Abstract
Background:
Nonalcoholic steatohepatitis (NASH) is associated with increased cardiovascular risk and mortality. No US Food and Drug Administration (FDA) approved therapies for NASH are available; clinical trials to date have not yet systematically assessed for changes in cardiovascular risk. This study examines the prospective utility of cardiovascular risk assessments, the Framingham risk score (FRS) and coronary artery calcium (CAC) score, as endpoints in a NASH randomized clinical trial, and assesses whether histologic improvements lead to lower cardiovascular risk.
Methods:
Secondary analysis of a 24-week randomized, double-blind, placebo-controlled trial (MOZART) in which 50 biopsy-proven NASH patients received oral ezetimibe 10 mg daily (n = 25) versus placebo (n = 25). Biochemical profiling, FRS, CAC scores, liver biopsies were obtained at baseline and endpoint.
Results:
Ezetimibe improved FRS whereas placebo did not (4.4 ± 6.2 to 2.9 ± 4.8, p = 0.038; 3.0 ± 4.4 to 2.9 ± 4.2, p = 0.794). CAC scores did not change with ezetimibe or placebo (180.4 ± 577.2 to 194.1 ± 623.9, p = 0.293; 151.4 ± 448.9 to 183.3 ± 555.7, p = 0.256). Ezetimibe improved FRS and CAC scores in more patients than placebo (48% versus 23%, p = 0.079, and 21% versus 0%, p = 0.090, respectively), though not significantly. No differences were noted in cardiovascular risk scores among histologic responders versus nonresponders.
Conclusions:
Ezetimibe improved FRS whereas placebo did not. FRS and CAC scores improved in a greater proportion of patients with ezetimibe; this trend did not reach significance. These findings indicate the utility and feasibility of monitoring cardiovascular risk in a NASH trial. The utility of CAC scores may be higher in trials of longer duration (⩾52 weeks) and with older patients (age ⩾45).
ClinicalTrials.gov registration: NCT01766713.
Keywords: coronary artery calcium, fatty liver, Framingham risk, hepatic steatosis, score
Introduction
Nonalcoholic steatohepatitis (NASH) is the more progressive form of nonalcoholic fatty liver disease (NAFLD) [Chalasani et al. 2012]. It is associated with increased cardiovascular risk [Gastaldelli et al. 2009; Williams et al. 2011; Kim et al. 2012; Fargion et al. 2014; Vanwagner et al. 2014], which is the most common cause of death in individuals with NASH [Lincoff et al. 2007; Targher et al. 2010; Chalasani et al. 2012]. Currently, there are no US Food and Drug Administration (FDA) approved therapies available for NASH [Sanyal et al. 2010; Chalasani et al. 2012]. Numerous agents have been investigated in clinical trials including vitamin E, pioglitazone, colesevelam, ezetimibe and obeticholic acid, all aimed at targeting various aspects of NASH pathogenesis [Sanyal et al. 2010; Chalasani et al. 2012; Farrell et al. 2012; Le et al. 2012; Zarrinpar and Loomba, 2012; Neuschwander-Tetri et al. 2014; Loomba et al. 2015b].
Current NAFLD practice guidelines recommend the use of vitamin E or pioglitazone for the treatment of NASH [Chalasani et al. 2012]. However, these therapies have been linked to worsened cardiovascular risk during treatment. Vitamin E is associated with an increased risk of hemorrhagic stroke [Schurks et al. 2010] and heart failure in diabetic patients [Lonn et al. 2005], and rosiglitazone is associated with an increased risk of myocardial infarction, heart failure and cardiovascular-related death [Nissen and Wolski, 2007]. Recently, the FLINT trial showed that obeticholic acid, a farnesoid X receptor ligand, improved histologic features of NASH. However, obeticholic acid also increased total cholesterol and low-density lipoprotein (LDL), and moderately decreased high-density lipoprotein (HDL) [Neuschwander-Tetri et al. 2014]. Moreover, cardiovascular risk was not formally assessed in FLINT. Thus, the clinical relevance of these lipid changes is unclear. It is therefore essential that cardiovascular risk be monitored in NASH clinical trials for risk neutrality or, ideally, risk reduction, as recommended in a recently published joint FDA–American Association for the Study of Liver Diseases (AASLD) symposium report on NASH clinical trial design [Sanyal et al. 2015].
However, no previous NASH trials have yet to systematically and prospectively assess cardiovascular risk assessments of therapies investigated in NASH trials [Corey et al. 2014; Neuschwander-Tetri et al. 2014; Tziomalos, 2014]. Ezetimibe, a gut luminal cholesterol absorption inhibitor that binds to and disrupts the Niemann-Pick C1-like 1 (NPC1L1) transporter, has been compared with placebo for the treatment of NASH in the MOZART randomized clinical trial (Magnetic resOnance imaging and elastography in eZetimibe versus placebo for the Assessment of Response to Treatment in NASH) [Loomba et al. 2015b]. This secondary analysis of the MOZART trial aims to: (1) examine the utility of cardiovascular risk assessment scores, including the Framingham risk score (FRS) and the coronary artery calcium (CAC) score, as secondary endpoints in a NASH randomized clinical trial; and (2) to assess whether histologic improvements in NASH lead to reduced cardiovascular risk.
