Abstract
Our study examined age-related differences on a new memory test assessing memory for “who,” “when,” and “where,” and associations among these elements. Participants were required to remember a sequence of pictures of different faces paired with different places. Older adults remembered significantly fewer correct face–place pairs in the correct sequence compared with young adults. Correlation analyses with standardized neuropsychological tests provide preliminary evidence for construct validity. Our results offer insight into age-related changes in the ability to remember associations between people and places at different points in time using a portable test that can be administered rapidly in various settings.
Episodic memory (EM) is the ability to remember a personally experienced event from one's past (Tulving 2002). Therefore, EM involves remembering events that occur in particular places and at particular times resulting in memory for “what,” “when,” and “where” (Tulving 2002). One key feature of EM that differentiates it from other types of memory is that the elements of an EM must be associated into a context to demarcate the episode in space and time. It is well-established that EM relies on the functioning of the medial temporal and frontal lobes (Wheeler et al. 1995; Squire et al. 2004; Kramer et al. 2005). The hippocampus may be important for memory accuracy, while the frontal lobes may be more important for decision-making and strategic aspects of EM (Kramer et al. 2005).
Deficits in EM have been well documented in older adults (Shing et al. 2010; Nyberg et al. 2012) and are one of the earliest and most prominent cognitive deficits in Alzheimer's disease (AD; Bondi et al. 2005; Fleisher et al. 2005). EM impairment also has been documented in older adults diagnosed with mild cognitive impairment (MCI) who are at risk for developing AD (Bennett et al. 2006). Studies have reported age-related impairments in spatial memory (Iachini et al. 2009; Bohbot et al. 2012; Bates and Wolbers 2014) and temporal order memory (Blachstein et al. 2012; Tolentino et al. 2012; Roberts et al. 2014; Rotblatt et al. 2015), which are important aspects of EM, supporting memory for “where” and “when” an event occurred. Older adults also show deficits on tests of associative memory (Castel and Craik 2003; Naveh-Benjamin et al. 2004). The encoding of contextual information may be critical to the formation of EMs due to the need to form associations among stimuli, actions, and places that compose an event (Eichenbaum 2004).
EM is often measured in the laboratory, and assessed clinically, using well-validated verbal memory tests such as the California Verbal Learning Test—Second Edition (CVLT-II; Delis 2000). As reviewed by Pause et al. (2013), a number of experimental tests also have been developed to measure episodic and episodic-like memory (also see Fouquet et al. 2010). The present study examined age-related differences on a new test assessing memory for “who,” “when,” and “where,” as well as associations among these elements. Our new test is portable and can be rapidly administered in a laboratory or clinical setting. One new aspect of our test is that it uses a spatial context (e.g., a grocery store) as the “where” component of the test rather than an arbitrary location on a grid, which has been used in previous studies. We hypothesized that the spatial context would resemble the spatial component of everyday EM. We also examined the relationship between performance on our test and a battery of standardized tests to provide preliminary evidence for construct validity.
The sample consisted of 40 young adults 18–25 yr of age (M = 21.20 yr, SD = 2.28) and 30 healthy older adults 65 yr of age and older (M = 69.87 yr, SD = 6.95). A χ2 analysis did not reveal a significant gender difference χ2 (1, N = 70) = 0.18, P = 0.67 between the young (65% female) and older (60% female) adults. The average years of education did not differ significantly F(1,68) = 2.80 P = 0.10 between young (M = 14.63 yr, SD = 1.58) and older (M = 15.30 yr, SD = 1.78) adults. The study was approved by the Institutional Review Boards at San Diego State University and all participants provided signed consent.
Older adults completed the Dementia Rating Scale-2 (DRS-2; Jurica et al. 2001) to assess general cognitive functioning. The average score was 140.52 (SD = 3.46) and all participants scored above 130. All participants completed the CVLT-II (Delis 2000), the Color Word Interference Test and the Trail Making Test from the Delis–Kaplan Executive Function System (D-KEFS; Delis et al. 2001), the Boston Naming Test (Kaplan et al. 2000), and the Benton Judgment of Line Orientation Test (Benton et al. 1983). These tests were administered following our new test. The mean (SD) raw scores of young and older adults on the neuropsychological tests are shown in Table 1 along with statistical comparisons between the groups and Cohen's d effect size estimates.
Table 1.
