Chan et al. describe a combination of alleles with hypomorphic and activating mutations in the T cell signaling molecule ZAP-70 in a patient with autoimmunity.
Abstract
A brother and sister developed a previously undescribed constellation of autoimmune manifestations within their first year of life, with uncontrollable bullous pemphigoid, colitis, and proteinuria. The boy had hemophilia due to a factor VIII autoantibody and nephrotic syndrome. Both children required allogeneic hematopoietic cell transplantation (HCT), which resolved their autoimmunity. The early onset, severity, and distinctive findings suggested a single gene disorder underlying the phenotype. Whole-exome sequencing performed on five family members revealed the affected siblings to be compound heterozygous for two unique missense mutations in the 70-kD T cell receptor ζ-chain associated protein (ZAP-70). Healthy relatives were heterozygous mutation carriers. Although pre-HCT patient T cells were not available, mutation effects were determined using transfected cell lines and peripheral blood from carriers and controls. Mutation R192W in the C-SH2 domain exhibited reduced binding to phosphorylated ζ-chain, whereas mutation R360P in the N lobe of the catalytic domain disrupted an autoinhibitory mechanism, producing a weakly hyperactive ZAP-70 protein. Although human ZAP-70 deficiency can have dysregulated T cells, and autoreactive mouse thymocytes with weak Zap-70 signaling can escape tolerance, our patients’ combination of hypomorphic and activating mutations suggested a new disease mechanism and produced previously undescribed human ZAP-70–associated autoimmune disease.
The adaptive immune system is tightly regulated to allow responses against invading pathogens while avoiding injurious hyperactivity and misdirected responses to self-proteins. Impairment of lymphocyte pathways by genetic defects in mediators of immune signaling and activation can lead to immunodeficiency, but also to immune dysregulation, autoimmunity, and malignancy (Notarangelo, 2014). Essential steps in T cell activation and signaling include antigen recognition by the TCR–CD3 complex; tyrosine phosphorylation of immunoreceptor activation motifs (ITAMs) of the CD3 and ζ-chains by the tyrosine kinase Lck; interaction between phosphorylated ITAMs and the cytoplasmic tyrosine kinase ZAP-70; phosphorylation of ZAP-70 by Lck to relieve its autoinhibition and promote its activation; and ZAP-70–mediated phosphorylation of its adaptor substrates, leading to downstream events, including activation of the Ras–MAPK pathway and increased intracellular calcium.
ZAP-70, a critical T cell signaling molecule, is expressed predominantly in T and NK cells. It exists in an autoinhibited state, which is relieved by a two-step process. The first step, binding of the ZAP-70 tandem SH2 domains to doubly phosphorylated ITAMs of the ζ-chain, requires dissociation of the SH2 linker from the back of the kinase domain and repositioning of the SH2 domains to align with ζ-chain ITAMs. This change in structure facilitates a second conformational change whereby ZAP-70 tyrosines Y315 and Y319 in interdomain B are exposed and phosphorylated by Lck, leading to stabilization of the active conformation of the ZAP-70 catalytic domain to permit phosphorylation of downstream signaling molecules (Au-Yeung et al., 2009; Yan et al., 2013; Klammt et al., 2015). The phosphorylation of Y319 is particularly important because, in the nonphosphorylated state, it interacts with the N-lobe of the catalytic domain to maintain its inactive conformation.
Deficiency of ZAP-70 in humans causes a profound combined immunodeficiency (CID) in which CD8 T cells are absent and CD4 T cells are defective (Arpaia et al., 1994; Elder et al., 1994; Roifman, 1995). Affected individuals are susceptible to life-threatening infections and require hematopoietic cell transplantation (HCT) to survive (Arpaia et al., 1994; Chan et al., 1994; Katamura et al., 1999; Elder et al., 2001; Turul et al., 2009; Fischer et al., 2010; Roifman et al., 2010). Some ZAP-70–deficient patients also have skin infiltration with dysfunctional CD4 T cells, elevated serum IgE, and eosinophilia (Katamura et al., 1999; Turul et al., 2009).
In contrast to humans, mice with complete Zap-70 deficiency manifest developmental arrest of both CD4 and CD8 T lineages. A hypomorphic murine Zap-70 mutation with reduced ζ-chain binding caused attenuated TCR signaling that permitted survival of autoreactive T cells normally deleted in the thymus (Tanaka et al., 2010). In response to innate stimuli, these self-reactive murine T cells contributed to the development of non–tissue-specific autoantibodies (such as rheumatoid factor and antibody to cyclic citrullinated peptide) and autoimmune arthritis (Sakaguchi et al., 2012). Other hypomorphic alleles of Zap-70 in the mouse have also been associated with nonspecific autoantibodies (e.g., antinuclear antibodies; Siggs et al., 2007). In contrast, antibody-mediated autoimmune disease due to hypomorphic ZAP-70 alleles in human patients has not been reported.
We present two siblings with unique mutations of ZAP70 who lacked clinical immunodeficiency, but instead had a novel constellation of early onset, severe autoimmune manifestations, including bullous pemphigoid. Compound heterozygosity for hypomorphic and hyperactive mutant ZAP70 alleles in these patients represents a new genetic mechanism underlying inappropriate T cell activation.
RESULTS AND DISCUSSION
Manifestations of autoimmune syndrome
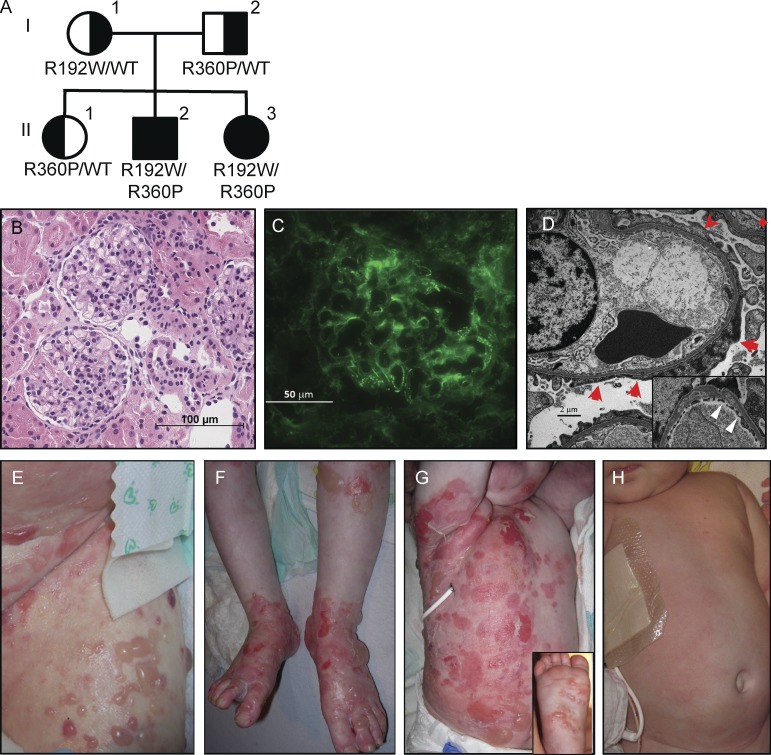
The two affected children were born at term to nonconsanguineous Caucasian parents with no family history of immune-mediated diseases. The parents and an older sibling were healthy (Fig. 1 A). At 9 mo of age, male II-2 developed nephrotic syndrome. A renal biopsy showed histologically normal glomeruli and mild IgG deposition, but electron microscopy revealed widespread foot process effacement most consistent with minimal change disease (Fig. 1, B–D). By 20 mo of age, blistering skin disease developed, involving his entire body and oral mucosa (Fig. 1, E and F); a biopsy showed bullous pemphigoid, with characteristic subepidermal clefting, infiltration with eosinophils and neutrophils, and linear basement membrane and intercellular deposition of IgG at a titer greater than 1:160 (not depicted). At age 2, he suffered bruising and a hemarthrosis as a result of an autoantibody to clotting factor VIII, and at 3 yr inflammatory colitis began. His autoimmune manifestations became refractory to high-dose steroids and multiple immunosuppressive regimens; therefore, he underwent a matched unrelated HCT at 5 yr of age that resolved his pretransplant autoimmunity.