Materials and methods
Study design and patients
This is a secondary analysis of a randomized clinical trial of 50 adult patients with biopsy-proven NASH. As such, data used for this study were obtained from the MOZART trial. The cardiovascular endpoints were changes in FRS and CAC scores between the beginning (week 0) and the completion of the trial (week 24). Histologic responders in this trial, defined as a decrease of ⩾2 points on NAFLD activity score (defined below), were also examined for changes in cardiovascular risk as assessed by these two scores.
The original MOZART trial study design and population is described elsewhere in detail [Loomba et al. 2015b]. Briefly, this randomized, double-blind, placebo-controlled clinical trial was designed to assess the efficacy of oral ezetimibe at 10 mg daily versus placebo over a 24 week period for the treatment of NASH. Patients first underwent clinical evaluation that included a detailed physical examination, clinical and biochemical evaluation, baseline magnetic resonance imaging proton density fat fraction (MRI-PDFF) and liver biopsy (see Supplemental Methods). The screening process also included an alcohol history assessment by completing both the Alcohol Use Disorders Identification Test (AUDIT) and Skinner Lifetime Drinking questionnaires. The study was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) Guidelines [Loomba et al. 2015b].
Details of study population, inclusion and exclusion criteria, baseline and clinical assessments, and the liver biopsy protocol are available as Supplementary Methods.
Cardiovascular risk scores
The FRS estimates the 10-year cardiovascular disease risk in an individual. It comprises age, sex, total cholesterol, HDL, systolic blood pressure, smoking status, and whether the individual is on treatment for blood pressure [Eichler et al. 2007]. Scores were calculated at week 0 and week 24 of the study by hand using a score sheet derived from the Adult Treatment Panel III (ATP III) guidelines [National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults, 2002]. The CAC scores of each patient were derived from a noncontrast computerized tomography (CT) scan of the thorax that combines the atherosclerotic burdens of the coronary arteries into a single score [McEvoy et al. 2010]. CT scans were also obtained at week 0 and 24.
The rationale for using the FRS is that it is readily available and well-studied, and has been validated in patients with fatty liver disease [Treeprasertsuk et al. 2012]. The CAC score was used because it has been found to be associated with fatty liver independent of visceral adiposity [Kim et al. 2012] and has been used in various clinical trials evaluating the effect of lipid-lowering therapy on atherosclerosis. Furthermore, it is a strong predictor of incident coronary heart disease across racial and ethnic groups [Detrano et al. 2008]. When CAC scores are used in conjunction with FRS, it provides the most robust improvement in risk prediction when compared with other combinations of cardiovascular risk assessment scores [Yeboah et al. 2012].
Coronary artery calcium assessment protocol
CAC images were obtained through noncontrast prospective electrocardiogram triggered volumetric CT of the heart utilizing a 320-slice scanner (Aquilion One, Toshiba Medical Systems, Otawara, Japan). No beta-blockers or nitroglycerin were administered. Scans were performed during a breathhold at the end of inspiration and the scan range extended from the carina to below the level of the heart. The collimation was 320 mm × 0.5 mm with field of view of 220 mm. Scans were performed using 120 kVp. The tube current was determined using Sure Exp. 3D software with a minimum of 40 mA, maximum of 580 mA (±10 for prospective, ±33 for retrospective scans). Rotation time was 0.35 s.
For image analysis, 3 mm axial slices were reconstructed using a filter convolution of 5. CAC scores were calculated using the Agatston method by a fellowship-trained cardiac radiologist (S.B.) using commercial postprocessing software (Vital Images, Inc., Minnetonka, MN). Criteria for the Agatston method has been described previously [Agatston et al. 1990]. Briefly, calcified plaques measuring ⩾130 Hounsfield units along the course of the coronary arteries with area of at least 3 voxels in the axial plane were included in the measurement. The area of each plaque found in the left main, left anterior descending, circumflex and right coronary arteries is multiplied by an intensity index to yield the Agatston score. A score of 0 is a normal artery; 100 confers mild calcification, while 300 or higher is considered high-risk atherosclerosis [McEvoy et al. 2010; Polonsky et al. 2014].
Statistical analysis
The two-tailed t-test was used for comparison of means for continuous variables across various groups in the study (paired within the ezetimibe and placebo arms, unpaired across the treatment arms). The Wilcoxon–Mann–Whitney test was performed on all non-normally distributed continuous variables. The Fisher’s exact or chi-square (χ2) tests were used for comparisons between categorical variables, as appropriate. All statistical analyses were performed using SAS version 9.3 (SAS Inc., Cary, NC) and Excel (Microsoft Corp., Redmond, WA) software. All p values ⩽0.05 were considered statistically significant.
Results
Baseline demographic, biochemical and cardiovascular risk scores
A total of 50 patients with biopsy-confirmed NASH were included in this secondary analysis of the MOZART trial, with 25 patients in each of the ezetimibe and placebo arms. The baseline demographic, biochemical, histologic and imaging characteristics of the study patients have been published previously [Loomba et al. 2015b]. Baseline cardiovascular risk scores are shown in Table 1. The mean FRS in the ezetimibe and placebo arm were 4.1 ± 6.0 and 2.9 ± 4.1 (p = 0.4375), respectively. In the ezetimibe and placebo arms, mean CAC scores were 131.9 ± 484.0 and 127.3 ± 393.0 (p = 0.9739), respectively. No significant difference was noted in the distribution of cardiovascular risk scores in either treatment arms. At baseline, the mean CAC score under the age of 45 was 0.3 (Figure 1). Between the ages of 45 and 54, the mean CAC score was 9.2; between the ages of 55 and 64, the mean CAC score was 213.1 and those aged 65 or older had mean CAC scores of 412.5.
