening/no change), >0% (any improvement) and then increasing thresholds of improvement from ⩾10% to ⩾40% in 5% increments. These categories were summarised as the number/percentage of patients meeting each threshold.
In order to characterise magnitude of the observed treatment effect relative to variance in the outcome measures, post hoc statistical testing compared multiple thresholds of improvement between treatment groups for MSWS-12 and TUG using a logistic regression adjusted for baseline. Multiple MSWS-12 thresholds of change were examined based on ranges previously identified as clinically meaningful (⩾4–6 points based on a 100-point scale) over three months.24 A post hoc analysis using the chi-square test compared the percentage of patients in each treatment group who reported any improvement in PGIC after two weeks of treatment. To assess magnitude of treatment effect over time, changes from baseline to each visit also were summarised for each efficacy endpoint, except for PGIC.
For missing data in MSWS-12, a visit in which ⩾50% of the component questions were answered but at least one question was missing, scores from unanswered questions were imputed using the respondent-specific mean score. MSWS-12 score was considered missing for a visit in which ⩾50% of the questions were unanswered. For the TUG test, two trials of the TUG were conducted at each visit and speed for any particular visit was calculated as the average for trials 1 and 2. If either trial was missing, then speed from the completed trial was used. For the BBS, if at least two questions were missing at a visit, the score was set to missing. If two or fewer questions were missing, these were imputed using the respondent-specific mean score. For MSIS-29 PHYS, if a patient had missing data for <10 of the 20 items, the mean of the non-missing items was used to impute the missing items. If ⩾10 items were missing, then the PHYS score was set to missing. For EQ-5D-5L, no imputation was used for missing values for the five summary scores or for VAS. A summary utility index value was calculated for patients with non-missing data for each of the five questions at a visit. The crosswalk method23 was used to map EQ-5D-5L to the EQ-5D 3-level United Kingdom value set, because value sets for EQ-5D-5L are still under development. Utility index value ranges from −0.594 (worst health state) to 1.000 (best health state).
Results
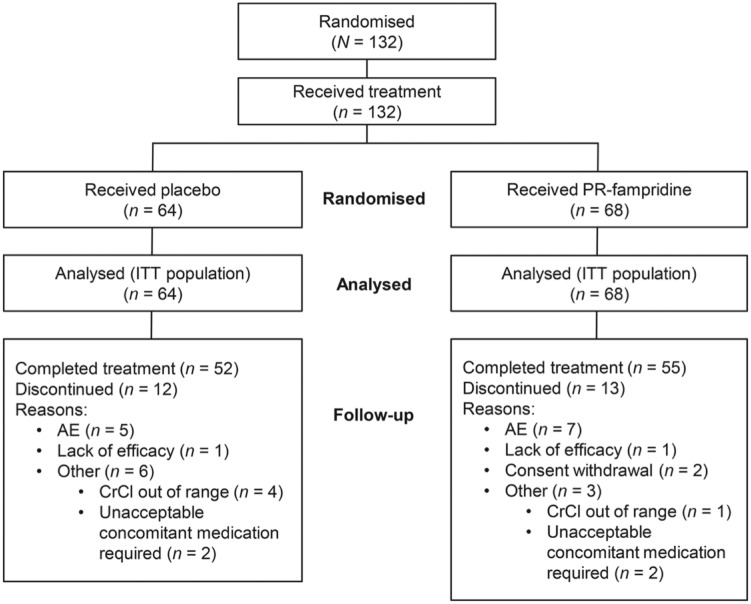
A total of 132 patients were randomised at 24 sites in Belgium, Canada, Italy, the Netherlands, Sweden and the United Kingdom. The first patient was treated on 30 August 2012 and the trial ended on 8 August 2013. All randomised patients were treated and included in the analysis: 64 were treated with placebo and 68 with PR-fampridine. In each group, 81% of patients completed treatment and 19% of patients discontinued treatment (Figure 1). AE was the most common reason for discontinuation and one patient in each treatment group discontinued owing to lack of efficacy (Figure 1). Patient characteristics were comparable between treatment groups at baseline (Table 1).
Figure 1.
MOBILE patient disposition. AE: adverse event; CrCl: creatinine clearance; ITT: intent-to-treat; PR: prolonged-release.
Table 1.