Mean (standard deviation) raw data from young and older adults on our new memory test (correct face–place pairs in correct sequence), Trials 1–5 Total Immediate Recall from the California Verbal Learning Test-Second Edition (CVLT II Trials 1–5 Total); the Trail Making Test Switching Condition (TMT Switching) from the Delis–Kaplan Executive Function System (D-KEFS); the Color Word Interference Test Inhibition Condition (CWIT Inhibition) from the D-KEFS; the total score from Benton Judgment of Line Orientation Test (JOLO); and the total score from the Boston Naming Test (BNT Total), along with statistical comparisons between the groups and Cohen's d effect size estimates.
Colored photographs of faces were acquired with written permission from the FACE database, which includes photographs of Caucasian adult models, validated for facial expression and perceived age (Ebner et al. 2010). We selected 12 female and 12 male middle-aged faces, displaying “happy” expressions. We used 24 colored photographs of common places that one is likely to visit in daily life (e.g., a park, a grocery store) and none featured any people (purchased and used with permission from Getty Images).
We developed our test to assess memory for the sequential order of face–place pairs. Over the course of a typical day, an individual often meets a series of different people in different places. This test was designed to simulate these everyday experiences. During the study phase, participants viewed pictures of faces and places. The pictures were presented in pairs for 5 sec on a sheet of paper with each pair consisting of one face and one place. Participants were shown a sequence of six face–place pairs one at a time in immediate succession. Participants were instructed to remember which face was paired with which place, similar to how one might remember meeting a person at a particular place in everyday life. In addition to remembering the face–place pairs, participants were instructed to remember the order in which the pairs were presented. This was designed to be analogous to remembering the order in which one met a series of people over the course of a typical day.
Once the six face–place pairs had been presented, participants were immediately given a stack of 12 face pictures and a separate stack 12 place pictures for the test phase. Half were the target stimuli presented during the study phase and half were distractor stimuli. During the test phase, participants were instructed to select and pair together the faces and places presented in the sample phase and to place the pairs in the order in which they were presented. A face–place pair was considered to be in the correct sequence if the pair was placed in the same ordinal position as it was presented during the study phase. The test phase was self-paced and no limit was placed on the time allowed to place the pairs in sequence. Gender and hair color were balanced in the face pictures between target and distractor stimuli.
Participants completed two trials of the test separated by a 1-min interval, with the first trial referred to as “Day 1” and the second trial as “Day 2.” None of the target or distractor stimuli from Day l were used in Day 2 and gender and hair color were balanced across trials. All items were chosen from the same set of stimuli but the target versus distractor stimuli for each sequence were chosen pseudo-randomly and were counterbalanced across trials.
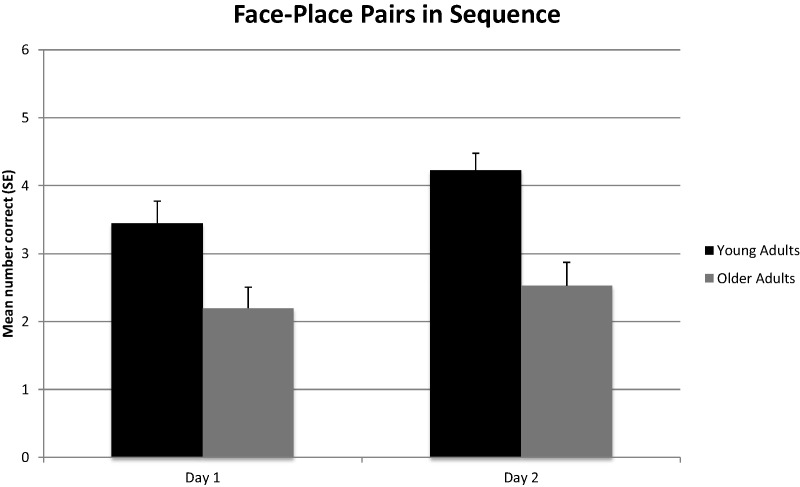
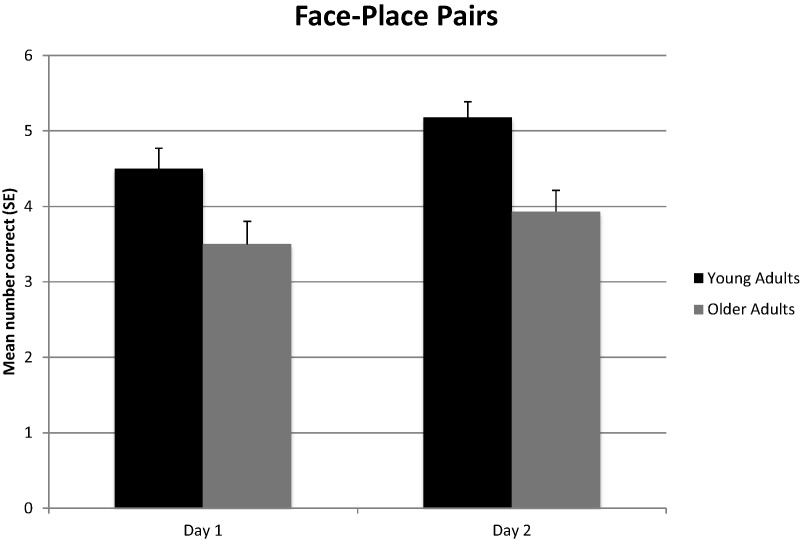