Table 1.
Baseline demographic, biochemical, histologic and cardiovascular characteristics of patients.
| Ezetimibe arm (n = 25) | Placebo arm (n = 25) | p value | |
|---|---|---|---|
| Demographics | |||
| Female participants | 14 (56%) | 17 (68%) | 0.3821 |
| Age (years) | 49.0 ± 14.9 | 49.5 ± 13.7 | 0.9139 |
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.9837 |
| Weight (kg) | 94.1 ± 18.1 | 91.8 ± 18.9 | 0.6662 |
| BMI (kg/m2) | 33.8 ± 5.2 | 32.9 ± 5.1 | 0.5432 |
| Race and ethnicity | |||
| White (versus non-White) | 19 (76%) | 21 (84%) | 0.7252 |
| Hispanic (versus non-Hispanic) | 8 (32%) | 9 (36%) | 0.7653 |
| Diabetes | 7 (28%) | 7 (28%) | 1.0000 |
| Biochemical profile | |||
| AST (IU/L) | 33.0 (23.0) | 32.0 (28.0) | 0.6572 |
| ALT (IU/L) | 51.0 (29.0) | 47.0 (26.0) | 0.9615 |
| Alkaline phosphatase (U/l) | 72.0 (29.0) | 72.0 (37.0) | 0.4584 |
| GGT (U/l) | 49.0 (32.0) | 32.5 (42.0) | 0.4049 |
| Total bilirubin (mg/dl) | 0.5 (0.4) | 0.4 (0.2) | 0.7167 |
| Direct bilirubin (mg/dl) | 0.1 (0.0) | 0.1 (0.1) | 0.3494 |
| Albumin (g/dl) | 4.5 (0.3) | 4.5 (0.4) | 0.3830 |
| Glucose (mg/dl) | 104.0 (25.0) | 106.0 (41.0) | 0.6504 |
| HbA1c (%) | 5.9 (0.7) | 6.1 (1.0) | 0.7015 |
| Insulin (mlU/l) | 23.0 (15.5) | 26.5 (18.0) | 0.2322 |
| Total cholesterol (mg/dl) | 182.0 (25.0) | 170.0 (54.0) | 0.5001 |
| Triglycerides (mg/dl) | 152.0 (58.0) | 149.0 (104.0) | 0.5567 |
| HDL (mg/dl) | 45.0 (11.0) | 44.0 (22.0) | 0.7209 |
| LDL (mg/dl) | 100.0 (32.0) | 90.0 (50.5) | 0.3831 |
| Other | |||
| MRI-PDFF (%) | 11.3 (10.9) | 19.6 (11.1) | 0.0978 |
| NAS | 5.0 (2.0) | 5.0 (2.0) | 0.2116 |
| Cardiovascular risk scores | |||
| CAC score | n = 20 | n = 21 | 0.6616 |
| 0 | 12 | 15 | |
| 1–100 | 4 | 2 | |
| >100 | 4 | 4 | |
| Mean CAC score ± SD | 131.9 ± 484.0 | 127.3 ± 393.0 | 0.9739 |
| Median CAC score (IQR) | 0 (58.5) | 0(65) | 0.6793 |
| FRS | n = 25 | n = 25 | 0.3487 |
| <10% | 21 | 24 | |
| ⩾10% | 4 | 1 | |
| Mean FRS ± SD | 4.1 ± 6.0 | 2.9 ± 4.1 | 0.4375 |
| Median FRS (IQR) | 1.0 (4.5) | 1.0 (3.5) | 0.7902 |
Mean ± standard deviation presented above for normally distributed variables, median (interquartile range) for non-normally distributed variables. The Wilcoxon–Mann–Whitney test was performed on all continuous variables. A chi-squared or Fisher’s exact test was used for categorical variables.
The NAS-CRN histologic scoring system was used for histology grading and staging of liver biopsies.
AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; CAC, coronary artery calcium; FRS, Framingham risk score; GGT, γ-glutamyl transferase; HDL, high-density lipoprotein; HgA1c, hemoglobin A1C; IQR, interquartile range; IU, international unit; LDL, low-density lipoprotein; MRI-PDFF, magnetic resonance imaging proton-derived fat fraction; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; SD, standard deviation.
Figure 1.
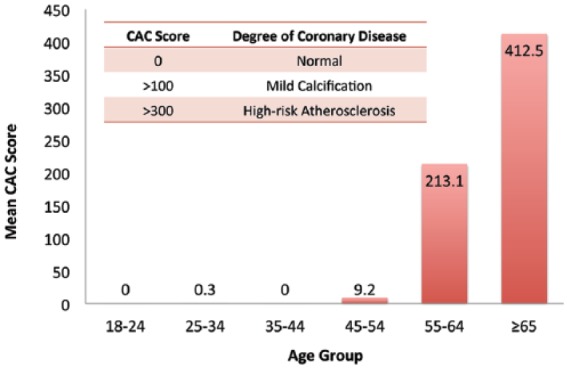
Mean baseline coronary artery calcium (CAC) scores by age groups.
At baseline, the mean CAC scores by age groups are as follows: 18–24, 0 (n = 4); 25–34, 0.3 (n = 3); 35–44, 0 (n = 9); 45–54, 9.2 (n = 12); 55–64, 213.1 (n = 9); ages ⩾65, 412.5(n = 8).
