Abstract
There has been increasing interest in understanding the role of the human gut microbiome to elucidate the therapeutic potential of its manipulation. Fecal microbiota transplantation (FMT) is the administration of a solution of fecal matter from a donor into the intestinal tract of a recipient in order to directly change the recipient’s gut microbial composition and confer a health benefit. FMT has been used to successfully treat recurrent Clostridium difficile infection. There are preliminary indications to suggest that it may also carry therapeutic potential for other conditions such as inflammatory bowel disease, obesity, metabolic syndrome, and functional gastrointestinal disorders.
Keywords: bacteriotherapy, Clostridium difficile infection, fecal microbiota transplantation, gut microbiome, inflammatory bowel disease
Function of the gut microbiome
The term ‘microbiota’ refers to the bacteria, archaea, microeukaryotes, and viruses that share the human body space, and these microorganisms may function in a commensal, symbiotic, or pathogenic relationship [Hollister et al. 2014]. The term ‘microbiome’ refers to the collective genomes of these microorganisms. The microbiome is often referenced instead of the microbiota, probably because much of our understanding of these bodily inhabitants has been derived from 16S rRNA and metagenomics, which have provided information for the genomic study of these microorganisms (many of which have proven difficult to isolate and culture). The human microbiota is estimated to contain 10–100 trillion microbial cells, and the intestinal microbiota accounts for the largest and most diverse population [Qin et al. 2010].
It has been estimated that the gut contains 1100 prevalent species and at least 160 species per individual [Qin et al. 2010]. The human microbiome project revealed variation in the composition of the human gut microbiota between sex, race/ethnicity, and age [Hollister et al. 2014]. Diet is also related to gut microbiota composition [David et al. 2014]. Large-scale, phylum-wide differences have been found between the fecal microbiota of children consuming predominantly plant carbohydrate-based diets compared with those consuming the typical Western diet [De Filippo et al. 2010]. The microbiota also varies in composition depending on the location along the gastrointestinal tract (esophageal, gastric, proximal intestinal, or distal intestinal) and axial depth (mucosal versus luminal) [Eckburg et al. 2005; Nava et al. 2011]. Despite the intra- and interindividual diversity of the microbiome, there seems to be conservation of core functions that are involved in carbohydrate, protein, and amino-acid metabolism [Harrell et al. 2012; Hollister et al. 2014]. Also, core organisms have been identified in the human gastrointestinal microbiota [Tap et al. 2009].
There has been increasing interest in understanding the role of the human gut microbiome to capitalize on the therapeutic potential of its manipulation. Metagenomic studies have indicated that the richness and diversity of bacterial species in the human gut may be an indicator of health [Gill et al. 2006; Wang et al. 2009; Claesson et al. 2012]. In addition, the presence of particular groups of bacteria may provide health advantages. Certain microbes have been shown to enhance metabolism, the immune system, cancer resistance, endocrine signaling, and brain function. Some bacterial taxa associated with these benefits include: Bacteroides, Bifidobacterium, Clostridium clusters XIVa/IV, and Lactobacillus [Mills, 2011; Tremaroli and Bäckhed, 2012; Hollister et al. 2014]. The gut microbiome appears to remain relatively resilient over time, however, antibiotic use, travel, and illness can lead to its variation. Studies have demonstrated the ability of the gut microbiome to recover from insults, however, continued perturbations can lead to a loss of this resilience and may have implications for human health [Dethlefsen and Relman, 2011; Jalanka-Tuovinen et al. 2011; Cho and Blaser, 2012; Hollister et al. 2014].
Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) is the administration of a solution of fecal matter from a donor into the intestinal tract of a recipient in order to directly change the recipient’s microbial composition and confer a health benefit [Bakken et al. 2011; Smits et al. 2013]. The first known description of the use of feces as therapy was described by Ge Hong in fourth-century China for the treatment of a variety of conditions including diarrhea [Zhang et al. 2012]. In 1958, Eiseman and colleagues described the use of fecal enemas as a treatment for pseudomembranous colitis, marking the introduction of FMT into mainstream medicine [Eiseman et al. 1958]. The process usually involves first selecting a donor without a family history of autoimmune, metabolic, and malignant diseases and screening for any potential pathogens. The feces are then prepared by mixing with water or normal saline, followed by a filtration step to remove any particulate matter. The mixture can be administered through a nasogastric tube, nasojejunal tube, esophagogastroduodenoscopy, colonoscopy, or retention enema. Most clinical experience with FMT has been derived from treating recurrent or refractory Clostridium difficile infection (CDI) [Smits et al. 2013]. This paper will review the therapeutic potential of FMT in treating CDI, inflammatory bowel disease (IBD), and several other conditions.
Clostridium difficile infection
C. difficile is a Gram-positive, anaerobic, spore-forming, and toxin-producing bacillus. It is a leading nosocomial infection, and is becoming an increasingly virulent and severe epidemic [Miller et al. 2011]. In 2008, it was estimated that the economic cost associated with CDI was greater than US$4.8 billion in acute-care facilities [Bartlett and Gerding, 2008; Kelly and Lamont, 2008; Dubberke and Olsen, 2012]. CDI causes symptoms ranging from mild watery diarrhea to potentially lethal conditions such as pseudomembranous colitis [Bauer et al. 2011]. The standard treatment is a course of vancomycin or metronidazole, however, a significant portion of patients with CDI goes on to develop recurrent CDI (rCDI), which can lead to significant morbidity and mortality. Antibiotics have been implicated in the pathogenesis of CDI. Antibiotic use can lead to dysbiosis (microbial imbalance), and this allows C. difficile to flourish [Kelly et al. 2015]. For rCDI, fidaxomicin may be used, as it has been shown to have a similar cure rate to vancomycin, but reportedly has lower recurrence rates (presumably due to a narrower spectrum of antimicrobial activity and more selective targeting of C. difficile) [Louie et al. 2011].