Baseline characteristics.
| Characteristic | Placebo (n = 64) | PR-fampridine (n = 68) | Total (N = 132) |
|---|---|---|---|
| Mean age, years | 49.8 | 49.8 | 49.8 |
| Female, n (%) | 33 (52) | 38 (56) | 71 (54) |
| Mean (SD) body mass index, kg/m2 | 26.5 (6.2) | 26.8 (4.9) | 26.6 (5.6) |
| Mean (median) time since first MS diagnosis, years | 12.4 (12.0), n = 63 | 10.9 (9.5) | 11.6 (10.0), n = 131 |
| Disease course, n (%) | |||
| RRMS | 20 (31) | 24 (35) | 44 (33) |
| SPMS | 37 (58) | 31 (46) | 68 (52) |
| PPMS | 6 (9) | 12 (18) | 18 (14) |
| PRMS | 1 (2) | 1 (1) | 2 (2) |
| Mean (median) time since most recent relapse, years | 3.3 (2.4), n = 56 | 4.2 (3.5), n = 56 | 3.8 (2.8), n = 112 |
| Mean (median) EDSS score | 5.9 (6.0) | 5.6 (6.0) | 5.7 (6.0) |
| Outcome measures: median (mean) [min, max] | |||
| MSWS-12 score | 81.3 (75.9) [8.3, 100.0] | 75.0 (71.7) [25.0, 100.0] | ND |
| TUG speed, m/s | 0.32 (0.34) [0.0, 0.8], n = 63 | 0.38 (0.38) [0.1, 0.7] | ND |
| BBS score | 41.0 (39.3) [5.0, 56.0],n = 63 | 43.8 (40.9) [6.5, 56.0] | ND |
| MSIS-29 PHYS score | 57.5 (53.0) [13.1, 91.9] | 50.0 (50.9) [8.1, 100.0] | ND |
| EQ-5D-5L utility index score | 0.547 (0.509) [–0.19, 1.00] | 0.584 (0.540) [0.04, 0.85] | ND |
| EQ-5D-5L VAS | 60.0 (59.1) [4.0, 90.0], n = 63 | 60.0 (61.6) [25.0, 90.0] | ND |
BBS: Berg Balance Scale; EDSS: Expanded Disability Status Scale; EQ-5D-5L: EuroQoL-5 Dimension 5-level; MSIS-29: 29-item Multiple Sclerosis Impact Scale; MSWS-12: 12-item Multiple Sclerosis Walking Scale; ND: not determined; PHYS: physical subscale; PPMS: primary-progressive multiple sclerosis; PR: prolonged-release; PRMS: progressive-relapsing multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary-progressive multiple sclerosis; TUG: Timed Up and Go; VAS: visual analogue scale.
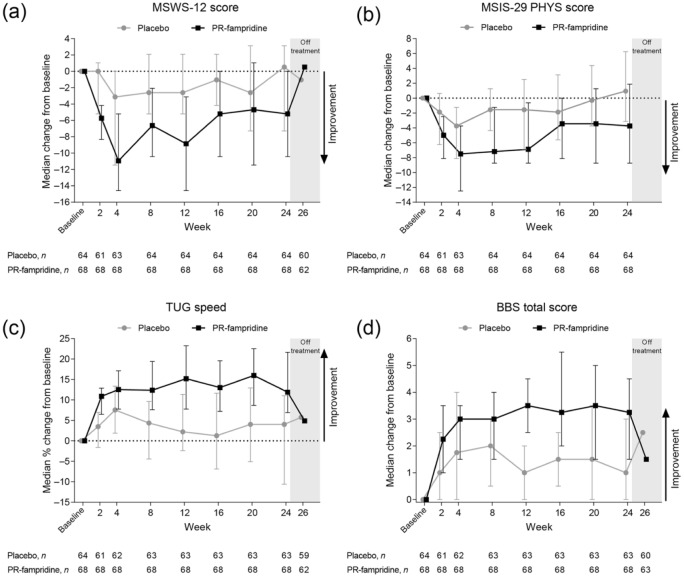
PR-fampridine therapy resulted in greater median improvements from baseline in TUG speed, BBS total score and MSWS-12 score compared with placebo during the 24-week treatment period (Figure 2(a), (c) and (d)). After treatment discontinuation at week 24, improvements declined and approached zero by the week 26 follow-up visit in the PR-fampridine group.
Figure 2.