As shown in Figure 1, a one-way analysis of variance (ANOVA) revealed that young adults placed significantly more correct face–place pairs in the correct sequence than older adults on Day 1, F(1,68) = 7.44, P < 0.01, η2 = 0.10 and Day 2, F(1,68) = 16.98, P < 0.001, η2 = 0.20. As shown in Figure 2, a one-way ANOVA also revealed that young adults correctly paired significantly more faces with the correct places (irrespective of sequence) than older adults on Day 1, F(1,68) = 6.15, P < 0.05, η2 = 0.08 and Day 2, F(1,68) = 13.45, P < 0.001, η2 = 0.17. As a follow-up analysis, the number of correct face–place pairs placed in sequence was divided by the number of total correct face–place pairs irrespective of sequence for each participant. A one-way ANOVA revealed that young adults placed significantly more correct face–place pairs in the correct sequence than older adults on Day 1, F(1,68) = 4.29, P < 0.05, η2 = 0.06 and Day 2, F(1,68) = 14.26, P < 0.001, η2 = 0.17, even when factoring in total correct face–place pairs. In addition, Cohen's d effect size estimates revealed that age-group differences in memory for face–place pairs in sequence were 15% larger than differences in memory for the face–place pairs irrespective of sequence. A within subjects ANOVA revealed that young adults placed significantly more correct face–place pairs in sequence on Day 2 compared to Day 1, F(1,39) = 4.62, P < 0.05, η2 = 0.11. However, older adults did not show a similar improvement across trials, F(1,29) = 0.91, P = 0.91.
Figure 1.
Mean (+SE) number of correct face–place pairs placed in correct sequence for young and older adults on two trials (Day 1 and Day 2).
Figure 2.
Mean (+SE) number of correct face–place pairs irrespective of sequence for young and older adults on two trials (Day 1 and Day 2).
ANOVA tests revealed that the number of face intrusion errors committed was significantly higher in older adults compared with young adults on Day 1, F(1,68) = 7.74, P < 0.01, η2 = 0.10 (Young M = 0.48 SD = 0.64; Old M = 1.03 SD = 1.03) and Day 2, F(1,68) = 12.91, P < 0.01, η2 = 0.16 (Young M = 0.28 SD = 0.45; Old M = 0.83 SD = 0.83). An ANOVA revealed that the number of place intrusion errors committed between groups was marginally significant on Day 1, F(1,68) = 3.92, P = 0.052, η2 = 0.05 (Young M = 0.05 SD = 0.22; Old M = 0.20 SD = 0.41) and reached significance on Day 2, F(1,68) = 4.59, P < 0.05, η2 = 0.06 (Young M = 0.03, SD = 0.16; Old M = 0.20 SD = 0.48). When total face and place intrusion errors were entered into the model as covariates, we still found that young adults placed significantly more total correct face–place pairs in the correct sequence than older adults with face intrusions as a covariate F(1,67) = 5.53, P < 0.05, η2 = 0.06 and place intrusions as a covariate F(1,67) = 11.32, P < 0.01, η2 = 0.13.
Bivariate correlations (Pearson r) were conducted to examine the relationship between performance on our EM test and the standardized neuropsychological tests. A Bonferroni correction was applied to control for multiple comparisons and the α-level for significance was set at 0.01. Performance on our test was significantly correlated with CVLT-II Total Immediate Recall (r = 0.53, P < 0.001), Trail Making Test Switching Condition (r = −0.45, P < 0.001), and Color Word Interference Test Inhibition Condition (r = −0.50, P < 0.001). However, our test was not significantly correlated with performance on the Boston Naming Test (r = −0.13, P = 0.30) or the Judgment of Line Orientation Test (r = 0.03, P = 0.78).