Cardiovascular risk assessment changes
FRS between ezetimibe versus placebo
During the 24 weeks of treatment of NASH, those treated with ezetimibe (n = 23) had improvements in mean FRS (4.4 ± 6.2 to 2.9 ± 4.8, p = 0.0383) (Figure 2a). Those treated with placebo did not have improvements in mean FRS (3.0 ± 4.4 to 2.9 ± 4.2, p = 0.7944). The mean difference between the ezetimibe and placebo arms was −0.79 ± 2.6 (p = 0.0759).
Figure 2.
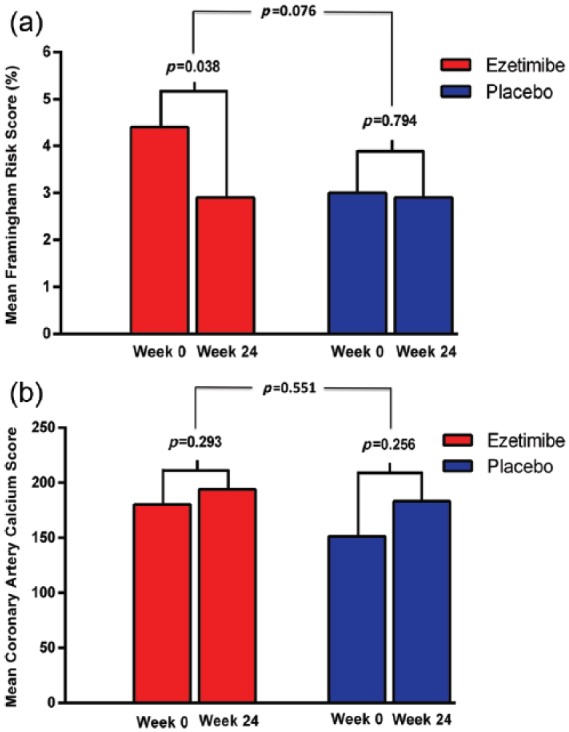
Changes in mean Framingham risk score (FRS) and coronary artery calcium (CAC) scores between treatment arms.
(a) Over 24 weeks of treatment for nonalcoholic steatohepatitis (NASH), ezetimibe (n = 23) improved mean FRS from 4.4 ± 6.2 to 2.9 ± 4.8, p = 0.0383, while no change was observed with placebo (n = 22, 3.0 ± 4.4 to 2.9 ± 4.2, p = 0.7944). The mean difference of FRS between the ezetimibe and placebo arms was −0.79 ± 2.6 (p = 0.0759). (b) In the ezetimibe arm (n = 14), mean CAC scores changed from 180.4 ± 577.2 to 194.1 ± 623.9, p = 0.2928. In the placebo arm (n = 16), mean CAC scores changed from 151.4 ± 448.9 to 183.3 ± 555.7, p = 0.2560. However, these changes were not statistically significant. The mean difference of CAC scores between the two treatment arms was 23.4 ± 84.2, p = 0.5507.
CAC scores between ezetimibe versus placebo
Mean CAC scores increased in the ezetimibe arm (n = 14) from 180.4 ± 577.2 to 194.1 ± 623.9, but this was not significant (p = 0.2928). Similarly, patients in the placebo arm also had an increase in mean CAC scores from 151.4 ± 448.9 to 183.3 ± 555.7, though this was also not statistically significant (p = 0.2560) (Figure 2b; Figure 3). The mean difference between the two treatment arms was 23.4 ± 84.2, p = 0.5507.
Figure 3.
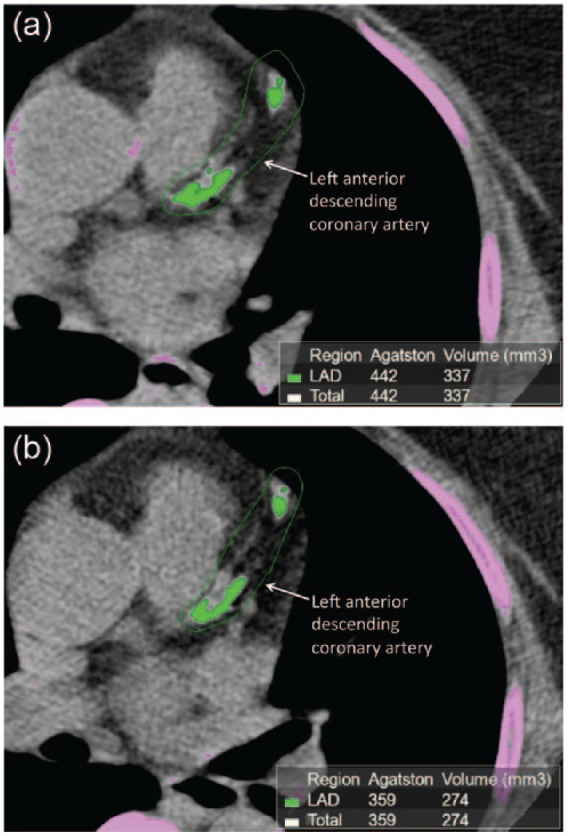
Coronary artery calcium (CAC) imaging of a patient prior to and after 24-week treatment with ezetimibe.
The green shaded areas denote the presence of coronary calcification in the left anterior descending (LAD) coronary artery before (a) and after (b) treatment with ezetimibe for 24 weeks. The green outline indicates the area drawn by the radiologist for the computer software to calculate the Agatston score for the LAD, which is added to the Agatston score of the other coronary arteries for a composite coronary artery calcium score. Note that the patient is slightly rotated during the second imaging session.
