Abstract
Background:
Self-expandable metal stent (SEMS) placement is a minimally invasive treatment for palliation of malignant colorectal strictures and as a bridge to surgery. However, the use of SEMS for benign colorectal diseases is controversial. The purpose of this retrospective study is to evaluate the efficacy and safety of fully covered SEMS (FCSEMS) placement in postsurgical colorectal diseases.
Methods:
From 2008 to 2014, 29 patients with 32 FCSEMS deployment procedures were evaluated. The indications for stent placement were: 17 anastomotic strictures (3/17 presented complete closure of the anastomosis); four anastomotic leaks; seven strictures associated with anastomotic leak; and one rectum-vagina fistula.
Results:
Clinical success was achieved in 18 out of 29 patients (62.1%) being symptom-free at an average of 19 months. In the remaining 11 patients (37.9%), a different treatment was needed: four patients required multiple endoscopic dilations, 4 patients colostomy confection, one patient definitive ileostomy and three patients revisional surgery. The FCSEMS were kept in place for a mean period of 34 (range: 6–65) days. Major complications occurred in 12 out of 29 patients (41.4%) and consisted of stent migration. Minor complications included two cases of transient fever, eight cases of abdominal or rectal pain, and one case of tenesmus.
Conclusion:
FCSEMS are considered a possible therapeutic option for treatment of postsurgical strictures and leaks. However, their efficacy in guaranteeing long-term anastomotic patency and leak closure is moderate. A major complication is migration. The use of FCSEMS for colonic postsurgical pathologies should be carefully evaluated for each patient.
Keywords: self-expandable metal stent, colorectum, postsurgical stricture, leak, migration
Introduction
Self-expandable metal stents (SEMS) have been widely used in malignant colonic obstruction both as palliative treatment in inoperable metastatic diseases and as bridge to surgery in case of acute obstruction [Baron et al. 2002]. SEMS for acute colonic obstruction have been used successfully even in case of benign pathologies as a bridge to surgery avoiding emergency surgery [Geiger et al. 2008]. However, there are limited data regarding temporary deployment of a covered stent for strictures or leaks related to postsurgical colorectal pathologies [Suzuki et al. 2004].
Benign colorectal strictures have numerous causes including diverticular disease, ischemia, inflammatory bowel disease, radiation and postoperative stenosis. The most common cause is postoperative anastomotic stricture, which occurs in 5–20% of all cases [Luchtefeld et al. 1989], and mainly involves the distal extraperitoneal rectum [Dai et al. 2010].
Factors underlying stenosis formation are not yet fully understood. Postoperative leakage, sepsis, radiotherapy and relative ischemia with reduced blood supply seem to play an important role [Orsay et al. 1995].
Surgical management with ‘re-do’ anastomosis was once considered the standard treatment for benign colorectal strictures. However, technical difficulties due to extensive fibrosis and inflammation led to minimally invasive endoscopic techniques being explored. Nowadays hydrostatic balloon dilation is considered the first-line treatment for benign strictures with reported satisfactory results [Araujo and Costa, 2008]. However, restenosis following dilation is common and repeated procedures are often necessary [Johansson, 1996]. Therefore in case of refractory stricture the use of covered SEMS has been proposed.
Anastomotic leakage is a serious complication of colorectal surgery, being responsible for an increase in short- and long-term mortality and morbidity [Ptok et al. 2007]. Moreover, anastomotic leaks have a strong influence on patient quality of life [Brown et al. 2014]. Risk factors for anastomotic leaks are low rectal surgery, malnutrition, preoperative radiotherapy, stoma confection and male gender [Kang et al. 2013]. Leak management is often cumbersome and a definitive stoma is often mandatory as rescue therapy. Although different endoscopic treatments have been proposed for upper gastrointestinal (GI) leaks, few studies have applied endoscopic techniques (endo-sponge, Ovesco™) for leaks following colorectal surgery [Strangioa et al. 2015; Donatelli et al. 2013].
The aim of this retrospective study is to report the results of consecutive series of 29 patients undergoing 32 cases of fully covered SEMS (FCSEMS) deployment for clinical significant anastomotic stenosis (3 cases of complete anastomotic closure) and/or anastomotic leakage.
Materials and methods
This was a retrospective study that included all patients referred at our institutions for endoscopic treatment of symptomatic postsurgical anastomotic stricture or leak. From 2008 to 2014, a total of 29 patients (19 males and 10 females) with a median age of 60 years (range: 19–82) were treated by means of FCSEMS deployment. All patients gave written informed consent to colonic stent procedure and authorized the use of their personal information.