FMT has been used to treat successfully rCDI, and although mostly based on case series, there has been an average 87–90% cure rate (defined by resolution of diarrhea) for the over 500 cases that have been reported in the literature to date [Kassam et al. 2013; Van Nood et al. 2013; Cammarota et al. 2014; Rossen et al. 2015]. Moreover, FMT leads to restoration of gut microbial communities in a sustained manner [Khoruts et al. 2010; Hamilton et al. 2013]. A randomized control trial showed that duodenal infusion of donor feces for rCDI had a cure rate of 81% versus a cure rate of 31% for patients treated with the standard course of oral vancomycin [Van Nood et al. 2013]. The literature to date supports FMT for use in CDI as a safe, well-tolerated, effective treatment with few adverse events [Kelly et al. 2015].
The efficacy of FMT for treating rCDI has been recognized by clinicians, and in 2011 a workgroup was formed to create a consensus on indications for FMT. In summary, it was concluded that FMT should be considered for recurrent or relapsing CDI when there is failure to respond to conventional antibiotic therapy or hospitalization and significant morbidity is involved. For moderate CDI, FMT is indicated when there is no response to standard therapy for at least 1 week. For severe CDI, it is indicated when there is no treatment response after appropriate maximal therapy for 48 h [Bakken et al. 2011]. FMT is also supported by the 2013 American College of Gastroenterology C. difficile-treatment guidelines as a therapeutic alternative for rCDI that has not responded to a pulse/tapered regimen of vancomycin [Surawicz et al. 2013]. Moreover, a small study from the Massachusetts General Hospital has indicated that administering 15 frozen pills with donor feces over 2 consecutive days in patients with relapsing CDI has a response rate of 90%, which is comparable with the previously reported cure rates through other routes of administration. Provided a patient has no dysphagia and no risk factors for aspiration, this approach has the potential to provide an even more cost-effective method of administering FMT that may present fewer procedural complications [Youngster et al. 2014]. Another alternative is the use of frozen donor feces administered by colonoscopy, which has also been shown to be effective [Hamilton et al. 2012].
Although FMT has been used to treat successfully CDI for some time now, the mechanisms by which it exerts its therapeutic effects have not yet been fully elucidated. However, the most likely scenario is competitive exclusion of the pathogen with the microbiota outcompeting C. difficile for nutrients and creating an environment that is unfavorable for its growth [Kelly et al. 2015]. Metagenomic analyses have shown that there is reduced richness and diversity in the gut microbiome of CDI patients compared with healthy controls. This dysbiosis is also characterized by increased levels of Proteobacteria species, and reduced levels of Firmicutes and Bacteroidetes species [Shahinas et al. 2012]. An important factor in the success of FMT is restoring the communities of Firmicutes and Bacteroidetes and decreasing Proteobacteria to favor out-competing C. difficile [Shahinas et al. 2012]. The efficacy of FMT for rCDI through competitive exclusion is thought to occur in part through the modulation of bile-salt metabolism, which affects C. difficile spore germination. Primary bile acids have been shown to stimulate germination of spores, while secondary bile acids such as lithocholate serve as potent inhibitors for spore germination. Several groups of gut microbes found in healthy microbiota (primarily within the Lachnospiraceae and Ruminococcaceae families, otherwise known as Clostridium clusters XIVa and IV, respectively) possess 7α-dehydroxylation activity, which allows them to convert primary bile acids to secondary bile acids [Stellwag and Hylemon, 1978; Hirano et al. 1981; Takamine and Imamura, 1995; Doerner et al. 1997; Kitahara et al. 2000; Ridlon et al. 2006; Sorg and Sonenshein, 2008]. In accordance with this hypothesis, pre-FMT feces of rCDI patients were extremely low in secondary bile acids, but high in primary bile acids. After FMT, this trend was reversed, and resembled that of healthy stools from patients without CDI [Weingarden et al. 2014]. Another important mechanism was recently described that involves sialic-acid metabolism. Ng and colleagues demonstrated through mouse models that antibiotic treatment disrupts the endogenous microbial community and leads to increased free mucosal sialic acid (a carbohydrate energy source for C. difficile), ultimately leading to C. difficile-colony expansion in the gut [Ng et al. 2013]. Therefore, FMT may also exert its therapeutic effect by increasing sialic-acid utilization by commensal bacteria, thus depriving C. difficile of a vital energy source.
