Abstract
Background:
Recently, the potential of L-arginine supplementation as a novel and effective strategy for weight loss and improving biochemical parameters in obese patients has been under consideration.
Objectives:
To evaluate the influence of 8-week oral L-arginine supplementation on body mass index (BMI), waist circumference (WC), triceps skinfold (TS), subscapular skinfold (SS), systolic blood pressure (SBP), diastolic blood pressure (DBP), plasma fasting blood sugar (FBS), glycated hemoglobin (HbA1c), triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and malondialdehyde (MDA) in patients with BMI values > 29.9 or visceral obesity (WC > 102 cm in men or > 88 cm in women).
Patients and Methods:
Ninety obese patients were included in a single-blind randomized controlled trial. Patients were randomized to receive either L-arginine (3 or 6 g thrice daily) or placebo for 8 weeks. Anthropometric and biochemical indices, dietary intake, and blood pressure values were measured at the baseline and after the 8-week intervention.
Results:
Significant decreases in anthropometric parameters, blood pressure (SBP, DBP), FBS, HbA1c, LDL, MDA (P < 0.001), TG (P = 0.02), and TC (P = 0.002) and a significant increase in HDL (P < 0.001) were observed in the intervention group, compared to the control group. In the control group, no significant differences were found between the baseline and end-of-intervention measurements.
Conclusions:
In conclusion, oral L-Arginine supplementation appears to improve anthropometric parameters, blood pressure values, and some blood biochemical indices associated with cardiovascular disease prevention.
Keywords: Obesity, Cardiovascular Systems, Risk Factors
1. Background
Obesity is associated with numerous comorbidities, such as type 2 diabetes, hypertension, and cardiovascular diseases (CVDs) (1). According to the world health organization (WHO) in 2015, globally, 2.3 billion people aged 15 years or older will be overweight, and more than 700 million people will be obese (2).
The national institutes of health (NIH) classifies overweight and obesity according to body mass index (BMI), waist circumference (WC), and the risks of associated diseases. Obesity, especially abdominal obesity, is associated with increased risks of morbidity and mortality, as well as CVDs. Of note, obesity is an independent risk factor for CVDs (3-6).
Importantly, obesity is associated with persistent insulin resistance, which can lead to hyperglycemia, lipid disorders, oxidative stress, and arterial hypertension (7, 8). Chronic hyperglycemia plays an important role in the etiology and progression of arterial pathology (9). The relationship between hyperglycemia and oxidative stress has been confirmed (10). High levels of free radicals are produced in patients with CVDs (11). These factors are responsible for the pathogenesis of and endothelium dysfunction associated with CVDs (12).
Numerous studies have demonstrated the effective use of L-arginine for the prevention of many diseases associated with endothelium dysfunction, such as atherosclerosis and diabetes (13-15). Piatti et al. reported that L-arginine treatment significantly improved peripheral and hepatic insulin sensitivity in patients with type 2 diabetes. The mechanism behind this result might have been due to the effect of L-arginine on nitric oxide (NO) synthesis (16). The proven antioxidative properties of L-arginine have been very significant (17). In vivo, L-arginine had an inhibitory effect on superoxide radicals and increased the concentrations of available antioxidants, which protect low-density lipoprotein (LDL) from oxidation, by decreasing the production of O2- (superoxide anion) (18).
2. Objectives
Few studies have assessed the potential positive effects of L-arginine supplementation on CVD-associated indices in obese patients. The aim of this study was to evaluate the influence of L-arginine supplementation on anthropometric and blood biochemical indices associated with CVDs in obese people (individuals with BMI values > 29.9 or WC values > 102 cm in men or > 88 cm in women).
3. Patients and Methods
3.1. Patients
The study protocol was approved by the local Bioethics committee of Shiraz university of medical sciences, IRCT (No 201501183236N6). Patients were recruited from October to December 2014. Ninety obese non-smokers and non-alcohol users were enrolled in this randomized clinical trial. The sample size was calculated based on the results of a study published by Lucotti et al. (19) for the effect of an intervention on participants’ weights with a standard deviation (SD) of 3 kg, intervention effect mean of 2.5 kg, statistical power of 80%, and significance level of < 0.05.