Data were collected in a prospectively maintained database and analyzed retrospectively.
Only patients with a history of previous colorectal surgery were included. All patients were referred for one of the following reasons: check endoscopy before temporary stoma closure; onset of subocclusive symptoms; or clinical suspicion of anastomotic leakage. A computerized tomography (CT) scan or barium enema was performed in all patients presenting with subocclusive symptoms or suspected leakage to confirm stenosis or leak. When necessary, surgical or radiologic drainage was performed before endoscopic insertion of a fully covered metal stent.
All procedures were carried out by expert endoscopists with the patients under conscious sedation with midazolam or deep sedation with propofol.
The procedures were carried out under fluoroscopic and endoscopic guidance with the patient in a supine position. A flexible scope CF130AI or GIFQ180 (OlympusTM, Tokyo, Japan) was used in all cases to reach the stricture/leak site. Before stent insertion, a water-soluble contrast agent was injected through the endoscope working channel to assess the location, the anatomy and the characteristics of the pathology such as the length and the degree of the stricture, and the evaluation of the leak (Figure 1a, b); biopsies were taken in all patients to rule out malignancy. Access across the lesion was gained using a 0.035 inch guidewire (RadiofocusTM plastic coated guidewire, Terumo, Tokyo, Japan; or JagwireTM Boston Scientific, Natick, MA, USA). Balloon dilation was not performed either before or after FCSEMS deployment to reduce the risk of intraprocedural perforation and stent migration. Deployed FCSEMS were the Niti-S enteral colonic covered stent (Tae Woong Medical, Gyeonggi-do, Korea) or the Hanarostent® (M.I.Tech Co. Ltd, Korea) with a diameter variable from 18 to 28 mm and a length from 6 to 15 cm. FCSEMS were delivered with an over the wire technique (OTW), choosing the dimensions of the stent according to leak site or stricture length (if present), degree, anatomy and distance from anal verge (Figure 2a, b, c, d).
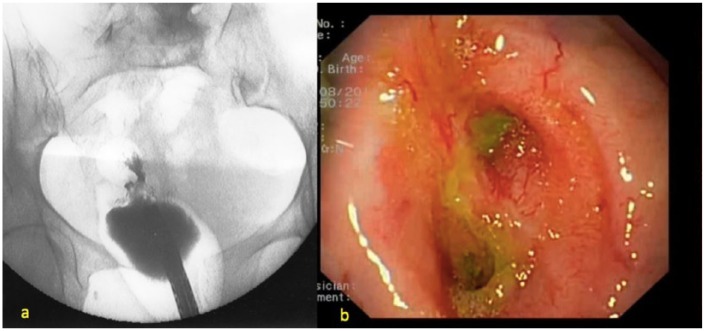
Figure 1.
(a) Contrast enema showing a rectal anastomotic severe stenosis coupled with leak. (b) Endoscopic view showing severe stenosis and leak’s orifice.
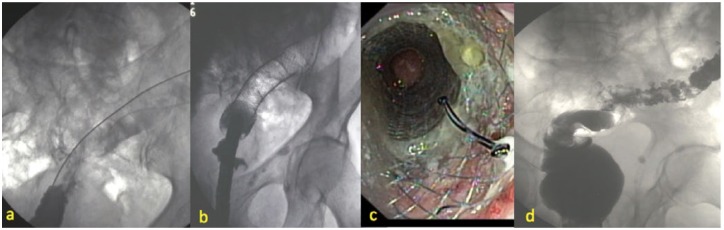
Figure 2.
(a) Positioning of the guidewire across the stenosis under fluoroscopic guidance. (b) Radiological view of FCSEMS before removal. Air filled colon above the FCSEMS is clearly visible. (c) Endoscopic view of FCSEMS before removal. (d) Contrast enema after FCSEMS removal. Residual diverticula may be seen.
FCSEMS, fully covered self-expanding metal stent.
As a general rule, longer stents were always delivered to assure a correct expansion above and below the lesion. After stent deployment, contrast fluoroscopy was performed to confirm stent efficacy and to rule out complications such as perforation.
Technical success was defined as a correctly positioned stent across the stenosis or across the leak demonstrated with a radiological image. Clinical success was defined as persistent relief from symptoms with good caliber of the anastomosis or watertight closure of the leak. Stent migration and perforation were considered as major complications, while self-limiting bleeding, abdominal pain, tenesmus and transient fever were labeled as minor complications. Procedural failure was considered any event which required performance of a different endoscopic technique or surgery.