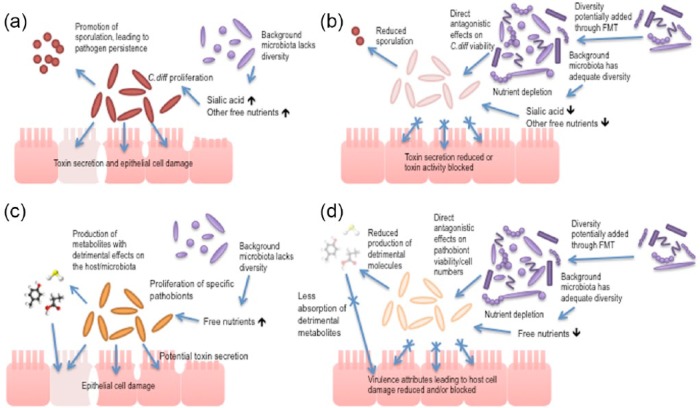
As well as the mechanisms described above, FMT likely exerts its therapeutic effect through other as-yet undetermined contributing mechanisms. These could include, for example, protease activity inactivating secreted C. difficile toxins, stimulation of host-cell defenses through release of small molecules such as short-chain fatty acids, and direct activity against C. difficile viability through bacteriocin-like mechanisms [Rea et al. 2010]. These potentially protective mechanisms of the human gut microbiota against C. difficile remain an area of active ongoing research and are summarized in Figure 1.
Figure 1.
(a), (b) Schematic to indicate the events that contribute to Clostridium difficile infection: (a) the contributions of the pathogen to disease driven by reduced gut microbiota diversity; (b) how these contributions are minimized when a more diverse microbiota is present. (c), (d) Schematic to indicate the events that contribute to the proliferation of pathobionts (resident bacteria that can contribute to disease pathology under the appropriate altered environmental conditions) in the gut microbiota. The potential contribution of the gut microbiota, and the reduction of diversity in the microbiota in particular, is highlighted as a driver for disease to draw a comparison against C. difficile infection, using C. difficile infection as a model for disease caused by reduction in host gut-microbiota diversity.
Inflammatory bowel disease
IBD is an intestinal disorder that includes ulcerative colitis (UC) and Crohn’s disease (CD). IBD is characterized by chronic inflammation of the gastrointestinal tract, and has a cyclic nature of disease progression and remission. During periods of disease activity (colloquially termed ‘flares’), patients may present with diarrhea, nausea, weight loss, loss of appetite, fever, and abdominal pain. The precise pathophysiology is unknown, but the cause is multifactorial, due to imbalances in the intestinal microbiota, gut epithelium, and immune system in genetically susceptible individuals. IBD is hypothesized to occur due to continuous inappropriate antigenic stimulation of gut mucosa-associated lymphatic tissue by commensal microbes [Loftus, 2004; Zhang and Li, 2014]. Dysbiosis of the gut has recently been considered as a possible pathologic contributor to IBD development. This idea is supported by observations that antibiotics such as amoxicillin/clavulanic acid and rifaximin can reduce intestinal inflammation and induce remission in some patients [Casellas et al. 1998; Sartor, 2004, 2008; Khan et al. 2011].
Metagenomic and metabolomics studies have characterized the IBD microbiota, and have found an overall reduced bacterial diversity, with specifically reduced members of the Bacteroidetes phylum and the Lachnospiraceae group within the Firmicutes phylum and an increase in Proteobacteria and Actinobacteria [Ott et al. 2004; Frank et al. 2007, 2011; Morgan et al. 2012]. Biopsy specimens from patients with CD were found to have a reduced population of the Clostridium cluster IV species, Faecalibacterium prausnitzii. F. prausnitzii is associated with anti-inflammatory properties in patients with CD, and increased levels of the bacterium are associated with maintenance of clinical remission in UC [Sokol et al. 2008, 2009; Willing et al. 2009; Varela et al. 2013]. Overall, the IBD microbiome was found to be inflammation promoting, with indications of increased oxidative stress, increased type II toxin secretion, and increased virulence-related bacterial genes [Erickson et al. 2012; Morgan et al. 2012]. Recently, it was shown that the transplantation of fecal ecosystems from patients with UC to germ-free mice increased sensitivity to dextran sodium sulfate-induced colitis, thus supporting the use of microbiota modification for the treatment of UC [Natividad et al. 2015].
The evidence that links gut microbial dysbiosis with IBD has led to the exploration of FMT as therapy for the disease [Damman et al. 2012]. A recent systematic review and meta-analysis looked at 18 studies including 122 patients with IBD treated with FMT, and found overall clinical remission rates of 36.2% (after case series were removed to control for publication bias). Subgroup analyses showed that the clinical remission rate in UC patients was 22%, whereas younger patients (aged 7–20 years) had a rate of 64.1%, and patients with CD had a rate of 60.5% [Colman and Rubin, 2014]. It appears that FMT may be more effective for CD and in younger patients than for UC infection, however it is difficult to draw definitive conclusions due to the small sample sizes, short follow-up times, and heterogeneous results [Kelly et al. 2015]. Recently, two randomized controlled trials exploring the use of FMT for treatment were published, with mixed results. The first study enrolled 75 patients with active UC and randomized them to weekly FMT or water enema for 6 weeks, and found remission (defined by Mayo score < 3 and complete mucosal healing) in 24% of patients treated with FMT compared with 5% treated with water control [Moayyedi et al. 2015]. The other study randomized 50 patients with mild to moderately active UC to donor or autologous FMT via nasoduodenal tube, which were administered once at the start of the trial and again 3 weeks later. Of the 37 patients that completed follow up, there was no difference in clinical and endoscopic remission between the two groups [Rossen et al. 2015]. These differing results may be due to differences in routes of administration, stool donors, dosing schedules, or concomitant therapies. In addition, the study by Rossen and colleagues may have been too underpowered to detect differences between the two groups [Kelly et al. 2015]. In the trial by Moayyedi and colleagues, the patients that benefitted most from FMT were those with a recent history of disease onset [Moayyedi et al. 2015]. This may indicate that FMT may be useful only in certain subsets of patients with UC.