Figure 1.
Pedigree and clinical phenotype of the affected siblings. (A) Family pedigree showing ZAP-70 genotypes. (B–D) Renal biopsy images from patient II-2. (B) Hematoxylin and eosin stain showing slightly increased glomerular mesangial matrix without hypercellularity or segmental sclerosis; no tubular, interstitial, or vascular abnormalities were noted. Bar, 100 µm. (C) Immunofluorescence demonstrating 1–2+ IgG staining in mesangium and capillary loops. Bar, 50 µm (D) Electron microscopy showing widespread foot process effacement (red arrows), with inset showing lucencies within the glomerular basement membrane (white triangles). Bar, 2 µm. (E–H) Bullous pemphigoid from patient II-2 (E and F); and from patient II-3 before (G) and 5 wk after (H) hematopoietic cell transplantation.
His sister II-3 experienced bullous pemphigoid beginning at 1 mo of age that rapidly became generalized (Fig. 1 G). She had poor weight gain that was initially attributed to a rotavirus infection but was later diagnosed by endoscopy as inflammatory colitis, similar to that of her brother. She also had proteinuria, although no renal biopsy was performed given the lack of progression to nephrotic syndrome. She received two HCTs at 6 and 28 mo of age from her HLA-matched healthy sister. Her pemphigoid resolved after the first HCT (Fig. 1 H), but a second HCT with myeloablative conditioning was performed after declining donor chimerism (37% donor CD3+ cells, 8% CD14+ cells, 7% CD19+ cells) indicated graft rejection and autoimmune hypothyroidism developed. At 3.5 yr of age, she is healthy with complete donor chimerism.
Neither child had infections suggesting immunodeficiency. Immunological workup revealed mild T and B cell lymphopenia in the brother and reduced CD8 T cells in both affected children, resulting in an elevated CD4/CD8 ratio (Table 1). Both children had normal newborn T cell receptor excision circles, as previously reported (Grazioli et al., 2014). The affected sister had increased CD19 B cells, and both children had elevated CD4/CD8 ratios, although not as high as generally found in patients with ZAP70 deficiency (Arpaia et al., 1994; Chan et al., 1994; Meinl et al., 2000; Elder et al., 2001; Picard et al., 2009; Turul et al., 2009; Newell et al., 2011; Karaca et al., 2013; Grazioli et al., 2014). The boy also had low total serum IgG, possibly attributable to nephrotic syndrome, but post-vaccine titers to type 14 pneumococcus and tetanus were protective. His proliferative responses were diminished to phytohemagglutinin (PHA) and absent of candida or tetanus antigens; however, he was receiving immunosuppressive medications when the immune studies were done. The sister’s cells, tested before institution of immunosuppressive medication, had diminished, but not absent, PHA responses.
Table 1. Immunological laboratory studies of affected siblings.
| Parameter | II-2 2 yr old (normal range, ages 2–5 yr) | II-3 2 mo old (normal range, age 1 wk–2 mo) |
|---|---|---|
| Lymphocyte subsets (cells × 10−6/l) | ||
| CD3 | 1,215 (1610–4,230) | 2,449 (2,070–6,540) |
| CD4 | 784 (900–2,860) | 2,041 (1,460–5,116) |
| CD8 | 294 (630–1,900) | 233 (650–2,490) |
| CD4/CD8 ratio | 2.7 (1–2.1) | 8.8 (1.3–3.5) |
| Naive CD4+CD45RA+ T cells | ND | 38% (64–95%) |
| Memory CD4+CD45RO+ T cells | ND | 27% (2–22%) |
| Naïve CD8+CD45RA+ T cells | ND | 50% (80–99%) |
| Memory CD8+CD45RO+ T cells | ND | 13% (1–9%) |
| CD19 | 216 (700–1,300) | 2215 (500–1,500) |
| NK | 490 (130–920) | 933 (170–1,100) |
| Newborn TRECsa (Normal >40 copies/µl of blood) | 99 | 75 |
| Serum immunoglobulin | ||
| IgA (mg/dl) | 37 (14–123) | 39 (3–47) |
| IgM (mg/dl) | 25 (48–168) | 9 (17–105) |
| IgG (mg/dl) | 276 (424–1051) | 334 (206–601) |
| IgE IU/ml | 99 (<64) | ND |
| Lymphocyte proliferation | ||
| PHA (as % CD45; Normal ≥ 49.9%) | 1% | 2.4% |
| Candida (Normal >3 SI) | 1 | ND |
| Tetanus (Normal >3 SI) | 1.1 | ND |
| Specific antibody titers | ||
| Pneumococcal IgG, Type 3 | 0.2 (>1.3 µg/ml) | ND |
| Pneumococcal IgG, Type 8 | 0.7 (>1.3 µg/ml) | ND |
| Pneumococcal IgG, Type 12 | 0.3 (>1.3 µg/ml) | ND |
| Pneumococcal IgG, Type 14 | 1.6 (>1.3 µg/ml) | ND |
| Antitetanus toxoid | 1.64 (>0.15 IU/ml) | ND |
ND, not done; PHA, phytohemagglutinin.
TREC: T cell receptor excision circle, measured in dried blood spots retrieved with parental consent from archived newborn specimens.
The immunological function of the healthy sister II-1 was normal; evaluations included leukocyte differential counts and lymphocyte subsets, T regulatory cells and B cell subsets, serum antibody concentrations, and proliferation to mitogens and antigens (unpublished data).