A full liquid diet was resumed the same day as the procedure. Analgesic drugs (paracetamol 1000 mg up to three times a day) were administered on patient demand. All patients were discharged the day after. Early discharge was possible because the surgical/radiological drainages were inserted days before stent insertion.
A total of three out of 29 patients presented with complete closure of the anastomosis, thus not allowing the passage of a guidewire across the anastomosis. Before FCSEMS deployment, these patients underwent a combined radiological (per rectum) and endoscopic (per ileostomy) rendezvous technique to gain access across the anastomosis and to re-establish the colic continuity as previously described by our team [Donatelli et al. 2008].
All patients underwent endoscopic control 6 weeks after deployment or before in case of the reappearance of symptoms, with an average time of FCSEMS in place of 34 (range: 6–65) days (Figure 3).
Figure 3.
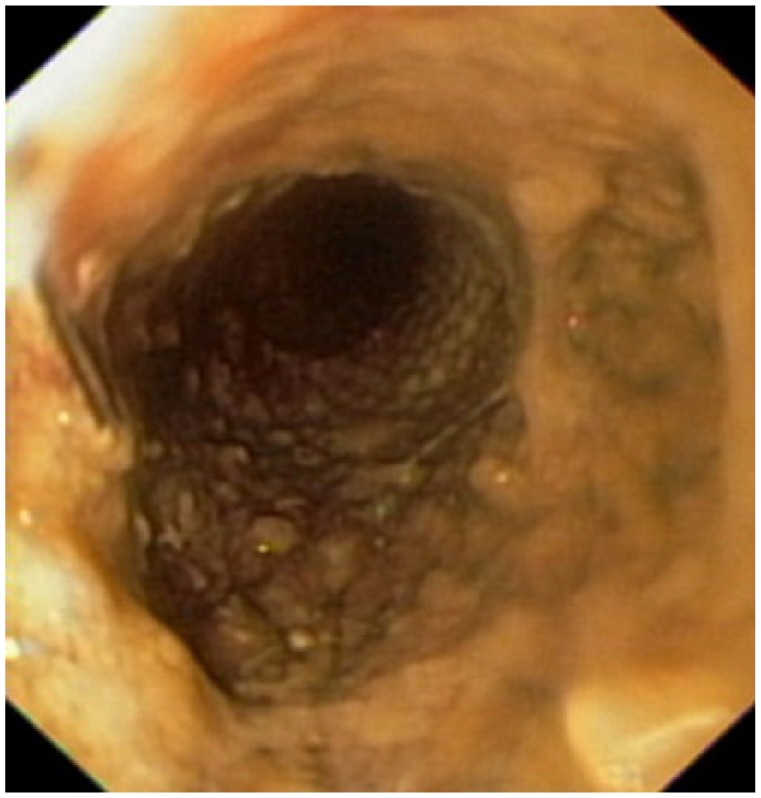
Endoscopic view of a partially obstructed FCSEMS before its removal at 65 days after deployment.
Patients with suspected complications (stent migration, etc.) underwent a plain abdominal X-ray or a CT scan.
Results
A total of 32 FCSEMS were inserted in 29 patients during the study period. Study population demographics, results and complications are listed in Table 1.
Table 1.
Study population demographics, results and complications.