Although no serious adverse events were noted during the short-term follow up of the IBD patients treated with FMT, some were reported to have developed fevers, chills, bloating, flatulence, vomiting, diarrhea, and abdominal tenderness [Suskind et al. 2015]. Also, there have been some reports of patients’ conditions worsening after FMT [Angelberger et al. 2013; De Leon et al. 2013]. Therefore, FMT should be used with caution until more high-quality, adequately powered trials assessing its efficacy in IBD are completed. However, it is clear that FMT is not as effective in IBD as it is in CDI (which has high cure rates regardless of method), and this is probably due to the multifactorial pathophysiology of IBD [Kelly et al. 2015].
Obesity and metabolic syndrome
Obesity is a disorder characterized by excessive adipose tissue deposition. Metabolic syndrome is characterized by a constellation of signs such as central obesity, hypertension, dyslipidemia, and hyperglycemia that increases one’s risk for developing heart disease and diabetes mellitus [Hoffman et al. 2015]. Recent studies indicate that the gut microbiota may be involved in the pathophysiology of obesity [Cho et al. 2012; Liou et al. 2013]. Metagenomic studies characterized the gut microbiome in lean and obese individuals, and reported marked differences between the two. The obese gut microbiota of the mice studied showed an increase in the Firmicutes to Bacteroidetes ratio, and had an increased capacity for energy extraction from dietary intake [Ley et al. 2006; Turnbaugh et al. 2006]. Moreover, Turnbaugh and colleagues showed that the colonization of germ-free mice with the obese microbiota resulted in a significantly greater increase in adiposity than those transplanted with the lean microbiota [Turnbaugh et al. 2006]. There was a lower prevalence of obesity amongst those with high gene counts (a measure of the richness of the gut microbiota). Obese individuals were found to have a relative abundance of genes involved in hydrogen and methane production, and a relative decrease in genes associated with hydrogen sulfide production [Le Chatelier et al. 2013]. Transfer of the gut microbiota from human twins discordant for obesity into germ-free mice led to greater adiposity and body mass in the mice transplanted with the obese microbiota. Moreover, when the obese-transplanted mice were co-housed with the lean-transplanted mice, the obese-transplanted mice were protected from developing the increased adiposity and body mass. This was found to occur through coprophagy and was associated with transfer of the lean microbiota (and Bacteroidetes, in particular) into the obese-transplanted mouse. The obese mice had less short-chain, fatty-acid oxidation, more branched-chain, amino-acid metabolism, and more bile-acid transformation than their lean counterparts [Ridaura et al. 2013]. The altered microbiota found in obese individuals may be predisposing them to obesity through increased energy extraction, or possibly through an interaction with the gut–brain access leading to decreased energy output or through influencing satiety [Kelly et al. 2015]. Recently, a small double-blind, randomized, controlled study found that fecal transplants from lean to obese (with metabolic syndrome) individuals resulted in improved insulin sensitivity, increased gut-microbial diversity, and increased butyrate-producing bacteria (Roseburia intestinalis) in the obese recipients [Vrieze et al. 2012]. This study demonstrates a proof of principle for the future study of FMT for the treatment of obesity, metabolic syndrome, and diabetes mellitus. Conversely, the potential of the gut microbiota to affect weight gain has led to the proposal that the body mass index of the donor may need to be taken into consideration when choosing candidate donors for FMT [Alang and Kelly, 2015].
Functional gastrointestinal disorders
Functional gastrointestinal disorders (FGID) are the most commonly diagnosed gastrointestinal disease in the Western hemisphere [Koloski et al. 2002]. They are characterized by the presence of gastrointestinal symptoms with the absence of any identifiable anatomic or biochemical abnormalities. Irritable bowel syndrome (IBS) is the most prevalent form of FGID, and affects 10–15% of the population and 20% of the North American population. IBS has a deleterious impact on a patient’s quality of life and places an economic burden on the healthcare system. There are four subtypes of IBS, based on the dominant symptoms experienced by the patient. IBS-D is diarrhea-predominant, IBS-C is constipation predominant, IBS-M is mixed diarrhea and constipation, and IBS-U is for those who are unsubtyped [Yao et al. 2012]. The pathophysiology is not well defined, but involves visceral hypersensitivity, altered barrier function, altered gastrointestinal motility, and an altered gut–brain axis. These changes may be related to changes in the gut microbiota [Pinn et al. 2015]. There have been small, limited case series published demonstrating the use of FMT to treat FGID. One study administered FMT to 45 patients with chronic constipation via colonoscopy and a subsequent retention enema, and found 89% of patients to have immediate symptom relief whilst 60% sustained benefit at 9–19 months [Andrews et al. 1995]. Another study administered FMT to 13 patients with IBS (9 with IBS-D, 3 with IBS-C, 1 with IBS-M) via esophagogastroduodenoscopy and found 70% had symptom relief at 6–18 months [Pinn et al. 2013]. It appears that FMT may have a therapeutic effect for the treatment of FGID, however, conclusions cannot be made because the available data are extremely limited and susceptible to bias. Well-designed trials should be pursued to determine whether there is indeed a link between the gut microbiota and FGID.