Identification of compound heterozygous mutations in ZAP70
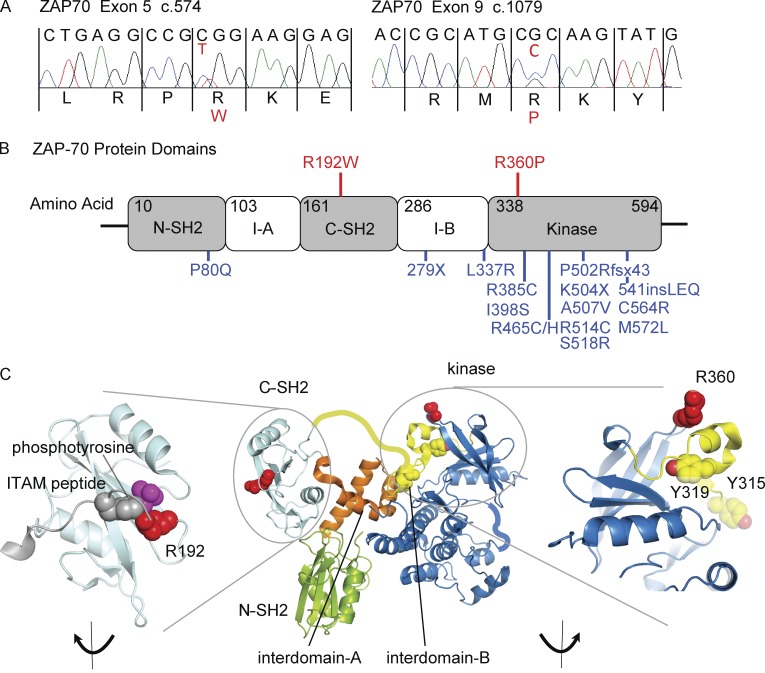
Analysis of whole-exome sequencing (WES) revealed no consanguinity and only one gene, ZAP70, with nonsynonymous coding variants potentially causing homozygous recessive immunological disease. Both affected children were compound heterozygous for ZAP70 c.574C>T, p.R192W, in exon 5; and c.1079G>C, p.R360P, in exon 9 (RefSeq accession nos. NM_001079.3 [ClinVar reference nos. SCV000258321 and SCV000258322] and NP_001070.2; Fig. 2 A). The mother carried the R192W allele, whereas the father and unaffected sister carried the R360P allele. Neither mutation had been reported previously, nor were they near mutations associated with human disease (Fig. 2 B; Matsuda et al., 1999; Turul et al., 2009; Newell et al., 2011; Hönig et al., 2012; Karaca et al., 2013; Ochs et al., 2014; Akar et al., 2015). The replacement of arginine with a bulky tryptophan residue in the phosphotyrosine-binding pocket of the ZAP-70 C-terminal SH2 domain (C-SH2; R192W mutation) was predicted to diminish ZAP-70 binding to the ζ-chain (Fig. 2 C, left; Deindl et al., 2007; Yan et al., 2013). In contrast, R360P lies in a loop of the N-lobe of the kinase domain in proximity to Y319, a key residue for autoinhibition of ZAP-70 (Fig. 2 C, right; Yan et al., 2013).
Figure 2.
ZAP-70 mutations. (A) Sanger sequence showing heterozygous mutations in II-2 (RefSeq accesssion nos. NM_001079.3 [ClinVar reference nos. SCV000258321 and SCV000258322] and NP_001070.2). (B) ZAP-70 protein diagram, showing N- and C-SH2 domains, interdomains I-A and I-B, and kinase domain. Mutation sites in the family are in red; previously reported mutations in combined immunodeficiency patients (all of which are loss-of-function) are in blue. (C) Three-dimensional structural representations of ZAP-70 (PyMOL software). Residues shown in red are R192 in the ITAM peptide phosphotyrosine binding site of the C-SH2 domain (magnified at left); and R360 in the kinase domain (blue), near amino acids Y315 and Y319 (yellow), known to participate in the autoinhibitory site (magnified at right).
Decreased binding of R192W ZAP-70 mutant to the ζ-chain
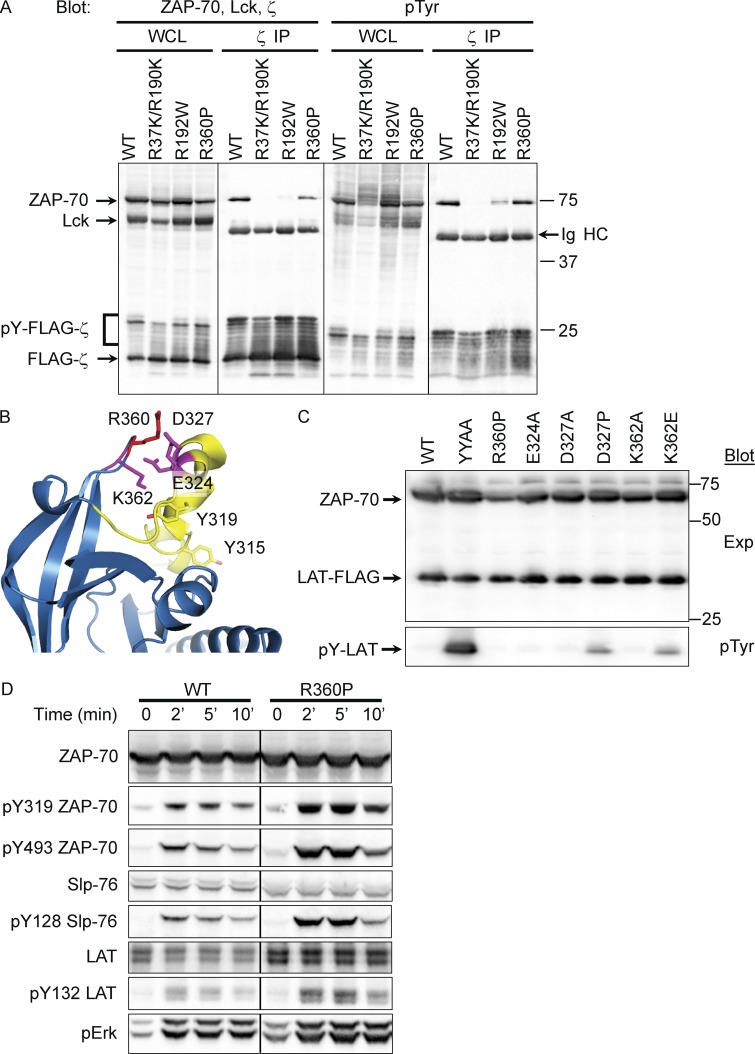
To assess the impact of the R192W mutation on high-affinity binding of ZAP-70 to phosphorylated ζ-chain ITAMs, 293T cells (which lack endogenous expression of Lck and ζ-chain) were transiently transfected with Lck, FLAG-tagged-ζ chain, and a panel of ZAP-70 constructs, including WT, R192W, R360P, and R37K/R190K; the latter construct is known to abrogate phosphotyrosine binding of both N- and C-SH2 domains (Labadia et al., 1997). Under these conditions, Lck phosphorylates the ζ-chain ITAM tyrosine residues, allowing recruitment of ZAP-70 to the signaling complex. Western blot analysis of whole-cell lysates demonstrated comparable expression of FLAG-ζ-chain, Lck, and each of the ZAP-70 constructs at the expected sizes (Fig. 3 A, far left).
Figure 3.
Association with the TCR ζ-chain and kinase activity of the mutant ZAP-70 proteins. (A) Blot of whole-cell lysates (WCL) and anti-TCR ζ-chain immunoprecipitates from 293T cells transfected with Lck (∼56 kD), FLAG-ζ-chain (∼18 kD), and WT, R37K/R190K, R192W, or R360P ZAP-70 (70kD). (left two panels) Protein expression levels of ZAP-70, Lck, or FLAG-ζ-chain from WCLs and levels of these proteins associated with ζ-chain immunoprecipitates. (right two panels) Phosphotyrosine (pTyr) immunoblots of WCL and ζ-chain immunoprecipitates. Ig heavy chain (Ig HC) is notated in the ζ-chain IPs. (B) Protein structure of the autoinhibitory site in the ZAP-70 kinase domain, highlighting residues known (Y315 and Y319) or proposed (E324, D327, R360, and K362) to participate in autoinhibition. R360, mutated in our patient, lies in the autoinhibitory region. (C) Immunoblots on WCLs from 293T cells transfected with LAT and WT and mutant ZAP-70 (mutants include YYAA, R360P, E324A, D327A, K362A, and K362E). (top) Expression of ZAP-70 and LAT; (bottom) pTyr blotting to show phosphorylated LAT. (D) After 24 h of doxycycline treatment to induce WT or R360P ZAP-70 protein expression in ZAP-70 deficient P116 rtTA cell lines, cells were stimulated with anti-TCR antibody for 0, 2, 5, or 10 min. Immunoblots of WCLs are shown for ZAP-70, pY319, and pY493 ZAP-70, Slp-76, pY128 Slp-76, LAT, pY132, and pErk. Data are representative of four independent experiments.