Our results show that young adults remembered significantly more correct face–place pairs in the correct sequence and more face–place pairs irrespective of sequence compared with older adults. Given that older adults were impaired in memory for face–place pairs in general, one might question whether the age-related deficits in sequence memory were due solely to impaired memory for the face–place associations. To address this issue, the number of correct face–place pairs place in correct sequence was divided by the number of correct face–place pairs overall. We found that young adults still significantly outperformed the older adults in memory for sequences of face–place pairs in this analysis. Our results provide evidence that older adults may experience deficits in multiple associative aspects of the present task, which might contribute to age-related changes in EM. Our findings are consistent with other studies reporting differences between young and older adults on experimental tests of memory for “what,” “where,” and “when” (Kessels et al. 2007; Kinugawa et al. 2013). In addition, we also assessed memory for the individual faces and places involved in the associations. Although we found significant group differences in intrusion errors for faces and places, the average number of errors committed was quite small. Furthermore, age group differences in memory for face–place pairs in sequence were still significant when face and place intrusion errors were entered into the model as covariates. Therefore, the age-related associative memory differences are not due solely to impaired memory for the individual items in the associations.
We also examined the relationship between performance on our test and a battery of standardized tests to provide preliminary evidence for construct validity. One type of construct validity, “convergent validity,” is established when scores on a test are correlated with variables that are theoretically related to the construct of interest. In contrast, “discriminant validity” is supported when scores on the measure demonstrate no relationships with variables that are theoretically distinct from the construct of interest (Groth-Marnat 2009). Our test correlated significantly with the CVLT-II Total Immediate Recall Index (a standardized memory measure), providing preliminary evidence for convergent validity. Our test also correlated significantly with performance on two executive function measures from the D-KEFS, which provides additional support for convergent validity and suggests that our test may involve strategic aspects related to executive function. Young adults showed a significant improvement in the ability to remember the face–place pairs in sequence across the two trials. It is possible that young individuals are developing strategies to better remember the stimuli after performing the first trial, which they are using on the second trial resulting in better performance. However, older adults did not show an improvement, suggesting they may not be using new strategies across trials. Our test did not correlate significantly with the Boston Naming Test or the Judgment of Line Orientation Test, which measure the constructs of language and visuospatial perception, respectively, providing preliminary evidence for discriminant validity. Given that performance on our test was correlated with scores on the CVLT-II (a measure thought to be sensitive to temporal lobe function) and scores on two executive function measures from the D-KEFS (indices thought to be sensitive to frontal lobe function), there is preliminary evidence that both cortical regions may play a role in performance on this test. Our findings offer preliminary evidence for construct validity and possible insight into the neural correlates of test performance; however, studies involving larger samples clearly are needed.
One difference between our test and everyday EM is that we informed participants about the subsequent memory test prior to encoding. Given that we wanted to compare performance across two different trials, it was necessary to provide instructions prior to the first trial. Future studies should examine performance when no encoding instructions are provided, rendering the test more analogous to everyday EM. The ability to remember associations between faces and places across time is critically important for EM. Our test was designed to simulate everyday experiences in which an individual meets a series of different people in different places during the course of a day. Clearly these experiences occur across much longer periods of time compared with the present task. Although the difference in the timeframe does limit the generalizability of the present findings to everyday EM, our results offer insight into age-related changes in the ability to remember associations between people and places at different points in time using a portable test that can be administered rapidly in a laboratory or clinical setting.
Acknowledgments
This research was supported by a National Institutes of Health Grant (R01AG034202) from the National Institute on Aging awarded to P.E.G. E.J.V.E. was supported by National Institutes of Health Grant R25AG043364. We thank Nicole DeFord and Charles Moreno for their assistance with data collection and entry. We also thank all of the participants for their contributions to this study.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.039313.115.