Safety
As mentioned previously, most clinical experience with FMT has come from its use in treating rCDI. There has been rapid uptake of FMT into medical treatment due to its reported efficacy for rCDI, however, most data are derived from case series. To date, long-term, follow-up studies (3–68 months post-FMT, average 17 months post-FMT) have found FMT to be relatively free of adverse effects [Brandt et al. 2012]. The only randomized control trial published to date studying FMT for treatment of rCDI found that of the 16 patients treated, 15 experienced diarrhea, 5 had abdominal cramping, 3 had belching, and 1 had nausea. These effects were not observed in the control group that received only a bowel lavage, however, the effects were all self-limiting and resolved within 3 h post-FMT [Van Nood et al. 2013]. In a pilot study, FMT was administered to four CDI patients via nasoduodenal tubes, and three patients experienced adverse effects including fever and abdominal tenderness that resolved within 2 days post-FMT [Vermeire et al. 2012]. Aside from these minor self-limiting adverse events, 3 of the 317 patients treated for CDI by FMT experienced serious adverse events possibly related to FMT, that is, upper gastrointestinal tract bleeding, peritonitis, and enteritis [Gough et al. 2011; Kassam et al. 2013]. There is also a report of a superficial mucosal tear that occurred after FMT colonoscopy [Kelly et al. 2015]. Two cases of norovirus infection and one case of Escherichia coli bacteremia were reported post-FMT, but were concluded to be unrelated to the treatment [Schwartz et al. 2013; Quera et al. 2014]. A multicenter retrospective study assessed immunosuppressed patients treated with FMT for CDI (who may be considered at risk of infection) and found no infections occurring due to FMT. However, one IBD patient died due to aspiration during sedation for FMT colonoscopy [Kelly et al. 2014]. In terms of long-term adverse effects of FMT, there exists a theoretical possibility of unrecognized infectious disease transfer or stimulation of chronic disease (e.g. obesity, diabetes, atherosclerosis, etc.) development due to alteration of the gut microbiota. However, long-term, follow-up studies are necessary to assess these risks. Advances in FMT delivery may reduce procedural complications in the future.
Regulation/policy
Health Canada released an interim policy regarding the regulation of FMT, which it currently regulates as a ‘new biologic drug’. It specifies that FMT may be used to treat CDI refractory to conventional treatments, provided the healthcare provider receives patient consent, and prepares the FMT from a single donor known to either the patient or provider. The donor stool must be screened for potential pathogens prior to administration. However, this provisional interpretation only applies to treatment of refractory CDI. To treat any other conditions with FMT, current regulations state that healthcare providers must first complete a clinical trial application including a risk–benefit analysis and on-site evaluation of techniques and facilities [Health Canada, 2015]. This policy limits the use of FMT for other conditions, given the paucity of high-quality clinical trials.
Similarly, the US Food and Drug Administration (FDA) considers stool as a biological product and drug, and mandates physicians to obtain an investigational new drug application (IND) to administer FMT. Recently, the FDA has stated that it will allow physicians to use their own discretion in administering FMT to patients with CDI that does not respond to conventional therapies (without an IND). The physician must obtain informed consent, explain the risks and benefits of the procedure, and explain that it is an investigational therapy. Also, the donor must be known to the patient or healthcare provider, and the stool must be screened for pathogens [FDA, 2014]. Despite this progress in public policy regarding FMT in the West, the European Medicines Agency has not yet regulated FMT for CDI, nor have regulatory bodies within China or Australia [Van Nood et al. 2014; Kelly et al. 2015].
Future directions
There is increasing uptake and acceptance for the therapeutic use of FMT, partially due to its perception as a ‘natural’ treatment, and its relatively inexpensive implementation [Kelly et al. 2015]. Despite this perception, there are fears of the infectious potential of the therapy. Few long-term studies have been undertaken to assess the safety of FMT and the theoretical risk remains. This has led research groups to explore the use of ‘synthetic stool’ products with defined bacterial populations to ameliorate such concerns [Petrof et al. 2013]. There are many efforts currently underway to explore a role for the gut microbiota in the pathophysiology of many other conditions, including necrotizing enterocolitis, liver disease, colorectal cancer, esophageal and gastric adenocarcinoma, and autism [Dicksved et al. 2009; Yang et al. 2009; Claud et al. 2013; Couturier-Maillard et al. 2013; Llopis et al. 2014; Buie, 2015]. In addition, observations from patients treated with FMT for functional bowel disorders have noted improvement in seemingly unrelated comorbidities, revealing a possible role for gut-microbiota modification in many other conditions [Borody and Khoruts, 2012]. Depending on the results of these investigations, FMT may be considered a potentially useful therapy for additional conditions in the future. Ongoing clinical trials will continue to provide insight into this growing field.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Shaan Gupta, Department of Medicine, Gastrointestinal Diseases Research Unit, Queen’s University, Kingston, ON, Canada.
Emma Allen-Vercoe, Department of Molecular and Cellular Biology, University of Guelph, Guelph, ON, Canada.
Elaine O. Petrof, Department of Medicine, Queen’s University, Gastrointestinal Diseases Research Unit Wing, Kingston General Hospital, 76 Stuart Street, Kingston, ON K7L 2V7, Canada.