The ability of the ZAP-70 constructs to bind phosphorylated ITAMs was tested using Western blots of immunoprecipitates of FLAG-ζ-chain blotted for associated ZAP-70 or anti-pTyr. Comparable FLAG-ζ-chain protein was immunoprecipated in each lane (Fig. 3 A, second from left). Small contributions of higher molecular weight signals representing phosphorylated ζ-chain were also detected (pY-FLAG-ζ). There was a reduction of R192W and R37K/R190K mutant ZAP-70 proteins associated with the FLAG-ζ-chain when compared with WT ZAP-70 (Fig. 3 A, second from left), but both WT and R360P were comparably coimmunoprecipitated with the FLAG-ζ chain. Phosphorylation of WT and R360P ZAP-70 coimmunoprecipitated with the FLAG-ζ-chain was readily detected, whereas only a small amount of R192W protein was phosphorylated (Fig. 3 A, far right). Thus, the data suggest that the R192W ZAP-70 mutant is a hypomorph with reduced ζ-chain association and phosphorylation compared with WT.
Mutations adjacent to R360P in ZAP-70 cause loss of autoinhibition
Based on its location, the R360P mutation was hypothesized to destabilize the autoinhibited conformation of ZAP-70 (Fig. 3 B; Yan et al., 2013). Mutations of tyrosines 315 and 319 to alanine (YYAA) in this region render ZAP-70 constitutively active, causing phosphorylation of substrates such as LAT, a critical T cell signaling adaptor, even in the absence of Lck (Brdicka et al., 2005; Deindl et al., 2007; Yan et al., 2013). An activating effect as robust as the YYAA mutant might be expected to confer a dominant phenotype in humans carrying a single mutant allele; the fact that the father I-2 and sister II-1 of our patients were asymptomatic heterozygous carriers suggested that R360P might be more weakly activating than YYAA.
To examine the possible activating effect of the R360P mutation on ZAP-70, 293T cells were transfected with FLAG-LAT and various ZAP-70 constructs, including WT, YYAA, R360P, and other substitutions at nearby residues proposed to participate in autoinhibition (Fig. 3 C; Yan et al., 2013). Immunoblotting showed the expected robust LAT phosphorylation by the YYAA mutant. Interestingly, a modest degree of LAT phosphorylation was observed with mutants D327P and K362E located near R360P (Fig. 3 C, bottom); however, spontaneous LAT phosphorylation was not detected in 293T cells transfected with WT ZAP-70 or with the R360P or other nearby mutants. Thus, although constitutive activity of R360P was not revealed in 293 cells, the increased activity of the nearby D327P and K362E mutants does support the notion that mutations in the region containing R360P might disrupt an important autoinhibitory mechanism.
Effects of ZAP-70 patient mutations in a human T cell line and primary human T cells
Although the R360P mutant did not constitutively phosphorylate LAT in transfected 293T cells, compromised autoinhibition might nonetheless be detected under more physiological conditions in T lymphocytes, in which Lck is expressed and contributes to TCR recruitment and activation of ZAP-70. P116 is a Jurkat-derived T cell line lacking endogenous ZAP-70 but expressing Lck and the full complement of regulators of the TCR signaling pathway (Williams et al., 1998). Although stable expression of the R360P allele in P116 cells was not achieved, possibly due to toxicity of the putative activated R360P allele, R360P and WT ZAP-70 were expressed using a Tet-inducible system (Gossen et al., 1995), as previously used to express PTEN and CD148 (Baker et al., 2001; Xu et al., 2002), proteins that were toxic as stable constructs. Comparison of cells induced to express comparable amounts of WT or R360P revealed higher TCR-induced phosphorylation of ZAP-70 at Y319 and Y493 (interdomain B and activation loop sites, respectively), as well as the immediate downstream ZAP-70 substrates SLP-76 and LAT (Fig. 3 D). Similarly, downstream induction of Erk phosphorylation was also higher in P116 cells induced to express R360P compared with cells induced to express WT ZAP-70. These results were consistent with the hypothesis that R360P ZAP-70 is a weakly hyperactive protein due to disruption of its autoinhibitory mechanism.
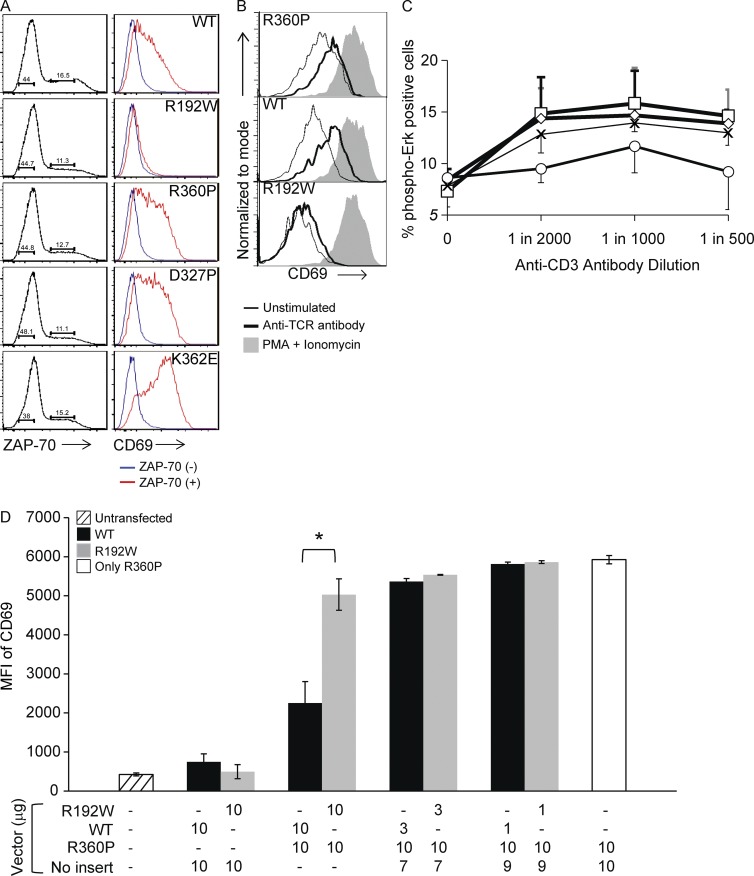
To evaluate the functional consequences of our patients’ mutations relative to other ZAP-70 mutants in P116 cells, we investigated whether the mutations in transiently transfected cells altered surface expression of CD69, a marker of TCR signaling mediated by the Ras–MAPK pathway (d’Ambrosio et al., 1994; Roose et al., 2005). Transiently transfected P116 cells expressing R360P ZAP-70 had higher basal CD69 surface expression than those expressing equal amounts of WT or R192W ZAP-70 (Fig. 4 A). Thus, although not evident in 293T lacking Lck, similar to the data obtained with the inducible stable system, the ZAP-70 R360P mutation indeed caused some degree of loss of autoinhibition when Lck was present, even without TCR stimulation. As might have been predicted from the 293 cell experiments in Fig. 3 C, P116 cells transfected with the mutants D327P and K362E showed even higher basal CD69 expression, consistent with their greater ability to prevent ZAP-70 autoinhibition.
Figure 4.