| N | Sex | Age | Surgical procedure | Pathology | Main problem | Delay between surgery and FCSEMS | Preoperative radiotherapy | Drainage | Ileostomy/ colostomy | Stricture degree | Days of stenting | Complications | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 79 | Sigmoidectomy | Diverticula | Fistula | 35 days | – | – | – | SS | 21 | Migration | Healed |
| 2 | F | 70 | ARR | ADK | Stricture + fistula | 22 days | Radiotherapy | Drainage | – | SS | 7 (+30) | Migration after 3 days (repositioning) | Endoscopic dilations |
| 3 | M | 62 | Sigmoidectomy | Diverticula | Stricture | 87 days | – | – | I | SS | 35 | – | Healed |
| 4 | M | 76 | Left hemicolectomy | ADK | Stricture + fistula | 21 days | – | Drainage | – | CS | 65 | – | Healed |
| 5 | M | 69 | Hartmann | Diverticula | Complete stenosis | 74 days | – | – | C | TS | 45 | – | Healed |
| 6 | F | 65 | LARR | ADK | Fistula | 21 days | Radiotherapy | – | – | NO | 10 | Migration | Colostomy |
| 7 | M | 73 | ARR | ADK | Stricture + fistula | 83 days | – | Drainage | I | SS | 22 | Pain | Healed |
| 8 | F | 65 | ARR | ADK | Stricture | 197 days | – | – | I | SS | 30 | – | Healed |
| 9 | M | 82 | Hartmann | Diverticula | Complete stenosis | 40 days | – | – | C | TS | 35 | Fever | Healed |
| 10 | M | 61 | Colectomy | Crohn’s disease | Fistula | 19 days | – | – | I | NO | 18 | Migration | Definitive ileostomy |
| 11 | M | 58 | LARR | ADK | Stricture | 157 days | – | – | I | SS | 31 | Tenesmus, pain | Healed |
| 12 | F | 34 | ARR | ADK | Stricture | 302 days | Radiotherapy | – | – | SS | 6 (+25) | Migration after 6 days (repositioning) | Healed |
| 13 | M | 69 | ARR | ADK | Stricture | 244 days | Radiotherapy | – | – | SS | 45 | – | Endoscopic dilations |
| 14 | M | 44 | Hartmann | Diverticula | Stricture | 71 days | – | – | C | SS | 25 | Fever | Healed |
| 15 | M | 75 | Sigmoidectomy | ADK | Fistula | 20 days | – | – | – | NO | 27 | Migration | Colostomy |
| 16 | M | 66 | Right hemicolectomy | ADK | Stricture | 139 days | – | – | – | SS | 20 | – | Healed |
| 17 | F | 19 | Colectomy | Crohn’s disease | Stricture + fistula | 31 days | – | – | – | CS | 34 | Pain | Healed |
| 18 | M | 55 | ARR | ADK | Stricture + fistula | 73 days | – | Drainage | – | SS | 39 | Pain | Healed |
| 19 | M | 73 | LARR | ADK | Fistula | 14 days | Radiotherapy | Drainage | I | NO | 40 | Migration | Colostomy |
| 20 | F | 62 | Hartmann | Diverticula | Stricture | 88 days | – | – | C | SS | 37 | – | Healed |
| 21 | M | 59 | Colectomy | Crohn’s disease | Stricture | 67 days | – | – | I | CS | 10 | Migration | Endoscopic dilations |
| 22 | M | 65 | ARR | ADK | Stricture | 302 days | Radiotherapy | – | – | SS | 30 | – | Healed |
| 23 | F | 24 | ARR | ADK | Complete stenosis | 65 days | Radiotherapy | – | C | TS | 30 | Pain | Healed |
| 24 | F | 63 | Left hemicolectomy | ADK | Stricture + fistula | 17 days | – | Drainage | – | CS | 28 | – | Healed |
| 25 | F | 60 | Left hemicolectomy | ADK | Stricture | 360 days | – | – | – | SS | 30 | – | Healed |
| 26 | M | 74 | LARR | ADK | Stricture | 92 days | – | – | I | SS | 60 | Pain | Endoscopic dilations |
| 27 | F | 54 | Hartmann | Diverticula | Stricture | 233 days | – | – | C | TS | 60 | Pain, migration | Colostomy |
| 28 | M | 30 | Sigmoidectomy | Diverticula | Stricture + fistula | 59 days | – | Drainage | – | SS | 30 | Pain, migration | Surgery |
| 29 | M | 55 | Sigmoidectomy | Diverticula | Stricture | 79 days | – | – | – | SS | 30 (+30) | Migration (two times) | Surgery |
ADK, adenocarcinoma; ARR, anterior rectal resection; C, colostomy; CS, clinical stricture (⩽12 mm); TS, total stenosis; F, female; FCSEMS, fully covered self-expandable metal stent; I, ileostomy; LARR, low anterior rectal resection; M. male; SS, severe stricture (⩽7 mm).
The initial indication for surgery was colorectal carcinoma in 17 patients, perforated diverticular disease in nine patients and nonresponsive Crohn’s disease in three patients. Among the first group, seven patients out of 17 underwent preoperative radiotherapy.
Patients underwent the following surgical procedures: eight anterior rectal resection (ARR); five sigmoidectomy; five Hartmann’s procedure, four low anterior rectal resection (LARR); three left hemicolectomy; three colectomy; and one right hemicolectomy.
Out of 29 patients, eight had an ileostomy (three LAAR, two ARR, two colectomy and one sigmoidectomy), while six patients had a colostomy (five Hartmann’s procedures and one ARR).
The SEMS procedures were undertaken for anastomotic stricture in 17 patients (three presented with complete closure of the anastomosis), for anastomotic leak/fistula in five patients and for concomitant anastomotic stricture and fistula in the remaining seven patients. Out of 12 patients presenting with a fistula, seven had a surgical/percutaneous drainage previously positioned.