The inclusion criteria were as follows: BMI > 29.9 kg/m2 or visceral obesity (BMI of 25 - 29.9 kg/m2 and WC > 102 cm in men or > 88 cm in women); stable body weight during the previous 3 months (based on self-reports); absence of any current regimen or supplement intake; absence of antihypertensive, antidiabetic, or antihyperlipidemic treatment or acute or chronic inflammation; no history of ischemic heart disease; and normal renal and liver function.
The exclusion criteria were as follows: use of any supplements other than the intervention or placebo, any diseases or physiological changes requiring special treatment, and failure to follow the designated intervention (not consuming the total amount of the dedicated supplement [or < 90% of the prescribed supplement] for > 3 days).
3.2. Methods
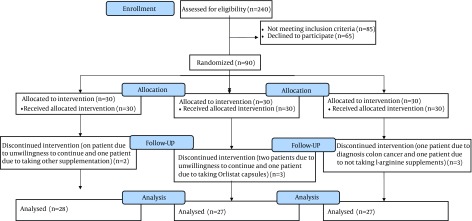
After reading and completing the informed consent form, eligible participants were assigned randomly in a 1:1:1 ratio into 1 of 3 subgroups using a block randomization design with 3 arms: group A, placebo (un-absorbable starch); group B, supplemented with 1 g of L-arginine thrice daily; and group C, supplemented with 2 g of L-arginine thrice daily. Randomization was performed using random allocation software (20) by an investigator with no clinical involvement in the study. L-arginine (purity, 99.9%) was obtained from Karen Pharma and Food Supplement Co. (Tehran, Iran). Placebo and L-arginine tablets were identical in shape, size, color, and packaging. The participants were not aware of the contents of the administered tablets (L-arginine or placebo). Participants were asked not to make major changes to their dietary and physical activity habits during the study and to report any other changes. Participants were visited biweekly to administer a 2-week supply of supplement and evaluate any possible side effects of the supplement. During the intervention and sample collection after the intervention phase, 7 patients were excluded (Figure 1): group A, 2 patients (1 because of unwillingness to continue and 1 because of other supplement usage); group B, 3 patients (2 patients because of unwillingness to continue and 1 patient because of orlistat capsule usage); and group C, 2 patients (1 patient because of diagnosed colon cancer and 1 patient for failure to take l-arginine supplements).
Figure 1. Flow Diagram of Participants in the Three Groups Throughout the Study.
Each patient completed an interview-administered questionnaire regarding demographic characteristics. Anthropometric and biochemical indices and dietary intake were determined at the baseline and after the 8-week intervention.
WC (cm) was measured at the level of the iliac crest at the end of normal expiration. This value was measured to the nearest 0.5 cm. Thicknesses of the triceps skinfold (TS) and subscapular skinfold (SS) were measured to the nearest 0.5 mm by using a Harpenden skinfold caliper (Baty International, Burgess Hill, West Sussex, UK). Recalled dietary intakes over 24-h periods (2 workdays and 1 weekend day) were recorded by a researcher at the baseline and the end of the study. Physical activity levels were determined using the international physical activity questionnaire (IPAQ) at the baseline and after the 8-week intervention. From each participant, 7-cc venous blood samples were collected after fasting. The blood was then distributed among tubes containing K2EDTA or heparin. Blood samples were centrifuged and to separate sera. Serum samples were stored at -72°C prior to biochemical measurements. The lipid profile, including total cholesterol (TC), LDL, high-density lipoprotein (HDL), triglyceride (TG), plasma fasting blood sugar (FBS), and malondialdehyde (MDA), was also assayed using routine enzymatic methods. The glycated hemoglobin (HbA1c) percentage was quantitatively analyzed in vitro using a Dimension® system (Dade Behring Inc., Milton Keynes, UK).
3.3. Statistical Analysis
Statistical analyses were performed with SPSS statistical software, version 16 (SPSS Inc., Chicago, IL, USA). Despite randomized allocation of the subjects, the groups were found to differ significantly in terms of the baseline BMI. Accordingly, after evaluating data normality, a repeated measurements analysis of covariance (ANCOVA) was used to control the results with respect to covariates (listed in Table 1). A post hoc test and Dunnett T3 test were used to compare changes in the outcome variables between groups. A P-value < 0.05 was considered statistically significant.