Median changes from baseline and corresponding 95% confidence intervals in efficacy measures by study visit. Outcome measures: (a) 12-item Multiple Sclerosis Walking Scale (MSWS-12); (b) 29-item Multiple Sclerosis Impact Scale (MSIS-29) physical subscale (PHYS); (c) Timed Up and Go (TUG) test; and (d) Berg Balance Scale (BBS) were assessed at baseline (mean over screening and day 1) and weeks 2, 4, 8, 12, 16, 20, 24 and week 26 (off-treatment visit; not assessed for MSIS-29). Error bars denote non-parametric 95% confidence interval for the median change at each visit. PR: prolonged-release.
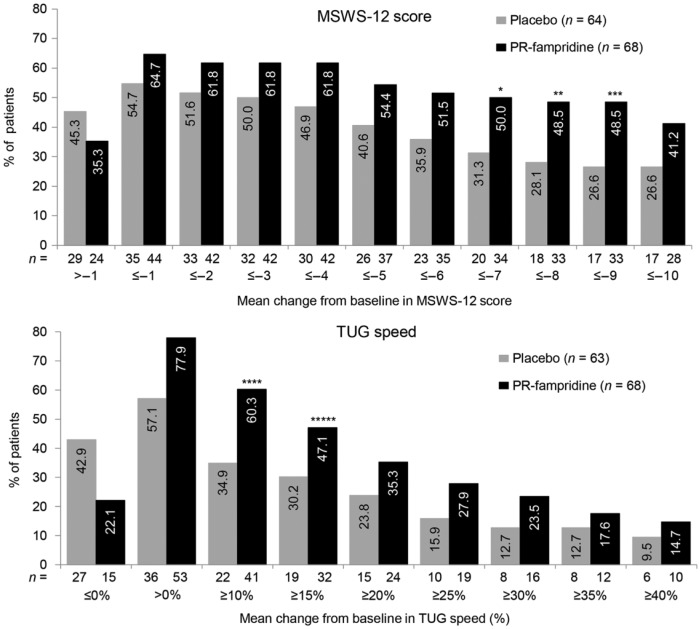
When changes from baseline were summarised as a change at pre-specified thresholds of improvement, a higher proportion of patients receiving PR-fampridine versus placebo met each threshold of improvement in the TUG test (>0% and from ⩾10% to ⩾40%), with statistically significant differences at thresholds ⩾10% (p = 0.0021) and ⩾15% (p = 0.0262; Figure 3). Similar results were observed for the MSWS-12; a higher proportion of patients receiving PR-fampridine versus placebo met each threshold of improvement in MSWS-12 (⩾1 to ⩾10 points) with statistically significant differences at thresholds ⩾7 (p = 0.0275), ⩾8 (p = 0.0153) and ⩾9 (p = 0.0088; Figure 3).
Figure 3.
Cumulative percentage of patients with mean improvement in 12-item Multiple Sclerosis Walking Scale (MSWS-12) and Timed Up and Go (TUG) speed over 24 weeks. MSWS-12 (upper panel): cumulative percentage of patients with increasing levels of improvement on the MSWS-12 over the on-treatment period (weeks 2–24) across multiple thresholds (thresholds ⩽–1 to ⩽–10 represent improvements in ⩾1 to ⩾10 points). TUG speed (lower panel): cumulative percentage of patients with average percent increase from baseline in TUG speed over the on-treatment period (weeks 2–24) across multiple thresholds. PR-fampridine versus placebo: *p = 0.0275; **p = 0.0153; ***p = 0.0088; ****p = 0.0021; *****p = 0.0262. PR: prolonged-release.
In a post hoc analysis, a significantly greater proportion of patients (n (%)) treated with PR-fampridine (31 (46%)) versus placebo (16 (26%)) also reported improvement on PGIC at the week 2 visit (p = 0.023).
PR-fampridine therapy also resulted in greater improvements from baseline in MSIS-29 PHYS score versus placebo (Figure 2(b)). These improvements were apparent throughout the 24-week trial duration. However, no apparent differences between treatment groups were observed in EQ-5D-5L results; median treatment difference (95% confidence interval) for PR-fampridine versus placebo for EQ-5D-5L VAS was 0.00 (–4.17, 4.67) and for utility index was 0.00 (–0.04, 0.04).