Histologic responders and cardiovascular risk scores
Among the pooled histologic responders (improvement of ⩾2 points on the NAFLD activity score) in the MOZART trial, 30% of patients had improvements in FRS (n = 10) versus 35% in nonresponders (n = 23); this was not statistically significant (Fisher’s p = 1.000). Compared with nonresponders, 20% of responders had decreases in CAC scores (n = 5) versus 11% in nonresponders (n = 18); this was also not a statistically significant association (Fisher’s p = 0.539).
Qualitative changes in cardiovascular risk scores
A greater proportion of patients treated with ezetimibe versus placebo had improved FRS (48%, n = 23, versus 23%, n = 22), though this trend did not reach significance (chi-squared p = 0.079) (Figure 4). Additionally, a higher proportion of patients treated with ezetimibe (n = 14) had improvements in CAC scores versus placebo (n = 16), though this trend also did not reach significance (21% versus 0%, Fisher’s p = 0.090).
Figure 4.
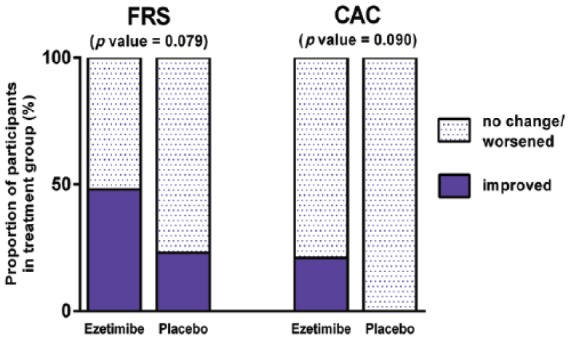
Proportion of patients with corresponding changes in cardiovascular risk scores.
Among 23 patients treated with ezetimibe, 11 (48%) had improved Framingham risk score (FRS), versus 5 of 22 patients (23%) in the placebo arm (chi-squared p value = 0.079). Coronary artery calcium (CAC) scores also improved in 3 of 14 patients (21%) in the ezetimibe arm, versus 0 of 16 (0%) in the placebo arm, though this was not a significant difference (Fisher’s p value = 0.090).
Discussion
In this secondary analysis of the MOZART trial, we demonstrate the utility and feasibility of two cardiovascular risk assessments, the FRS and CAC scores, as additional endpoints for monitoring cardiovascular risk in a NASH clinical trial. This is the first NASH clinical trial, to our knowledge, that systematically and prospectively follows cardiovascular risk assessments longitudinally. Our data suggest that ezetimibe improved the FRS whereas placebo did not. Both ezetimibe and placebo did not improve CAC scores. Qualitatively, ezetimibe improved FRS and CAC scores in a greater proportion of patients than placebo, but this trend did not reach statistical significance. No proportional differences in changes of either risk scores were found between histologic responders versus nonresponders. These trends and changes in cardiovascular endpoints provide base case estimates as well as placebo arm data that will be critical for sample size estimation to help design future NASH trials utilizing these risk scores.
Interpreting changes of cardiovascular risk scores
The improvements in FRS seen in the ezetimibe arm most likely reflect the risk calculation taking into consideration total cholesterol and HDL. Indeed, in the MOZART trial, the mean change of total cholesterol of the ezetimibe arm was a 30 mg/dl reduction [Loomba et al. 2015b], which translates as a 1–2% decrease in FRS [National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults, 2002]. This is reflected in our findings of a mean improvement of 1.5% within the ezetimibe arm. During this 24-week trial, we also found no significant difference in changes of CAC scores between ezetimibe and placebo. The interpretation of an unchanged mean CAC score in the treatment arm can be challenging, especially given the isolated benefit of ezetimibe on improving total cholesterol and LDL. However, unchanged CAC scores in both treatment arms indicates that the tested therapy (ezetimibe) is at least, from a coronary cardiovascular risk perspective, risk neutral – a recommendation set forth recently by the FDA–AASLD symposium on tested therapies in a NASH trial [Sanyal et al. 2015]. Thus, the lack of progression of atherosclerosis during a NASH trial through monitoring of CAC scores may have utility with regards to assessing pharmacologic safety (i.e. treatment does not rapidly accelerate atherosclerosis). Improvements in CAC scores, nonetheless, would be the more desirable endpoint.
Histologic response and cardiovascular risk scores
When comparing histologic responders (those with improvements of ⩾2 points on the NAFLD activity score after 24 weeks of ezetimibe) to nonresponders, there was no difference observed between the proportions of patients with improved FRS or CAC scores. This lack of change in cardiovascular endpoints between responders and nonresponders may be inherent to the investigational drug ezetimibe, suggesting that improving cardiovascular risk may involve more than just decreasing luminal cholesterol absorption and lowering of serum LDL levels.
Context of findings in literature
The monitoring of cardiovascular risk in the treatment of NASH is important due to cardiovascular disease being the most common cause of death in this population [Lincoff et al. 2007; Gastaldelli et al. 2009; Targher et al. 2010; Kim et al. 2012]. Recent studies of potential NASH treatments show significant variations in lipid profile changes and histologic response, but the cardiovascular significance of these findings is unknown. For example, colesevelam, a bile acid sequesterant, lowers LDL compared with placebo in the absence of histologic response [Le et al. 2012]. In a phase II trial, eicosapentanoic acid (EPA-E), a synthetic polyunsaturated fatty acid, showed reductions in triglycerides over placebo, also without significant effect on histologic features [Sanyal et al. 2014]. In the FLINT trial, a 72-week study conducted by the NASH Clinical Research Network (CRN), obeticholic acid improved histologic features of NASH but this accompanied an increase in total cholesterol and LDL, and a moderate decrease in HDL [Neuschwander-Tetri et al. 2014]. These findings raise concerns regarding the safety profile of new potential therapeutics on cardiovascular outcomes, emphasizing the need to monitor cardiovascular endpoints.