Table 1. Participants’ Baseline Characteristics and Important Anthropometrical Measurements a.
| Parameter | Placebo (n = 28) | L-arginine 3, g/d (n = 27) | L-arginine 6, g/d (n = 27) | P Value |
|---|---|---|---|---|
| N (Female/Male) | 28 (13/15) | 27 (14/13) | 28 (13/15) | 0.874 |
| Age, y b | 42.39 ± 7.21 | 42.33 ± 7.91 | 44.07 ± 7.82 | 0.751 |
| Weight, kg b | 96.03 ± 13.78 | 96.48 ± 9.41 | 93.21 ± 11.51 | 0.533 |
| BMI, kg/m 2b | 35.03 ± 3.48 | 35.85 ± 4.70 | 33.16 ± 1.72 | 0.017 |
| WC, cm b | 109.9 ± 10.28 | 108.85 ± 7.96 | 111.21 ± 6.23 | 0.577 |
aAbbreviations: BMI, body mass index; WC, waist circumference.
b Data are presented as means ± standard deviations.
4. Results
Table 1 lists the baseline characteristics and anthropometrical data of participants in the three study groups.
A comparison of pre- and post-intervention measurements within each group revealed significant decreases in weight, BMI, WC, TS, systolic blood pressure (SBP; all P < 0.001), and SS (P = 0.01) in group B, and significant decreases in all parameters in group C (all P < 0.001).
According to a paired t-test, a significant decrease in TC (P = 0.041) was observed in group A, significant decreases in LDL, MDA (both P < 0.001), and TC (P = 0.07) were observed in group B, and significant decreases in FBS, HbA1c, TC, LDL, MDA (all P < 0.001), and TG (P = 0.0.01) and a significant increase in HDL were observed in group C (P < 0.001).
Table 2 lists the mean pre- and post-intervention anthropometric (weight, BMI, WC, TS, SS) and blood pressure (SBP, diastolic [DBP]) parameter values for each study group. Among the study groups, significant decreases were observed in all parameters (P < 0.001). The results of a post hoc analysis are presented in the appendix.
Table 2. Pre- and post-intervention anthropometrical and blood pressure parameters in the study groups a,b,c.
| Parameter | Groups | P Value d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 28) | L-arginine 3, g/d (n = 27) | L-arginine 6, g/d (n = 28) | ||||||||
| Before | After | P Value e | Before | After | P Value e | Before | After | P Value e | ||
| Weight, kg | 96.03 ± 13.78 | 95.80 ± 14.59 | 0.641 | 96.48 ± 9.41 | 93.76 ± 9.34 | < 0.001 | 93.21 ± 11.51 | 84.90 ± 11.28 | < 0.001 | < 0.001 |
| BMI, kg/m 2 | 35.03 ± 3.480 | 34.90 ± 3.43 | 0.462 | 35.85 ± 4.70 | 34.85 ± 4.68 | < 0.001 | 33.16 ± 1.720 | 30.22 ± 2.29 | < 0.001 | < 0.001 |
| WC, cm | 109.9 ± 10.280 | 109.61 ± 11.18 | 0.484 | 108.85 ± 7.96 | 106.57 ± 8.14 | < 0.001 | 111.21 ± 6.23 | 104.05 ± 5.24 | < 0.001 | < 0.001 |
| TS, mm | 34.93 ± 3.81 | 35.18 ± 3.59 | 0.165 | 34.11 ± 2.92 | 33.19 ± 3.05 | < 0.001 | 34.79 ± 2.63 | 32.00 ± 2.80 | < 0.001 | < 0.001 |
| SS, mm | 29.71 ± 2.93 | 29.89 ± 3.531 | 0.502 | 28.96 ± 2.26 | 28.33 ± 2.30 | < 0.001 | 29.64 ± 1.33 | 27.11 ± 1.61 | < 0.001 | < 0.001 |
| SBP, mm Hg | 130.89 ± 4.31 | 129.46 ± 4.58 | 0.058 | 130.93 ± 4.81 | 126.85 ± 4.63 | < 0.001 | 132.14 ± 4.79 | 124.29 ± 3.52 | < 0.001 | < 0.001 |
| DBP, mm Hg | 86.43 ± 5.41 | 87.61 ± 5.15 | 0.095 | 87.04 ± 4.22 | 85.93 ± 4.16 | 0.110 | 88.21 ± 4.75 | 82.68 ± 4.19 | < 0.001 | < 0.001 |
aAbbreviations: BMI, body mass index; DBP diastolic blood pressure; SS, subscapular skinfold; TS, triceps skinfold; SBP, systolic blood pressure; WC, waist circumference.