Activation states of cells transfected with mutant ZAP-70. (A) Basal CD69 expression in P116 cells transfected with ZAP-70 constructs. Cell populations gated on ZAP-70 expressing cells (left) and their corresponding CD69 expression (right, blue, ZAP-70−; red, ZAP-70+). (B) CD69 expression in transfected cells with equal ZAP-70 expression, unstimulated (thin lines), or stimulated (bold lines) with anti-TCR antibody C305 or PMA plus ionomycin (shading). (C) Phospho-Erk expression in PBMCs from the father (open squares), healthy sister (open diamonds), and mother (open circles) of the affected patients and from four controls (Xs), stimulated with graded dilutions of anti-CD3 antibody for 15 min. Error bars, SEM. Data are representative of four independent experiments. Note that affected patients’ cells were not available due to their having received hematopoietic stem cell transplants before identification of their gene defect. (D) CD69 expression by mean fluorescence intensity (MFI) in P116 cells either untransfected (hatched bar) or transfected with indicated amounts of plasmid vectors containing no insert or ZAP-70 cDNA constructs, as shown. All transfections contained 20 µg of plasmid DNA. Black bars, WT with or without R360P; gray bars, R192W with or without R360P. White bar, R360P plus vector with no insert. *, P < 0.05, unpaired Student’s t test. Data are representative of three independent experiments.
We also assessed CD69 expression after stimulation (Fig. 4 B). All transfected P116 cells up-regulated CD69 in response to stimulation with PMA plus ionomycin, demonstrating intact activation when bypassing TCR-dependent pathways. After stimulation with anti-TCR antibody, however, transfected cells expressing WT or R360P ZAP-70 up-regulated CD69 to equivalent levels, whereas those expressing R192W showed only a minimal increase. Thus, the response of cells expressing R360P was inducible and distinct from that of the hypomorphic mutant.
Although direct assessment of the patients’ T cells was not possible due to their prior HCT treatment, further evidence of the effects of the ZAP70 mutations in this family was sought by stimulating with anti-CD3 PBMCs from healthy controls and the patients’ heterozygous carrier relatives (Fig. 4 C). Maternal cells (WT/R192W) showed decreased phosphorylation of the MAP kinases Erk1 and Erk2 in an anti-CD3 dose dependent manner, whereas cells from the father and healthy sister (WT/R360P) tended to have higher Erk phosphorylation. This was consistent with the transfection experiments showing that R192W is a hypoactive mutation, whereas R360P is weakly hyperactive.
Finally, to reveal any influence that either the WT or R192W ZAP-70 proteins might have on the function of the hyperactive R360P allele, P116 cells were transfected with fixed total amounts of plasmids encoding ZAP-70 R360P with decreasing amounts of either WT or R192W. The cells expressing only R360P had a high basal expression of CD69 (Fig. 4 D, clear bar). When equal amounts of R360P and either WT or R192W were transfected, the basal CD69 levels of cells coexpressing WT and R360P were lower than those of cells coexpressing R192W and R360P (Fig. 4 D). Thus, the hyperactive R360P phenotype was masked by WT ZAP-70 and revealed only in the presence of the hypoactive R192W allele.
New phenotype and new inheritance mechanism for ZAP-70–associated disease
Our WES approach and cellular analysis revealed not only unexpected mutations in ZAP70, but also a new mechanism of inheritance causing a previously unreported constellation of severe autoimmune features. In particular, autoantibody-mediated bullous pemphigoid usually occurs in the elderly, and unlike in our patients, when found in young children it is very responsive to steroid treatment (Waisbourd-Zinman et al., 2008). Patients with previously reported ZAP-70 mutations have all had two recessive heterozygous loss-of-function mutations, which disrupted catalytic activity, led to early truncation, or abrogated the N-SH2 domain required for binding to ζ-chain ITAM (Fig. 2 B; Arpaia et al., 1994; Chan et al., 1994; Elder et al., 1994; Roifman, 1995; Katamura et al., 1999; Matsuda et al., 1999; Turul et al., 2009; Fischer et al., 2010; Roifman et al., 2010). Although some patients exhibited nonspecific autoinflammation, eosinophilia, and elevated IgE reminiscent of Omenn syndrome or other leaky SCID and CID disorders, the predominant phenotype was absence of CD8 T cells and impaired CD4 T cell function, leading to severe and opportunistic infections. In marked contrast, our patients had a distinct phenotype with early onset, severe to life-threatening autoimmune disease and only minor lymphocyte abnormalities and no evidence of immune deficiency (Table 1).
In mice, defects in both central and peripheral tolerance have been associated with hypomorphic Zap-70 defects and autoimmune features (Siggs et al., 2007; Au-Yeung et al., 2010; Tanaka et al., 2010). In humans, ZAP70 deficiency can alter the thymic stromal compartment, reduce thymic T reg cell numbers, and develop an autoimmune-prone gene expression profile in CD4 T cells (Roifman et al., 2010; Poliani et al., 2013). However, autoantibody-associated disorders such as those of our patients were not present. Our patients’ ZAP70 defects alter T cell signaling, a recognized cause of autoimmunity (Moulton and Tsokos, 2015). Altered signaling could allow autoreactive T cells to escape thymic deletion and emigrate into the periphery, where they could activate potentially autoreactive B cells, thereby leading to production of autoantibodies to antigens such as skin proteins (bullous pemphigoid) and clotting factors (autoimmune hemophilia). ZAP-70 has also been linked to B cell malignancy, and our mutations could also affect early B cell tolerance directly if, as in the mouse, ZAP-70 is expressed at low levels in human B cells (Fallah-Arani et al., 2008).
Although most human primary immunodeficiencies are caused by recessive autosomal or X-linked gene mutations, dominant mutations are increasingly recognized, with mechanisms including haploinsufficiency, as in DiGeorge syndrome with hemizygous deletion of chromosome 22q11 or intragenic TBX1 loss of function mutations (Baldini, 2006; McDonald-McGinn and Sullivan, 2011); dominant-negative effects, such as in autoimmune lymphoproliferative syndrome (Wang et al., 2010); and toxic gain-of-function or hypermorphic alleles (Boisson et al., 2015). The latter category is typified by STAT3-activating mutations (Flanagan et al., 2014), which lead to early onset autoimmunity, in contrast to the dominant-negative defects in the same gene that cause hyper-IgE syndrome with boils and necrotizing pneumonias. Our patients are the first humans, to our knowledge, to have a constitutively activating mutation of ZAP-70 with a disease-causing gain of function; but, the syndrome caused by the weakly activating allele requires pairing with the hypomorphic allele to produce the phenotypic manifestations.
We report a previously undescribed form of compound heterozygosity in which autoimmunity arose from the combination of the ZAP-70 R192W allele with reduced ζ-chain binding together with the constitutively active R360P allele with reduced autoinhibition. In the father and sister who carried only R360P, the effects of the activating allele were neutralized by the presence of WT ZAP-70. Although both the weakly activating and WT alleles bind similarly to the phospho-ζ-chain, the presence of a WT ZAP-70 allele in this context was able to maintain an appropriate balance in controlling T cell activation. Stronger activating alleles, such as the experimental YYAA or K362E mutants, could potentially manifest a dominant phenotype in humans. Overall, our findings expand the clinical phenotype and inheritance mechanism of ZAP-70 defects.
MATERIALS AND METHODS
Patients
With informed consent, individuals depicted in Fig. 1 A enrolled in a UCSF-approved protocol and submitted samples for immunological assessment and WES.