The median timespan between surgery and diagnosis of stricture/leak was 104 days (range: 14–360 days). The median period considering the fistula group only was shorter (34.6 days versus 152.7 days) than for the stricture group.
Considering the 24 patients with anastomotic stricture, the degree of the stenosis was as follows: four presented with low-grade stricture (residual lumen ⩽12 mm), 17 with high-grade stricture (residual lumen ⩽7 mm) and three with complete closure of the anastomosis.
The length of the stricture was preoperatively evaluated by CT scan or enema study, and intraprocedurally confirmed with a water contrast agent. In all cases, the stricture’s length did not exceed 3 cm. Longer stenosis were considered not eligible for SEMS treatment due to their presumable ischemic nature and endoscopic treatment inefficiency.
Technical success was achieved in all cases allowing a correct SEMS deployment in all 32 stent procedures.
Overall clinical success was 62.1%, corresponding in the healing of the colorectal anastomotic stricture/leak in 18 patients out of 29; the mean period with the stent in place was 32.6 days (range: 6–65). These 18 patients after a mean follow up of 19 months (range: 4–29) were symptom free and did not required any further treatment.
Considering group-specific clinical success (stricture only group, stricture/leak group and leak without stricture group), we reported a similar percentage of success in the first two groups amounting respectively to 70.6% (12/17 patients) and 71.4% (5/7 patients) while the success rate dropped to an unsatisfactory 20% (1/5 patients) when a stricture was not present as for Group 3.
No FCSEMS related perforation occurred. Migration after a mean period of 28.3 days (range: 6–60) occurred in 12 cases (41.4%). Considering the fistula only subset of patients (Group 3), migration took place in all cases (100%). In three patients, a second stent was repositioned after migration achieving final clinical success in one.
Minor complications occurred in 11/29 patients (37.9%) consisting of eight abdominal/rectal pain, two fever and one tenesmus. Six out of eight patients reporting pain presented with a low colorectal anastomosis (LARR or ARR).
Procedural failure was reported in 11 out of 29 patients (37.9%) requiring subsequent multiple endoscopic dilations in four patients, colostomy confection in four cases, definitive ileostomy in one case and revisional surgery in the remaining two patients.
Discussion
Benign colorectal strictures occur frequently after colorectal surgery both for malignant and benign disease [Baron et al. 1998]. Subclinical anastomotic leakage, radiotherapy, relative ischemia and pelvic sepsis have been suggested to play a pivotal role in the development of postoperative stricture [Påhlman et al. 1989]. Strictures are more frequent after surgery for cancer than for benign disease, probably due to more radical removal of lymphatic and vascular tissue. Diverting stoma also seems to predispose to stricture formation mainly due to absence of fecal dilation; the extraperitoneal rectum is the most frequent location.
Up to now benign strictures have been mainly treated initially by balloon dilation [Virgilio et al. 1995] and, in case of endoscopic failure and stricture recurrence, by revisional surgery. However, balloon dilation requires multiple sessions and fails to guarantee long-term potency especially in cases of strictures longer than 2 cm; this is most probably due to the small period of time in which radial force is applied to the stricture. Similarly, revisional surgery may be very difficult to carry out given the adhesions, local inflammation and stricture frequent location in the lower rectum.
Therefore considering the growing experience of the use of FCSEMS in management of upper GI benign pathologies [Donatelli et al. 2012], fully covered stents have been proposed even in the case of benign colon stricture. The rationale of SEMS deployment is that the stent is able to apply a constant radial force to the stricture for a longer period of time, thus inducing a remodeling of the stenosis. However, the results of FCSEMS are discordant [Forshaw et al. 2006; Caruso et al. 2015]. According to the literature, the efficacy of FCSEMS depends mainly on the etiology of the stricture. Diverticular, Crohn’s disease and ischemic stenosis appear to be an incorrect indication for stent deployment; on contrast, a stent was effective in postsurgical anastomotic strictures. Nonetheless, FCSEMS showed different shortcomings. Firstly, the FCSEMS procedure is not free of complications. Risk of perforation increases if pre- or post-deployment balloon dilation is performed [Bonin and Baron, 2010]. Secondly, FCSEMS are burdened with an high rate of migration. Even though some authors report that migration occurs only after successful stricture dilation [Vanbiervliet et al. 2013], the majority of experts concur with the fact that migration in most cases results in treatment failure requiring additional endoscopic procedures. Moreover, cases of obstruction due to granulation tissue overgrowth at the edge of the stent, mucosal erosion and stent fracture have been described [Odurny, 2001].