b Data are presented as means ± standard deviations.
c Significance was defined as < 0.05.
d Repeated-measures analysis of covariance after adjusting for BMI. All covariates in Table 1 were included in the initial model; of these, only baseline BMI was found to have a significant interaction with the group effect.
e Paired t-test P value.
Table 3 shows the mean biochemical parameter values (FBS, HbA1c, TG, TC, LDL, HDL, and MDA) before and after supplementation in each study groups. Among the groups, significant decreases in FBS, HbA1c, LDL, MDA (all P < 0.001), TG (P = 0.02), and TC (P = 0.002) and a significant increase in HDL (P < 0.001) were observed, and post hoc tables are presented in the appendix.
Table 3. Pre- and post-intervention biochemical parameters a,b,c.
| Parameter | Groups | P Value d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 28) | L-Arginine 3, g/d (n = 27) | L-Arginine 6, g/d (n = 28) | ||||||||
| Before | After | P Value e | Before | After | P Value e | Before | After | P Value e | ||
| FBS, mg/dL | 97.39 ± 5.55 | 98.46 ± 4.41 | 0.265 | 97.41 ± 9.61 | 95.85 ± 7.61 | 0.171 | 99.25 ± 7.66 | 92.14 ± 7.47 | < 0.001 | < 0.001 |
| HbA1c, % | 5.504 ± 0.38 | 5.48 ± 0.37 | 0.460 | 5.544 ± 0.30 | 5.50 ± 0.27 | 0.086 | 5.632 ± 0.25 | 5.39 ± 0.29 | < 0.001 | < 0.001 |
| TG, mg/dL | 221.21 ± 43.99 | 221.43 ± 43.84 | 0.933 | 222.59 ± 52.56 | 223.48 ± 52.75 | 0.859 | 224.04 ± 48.26 | 210.54 ± 52.76 | < 0.001 | 0.02 |
| TC, mg/dL | 229.11 ± 28.19 | 223.93 ± 31.02 | 0.041 | 231.30 ± 28.85 | 224.81 ± 29.98 | 0.007 | 237.25 ± 32.693 | 221.00 ± 31.63 | < 0.001 | 0.002 |
| LDL, mg/dL | 134.11 ± 27.56 | 134.36 ± 26.68 | 0.862 | 138.41 ± 23 | 132.78 ± 22.86 | < 0.001 | 130.39 ± 23.58 | 121.89 ± 23.50 | < 0.001 | < 0.001 |
| HDL, mg/dL | 39.21 ± 2.60 | 38.75 ± 2.92 | 0.445 | 39.04 ± 3.67 | 40.11 ± 3.46 | 0.063 | 40.75 ± 2.79 | 44.46 ± 2.71 | < 0.001 | < 0.001 |
| MDA, mmol/L | 4.83 ± 0.62 | 4.8712 ± 0.59 | 0.624 | 4.89 ± 0.52 | 3.6718 ± 0.37 | <0.001 | 4.85 ± 0.43 | 3.6492 ± 0.98 | <0.001 | <0.001 |
a Abbreviations: FBS, fasting blood sugar; HbA1c, glycated hemoglobin; HDL, high density lipoprotein; MDA, malondialdehyde; TG, triglyceride; TC, total cholesterol.
b Data are presented as means ± standard deviations.
c Significance was defined at < 0.05.
d Repeated-measures analysis of covariance after adjusting for BMI. All covariates in Table 1 were included in the initial model; of these, only baseline BMI was found to have significant an interaction with the group effect.
e Paired t-test P value.
5. Discussion
The prevalence of CVDs is increasing dramatically, and prior studies have shown that L-arginine supplementation can ameliorate some associated risk factors (11, 14, 16). The present study evaluated the effect of L-arginine supplementation (3 or 6 g/d) on BP, as well as some anthropometrical and biochemical factors associated with CVDs, in obese subjects.