To our knowledge, this study is the first to prospectively follow FRS, which we have shown to reflect lipid profile changes longitudinally. CAC scores have been used in studies investigating statins [Callister et al. 1998; Schmermund et al. 2006], lifestyle modification [Lehmann et al. 2011], the role family history [Pandey et al. 2014] and ethnicity [Detrano et al. 2008], as well as in assessing risk in individuals with low FRS [Okwuosa et al. 2012]. However, CAC scores have not yet been utilized in a NASH trial. Current American College of Cardiology (ACC)/American Heart Association (AHA) guidelines indicate use of CAC scores as Class IIb, for when even after quantitative risk assessment (i.e. FRS) the exact cardiovascular risk is still uncertain [Andrus and Lacaille, 2014]. The combined use of CAC scores with FRS has been shown to provide significant improvements in cardiovascular risk prediction [Yeboah et al. 2012]. Thus, the proof-of-concept MOZART trial provides data to support the use of FRS and CAC scores in this setting.
Other modalities of assessing cardiovascular risk in NASH
The phenotypic manifestations of fatty liver and cardiovascular risk vary, and include serologic (e.g. cholesterol, insulin resistance, glycemic control) and physical properties (e.g. anthropometrics, vascular characteristics, quantification of liver and epicardial fat). Thus, cardiovascular risk assessments in NASH should reflect these measures. The numerous potential therapies tested for the treatment of NASH often augment a patient’s cholesterol profile; thus, a single risk assessment (i.e. FRS) should be supplemented by an additional form of assessment, such as an imaging modality, to assess risk differently and in a way not directly impacted by pharmacologic intervention.
This study employs the first use of CAC scores in a NASH trial to assess atherosclerotic changes. While no changes were observed between the treatment arms, our results suggest that ezetimibe does not accelerate or worsen cardiovascular risk. The findings also suggest that CAC scores may be more appropriate for use at longer intervals, such as every 1–7 years [Callister et al. 1998; Schmermund et al. 2006; McEvoy et al. 2010; Lehmann et al. 2011], and useful in a cohort of older patients over the age of 45. Furthermore, there may be a role for CAC scores in assessing long-term, longitudinal cardiovascular risk among patients with NASH [Kim et al. 2012; Budoff et al. 2013]. Other imaging modalities to assess cardiovascular risk, such as cardiac MRI or quantitative ultrasound [Lin et al. 2014] to quantify hepatic steatosis, which has been associated with cardiovascular disease [Arulanandan et al. 2015], may be utilized in future trials. Measures of vascular and endothelial dysfunction, such as the ankle-brachial index, measures of flow-mediated dilation and carotid intima-media thickness [Sookoian and Pirola, 2008; Kozakova et al. 2012], may be potential cardiovascular endpoints in future NASH trials. The use and limitations of these other modalities in a prospective NASH trial, however, is currently unknown.
Strengths and limitations
One strength of this study is that it is analysis based on a randomized, allocation-concealed, double-blind and placebo-controlled study design of the MOZART trial. This study also has assessments of cardiovascular risk designed into the protocol, suggesting the feasibility of FRS and CAC score assessments in NASH trials. Additionally, two types of cardiovascular risk assessments are used to capture risk based on different components of a patient’s phenotype, such as anthropometric and biochemical profile, as well as imaging characteristics, thus providing a more comprehensive assessment of cardiovascular risk. Histologic changes also provide an additional comparison against cardiovascular endpoints.
One important limitation of our study is that, while our data shows movement of the cardiovascular risk scores, the small sample size provides insufficient power to reach significance. However, this provides sample size estimation for future NASH trials. Furthermore, each of the risk scores has shortcomings. The FRS reflects changes in lipid profile; thus, its sole use in a NASH trial with an agent that primarily improves cholesterol may not provide a comprehensive assessment of risk. Additionally, it does not consider elements of ethnicity or family history, in light of emerging evidence for the heritability of hepatic steatosis and fibrosis [Schwimmer et al. 2009; Loomba et al. 2010, 2015a]. CAC assessments also have limitations. We demonstrate that coronary calcium is rare among individuals with NASH under the age of 45, which is of similar distribution in individuals without NASH [Polonsky et al. 2014]; thus, its use may be limited to trials with an older age group and is of no use in pediatric NASH trials. An additional limitation is the short duration of this trial, which has two important implications. Firstly, important improvements in CAC scores that may have occurred at 1 year, for example, may not be observed in this 24-week period. Secondly, the utility of CAC assessments may be more applicable to trials of longer durations, those greater than 24 weeks.
Conclusion
This proof-of-concept study demonstrates the feasibility and utility of the FRS and CAC score as secondary endpoints for monitoring cardiovascular risk in a NASH trial. CAC scores can be used to assess atherosclerotic safety of a tested NASH therapy, and have further potential in longer duration trials and in cohorts of patients over age 45. Future studies may include prospective designs that follow patients and their cardiovascular risks during and beyond NASH trials. Other cardiovascular risk assessment modalities may be tested and be compared prospectively against cardiovascular mortality endpoints. With the inclusion of these risk scores in NASH trials, potential therapies can be monitored for risk reduction or, at the very least, risk neutrality.