References
- Alang N., Kelly C. (2015) Weight gain after fecal microbiota transplantation. Open Forum Infect Dis 2 DOI: 10.1093/ofid/ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P., Borody T., Shortis N. (1995) Bacteriotherapy for chronic constipation – long term follow up. Gastroenterology 108: A563. [Google Scholar]
- Angelberger S., Reinisch W., Makristathis A., Lichtenberger C., Dejaco C., Papay P., et al. (2013) Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol 108: 1620–1630. [DOI] [PubMed] [Google Scholar]
- Bakken J., Borody T., Brandt L., Brill J., Demarco D., Franzos M., et al. (2011) Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 9: 1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J., Gerding D. (2008) Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis 46(Suppl. 1): S12–S18. [DOI] [PubMed] [Google Scholar]
- Bauer M., Notermans D., Van Benthem B., Brazier J., Wilcox M., Rupnik M., et al. (2011) Clostridium difficile infection in Europe: a hospital-based survey. Lancet 377: 63–73. [DOI] [PubMed] [Google Scholar]
- Borody T., Khoruts A. (2012) Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 9: 88–96. [DOI] [PubMed] [Google Scholar]
- Brandt L., Aroniadis O., Mellow M., Kanatzar A., Kelly C., Park T., et al. (2012) Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 107: 1079–1087. [DOI] [PubMed] [Google Scholar]
- Buie T. (2015) Potential etiologic factors of microbiome disruption in autism. Clin Ther 37: 976–983. [DOI] [PubMed] [Google Scholar]
- Cammarota G., Ianiro G., Gasbarrini A. (2014) Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol 48: 693–702. [DOI] [PubMed] [Google Scholar]
- Casellas F., Borruel N., Papo M., Guarner F., Antolín M., Videla S., et al. (1998) Antiinflammatory effects of enterically coated amoxicillin-clavulanic acid in active ulcerative colitis. Inflamm Bowel Dis 4: 1–5. [DOI] [PubMed] [Google Scholar]
- Cho I., Blaser M. (2012) The human microbiome: at the interface of health and disease. Nat Rev Genet 13: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I., Yamanishi S., Cox L., Methé B., Zavadil J., Li K., et al. (2012) Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson M., Jeffery I., Conde S., Power S., O’Connor E., Cusack S., et al. (2012) Gut microbiota composition correlates with diet and health in the elderly. Nature 488: 178–184. [DOI] [PubMed] [Google Scholar]
- Claud E., Keegan K., Brulc J., Lu L., Bartels D., Glass E., et al. (2013) Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome 1: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R., Rubin D. (2014) Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 8: 1569–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier-Maillard A., Secher T., Rehman A., Normand S., De Arcangelis A., Haesler R., et al. (2013) NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest 123: 700–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damman C., Miller S., Surawicz C., Zisman T. (2012) The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol 107: 1452–1459. [DOI] [PubMed] [Google Scholar]
- David L., Maurice C., Carmody R., Gootenberg D., Button J., Wolfe B., et al. (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J., Massart S., et al. (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and Rural Africa. Proc Natl Acad Sci U S A 107: 14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon L., Watson J., Kelly C. (2013) Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol 11: 1036–1038. [DOI] [PubMed] [Google Scholar]
- Dethlefsen L., Relman D. (2011) Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 108(Suppl. 1): 4554–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicksved J., Lindberg M., Rosenquist M., Enroth H., Jansson J., Engstrand L. (2009) Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. J Med Microbiol 58: 509–516. [DOI] [PubMed] [Google Scholar]
- Doerner K., Takamine F., Lavoie C., Mallonee D., Hylemon P. (1997) Assessment of fecal bacteria with bile acid 7 alpha-dehydroxylating activity for the presence of bai-like genes. Appl Environ Microbiol 63: 1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubberke E., Olsen M. (2012) Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 55(Suppl. 2): S88–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg P., Bik E., Bernstein C., Purdom E., Dethlefsen L., Sargent M., et al. (2005) Diversity of the human intestinal microbial flora. Science 308: 1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiseman B., Silen W., Bascom G., Kauvar A. (1958) Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44: 854–859. [PubMed] [Google Scholar]
- Erickson A., Cantarel B., Lamendella R., Darzi Y., Mongodin E., Pan C., et al. (2012) Integrated metagenomics/metaproteomics reveals human host-microbiota signatures of Crohn’s disease. PLoS One 7: e49138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. (2014) Draft Guidance for Industry: Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. Silver Spring, MD: US Food and Drug Administration. [Google Scholar]
- Frank D., Robertson C., Hamm C., Kpadeh Z., Zhang T., Chen H., et al. (2011) Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis 17: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D., St Amand A., Feldman R., Boedeker E., Harpaz N., Pace N. (2007) Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 104: 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S., Pop M., Deboy R., Eckburg P., Turnbaugh P., Samuel B., et al. (2006) Metagenomic analysis of the human distal gut microbiome. Science 312: 1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough E., Shaikh H., Manges A. (2011) Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 53: 994–1002. [DOI] [PubMed] [Google Scholar]
- Hamilton M., Weingarden A., Sadowsky M., Khoruts A. (2012) Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol 107: 761–767. [DOI] [PubMed] [Google Scholar]