The present study revealed significant decreases in anthropometrical parameters. Previous study have reported excess activation of tissue necrosis factor (TNF)-alpha in obese patients. The role of TNF-alpha in the pathogenesis of insulin resistance has been demonstrated in obese patients (21). This cytokine reduces insulin activity in various tissues. In adipose tissue, TNF-alpha overstimulates lipolysis by activating hormone-sensitive lipase and increases insulin resistance by increasing serine phosphorylation and inhibiting tyrosine phosphorylation in insulin receptor substrate-1 (IRS-1), thus decreasing insulin receptor activity; in muscle cells, this cytokine also reduces the expression of GLUT-4. Weight loss can downregulate the expression of TNF-alpha and improve insulin sensitivity (22). Catabolic changes in HDL concomitant with weight loss might be related to an increase in HDL particle size, which might itself be consequent to a reduction in the pool of plasma very LDL TGs available for exchange with HDL (23). Weight reduction increases adiponectin, which can inhibit hepatic lipase activity (24); this might account for the partial increase in HDL, and likely accounts for the increased HDL levels observed after weight loss (25).
Our study observed significant decreases in BP parameters (SBP, DBP) after the 8-week intervention. Similar findings were reported in previous studies (13, 26). L-arginine supplementation was found to be associated with significant decreases in SBP and DBP in patients with essential hypertension (13). A possible partial association has been suggested between impaired L-arginine transport and the risk of hypertension development, possibly indicating an association between defective L-arginine/NO pathway signaling and the onset of hypertension (27).
As obesity can cause insulin resistance, elevated FBS and HbA1c levels were expected as typical consequences in the participants. The present study showed significant improvements in FBS and HbA1c in groups treated with L-arginine. In a previous study, an FBS level > 109.8 mg/dL and a 1% increase in HbA1c were found to increase the risk of death from ischemic heart disease by 38% and 30%, respectively (28). The availability of NO can also improve glucose metabolism (24). NO per se can influence muscle glucose metabolism; in a previous study, insulin-resistant obese rats were found to harbor a defect in the NO metabolic pathway. Furthermore, NO increased glucose transport both in the absence and in the presence of insulin in rat muscles in vitro (29).
L-arginine restores NO bioavailability through the competitive inhibition of NO synthase (NOS) by asymmetric dimethylarginine (ADMA), the main endogenic inhibitor of NOS (30). ADMA was found to improve the clinical statuses of patients with CVDs by reducing the generation of O2- (31). In a previous study, L-arginine increased the amount of available antioxidants, which protect LDL from oxidation (18). Free radicals cause lipid oxidation as well as biomembrane damage, which is reflected by lipid peroxidation and consequently increases the levels of MDA (32). The present study observed a significant decrease in the MDA level. Previous studies have reported similar results with L-arginine supplementation, including improved antioxidant statuses indicated by an increased total antioxidant status (33), glutathione and superoxide dismutase levels, (19) and circulating plasma ascorbic acid levels, all of which have been shown to protect against peroxidative lipid damage (34); therefore, increased cell membrane permeability and decreased lipid oxidation lead to decreased MDA levels (35).
We recommend additional clinical trials with larger sample sizes and longer intervention periods to facilitate a better understanding of the role of L-arginine supplementation for weight management in obese people at risk of CVDs. This study suggests L-arginine as a promising, safe, and beneficial supplement with beneficial effects on some risk factors of CVDs.
In conclusion, an 8-week course of oral L-arginine supplementation decreased anthropometric parameters and blood pressure values and improved some biochemical factors associated with CVDs in patients with BMI values indicating obesity or visceral obesity.
Acknowledgments
The authors would like to thank all patients for participating in this study. This article was based on a data set used in an MSc thesis written by Arash Dashtabi and registered at the Shiraz University of Medical Sciences.
Footnotes
Authors’ Contributions:Study concept and design: Arash Dashtabi; Acquisition of data: Arash Dashtabi; Analysis and interpretation of data: Mohammad Fararouei, Najmeh Hejazi; Drafting of the manuscript: Arash Dashtabi, Najmeh Hejazi; Critical revision of the manuscript for important intellectual content: Mohammad Fararouei, Zohreh Mazloom; Statistical analysis: Mohammad Fararouei, Najmeh Hejazi; Administrative, technical, and material support: Zohreh Mazloom; Study supervision: Zohreh Mazloom.