Exome sequencing and analysis
Genomic DNA from blood from parents and children (Fig. 1 A) was subjected to WES (HiSeq2500; Illumina) and analysis to identify nonsynonymous, rare variants present in both affected siblings. Read alignment and variant calling were performed using a previously described protocol (Patel et al., 2015). The variants were reconfirmed with updated software versions BWA version 0.7.10 for read mapping and GATK version 3.1–1 for base quality score recalibration, realignment around known indels, variant calling, and variant quality score recalibration. Average coverage across the capture region in the five samples was 43–113×, ≥45× in 85–96% of capture sites. Variants were annotated using an in-house annotation tool, Varant (Brenner, 2015), to identify stop-gain, stop-loss, start-loss, nonsynonymous, frameshift-insert, frameshift-delete, nonframeshift-insert, nonframeshift-delete, splice-donor, and splice-acceptor variants. Variants marked as high quality by GATK (truth sensitivity level 99.9 for SNPs, 99.0 for indels), with genotype quality (GQ) ≥30 and minor allele frequency (MAF) ≤0.05 in both 1000 Genomes (Abecasis et al., 2012) and ExAC database (http://exac.broadinstitute.org) were retained for downstream analysis. Additionally, variants in the affected siblings were required to follow a compound heterozygous, homozygous recessive, X-linked recessive, or uniparental disomy inheritance model or to be absent in both parents. These steps shortlisted 88 variants in 30 genes for further analysis (Fig. S1). Priority was given to variants in genes known to be associated with human immune disorders (Al-Herz et al., 2011).
The affected siblings shared a maternal rare ZAP70 variant, c.574C>T, p.R192W (chr2: 98349356, NM_001079.3; NP_001070.2), with allele frequency 0.00003 (ExAC), predicted deleterious by SIFT (Ng and Henikoff, 2001), benign by Polyphen2 (Adzhubei et al., 2010), and damaging by CADD (Kircher et al., 2014). They also shared with their father and unaffected sibling a novel ZAP70 variant, c.1079G>C, p.R360P (chr2: 98351172), predicted probably damaging by SIFT, Polyphen2, and CADD. ZAP70 variants were verified by Sanger sequencing.
The affected siblings had one de novo coding variant of unknown significance in the histone deacetylase gene HDAC4 (Ch2, 240016733G>T; p.F746L; EXAC frequency 6.32 × 10−5). No immune phenotype is associated with this gene, which was not investigated further.
Consanguinity calculated using the KING toolset (Manichaikul et al., 2010) showed parental kinship coefficient 0.005, indicating that the parents were unrelated. Details are available from the authors upon request.
Rare coding region variants (<1% frequency in 1000 Genomes and EXAC databases) that were compound heterozygous in at least one affected individual are available from the authors upon request.
Plasmid constructs and protein detection
WT ZAP70 cDNA and mutants R192W, R360P, YYAA (Brdicka et al., 2005; Deindl et al., 2007), E324A, D327A, D327P, K362A, and K362E (prepared with GENEART site-directed mutagenesis kits) in vector pEF6 (Invitrogen), pEF6 Lck, and pEF ζ-chain, or pEF FLAG-tagged LAT were expressed in 293T cells using Lipofectamine and PLUS reagents (Invitrogen). The pEF ζ-chain construct also contained FLAG and a CD2 transmembrane domain, with the latter helping to localize the ζ-chain to the cell membrane, as the TCR complex is lacking in the in 293T cells. Lysis, immunoprecipitation, SDS PAGE, and Western blots were performed as previously described (Deindl et al., 2007) with monoclonal 2F3.2 (anti–ZAP-70), 4G10 (anti-phosphotyrosine), M2 (anti-FLAG; Sigma-Aldrich), 1F6 (anti-Lck), and 6B10 (anti-ζ).
The ZAP-70–deficient Jurkat-derived T cell line P116 (from R. Abraham, Burnham Institute, La Jolla, CA; Williams et al., 1998) was stably transfected with the reverse tetracycline transactivator (rtTA) pUHD 172–1 neo rtTA (a gift from H. Bujard, Zentrum für Molekulare Biologie der Universität Heidelberg, Heidelberg, Germany), and subsequently stably transfected with either ZAP-70 WT or R360P cloned into the tetracycline response element (TRE) construct pUHD 10–3 mcs. WT or R360P ZAP-70 proteins were induced in the tetracycline-responsive stable cell lines by addition of 1 µg/ml doxycycline for 24 h. Cells were pelleted and resuspended at 108/ml in PBS (with calcium and magnesium) and rested for 15 min at 37°C, then stimulated for 0, 2, 5, and 10 min with 1.7 µg/ml anti–human TCR Vβ8 antibody C305 (Brdicka et al., 2005). Cells were lysed at 108/ml in 1% NP-40 lysis buffer containing protease and phosphatase inhibitors, and lysates were run on TRIS/BIS gels. After transfer to Immobilon (EMD Millipore), Western blotting was performed using the following antibodies: ZAP-70: 2F3.2 (Brdicka et al., 2005); anti-pY319 ZAP-70 (2717S), anti-pY493 ZAP-70 (2704S), and anti-pErk (4377S; Cell Signaling Technology); anti-pY128 Slp-76 (558367; BD); anti–Slp-76 (sc-9062), and anti-LAT (sc-7948; Santa Cruz Biotechnology, Inc.); and anti-pY132 LAT (ab4476; Abcam).
P116 cells were transiently transfected by electroporation with a constant total amount of DNA consisting of combinations of empty plasmid, WT, and mutant ZAP70 cDNA expression constructs. The cells were rested for 8 h, stimulated overnight with anti-TCR antibody C305 1:1,000 ascites, or PMA 20 ng/µl + ionomycin 1 µM, and assayed by flow cytometry. Surface expression of CD69 (CD69-PE; BD) and intracellular ZAP-70 (clone 1E7.2-Alexa Fluor 488; eBioscience) were analyzed 16 h after transfection.
Human T cell activation assays
Peripheral blood mononuclear cells (PBMCs) from family members and controls were stimulated with anti-CD3 (235) antibody dilutions for 15 min at 37°C, and then fixed and permeabilized with prewarmed Cytofix (BD), followed by ice-cold 90% MeOH. Cells were then stained with rabbit anti-Erk p44/42 (Cell Signaling Technology) and PE-conjugated anti–rabbit antibody (Jackson ImmunoResearch Laboratories); and analyzed by flow cytometry. Surface expression of CD69 and intracellular ZAP-70 were measured as above.
Online supplemental material
Fig. S1 shows our exome analysis. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20150888/DC1.
Supplementary Material
Acknowledgments
The authors thank Drs. Christopher Dvorak, Biljana Horn, James Huang, and Amy Gilliam for outstanding clinical care and Diana Gonzalez, Karly Kondratowicz, Jacob Mallott, and Yanning Wang for expert technical contributions.