- Hamilton M., Weingarden A., Unno T., Khoruts A., Sadowsky M. (2013) High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes 4: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell L., Wang Y., Antonopoulos D., Young V., Lichtenstein L., Huang Y., et al. (2012) Standard colonic lavage alters the natural state of mucosal-associated microbiota in the human colon. PLoS One 7: e32545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. (2015) Guidance Document: Fecal Microbiota Therapy Used in the Treatment of Clostridium difficile Infections. Ottawa, ON: Health Canada. [Google Scholar]
- Hirano S., Nakama R., Tamaki M., Masuda N., Oda H. (1981) Isolation and characterization of thirteen intestinal microorganisms capable of 7 alpha-dehydroxylating bile acids. Appl Environ Microbiol 41: 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E., Vonwald T., Hansen K. (2015) The metabolic syndrome. S D Med Spec. No.: 24–28. [PubMed] [Google Scholar]
- Hollister E., Gao C., Versalovic J. (2014) Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology 146: 1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalanka-Tuovinen J., Salonen A., Nikkilä J., Immonen O., Kekkonen R., Lahti L., et al. (2011) Intestinal microbiota in healthy adults: temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS One 6: e23035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassam Z., Lee C., Yuan Y., Hunt R. (2013) Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol 108: 500–508. [DOI] [PubMed] [Google Scholar]
- Kelly C., Ihunnah C., Fischer M., Khoruts A., Surawicz C., Afzali A., et al. (2014) Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 109: 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C., Kahn S., Kashyap P., Laine L., Rubin D., Atreja A., et al. (2015) Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 149: 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C., Lamont J. (2008) Clostridium difficile – more difficult than ever. N Engl J Med 359: 1932–1940. [DOI] [PubMed] [Google Scholar]
- Khan K., Ullman T., Ford A., Abreu M., Abadir A., Marshall J., et al. (2011) Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 106: 661–673. [DOI] [PubMed] [Google Scholar]
- Khoruts A., Dicksved J., Jansson J., Sadowsky M. (2010) Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol 44: 354–360. [DOI] [PubMed] [Google Scholar]
- Kitahara M., Takamine F., Imamura T., Benno Y. (2000) Assignment of Eubacterium sp. vpi 12708 and related strains with high bile acid 7alpha-dehydroxylating activity to Clostridium scindens and proposal of Clostridium hylemonae sp. nov., isolated from human faeces. Int J Syst Evol Microbiol 50: 971–978. [DOI] [PubMed] [Google Scholar]
- Koloski N., Talley N., Boyce P. (2002) Epidemiology and health care seeking in the functional GI disorders: a population-based study. Am J Gastroenterol 97: 2290–2299. [DOI] [PubMed] [Google Scholar]
- Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G., et al. (2013) Richness of human gut microbiome correlates with metabolic markers. Nature 500: 541–546. [DOI] [PubMed] [Google Scholar]
- Ley R., Turnbaugh P., Klein S., Gordon J. (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- Liou A., Paziuk M., Luevano J., Machineni S., Turnbaugh P., Kaplan L. (2013) Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 5: 178ra141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopis M., Cassard-Doulcier A., Boschat L., Bruneau A., Cailleux F., Rabot S., et al. (2014) Intestinal dysbiosis explains inter-individual differences in susceptibility to alcoholic liver disease. Abstract presented at the International Liver Congress. J Hepatol 60(Suppl.): S1–S598. [Google Scholar]
- Loftus E. (2004) Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 126: 1504–1517. [DOI] [PubMed] [Google Scholar]
- Louie T., Miller M., Mullane K., Weiss K., Lentnek A., Golan Y., et al. (2011) Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 364: 422–431. [DOI] [PubMed] [Google Scholar]
- Miller B., Chen L., Sexton D., Anderson D. (2011) Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol 32: 387–390. [DOI] [PubMed] [Google Scholar]
- Mills K. (2011) TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol 11: 807–822. [DOI] [PubMed] [Google Scholar]
- Moayyedi P., Surette M., Kim P., Libertucci J., Wolfe M., Onischi C., et al. (2015) Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized, controlled trial. Gastroenterology 149: 102–109. [DOI] [PubMed] [Google Scholar]
- Morgan X., Tickle T., Sokol H., Gevers D., Devaney K., Ward D., et al. (2012) Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13: R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad J., Pinto-Sanchez M., Galipeau H., Jury J., Jordana M., Reinisch W., et al. (2015) Ecobiotherapy rich in firmicutes decreases susceptibility to colitis in a humanized gnotobiotic mouse model. Inflamm Bowel Dis 21: 1883–1893. [DOI] [PubMed] [Google Scholar]
- Nava G., Friedrichsen H., Stappenbeck T. (2011) Spatial organization of intestinal microbiota in the mouse ascending colon. ISME J 5: 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K., Ferreyra J., Higginbottom S., Lynch J., Kashyap P., Gopinath S., et al. (2013) Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502: 96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott S., Musfeldt M., Wenderoth D., Hampe J., Brant O., Fölsch U., et al. (2004) Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 53: 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof E., Gloor G., Vanner S., Weese S., Carter D., Daigneault M., et al. (2013) Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘repopulating’ the gut. Microbiome 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinn D., Aroniadis O., Brandt L. (2013) Follow-up study of fecal microbiota transplantation (FMT) for the treatment of refractory irritable bowel syndrome (IBS). Presented at the American College of Gastroenterology Annual Meeting; San Diego, CA, abstract 2013: P1688. [Google Scholar]
- Pinn D., Aroniadis O., Brandt L. (2015) Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroenterol Motil 27: 19–29. [DOI] [PubMed] [Google Scholar]
- Qin J., Li R., Raes J., Arumugam M., Burgdorf K., Manichanh C., et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quera R., Espinoza R., Estay C., Rivera D. (2014) Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J Crohns Colitis 8: 252–253. [DOI] [PubMed] [Google Scholar]