References
- 1.Poirier P, Eckel RH. Obesity and cardiovascular disease. Curr Atheroscler Rep. 2002;4(6):448–53. doi: 10.1007/s11883-002-0049-8. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Media centre Obesity and Overweight. WHO; 2009. [Google Scholar]
- 3.Pi-Sunyer FX, Becker DM, Bouchard C, Carleton RA, Colditz GA, Dietz WH. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. Am J Clin Nutr. 1998;68(4):899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Vaz M, Pais P. Tackling the challenge of cardiovascular disease burden in developing countries. Am Heart J. 2004;148(1):1–4. doi: 10.1016/j.ahj.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–15. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poirier P, Despres JP. Waist circumference, visceral obesity, and cardiovascular risk. J Cardiopulm Rehabil. 2003;23(3):161–9. doi: 10.1097/00008483-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Leslie RDG, Cohen RM. Biologic Variability in Plasma Glucose, Hemoglobin A1c, and Advanced Glycation End Products Associated with Diabetes Complications. J Diabetes Sci Technol. 2009;3(4):635–43. doi: 10.1177/193229680900300403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giardino I, Edelstein D, Brownlee M. BCL-2 expression or antioxidants prevent hyperglycemia-induced formation of intracellular advanced glycation endproducts in bovine endothelial cells. J Clin Invest. 1996;97(6):1422–8. doi: 10.1172/JCI118563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jablecka A, Bogdanski P, Balcer N, Cieslewicz A, Skoluda A, Musialik K. The effect of oral L-arginine supplementation on fasting glucose, HbA1c, nitric oxide and total antioxidant status in diabetic patients with atherosclerotic peripheral arterial disease of lower extremities. Eur Rev Med Pharmacol Sci. 2012;16(3):342–50. [PubMed] [Google Scholar]
- 12.Roberts W, Riba R, Homer-Vanniasinkam S, Farndale RW, Naseem KM. Nitric oxide specifically inhibits integrin-mediated platelet adhesion and spreading on collagen. J Thromb Haemost. 2008;6(12):2175–85. doi: 10.1111/j.1538-7836.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- 13.Gatto C. NO control: nitric oxide directly regulates substrate delivery to NOS. Focus on "Nitric oxide can acutely modulate its biosynthesis through a negative feedback mechanism on L-arginine transport in cardiac myocytes". Am J Physiol Cell Physiol. 2010;299(2):C213–5. doi: 10.1152/ajpcell.00191.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martina V, Masha A, Gigliardi VR, Brocato L, Manzato E, Berchio A, et al. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care. 2008;31(5):940–4. doi: 10.2337/dc07-2251. [DOI] [PubMed] [Google Scholar]
- 15.Bruckdorfer KR, Jacobs M, Rice-Evans C. Endothelium-derived relaxing factor (nitric oxide), lipoprotein oxidation and atherosclerosis. Biochem Soc Trans. 1990;18(6):1061–3. doi: 10.1042/bst0181061. [DOI] [PubMed] [Google Scholar]
- 16.Piatti PM, Monti LD, Valsecchi G, Magni F, Setola E, Marchesi F, et al. Long-term oral L-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care. 2001;24(5):875–80. doi: 10.2337/diacare.24.5.875. [DOI] [PubMed] [Google Scholar]
- 17.Jablecka A, Checinski P, Krauss H, Micker M, Ast J. The influence of two different doses of L-arginine oral supplementation on nitric oxide (NO) concentration and total antioxidant status (TAS) in atherosclerotic patients. Med Sci Monit. 2004;10(1):CR29–32. [PubMed] [Google Scholar]
- 18.Boger RH, Bode-Boger SM, Phivthong-ngam L, Brandes RP, Schwedhelm E, Mugge A, et al. Dietary L-arginine and alpha-tocopherol reduce vascular oxidative stress and preserve endothelial function in hypercholesterolemic rabbits via different mechanisms. Atherosclerosis. 1998;141(1):31–43. doi: 10.1016/s0021-9150(98)00145-2. [DOI] [PubMed] [Google Scholar]