The proportion of patients with any AE was similar in placebo-treated patients (49 (77%)) versus patients receiving PR-fampridine (51 (75%)). Nasopharyngitis and urinary tract infections (UTIs) were the most frequently reported AEs in patients treated with PR-fampridine and placebo, respectively (Table 2). Incidence of falls was higher in patients receiving placebo versus PR-fampridine, whereas incidences of balance disorder, gait disturbance and dizziness were higher in patients receiving PR-fampridine. A lower proportion of patients receiving PR-fampridine (3%) versus placebo (8%) reported SAEs. In the PR-fampridine group, SAEs were considered unrelated to treatment and included moderate MS relapse in one patient and moderate MS relapse and severe paraparaesis (worsened MS symptoms) in the second patient. No seizures were reported during treatment.
Table 2.
Incidence of treatment-emergent AEs reported for ⩾5% of patients.
| n (%) | Placebo (n = 64) | PR-fampridine (n = 68) |
|---|---|---|
| No. of patients with AE | 49 (77) | 51 (75) |
| No. of patients with SAE | 5 (8) | 2 (3) |
| AEs | ||
| Nasopharyngitis | 9 (14) | 11 (16) |
| Urinary tract infection | 12 (19) | 6 (9) |
| Back pain | 3 (5) | 6 (9) |
| Balance disorder | 1 (2) | 6 (9) |
| Headache | 5 (8) | 5 (7) |
| Gait disturbance | 2 (3) | 5 (7) |
| Fall | 8 (13) | 4 (6) |
| Arthralgia | 4 (6) | 4 (6) |
| Fatigue | 4 (6) | 4 (6) |
| Nausea | 3 (5) | 4 (6) |
| Dizziness | 1 (2) | 4 (6) |
| MS relapse | 3 (5) | 3 (4) |
| Influenza | 4 (6) | 2 (3) |
| Muscle spasms | 3 (5) | 1 (1) |
AE: adverse event; PR: prolonged-release; SAE: serious adverse event.
Discussion
In this trial, PR-fampridine therapy resulted in greater/sustained improvements in mobility/balance over the six-month trial duration compared with placebo as measured by the TUG test and BBS. Benefits were seen as early as two weeks after treatment initiation and were maintained throughout the trial. Upon discontinuation of PR-fampridine therapy, improvements in outcome measures reversed and approached pre-treatment levels within two weeks.
Early benefits also were seen in walking ability measured by MSWS-12, and although MSWS-12 score fluctuated between weeks 4 and 12, it stabilised after week 16 and benefits were maintained to the end of the study in patients receiving PR-fampridine therapy. In addition to greater median improvements in TUG speed and MSWS-12 score, a higher cumulative proportion of patients receiving PR-fampridine versus placebo met each threshold of improvement in TUG speed (>0% and ⩾10% to ⩾40%) and MSWS-12 score (⩾1 to ⩾10 points), including thresholds in a range (⩾4 to ⩾6 points) previously identified as clinically meaningful for MSWS-12 with different data sources.24
The MOBILE study was the first randomised, placebo-controlled study that assessed the effect of PR-fampridine on dynamic and static balance. Walking is a complex activity10,11 and individual patients with MS may improve in one or more domains, such as speed and/or balance. The findings of MOBILE suggested that the previous definition of PR-fampridine responder that was based on walking speed alone6,7 may be too narrowly defined and may not identify some patients who received treatment benefits associated with improved balance.
PR-fampridine therapy also demonstrated improvements on MSIS-29 PHYS, a patient-reported measure of the physical impact of MS, but no clear differences between treatment groups were observed in EQ-5D-5L VAS and utility index results over time. The discrepancy observed in this trial between the MSIS-29 and EQ-5D-5L may be related to the insensitivity of generic measures to assess MS-related changes in QoL. The MSIS-29 PHYS results in this trial supported those observed in the ENABLE study, in which PR-fampridine therapy demonstrated significant/clinically meaningful improvements on QoL/health state, measured by a broad range of MS-specific and generic patient-reported endpoints, over 48 weeks in >600 patients who received PR-fampridine.25
Safety findings from MOBILE were consistent with the known safety profile of PR-fampridine,6,7,26 with the exception that incidence of UTIs was higher in placebo-treated patients compared with patients receiving PR-fampridine. This is in contrast to that observed in the pivotal studies,6,7 in which incidence of UTIs was higher for the PR-fampridine group. In the current study, UTIs were confirmed by culture. In contrast, the pivotal trials did not require confirmation of UTIs, which may have resulted in an overestimation of the rate of UTIs. No seizures were observed in MOBILE and although more patients receiving PR-fampridine reported balance disorders, gait disturbance and dizziness versus placebo, the incidence of falls was higher in the placebo group. Balance disorders, gait disturbance and dizziness are broad, non-specific AEs, and increases in these events may not result in an increase in falls, as was the case in the MOBILE study. Furthermore, the improvement in TUG and BBS could have manifested in fewer falls among the patients treated with PR-fampridine.