- Rea M., Sit C., Clayton E., O’Connor P., Whittal R., Zheng J., et al. (2010) Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci U S A 107: 9352–9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura V., Faith J., Rey F., Cheng J., Duncan A., Kau A., et al. (2013) Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341: 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridlon J., Kang D., Hylemon P. (2006) Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47: 241–259. [DOI] [PubMed] [Google Scholar]
- Rossen N., Fuentes S., Van Der Spek M., Tijssen J., Hartman J., Duflou A., et al. (2015) Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 149: 110–118. [DOI] [PubMed] [Google Scholar]
- Rossen N., Macdonald J., De Vries E., D’Haens G., De Vos W., Zoetendal E., et al. (2015) Fecal microbiota transplantation as novel therapy in gastroenterology: a systematic review. World J Gastroenterol 21: 5359–5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R. (2008) Microbial influences in inflammatory bowel diseases. Gastroenterology 134: 577–594. [DOI] [PubMed] [Google Scholar]
- Sartor R. (2004) Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 126: 1620–1633. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Gluck M., Koon S. (2013) Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol 108: 1367. [DOI] [PubMed] [Google Scholar]
- Shahinas D., Silverman M., Sittler T., Chiu C., Kim P., Allen-Vercoe E., et al. (2012) Toward an understanding of changes in diversity associated with fecal microbiome transplantation based on 16s RRNA gene deep sequencing. MBio 3: e00338–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits L., Bouter K., De Vos W., Borody T., Nieuwdorp M. (2013) Therapeutic potential of fecal microbiota transplantation. Gastroenterology 145: 946–953. [DOI] [PubMed] [Google Scholar]
- Sokol H., Pigneur B., Watterlot L., Lakhdari O., Bermúdez-Humarán L., Gratadoux J., et al. (2008) Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 105: 16731–16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H., Seksik P., Furet J., Firmesse O., Nion-Larmurier I., Beaugerie L., et al. (2009) Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis 15: 1183–1189. [DOI] [PubMed] [Google Scholar]
- Sorg J., Sonenshein A. (2008) Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 190: 2505–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwag E., Hylemon P. (1978) Characterization of 7-alpha-dehydroxylase in Clostridium leptum. Am J Clin Nutr 31: S243–S247. [DOI] [PubMed] [Google Scholar]
- Surawicz C., Brandt L., Binion D., Ananthakrishnan A., Curry S., Gilligan P., et al. (2013) Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 108: 478–498. [DOI] [PubMed] [Google Scholar]
- Suskind D., Brittnacher M., Wahbeh G., Shaffer M., Hayden H., Qin X., et al. (2015) Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm Bowel Dis 21: 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamine F., Imamura T. (1995) Isolation and characterization of bile acid 7-dehydroxylating bacteria from human feces. Microbiol Immunol 39: 11–18. [DOI] [PubMed] [Google Scholar]
- Tap J., Mondot S., Levenez F., Pelletier E., Caron C., Furet J., et al. (2009) Towards the human intestinal microbiota phylogenetic core. Environ Microbiol 11: 2574–2584. [DOI] [PubMed] [Google Scholar]
- Tremaroli V., Bäckhed F. (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P., Ley R., Mahowald M., Magrini V., Mardis E., Gordon J. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- Van Nood E., Speelman P., Nieuwdorp M., Keller J. (2014) Fecal microbiota transplantation: facts and controversies. Curr Opin Gastroenterol 30: 34–39. [DOI] [PubMed] [Google Scholar]
- Van Nood E., Vrieze A., Nieuwdorp M., Fuentes S., Zoetendal E., De Vos W., et al. (2013) Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368: 407–415. [DOI] [PubMed] [Google Scholar]
- Varela E., Manichanh C., Gallart M., Torrejón A., Borruel N., Casellas F., et al. (2013) Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment Pharmacol Ther 38: 151–161. [DOI] [PubMed] [Google Scholar]
- Vermeire S., Joossens M., Verbeke K., Hildebrand F., Machiels K., Van Den Broeck K., et al. (2012) Sa1922 pilot study on the safety and efficacy of faecal microbiota transplantation in refractory Crohn’s disease. Gastroenterology 142: S360. [Google Scholar]
- Vrieze A., Van Nood E., Holleman F., Salojärvi J., Kootte R., Bartelsman J., et al. (2012) Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143: 913–916. [DOI] [PubMed] [Google Scholar]
- Wang Y., Hoenig J., Malin K., Qamar S., Petrof E., Sun J., et al. (2009) 16s rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 3: 944–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarden A., Chen C., Bobr A., Yao D., Lu Y., Nelson V., et al. (2014) Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol 306: G310–G319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing B., Halfvarson J., Dicksved J., Rosenquist M., Järnerot G., Engstrand L., et al. (2009) Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn’s disease. Inflamm Bowel Dis 15: 653–660. [DOI] [PubMed] [Google Scholar]
- Yang L., Lu X., Nossa C., Francois F., Peek R., Pei Z. (2009) Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 137: 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Yang Y., Cui L., Zhao K., Zhang Z., Peng L., et al. (2012) Subtypes of irritable bowel syndrome on Rome III criteria: a multicenter study. J Gastroenterol Hepatol 27: 760–765. [DOI] [PubMed] [Google Scholar]
- Youngster I., Russell G., Pindar C., Ziv-Baran T., Sauk J., Hohmann E. (2014) Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 312: 1772–1778. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li Y. (2014) Inflammatory bowel disease: pathogenesis. World J Gastroenterol 20: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Luo W., Shi Y., Fan Z., Ji G. (2012) Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol 107: 1755–1756. [DOI] [PubMed] [Google Scholar]