References
- Bates SL, Wolbers T. 2014. How cognitive aging affects multisensory integration of navigational cues. Neurobiol Aging 35: 2761–2769. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Golob EJ, Parker ES, Starr A. 2006. Memory evaluation in mild cognitive impairment using recall and recognition tests. J Clin Exp Neuropsychol 28: 1408–1422. [DOI] [PubMed] [Google Scholar]
- Benton A, Sivan A, Hamsher K, Varney N, Spreen O. 1983. Benton judgment of line orientation. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Blachstein H, Greenstein Y, Vakil E. 2012. Aging and temporal order memory: a comparison of direct and indirect measures. J Clin Exp Neuropsychol 34: 107–112. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, McKenzie S, Konishi K, Fouquet C, Kurdi V, Schachar R, Boivin M, Robaey P. 2012. Virtual navigation strategies from childhood to senescence: evidence for changes across the life span. Front Aging Neurosci 4: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. 2005. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64: 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Craik FI. 2003. The effects of aging and divided attention on memory for item and associative information. Psychol Aging 18: 873–885. [DOI] [PubMed] [Google Scholar]
- Delis DC, ed. 2000. California verbal learning test, second edition: CVLT-II. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. 2001. Delis-Kaplan executive function system: D-KEFS. Pearson, San Antonio, TX. [Google Scholar]
- Ebner NC, Riediger M, Lindenberger U. 2010. FACES—a database of facial expressions in young, middle-aged, and older women and men: development and validation. Behav Res Methods 42: 351–362. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. 2004. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron 44: 109–120. [DOI] [PubMed] [Google Scholar]
- Fleisher A, Grundman M, Jack CR Jr, Petersen RC, Taylor C, Kim HT, Schiller DH, Bagwell V, Sencakova D, Weiner MF, et al. 2005. Sex, apolipoprotein E ε4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol 62: 953–957. [DOI] [PubMed] [Google Scholar]
- Fouquet C, Tobin C, Rondi-Reig L. 2010. A new approach for modeling episodic memory from rodents to humans: the temporal order memory. Behav Brain Res 215: 172–179. [DOI] [PubMed] [Google Scholar]
- Groth-Marnat G. 2009. Handbook of psychological assessment, 5th ed John Wiley & Sons, Inc, Hoboken, NJ. [Google Scholar]
- Iachini I, Iavarone A, Senese VP, Ruotolo F, Ruggiero G. 2009. Visuospatial memory in healthy elderly, AD and MCI: a review. Curr Aging Sci 2: 43–59. [DOI] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. 2001. Dementia Rating Scale-2. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. 2000. Boston Naming Test. Pro-Ed, Austin, TX. [Google Scholar]
- Kessels RP, Hobbel D, Postma A. 2007. Aging, context memory and binding: a comparison of “what, where and when” in young and older adults. Int J Neurosci 117: 795–810. [DOI] [PubMed] [Google Scholar]
- Kinugawa K, Schumm S, Pollina M, Depre M, Jungbluth C, Doulazmi M, Sebban C, Zlomuzica A, Pietrowsky R, Pause B, et al. 2013. Aging-related episodic memory decline: are emotions the key? Front Behav Neurosci 7: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Rosen HJ, Du AT, Schuff N, Hollnagel C, Weiner MW, Miller BL, Delis DC. 2005. Dissociations in hippocampal and frontal contributions to episodic memory performance. Neuropsychology 19: 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Kilb A, Reedy S. 2004. The associative memory deficit of older adults: further support using face–name associations. Psychol Aging 19: 541–546. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. 2012. Memory aging and brain maintenance. Trends Cogn Sci 16: 292–305. [DOI] [PubMed] [Google Scholar]
- Pause BM, Zlomuzica A, Kinugawa K, Mariani J, Pietrowsky R, Dere E. 2013. Perspectives on episodic-like and episodic memory. Front Behav Neurosci 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM, Ly M, Murray E, Yassa MA. 2014. Temporal discrimination deficits as a function of lag interference in older adults. Hippocampus 24: 1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotblatt LJ, Sumida CA, Van Etten EJ, Turk EP, Tolentino JC, Gilbert PE. 2015. Differences in temporal order memory among young, middle-aged, and older adults may depend on the level of interference. Front Aging Neurosci 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing YL, Werkle-Bergner M, Brehmer Y, Müller V, Li SC, Lindenberger U. 2010. Episodic memory across the lifespan: the contributions of associative and strategic components. Neurosci Biobehav Rev 34: 1080–1091. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. 2004. The medial temporal lobe. Annu Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- Tolentino JC, Pirogovsky E, Luu T, Toner CK, Gilbert PE. 2012. The effect of interference on temporal order memory for random and fixed sequences in nondemented older adults. Learn Mem 19: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. 2002. Episodic memory: from mind to brain. Ann Rev Psychol 53: 1–25. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. 1995. Frontal lobe damage produces episodic memory impairment. J Int Neuropsychol Soc 1: 525–536. [DOI] [PubMed] [Google Scholar]