Dr. Brenner received support from National Institutes of Health (NIH) R01 grant AI105776, Tata Consultancy Services (TCS), and unrestricted research funds from Celera. Dr. Puck received support from NIH R01 grants AI078248 and AI105776, NCATS TL1TR000144 to the UCSF CTSI, the Lisa and Douglas Goldman Foundation, and the UCSF Jeffrey Model Diagnostic Center for Primary Immunodeficiencies. Drs. Weiss and Kuriyan received support from the Howard Hughes Medical Institute and NIH P01 AI091580. Drs. Cowan and Puck received support from U54 AI082973.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- CID
- combined immunodeficiency
- HCT
- hematopoietic cell transplantation
- ZAP-70
- ζ-chain associated protein 70-kD T cell receptor
References
- Abecasis G.R., Auton A., Brooks L.D., DePristo M.A., Durbin R.M., Handsaker R.E., Kang H.M., Marth G.T., and McVean G.A.. 1000 Genomes Project Consortium . 2012. An integrated map of genetic variation from 1,092 human genomes. Nature. 491:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., and Sunyaev S.R.. 2010. A method and server for predicting damaging missense mutations. Nat. Methods. 7:248–249. 10.1038/nmeth0410-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akar H.H., Patiroglu T., Akyildiz B.N., Tekerek N.U., Doğan M.S., Doğanay S., van der Burg M., and Dusunsel R.. 2015. Silent brain infarcts in two patients with zeta chain-associated protein 70 kDa (ZAP70) deficiency. Clin. Immunol. 158:88–91. 10.1016/j.clim.2015.03.014 [DOI] [PubMed] [Google Scholar]
- Al-Herz W., Bousfiha A., Casanova J.L., Chapel H., Conley M.E., Cunningham-Rundles C., Etzioni A., Fischer A., Franco J.L., Geha R.S., et al. 2011. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front. Immunol. 2:54 10.3389/fimmu.2011.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia E., Shahar M., Dadi H., Cohen A., and Roifman C.M.. 1994. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell. 76:947–958. 10.1016/0092-8674(94)90368-9 [DOI] [PubMed] [Google Scholar]
- Au-Yeung B.B., Deindl S., Hsu L.Y., Palacios E.H., Levin S.E., Kuriyan J., and Weiss A.. 2009. The structure, regulation, and function of ZAP-70. Immunol. Rev. 228:41–57. 10.1111/j.1600-065X.2008.00753.x [DOI] [PubMed] [Google Scholar]
- Au-Yeung B.B., Levin S.E., Zhang C., Hsu L., Cheng D.A., Killeen N., and Weiss A.. 2010. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat. Immunol. 11:1085–1092. 10.1038/ni.1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.E., Majeti R., Tangye S.G., and Weiss A.. 2001. Protein tyrosine phosphatase CD148-mediated inhibition of T-cell receptor signal transduction is associated with reduced LAT and phospholipase Cgamma1 phosphorylation. Mol. Cell. Biol. 21:2393–2403. 10.1128/MCB.21.7.2393-2403.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini A. 2006. The 22q11.2 deletion syndrome: a gene dosage perspective. ScientificWorldJournal. 6:1881–1887. 10.1100/tsw.2006.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson B., Quartier P., and Casanova J.L.. 2015. Immunological loss-of-function due to genetic gain-of-function in humans: autosomal dominance of the third kind. Curr. Opin. Immunol. 32:90–105. 10.1016/j.coi.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brdicka T., Kadlecek T.A., Roose J.P., Pastuszak A.W., and Weiss A.. 2005. Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases. Mol. Cell. Biol. 25:4924–4933. 10.1128/MCB.25.12.4924-4933.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S.E.V. 2015. Brenner Lab, University of California, San Francisco: http://compbio.berkeley.edu/proj/varant/Home.html. (Accessed May 20, 2015) [Google Scholar]
- Chan A.C., Kadlecek T.A., Elder M.E., Filipovich A.H., Kuo W.L., Iwashima M., Parslow T.G., and Weiss A.. 1994. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 264:1599–1601. 10.1126/science.8202713 [DOI] [PubMed] [Google Scholar]
- d’Ambrosio D., Cantrell D.A., Frati L., Santoni A., and Testi R.. 1994. Involvement of p21ras activation in T cell CD69 expression. Eur. J. Immunol. 24:616–620. 10.1002/eji.1830240319 [DOI] [PubMed] [Google Scholar]
- Deindl S., Kadlecek T.A., Brdicka T., Cao X., Weiss A., and Kuriyan J.. 2007. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell. 129:735–746. 10.1016/j.cell.2007.03.039 [DOI] [PubMed] [Google Scholar]
- Elder M.E., Lin D., Clever J., Chan A.C., Hope T.J., Weiss A., and Parslow T.G.. 1994. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 264:1596–1599. 10.1126/science.8202712 [DOI] [PubMed] [Google Scholar]
- Elder M.E., Skoda-Smith S., Kadlecek T.A., Wang F., Wu J., and Weiss A.. 2001. Distinct T cell developmental consequences in humans and mice expressing identical mutations in the DLAARN motif of ZAP-70. J. Immunol. 166:656–661. 10.4049/jimmunol.166.1.656 [DOI] [PubMed] [Google Scholar]
- Fallah-Arani F., Schweighoffer E., Vanes L., and Tybulewicz V.L.. 2008. Redundant role for Zap70 in B cell development and activation. Eur. J. Immunol. 38:1721–1733. 10.1002/eji.200738026 [DOI] [PubMed] [Google Scholar]
- Fischer A., Picard C., Chemin K., Dogniaux S., le Deist F., and Hivroz C.. 2010. ZAP70: a master regulator of adaptive immunity. Semin. Immunopathol. 32:107–116. 10.1007/s00281-010-0196-x [DOI] [PubMed] [Google Scholar]
- Flanagan S.E., Haapaniemi E., Russell M.A., Caswell R., Lango Allen H., De Franco E., McDonald T.J., Rajala H., Ramelius A., Barton J., et al. 2014. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat. Genet. 46:812–814. 10.1038/ng.3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Freundlieb S., Bender G., Müller G., Hillen W., and Bujard H.. 1995. Transcriptional activation by tetracyclines in mammalian cells. Science. 268:1766–1769. 10.1126/science.7792603 [DOI] [PubMed] [Google Scholar]
- Grazioli S., Bennett M., Hildebrand K.J., Vallance H., Turvey S.E., and Junker A.K.. 2014. Limitation of TREC-based newborn screening for ZAP70 severe combined immunodeficiency. Clin. Immunol. 153:209–210. 10.1016/j.clim.2014.04.015 [DOI] [PubMed] [Google Scholar]
- Hönig M., Schuetz C., Schwarz K., Rojewski M., Jacobsen E., Lahr G., Debatin K.M., Schulz A., and Friedrich W.. 2012. Immunological reconstitution in a patient with ZAP-70 deficiency following transfusion of blood lymphocytes from a previously transplanted sibling without conditioning. Bone Marrow Transplant. 47:305–307. 10.1038/bmt.2011.71 [DOI] [PubMed] [Google Scholar]
- Karaca E., Karakoc-Aydiner E., Bayrak O.F., Keles S., Sevli S., Barlan I.B., Yuksel A., Chatila T.A., and Ozen M.. 2013. Identification of a novel mutation in ZAP70 and prenatal diagnosis in a Turkish family with severe combined immunodeficiency disorder. Gene. 512:189–193. 10.1016/j.gene.2012.10.062 [DOI] [PubMed] [Google Scholar]