Supplementary Material
Acknowledgments
The authors would like to thank and acknowledge all members of the San Diego Integrated NAFLD Research Consortium (SINC). The members of the San Diego Integrated NAFLD Research Consortium (SINC) are:
University of California San Diego, La Jolla, CA: Rohit Loomba, MD, MHSc (Principal Investigator of SINC),Ottar Lunde, MD; Heather Hofflich, DO; Yuko Kono, MD; Alexander Kuo, MD; Heather Patton, MD, Lisa Richards, NP; Joanie Salotti, NP; Linda Soaft, NP.
Sharp Health System, San Diego, CA: Tommy Yen, MD; Michael Bennett, MD; John Person, MD; Cynthia Behling, MD; James Wolosin, MD; Steven Brozinsky, MD.
Kaiser Permanente of Southern California, San Diego, CA: Lisa Nyberg, MD; Anders Nyberg, MD; Mamie Dong, MD.
Balboa Naval Medical Center, San Diego, CA: Lt. CMDR William Shields, MD, and Len Philo, MD.
Footnotes
Funding: This work was supported by an investigator initiated study grant to R.L. by Merck & Co. Inc. The study was conducted at the Clinical and Translational Research Institute, University of California at San Diego. R.L. is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Association of Specialty Professors, and the American Gastroenterological Association; and grant K23-DK090303. The funding agencies did not have any role in the design and conduct of the study, collection, management, analysis or interpretation of the data; preparation, review, or approval of the manuscript.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Steven C. Lin, NAFLD Translational Research Unit, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA
Brandon Ang, NAFLD Translational Research Unit, Division of Epidemiology, Department of Family and Preventive Medicine, University of California at San Diego, La Jolla, CA, USA.
Carolyn Hernandez, NAFLD Translational Research Unit, Division of Epidemiology, Department of Family and Preventive Medicine, University of California at San Diego, La Jolla, CA, USA.
Ricki Bettencourt, NAFLD Translational Research Unit, Division of Epidemiology, Department of Family and Preventive Medicine, University of California at San Diego, La Jolla, CA, USA.
Rashmi Jain, NAFLD Translational Research Unit, Division of Epidemiology, Department of Family and Preventive Medicine, University of California at San Diego, La Jolla, CA, USA.
Joanie Salotti, NAFLD Translational Research Unit, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA.
Lisa Richards, NAFLD Translational Research Unit, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA.
Yuko Kono, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA.
Archana Bhatt, NAFLD Translational Research Unit, Division of Epidemiology, Department of Family and Preventive Medicine, University of California at San Diego, La Jolla, CA, USA.
Hamed Aryafar, Department of Radiology, University of California at San Diego, La Jolla, CA, USA.
Grace Y. Lin, Department of Pathology, University of California at San Diego, La Jolla, CA, USA
Mark A. Valasek, Department of Pathology, University of California at San Diego, La Jolla, CA, USA
Claude B. Sirlin, Department of Pathology and Liver Imaging Group, University of California at San Diego, La Jolla, CA, USA
Sharon Brouha, Department of Radiology, University of California at San Diego, La Jolla, CA, USA.
Rohit Loomba, Professor of Medicine and Associate Director of Clinical Research, Division of Gastroenterology, Adjunct Professor, Division of Epidemiology, University of California at San Diego, Biomedical Research Facility II, 4A18, 9500 Gilman Drive, La Jolla, CA 92093, USA.
References
- Agatston A., Janowitz W., Hildner F., Zusmer N., Viamonte M., Jr., Detrano R. (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832. [DOI] [PubMed] [Google Scholar]
- Andrus B., Lacaille D. (2014) 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol 63: 2886. [DOI] [PubMed] [Google Scholar]
- Arulanandan A., Ang B., Bettencourt R., Hooker J., Behling C., Lin G., et al. (2015) Association between quantity of liver fat and cardiovascular risk in patients with nonalcoholic fatty liver disease independent of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 13: 1513–1520. [DOI] [PubMed] [Google Scholar]
- Budoff M., Young R., Lopez V., Kronmal R., Nasir K., Blumenthal R., et al. (2013) Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 61: 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callister T., Raggi P., Cooil B., Lippolis N., Russo D. (1998) Effect of HMG-COA Reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med 339: 1972–1978. [DOI] [PubMed] [Google Scholar]
- Chalasani N., Younossi Z., Lavine J., Diehl A., Brunt E., Cusi K., et al. (2012) The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55: 2005–2023. [DOI] [PubMed] [Google Scholar]
- Corey K., Vuppalanchi R., Wilson L., Cummings O., Chalasani N. NASH Clinical Research Network (2014) NASH resolution is associated with improvements in HDL and triglyceride levels but not improvement in LDL or non-HDL-C levels. Aliment Pharmacol Ther 41: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrano R., Guerci A., Carr J., Bild D., Burke G., Folsom A., et al. (2008) Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 358: 1336–1345. [DOI] [PubMed] [Google Scholar]
- Eichler K., Puhan M., Steurer J., Bachmann L. (2007) Prediction of first coronary events with the Framingham score: a systematic review. Am Heart J 153: 722–731, 731 e721–728. [DOI] [PubMed] [Google Scholar]
- Fargion S., Porzio M., Fracanzani A. (2014) Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol 20: 13306–13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell G., Van Rooyen D., Gan L., Chitturi S. (2012) NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver 6: 149–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldelli A., Kozakova M., Hojlund K., Flyvbjerg A., Favuzzi A., Mitrakou A., et al. (2009) Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 49: 1537–1544. [DOI] [PubMed] [Google Scholar]