The findings of MOBILE confirm and expand findings of previous controlled studies with a longer treatment period, geographically different study population and broader range of objective and patient-reported measures of mobility and balance to assess different domains of walking.6,7 MOBILE was exploratory in design and its findings require confirmation in a prospective trial that is underway.27 Nevertheless, the results of MOBILE support that PR-fampridine therapy provides significant and sustained improvements in walking characteristics beyond walking speed; in particular, PR-fampridine therapy resulted in early and sustained benefits on measures of dynamic and static balance and mobility as well as patient-reported walking disability. Overall, these findings provided additional support for the potential of PR-fampridine to result in clinically meaningful improvements in walking quality/ambulatory function in patients with MS with walking disability.
Supplementary Material
Supplementary Material
Acknowledgments
This study was funded by Biogen, who provided funding for editorial support in the development of this manuscript; Maria Hovenden from Excel Scientific Solutions wrote the first draft of the manuscript based on input from other authors, and Kristen DeYoung from Excel Scientific Solutions copy-edited and styled the manuscript per journal requirements. Biogen reviewed and provided feedback on the manuscript to the authors. The authors had full editorial control of the manuscript, and provided their final approval of all content. Please see electronic Supplemen-tary Materials, Appendix 1 for a full list of study investigators.
Footnotes
Conflict of Interest: Dr Hupperts has received compensation for consulting, advisory boards and research grants and as a speaker for lectures from Biogen, Genzyme, Merck, Novartis and Teva.
Dr Lycke has received compensation for consulting, serving on scientific advisory boards and as a speaker for lectures from Biogen, Genzyme, Novartis and Teva.
Dr Short has received compensation for serving on scientific advisory boards and as a speaker for lectures from Biogen.
Dr Gasperini has received compensation for consulting from Bayer HealthCare and Biogen and as a speaker for lectures from Biogen, Bayer HealthCare, Genzyme, Merck Serono, Novartis and Teva.
M McNeill, Dr Tofil-Kaluza, Dr Mehta and Dr Elkins are full-time employees of Biogen.
Dr Medori was a full-time employee of Biogen at the time of this study and manuscript development.
Dr Hovenden is a full-time employee of Excel Scientific Solutions.
Funding: This study was sponsored by Biogen.
Contributor Information
Raymond Hupperts, Orbis Medical Center, Sittard-Geleen, the Netherlands.
Jan Lycke, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, Gothenburg, Sweden.
Christine Short, Queen Elizabeth II Health Sciences Centre, Halifax, Canada.
Claudio Gasperini, Hospital San Camillo Forlanini, Rome, Italy.
Manjit McNeill, Biogen, Maidenhead, UK.
Rossella Medori, Biogen, Cambridge, MA, USA.
Agata Tofil-Kaluza, Biogen, Maidenhead, UK.
Maria Hovenden, Excel Scientific Solutions, Southport, CT, USA.
Lahar R Mehta, Biogen, Cambridge, MA, USA.
Jacob Elkins, Biogen, Cambridge, MA, USA.