- Katamura K., Tai G., Tachibana T., Yamabe H., Ohmori K., Mayumi M., Matsuda S., Koyasu S., and Furusho K.. 1999. Existence of activated and memory CD4+ T cells in peripheral blood and their skin infiltration in CD8 deficiency. Clin. Exp. Immunol. 115:124–130. 10.1046/j.1365-2249.1999.00759.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher M., Witten D.M., Jain P., O’Roak B.J., Cooper G.M., and Shendure J.. 2014. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 46:310–315. 10.1038/ng.2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klammt C., Novotná L., Li D.T., Wolf M., Blount A., Zhang K., Fitchett J.R., and Lillemeier B.F.. 2015. T cell receptor dwell times control the kinase activity of Zap70. Nat. Immunol. 16:961–969. 10.1038/ni.3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labadia M.E., Jakes S., Grygon C.A., Greenwood D.J., Schembri-King J., Lukas S.M., Warren T.C., and Ingraham R.H.. 1997. Interaction between the SH2 domains of ZAP-70 and the tyrosine-based activation motif 1 sequence of the ζ subunit of the T-cell receptor. Arch. Biochem. Biophys. 342:117–125. 10.1006/abbi.1997.0118 [DOI] [PubMed] [Google Scholar]
- Manichaikul A., Mychaleckyj J.C., Rich S.S., Daly K., Sale M., and Chen W.M.. 2010. Robust relationship inference in genome-wide association studies. Bioinformatics. 26:2867–2873. 10.1093/bioinformatics/btq559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S., Suzuki-Fujimoto T., Minowa A., Ueno H., Katamura K., and Koyasu S.. 1999. Temperature-sensitive ZAP70 mutants degrading through a proteasome-independent pathway. Restoration of a kinase domain mutant by Cdc37. J. Biol. Chem. 274:34515–34518. 10.1074/jbc.274.49.34515 [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn D.M., and Sullivan K.E.. 2011. Chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Medicine (Baltimore). 90:1–18. 10.1097/MD.0b013e3182060469 [DOI] [PubMed] [Google Scholar]
- Meinl E., Lengenfelder D., Blank N., Pirzer R., Barata L., and Hivroz C.. 2000. Differential requirement of ZAP-70 for CD2-mediated activation pathways of mature human T cells. J. Immunol. 165:3578–3583. 10.4049/jimmunol.165.7.3578 [DOI] [PubMed] [Google Scholar]
- Moulton V.R., and Tsokos G.C.. 2015. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J. Clin. Invest. 125:2220–2227. 10.1172/JCI78087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell A., Dadi H., Goldberg R., Ngan B.Y., Grunebaum E., and Roifman C.M.. 2011. Diffuse large B-cell lymphoma as presenting feature of Zap-70 deficiency. J. Allergy Clin. Immunol. 127:517–520. 10.1016/j.jaci.2010.09.016 [DOI] [PubMed] [Google Scholar]
- Ng P.C., and Henikoff S.. 2001. Predicting deleterious amino acid substitutions. Genome Res. 11:863–874. 10.1101/gr.176601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo L.D. 2014. Immunodeficiency and immune dysregulation associated with proximal defects of T cell receptor signaling. Curr. Opin. Immunol. 31:97–101. 10.1016/j.coi.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs H. D., Smith C. I. E., and Puck J. M.. 2014. Primary immunodeficiency diseases: A molecular and genetic approach. Third edition Oxford University Press, New York: 911 pp. [Google Scholar]
- Patel J.P., Puck J.M., Srinivasan R., Brown C., Sunderam U., Kundu K., Brenner S.E., Gatti R.A., and Church J.A.. 2015. Nijmegen breakage syndrome detected by newborn screening for T cell receptor excision circles (TRECs). J. Clin. Immunol. 35:227–233. 10.1007/s10875-015-0136-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C., Dogniaux S., Chemin K., Maciorowski Z., Lim A., Mazerolles F., Rieux-Laucat F., Stolzenberg M.C., Debre M., Magny J.P., et al. 2009. Hypomorphic mutation of ZAP70 in human results in a late onset immunodeficiency and no autoimmunity. Eur. J. Immunol. 39:1966–1976. 10.1002/eji.200939385 [DOI] [PubMed] [Google Scholar]
- Poliani P.L., Fontana E., Roifman C.M., and Notarangelo L.D.. 2013. ζ Chain-associated protein of 70 kDa (ZAP70) deficiency in human subjects is associated with abnormalities of thymic stromal cells: Implications for T-cell tolerance. J. Allergy Clin. Immunol. 131:597 10.1016/j.jaci.2012.11.002 [DOI] [PubMed] [Google Scholar]
- Roifman C.M. 1995. A mutation inzap-70 protein tyrosine kinase results in a selective immunodeficiency. J. Clin. Immunol. 15:S52–S62. 10.1007/BF01540894 [DOI] [PubMed] [Google Scholar]
- Roifman C.M., Dadi H., Somech R., Nahum A., and Sharfe N.. 2010. Characterization of ζ-associated protein, 70 kd (ZAP70)-deficient human lymphocytes. J. Allergy Clin. Immunol. 126:1226–1233: 1233.e1. 10.1016/j.jaci.2010.07.029 [DOI] [PubMed] [Google Scholar]
- Roose J.P., Mollenauer M., Gupta V.A., Stone J., and Weiss A.. 2005. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol. Cell. Biol. 25:4426–4441. 10.1128/MCB.25.11.4426-4441.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Benham H., Cope A.P., and Thomas R.. 2012. T-cell receptor signaling and the pathogenesis of autoimmune arthritis: insights from mouse and man. Immunol. Cell Biol. 90:277–287. 10.1038/icb.2012.4 [DOI] [PubMed] [Google Scholar]
- Siggs O.M., Miosge L.A., Yates A.L., Kucharska E.M., Sheahan D., Brdicka T., Weiss A., Liston A., and Goodnow C.C.. 2007. Opposing functions of the T cell receptor kinase ZAP-70 in immunity and tolerance differentially titrate in response to nucleotide substitutions. Immunity. 27:912–926. 10.1016/j.immuni.2007.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Maeda S., Hashimoto M., Fujimori C., Ito Y., Teradaira S., Hirota K., Yoshitomi H., Katakai T., Shimizu A., et al. 2010. Graded attenuation of TCR signaling elicits distinct autoimmune diseases by altering thymic T cell selection and regulatory T cell function. J. Immunol. 185:2295–2305. 10.4049/jimmunol.1000848 [DOI] [PubMed] [Google Scholar]
- Turul T., Tezcan I., Artac H., de Bruin-Versteeg S., Barendregt B.H., Reisli I., Sanal O., van Dongen J.J., and van der Burg M.. 2009. Clinical heterogeneity can hamper the diagnosis of patients with ZAP70 deficiency. Eur. J. Pediatr. 168:87–93. 10.1007/s00431-008-0718-x [DOI] [PubMed] [Google Scholar]
- Waisbourd-Zinman O., Ben-Amitai D., Cohen A.D., Feinmesser M., Mimouni D., Adir-Shani A., Zlotkin M., and Zvulunov A.. 2008. Bullous pemphigoid in infancy: Clinical and epidemiologic characteristics. J. Am. Acad. Dermatol. 58:41–48. 10.1016/j.jaad.2007.08.010 [DOI] [PubMed] [Google Scholar]
- Wang L., Yang J.K., Kabaleeswaran V., Rice A.J., Cruz A.C., Park A.Y., Yin Q., Damko E., Jang S.B., Raunser S., et al. 2010. The Fas-FADD death domain complex structure reveals the basis of DISC assembly and disease mutations. Nat. Struct. Mol. Biol. 17:1324–1329. 10.1038/nsmb.1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.L., Schreiber K.L., Zhang W., Wange R.L., Samelson L.E., Leibson P.J., and Abraham R.T.. 1998. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol. Cell. Biol. 18:1388–1399. 10.1128/MCB.18.3.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Stokoe D., Kane L.P., and Weiss A.. 2002. The inducible expression of the tumor suppressor gene PTEN promotes apoptosis and decreases cell size by inhibiting the PI3K/Akt pathway in Jurkat T cells. Cell Growth Differ. 13:285–296. [PubMed] [Google Scholar]
- Yan Q., Barros T., Visperas P.R., Deindl S., Kadlecek T.A., Weiss A., and Kuriyan J.. 2013. Structural basis for activation of ZAP-70 by phosphorylation of the SH2-kinase linker. Mol. Cell. Biol. 33:2188–2201. 10.1128/MCB.01637-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.