- Kim D., Choi S., Park E., Lee W., Kang J., Kim W., et al. (2012) Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 56: 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozakova M., Palombo C., Eng M., Dekker J., Flyvbjerg A., Mitrakou A., et al. (2012) Fatty liver index, gamma-glutamyltransferase, and early carotid plaques. Hepatology 55: 1406–1415. [DOI] [PubMed] [Google Scholar]
- Le T.A., Chen J., Changchien C., Peterson M., Kono Y., Patton H., et al. (2012) Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 56: 922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann N., Paul A., Moebus S., Budde T., Dobos G., Michalsen A. (2011) Effects of lifestyle modification on coronary artery calcium progression and prognostic factors in coronary patients – 3-year results of the randomized safe-life trial. Atherosclerosis 219: 630–636. [DOI] [PubMed] [Google Scholar]
- Lin S., Heba E., Wolfson T., Ang B., Gamst A., Han A., et al. (2014) Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clin Gastroenterol Hepatol 13: 1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoff A., Wolski K., Nicholls S., Nissen S. (2007) Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298: 1180–1188. [DOI] [PubMed] [Google Scholar]
- Lonn E., Bosch J., Yusuf S., Sheridan P., Pogue J., Arnold J., et al. (2005) Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 293: 1338–1347. [DOI] [PubMed] [Google Scholar]
- Loomba R., Rao F., Zhang L., Khandrika S., Ziegler M., Brenner D., et al. (2010) Genetic covariance between gamma-glutamyl transpeptidase and fatty liver risk factors: role of beta2-adrenergic receptor genetic variation in twins. Gastroenterology 139: 836–845, 845 e831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R., Schork N., Chen C., Bettencourt R., Bhatt A., Ang B., et al. (2015a) Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology. 20 August 2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R., Sirlin C., Ang B., Bettencourt R., Jain R., Salotti J., et al. (2015b) Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 61: 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy J., Blaha M., Defilippis A., Budoff M., Nasir K., Blumenthal R., et al. (2010) Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol 56: 1613–1622. [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol In Adults (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 106: 3143–3421. [PubMed] [Google Scholar]
- Neuschwander-Tetri B., Loomba R., Sanyal A., Lavine J., Van Natta M., Abdelmalek M., et al. (2014) Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385: 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen S., Wolski K. (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356: 2457–2471. [DOI] [PubMed] [Google Scholar]
- Okwuosa T., Greenland P., Burke G., Eng J., Cushman M., Michos E., et al. (2012) Prediction of Coronary artery calcium progression in individuals with low Framingham risk score: the multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging 5: 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A., Blaha M., Sharma K., Rivera J., Budoff M., Blankstein R., et al. (2014) Family History of coronary heart disease and the incidence and progression of coronary artery calcification: multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 232: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky T., Blumenthal R., Greenland P. (2014) Coronary artery calcium score. JAMA 312: 837–838. [DOI] [PubMed] [Google Scholar]
- Sanyal A., Abdelmalek M., Suzuki A., Cummings O., Chojkier M. EPE-A Study Group (2014) No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 147: 377–384. [DOI] [PubMed] [Google Scholar]
- Sanyal A., Chalasani N., Kowdley K., Mccullough A., Diehl A., Bass N., et al. (2010) Pioglitazone, vitamin E, or Placebo for nonalcoholic steatohepatitis. N Engl J Med 362: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A., Friedman S., Mccullough A., Dimick-Santos L. (2015) Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases–U.S. Food and Drug Administration Joint Workshop. Hepatology 61: 1392–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmermund A., Achenbach S., Budde T., Buziashvili Y., Forster A., Friedrich G., et al. (2006) Effect of Intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation 113: 427–437. [DOI] [PubMed] [Google Scholar]
- Schurks M., Glynn R.J., Rist P., Tzourio C., Kurth T. (2010) Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ 341: c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer J., Celedon M., Lavine J., Salem R., Campbell N., Schork N., et al. (2009) Heritability of nonalcoholic fatty liver disease. Gastroenterology 136: 1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookoian S., Pirola C. (2008) Non-Alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol 49: 600–607. [DOI] [PubMed] [Google Scholar]
- Targher G., Day C., Bonora E. (2010) Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 363: 1341–1350. [DOI] [PubMed] [Google Scholar]
- Treeprasertsuk S., Leverage S., Adams L., Lindor K., St Sauver J., Angulo P. (2012) The Framingham risk score and heart disease in nonalcoholic fatty liver disease. Liver Int 32: 945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziomalos K. (2014) Lipid-lowering agents in the management of nonalcoholic fatty liver disease. World J Hepatol 6: 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanwagner L., Ning H., Lewis C., Shay C., Wilkins J., Carr J., et al. (2014) Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis 235: 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C., Stengel J., Asike M., Torres D., Shaw J., Contreras M., et al. (2011) Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140: 124–131. [DOI] [PubMed] [Google Scholar]
- Yeboah J., Mcclelland R., Polonsky T., Burke G., Sibley C., O’Leary D., et al. (2012) Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 308: 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A., Loomba R. (2012) Review article: the emerging interplay among the gastrointestinal tract, bile acids and incretins in the pathogenesis of diabetes and non-alcoholic fatty liver disease. Aliment Pharmacol Ther 36: 909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