- 19.Lucotti P, Setola E, Monti LD, Galluccio E, Costa S, Sandoli EP, et al. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2006;291(5):E906–12. doi: 10.1152/ajpendo.00002.2006. [DOI] [PubMed] [Google Scholar]
- 20.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson NC, Callas PW, Hanley AJ, Festa A, Haffner SM, Wagenknecht LE, et al. Circulating levels of TNF-alpha are associated with impaired glucose tolerance, increased insulin resistance, and ethnicity: the Insulin Resistance Atherosclerosis Study. J Clin Endocrinol Metab. 2012;97(3):1032–40. doi: 10.1210/jc.2011-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cartier A, Cote M, Bergeron J, Almeras N, Tremblay A, Lemieux I, et al. Plasma soluble tumour necrosis factor-alpha receptor 2 is elevated in obesity: specific contribution of visceral adiposity. Clin Endocrinol (Oxf). 2010;72(3):349–57. doi: 10.1111/j.1365-2265.2009.03671.x. [DOI] [PubMed] [Google Scholar]
- 23.Lamarche B, Uffelman KD, Carpentier A, Cohn JS, Steiner G, Barrett PH, et al. Triglyceride enrichment of HDL enhances in vivo metabolic clearance of HDL apo A-I in healthy men. J Clin Invest. 1999;103(8):1191–9. doi: 10.1172/JCI5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol (1985). 1994;77(6):2519–21. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- 25.Schneider JG, von Eynatten M, Schiekofer S, Nawroth PP, Dugi KA. Low plasma adiponectin levels are associated with increased hepatic lipase activity in vivo. Diabetes Care. 2005;28(9):2181–6. doi: 10.2337/diacare.28.9.2181. [DOI] [PubMed] [Google Scholar]
- 26.Ast J, Jabłecka A, Bogdański P, Smolarek I, Krauss H, Chmara E, et al. Evaluation of antihypertensive effect of l-arginine supplementation in patients with mild hypertension assessed with ambulatory blood pressure monitoring. Pharmacol Rep. 2010;62:73. doi: 10.1016/s1734-1140(10)71182-8. [DOI] [PubMed] [Google Scholar]
- 27.Schlaich MP, Parnell MM, Ahlers BA, Finch S, Marshall T, Zhang WZ, et al. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation. 2004;110(24):3680–6. doi: 10.1161/01.CIR.0000149748.79945.52. [DOI] [PubMed] [Google Scholar]
- 28.Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ. 2001;322(7277):15–8. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young ME, Leighton B. Evidence for altered sensitivity of the nitric oxide/cGMP signalling cascade in insulin-resistant skeletal muscle. Biochem J. 1998;329 ( Pt 1):73–9. doi: 10.1042/bj3290073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooke JP. Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol. 2000;20(9):2032–7. doi: 10.1161/01.atv.20.9.2032. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell AJ, Anderson BE, Cooke JP. Nutritional therapy for peripheral arterial disease: a double-blind, placebo-controlled, randomized trial of HeartBar. Vasc Med. 2000;5(1):11–9. doi: 10.1177/1358836X0000500103. [DOI] [PubMed] [Google Scholar]
- 32.Lubec B, Hayn M, Kitzmuller E, Vierhapper H, Lubec G. L-Arginine reduces lipid peroxidation in patients with diabetes mellitus. Free Radic Biol Med. 1997;22(1-2):355–7. doi: 10.1016/s0891-5849(96)00386-3. [DOI] [PubMed] [Google Scholar]
- 33.Lantos J, Roth E, Czopf L, Nemes J, Gal I. Monitoring of plasma total antioxidant status in different diseases. Acta Chir Hung. 1997;36(1-4):188–9. [PubMed] [Google Scholar]
- 34.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41(12 Pt 2):1819–28. [PubMed] [Google Scholar]
- 35.Santillo M, Mondola P, Milone A, Gioielli A, Bifulco M. Ascorbate administration to normal and cholesterol-fed rats inhibits in vitro TBARS formation in serum and liver homogenates. Life Sci. 1996;58(14):1101–8. doi: 10.1016/0024-3205(96)00068-9. [DOI] [PubMed] [Google Scholar]