References
- 1. Souza A, Kelleher A, Cooper R, et al. Multiple sclerosis and mobility-related assistive technology: Systematic review of literature. J Rehabil Res Dev 2010; 47: 213–223. [DOI] [PubMed] [Google Scholar]
- 2. van Asch P. Impact of mobility impairment in multiple sclerosis 2 – patients’ perspectives. Eur Neurol Rev 2011; 6: 115–120. [Google Scholar]
- 3. Martin CL, Phillips BA, Kilpatrick TJ, et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler 2006; 12: 620–628. [DOI] [PubMed] [Google Scholar]
- 4. Heesen C, Böhm J, Reich C, et al. Patient perception of bodily functions in multiple sclerosis: Gait and visual function are the most valuable. Mult Scler 2008; 14: 988–991. [DOI] [PubMed] [Google Scholar]
- 5. Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin 2010; 26: 109–119. [DOI] [PubMed] [Google Scholar]
- 6. Goodman AD, Brown TR, Edwards KR, et al. ; MSF204 Investigators. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol 2010; 68: 494–502. [DOI] [PubMed] [Google Scholar]
- 7. Goodman AD, Brown TR, Krupp LB, et al. ; Fampridine MS-F203 Investigators. Sustained-release oral fampridine in multiple sclerosis: A randomised, double-blind, controlled trial. Lancet 2009; 373: 732–738. [DOI] [PubMed] [Google Scholar]
- 8. Fischer JS, Rudick RA, Cutter GR, et al. ; National MS Society Clinical Outcomes Assessment Task Force. The Multiple Sclerosis Functional Composite Measure (MSFC): An integrated approach to MS clinical outcome assessment. Mult Scler 1999; 5: 244–250. [DOI] [PubMed] [Google Scholar]
- 9. Hobart JC, Riazi A, Lamping DL, et al. Measuring the impact of MS on walking ability: The 12-item MS Walking Scale (MSWS-12). Neurology 2003; 60: 31–36. [DOI] [PubMed] [Google Scholar]
- 10. Nogueira LA, Dos Santos LT, Sabino PG, et al. Factors for lower walking speed in persons with multiple sclerosis. Mult Scler Int 2013; 2013: 875648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nieuwenhuis MM, Van Tongeren H, Sorensen PS, et al. The Six Spot Step Test: A new measurement for walking ability in multiple sclerosis. Mult Scler 2006; 12: 495–500. [DOI] [PubMed] [Google Scholar]
- 12. Sandroff BM, Sosnoff JJ, Motl RW. Physical fitness, walking performance, and gait in multiple sclerosis. J Neurol Sci 2013; 328: 70–76. [DOI] [PubMed] [Google Scholar]
- 13. Cameron MH, Lord S. Postural control in multiple sclerosis: Implications for fall prevention. Curr Neurol Neurosci Rep 2010; 10: 407–412. [DOI] [PubMed] [Google Scholar]
- 14. Paltamaa J, Sarasoja T, Leskinen E, et al. Measures of physical functioning predict self-reported performance in self-care, mobility, and domestic life in ambulatory persons with multiple sclerosis. Arch Phys Med Rehabil 2007; 88: 1649–1657. [DOI] [PubMed] [Google Scholar]
- 15. Prosperini L, Giannì C, Fortuna D, et al. Oral dalfampridine improves standing balance detected at static posturography in multiple sclerosis. Mult Scler Int 2014; 2014: 802307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 17. Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil 2006; 28: 789–795. [DOI] [PubMed] [Google Scholar]
- 18. Cattaneo D, Jonsdottir J, Repetti S. Reliability of four scales on balance disorders in persons with multiple sclerosis. Disabil Rehabil 2007; 29: 1920–1925. [DOI] [PubMed] [Google Scholar]
- 19. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 2001; 50: 121–127. [DOI] [PubMed] [Google Scholar]
- 20. Polman CH, Wolinsky JS, Reingold SC. Multiple sclerosis diagnostic criteria: Three years later. Mult Scler 2005; 11: 5–12. [DOI] [PubMed] [Google Scholar]
- 21. Guy W. ECDEU assessment manual for psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976. [Google Scholar]
- 22. Hobart J, Lamping D, Fitzpatrick R, et al. The Multiple Sclerosis Impact Scale (MSIS-29): A new patient-based outcome measure. Brain 2001; 124: 962–973. [DOI] [PubMed] [Google Scholar]
- 23. EuroQoL Group. EQ-5D: A standardized instrument for use as a measurement of health outcome. Available at: http://www.euroqol.org/, 2014. (accessed 24 October 2014).
- 24. Hobart J. Prolonged-release fampridine for multiple sclerosis: Was the effect on walking ability clinically significant? Mult Scler 2010; 16(10 suppl): P509. [Google Scholar]
- 25. Macdonell R, Sorensen PS, Pozzilli C, et al. Long-term prolonged-release fampridine treatment and health-related quality of life outcomes: 9-month interim analysis of the ENABLE study. Mult Scler 2013; 19(11 suppl): P665. [Google Scholar]
- 26. Jara M, Barker G, Henney HR., 3rd Dalfampridine extended release tablets: 1 year of postmarketing safety experience in the US. Neuropsychiatr Dis Treat 2013; 9: 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Efficacy and Safety Study of Prolonged-Release Fampridine in Subjects With Multiple Sclerosis (ENHANCE). Available at: https://clinicaltrials.gov/ct2/show/NCT02219932, 2014. (accessed 24 October 2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.