Lately FCSEMS have also acquired a primary role in the management of upper GI leaks, especially those following bariatric surgery. The rationale of a covered stent coupled with drainage of any fluid collection is to span the leak, thus facilitating leak closure. Several case series are available in the literature describing variable results [Nedelcu et al. 2015; Edwards et al. 2008]. However, FCSEMS cannot be considered a gold standard due to the not irrelevant complication rate [Iossa et al. 2014]. Therefore different endoscopic techniques have been proposed [Donatelli et al. 2015]. Given the role of FCSEMS in the upper GI, some authors have proposed their use even for leakage of colonic anastomosis [Gürbalak et al. 2015]. However in literature only few case reports are present. Moreover in comparison with an upper GI leak, fecal contamination in a colon leak could play a detrimental factor.
In this study we reported our results on the efficacy of FCSEMS for postanastomotic strictures and leakage with or without an associated stricture. Based on our experience, we highlighted how the use of FCSEMS for the management of leakage without stricture should always be avoided because early migration occurs in most cases with no effect on leak closure. Some authors have tried fixing the FCSEMS by means of different kind of clips with no real success, or using partially covered SEMS to allow tissue ingrowth at the noncovered edges of the stent and thus fixing it to the lumen. However, reported results showed a high complication rate when removing the stent (massive bleeding, perforation) [Small et al. 2008] or the need to deploy a FCSEMS for 48 hours in order to remove both of them (stent-in-stent technique) [Yang et al. 2014]. Therefore we believe that, even for colonic leak, the endoscopic rationale should be switched, as happened for bariatric surgery, from stenting to complete internal drainage and transorificeal traumatism by plastic double pigtail stent to promote healing. As yet, there are no published articles describing such a technique, but Calzolari and colleagues interestingly applied endoscopic transluminal drainage in case of diverticular abscess [Calzolari et al. 2014].
FCSEMS may have a role in the endoscopic treatment of benign anastomotic stricture, associated or not with leaks. A covered stent proved efficient in producing a longer lasting and more constant radial force compared with balloon dilation. However, the results reported in our study are suboptimal. Moreover, the complication rate especially considering migration is unacceptably high. Erosion, pressure necrosis and bleeding have also been reported [Oz and Forde, 2014].
Furthermore, on the basis of our results and experience, we believe that a different endoscopic approach should be advised to treat postsurgical stricture. An interesting innovation was reported by Rejchrt and colleagues with the use of biodegradable stents [Rejchrt et al. 2011]. Stents made of biodegradable materials seem to overcome the shortcoming of metal stents, in particular concerning migration, mucosal hyperplastic reaction and shorter potency. Moreover, biodegradable stents do not require any removal procedure. Biodegradable stents were first used for upper GI stricture, but Rejchrt and colleagues showed good results even for colonic and small intestine stricture. Future development of biodegradable stents may be patient-tailored stent production on the basis of stricture characteristic thanks to three-dimensional printing.
A different innovative and promising approach for benign colorectal strictures was proposed by Chen and Hsu who achieved good long-term results by means of sphincterotome multiple shallow incision of the stricture [Chen and Hsu, 2014] . The rationale was to break down the membranous circular scar allowing release of the stricture and subsequent stool passage dilation.
Conclusion
Postsurgical colorectal anastomotic stricture may be treated by means of FCSEMS. Strict patient selection is mandatory. FCSEMS seems to induce moderate long term patency in an acceptable percentage of cases even if burdened by a high percentage of migration.
Anastomotic leakage without stricture appears to be not a good indication to FCSEMS deployment due to poor clinical results.
New concepts are needed for the endoscopic management of such colorectal postsurgical pathologies.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Fabrizio Cereatti, Emergency Endoscopic Unit, Policlinico Umberto I, ‘SAPIENZA’ University of Rome, Rome, Italy.
Fausto Fiocca, Emergency Endoscopic Unit, Policlinico Umberto I, ‘SAPIENZA’ University of Rome, Rome, Italy.
Jean-Loup Dumont, Unité d’Endoscopie Interventionnelle, RamsayGénérale de Santé, Hôpital Privé des Peupliers, Paris, France.
Vincenzo Ceci, Emergency Endoscopic Unit, Policlinico Umberto I, ‘SAPIENZA’ University of Rome, Rome, Italy.
Bertrand-Marie Vergeau, Unité d’Endoscopie Interventionnelle, RamsayGénérale de Santé, Hôpital Privé des Peupliers, Paris, France.
Thierry Tuszynski, Unité d’Endoscopie Interventionnelle, RamsayGénérale de Santé, Hôpital Privé des Peupliers, Paris, France.
Bruno Meduri, Unité d’Endoscopie Interventionnelle, RamsayGénérale de Santé, Hôpital Privé des Peupliers, Paris, France.
Gianfranco Donatelli, Unité d’Endoscopie Interventionnelle, Ramsay Générale de Santé, Hôpital Privé des Peupliers, 8 Place de l’Abbé G. Hénocque, 75013 Paris, France.
References
- Araujo S., Costa A. (2008) Efficacy and safety of endoscopic balloon dilation of benign anastomotic strictures after oncologic anterior rectal resection: report on 24 cases. Surg Laparosc Endosc Percutan Tech 18: 565–568. [DOI] [PubMed] [Google Scholar]
- Baron T., Dean P., Yates M., III, Canon C., Koehler R. (1998) Expandable metal stents for the treatment of colonic obstruction: techniques and outcomes. Gastrointest Endosc 47: 277–286. [DOI] [PubMed] [Google Scholar]
- Baron T., Rey J., Spinelli P. (2002) Expandable metal stent placement for malignant colorectal obstruction. Endoscopy 34: 823–830. [DOI] [PubMed] [Google Scholar]
- Bonin E., Baron T. (2010) Update on the indications and use of colonic stent. Curr Gastroenterol Rep 12: 374–382. [DOI] [PubMed] [Google Scholar]
- Brown S., Mathew R., Keding A., Marshall H., Brown J., Jayne D. (2014) The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg 259: 916–923. [DOI] [PubMed] [Google Scholar]
- Calzolari C., Barret M., Rahmi G., Samaha E., Jian R., de’Angelis G., et al. (2014) Diverticular abscess successfully treated by endoscopic transluminal drainage. Clin Res Hepatol Gastroenterol 38: e17–e18. [DOI] [PubMed] [Google Scholar]
- Caruso A., Conigliaro R., Manta R., Bertani H., Manno M., Zullo A., et al. (2015) Fully covered self-expanding metal stents for refractory anastomotic colorectal strictures. Surg Endosc 29: 1175–1178. [DOI] [PubMed] [Google Scholar]
- Chen T., Hsu W. (2014) Successful treatment of colorectal anastomosis stricture by using sphincterotomes. Front Surg 1: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Chopra S., Wysocki W., Hünerbein M. (2010) Treatment of benign colorectal strictures by temporary stenting with self-expanding stents. Int J Colorectal Dis 25: 1475–1479. [DOI] [PubMed] [Google Scholar]
- Donatelli G., Ceci V., Cereatti F., Bruni A., Salvatori F., Minervini S., et al. (2008) Minimally invasive treatment of benign complete stenosis of colorectal anastomosis. Endoscopy 40(Suppl. 2): E263–E264. [DOI] [PubMed] [Google Scholar]
- Donatelli G., Dhumane P., Perretta S., Dallemagne B., Vix M., Mutt D., et al. (2012) Endoscopic placement of fully covered self expanding metal stents for management of postoperative foregut leaks. J Minim Access Surg 8: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatelli G., Dumont J., Cereatti F., Ferretti S., Vergeau B., Tuszynski B., et al. (2015) Treatment of leaks following sleeve gastrectomy by endoscopic internal drainage (EID). Obes Surg 25: 1293–1301. [DOI] [PubMed] [Google Scholar]
- Donatelli G., Leblanc S., Vienne A., Gaudric M., Mangialavori L., Duchmann J.-C., et al. (2013) Is over-the-scope clip a permanently implanted device? Outcome and follow up of clip delivery for fistulas, perforations and bleeding. Gastroint Endosc 77(Suppl.): AB207–AB208. [Google Scholar]
- Edwards C., Bui T., Astudillo J., de la Torre R., Miedema B., Ramaswami A., et al. (2008) Management of anastomotic leaks after Roux-en-Y bypass using self-expanding polyester stents. Surg Obes Relat Dis. 4: 594–599. [DOI] [PubMed] [Google Scholar]
- Forshaw M., Sankararajah D., Stewart M., Parker M. (2006) Self-expanding metallic stents in the treatment of benign colorectal disease: indications and outcomes. Colorectal Dis 8: 102–111. [DOI] [PubMed] [Google Scholar]
- Geiger T., Miedema B., Tsereteli Z., Sporn G., Thaler K., et al. (2008) Stent placement for benign colonic stenosis: case report, review of the literature, and animal pilot data. Int J Colorectal Dis 23: 1007–1012. [DOI] [PubMed] [Google Scholar]
- Gürbalak E., Akgün I., Oz A., Omeroglu S., Battal M., Celayir F., et al. (2015) Minimal invasive management of anastomosis leakage after colon resection. Case Rep Med (2015): article ID 374072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossa A., Fiocca F., Corona M., Cavallaro G., Cereatti F., Rizzello M., et al. (2014) Severe events related to use of stents in case of bariatric complications. CRSLS e2014.002234. DOI: 10.4293/CRSLS.2014.002234 [DOI] [Google Scholar]
- Johansson C. (1996) Endoscopic dilation of rectal strictures: a prospective study of 18 cases. Dis Colon Rectum 39: 423–428. [DOI] [PubMed] [Google Scholar]
- Kang C., Halabi W., Chaudhry O., Nguyen V., Pigazzi A., Carmichael J., et al. (2013) Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg 148: 65–71. [DOI] [PubMed] [Google Scholar]
- Luchtefeld M., Milsom J., Senagore A., Surrell J., Mazier W. (1989) Colorectal anastomotic stenosis. Results of a survey of the ASCRS membership. Dis Colon Rectum 32: 733. [DOI] [PubMed] [Google Scholar]
- Nedelcu M., Manos T., Cotirlet A., Noel P., Gagner M. (2015) Outcome of leaks after sleeve gastrectomy based on a new algoritm addressing leak size and gastric stenosis. Obes Surg 25: 559–563. [DOI] [PubMed] [Google Scholar]
- Odurny A. (2001) Colonic anastomotic stenoses and Memotherm stent fracture: a report of three cases. Cardiovasc Intervent Radiol 24: 336–339. [DOI] [PubMed] [Google Scholar]
- Orsay C., Bass E., Firfer B., et al. (1995) Blood flow in colon anastomotic stricture formation. Dis Colon Rectum 38: 202–206. [DOI] [PubMed] [Google Scholar]
- Oz M., Forde K. (1990) Endoscopic alternatives in the management of colonic strictures. Surgery 108: 513–519. [PubMed] [Google Scholar]
- Påhlman L., Glimelius B., Frykholm G. (1989) Ischaemic strictures in patients treated with a low anterior resection and perioperative radiotherapy for rectal carcinoma. Br J Surg 76: 605–606. [DOI] [PubMed] [Google Scholar]
- Ptok H., Marusch F., Meyer F., Schubert D., Gastinger I., Lippert H., et al. (2007) Impact of anastomotic leakage on onco-logical outcome after rectal cancer resection. Br J Surg 94: 1548–1554. [DOI] [PubMed] [Google Scholar]
- Rejchrt S., Kopacova M., Brozik J., Bures J., et al. (2011) Biodegradable stents for the treatment of benign stenosis of the small and large intestines. Endoscopy 43: 911–917. [DOI] [PubMed] [Google Scholar]
- Small A., Young-Fadok T., Baron T. (2008) Expandable metal stent placement for benign colorectal obstruction: outcomes for 23 cases. Surg Endosc 22: 454–462. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Saunders B., Thomas-Gibson S., Akle C., Marshall M., Halligan S. (2004) Colorectal stenting for malignant and benign disease: outcomes in colorectal stenting. Dis Colon Rectum; 47: 1201–1207. [DOI] [PubMed] [Google Scholar]
- Strangioa G., Zullo A., Ferrara E., Anderlonia A., Carlino A., Jovani M., et al. (2015) Endo-sponge therapy for management of anastomotic leakages after colorectal surgery: a case series and review of literature. Dig Liver Dis 47: 465–469. [DOI] [PubMed] [Google Scholar]
- Vanbiervliet G., Bichard P., Demarquay J., Ben-Soussan E., Lecleire S., Barange K., et al. (2013) Fully covered selfexpanding metal stents for benign colonic strictures. Endoscopy 45: 35–41. [DOI] [PubMed] [Google Scholar]
- Virgilio C., Cosentino S., Favara C., Russo V., Russo A. (1995) Endoscopic treatment of postoperative colonic strictures using an achalasia dilator: short-term and long-term results. Endoscopy 27: 219–222. [DOI] [PubMed] [Google Scholar]
- Yang D., Seo M., Lee H., Park S., Kim K., Ye B., et al. (2014) Stent-in stent technique and endoscopic resection of granulation tissue to remove a migrated metal duodenal stent embedded in the colon. Endoscopy 46: E159–E160. [DOI] [PubMed] [Google Scholar]