Highlights
-
•
Uterine tumors with sex-cord elements are very rare.
-
•
UTROSCT can present with infiltration similar to endometrial stromal sarcomas.
-
•
Case report of a locally advanced UTROSCT.
1. Introduction
Uterine tumors with sex-cord elements are very rare. Clement and Scully described 14 patients in 1976 (Clement and Scully, 1976). The authors divided these tumors into two groups: one corresponding to endometrial stromal neoplasms with focal areas resembling ovarian sex-cord elements (also called endometrial stromal tumors with sex cord-like elements — ESTSCLE) and the other corresponding to intramural uterine tumors with a predominant or exclusive pattern similar to ovarian sex-cord tumor (also called uterine tumors resembling ovarian sex-cord tumor — UTROSCT) (Clement and Scully, 1976, Czernobilsky, 2008). We describe a patient diagnosed with locally advanced UTROSCT with an unusual pattern of infiltration and growth, similar to endometrial stromal sarcomas.
2. Case report
This is a 53-year-old female, heavy smoker, who had a history of irregular vaginal bleeding for the past two years, which led to a supracervical hysterectomy in March 2010. Pathology revealed a myometrial neoplasia with extensive sex-cord pattern with positive surgical margins.
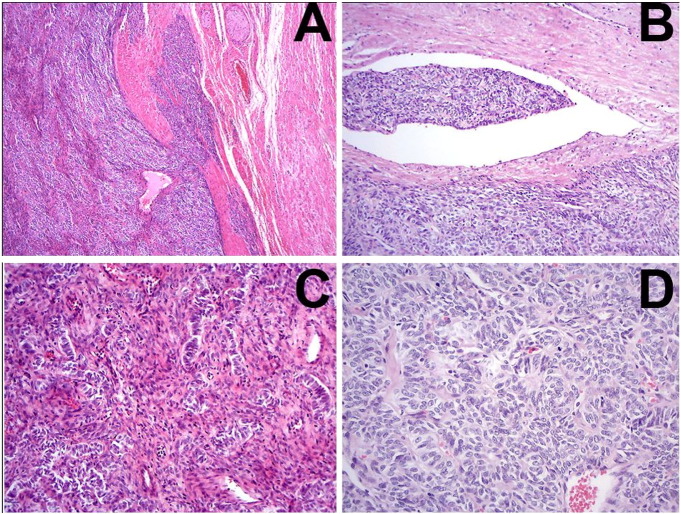
Few weeks later the patient had subsequent complete staging including bilateral salpingo-oophorectomy, omentectomy, parametrectomy, pelvic lymphadenectomy and uterine cervical resection. A central pathology review revealed a 12.0 cm sex-cord uterine tumor resembling an ovarian sex-cord tumor with Sertoli cell-like differentiation. The neoplasia was characterized by epithelioid polygonal or cylindrical cells in solid cordonal architecture or anastomosing trabecule disposed in a dense colagenic stroma. No stromal endometrioid areas were identified. The tumor had fingerlike projections and endovascular growth, with multifocal myometrial vascular embolization and infiltration of the entire myometrial layer up to the serosal layer (Fig. 1). Pathology analysis also revealed that the tumor extended to uterine cervix, right parametrium and right ovarium hilum. There were extense ischemic areas. The right parametrial surgical margin was positive. Pelvic lymph nodes were not involved.
Fig. 1.
Uterine tumor sections showing trabeculae and anastomosing cords of epithelioid cells (A) with areas of sertoliform (B) and retiform structures (C). Endovascular growth was evident (D) (hematoxylin–eosin).
Immunohistochemistry study (IHC) revealed strong positivity for vimentin, CD99, cytokeratins AE1/AE3, estrogen and progesterone receptors and focal positivity for WT-1. Weak immunoreactivity was present for CD10, melan-A, inhibin and desmin. IHC was negative for alpha-smooth muscle actin, muscle actin (HHF35) and epithelial membrane antigen (EMA).
Computed tomography of thorax, abdomen and pelvis revealed no evidence of metastatic disease. She was treated with 4 cycles of adjuvant modified BEP every 3 weeks (bleomycin 20 U/m2 (intravenous) IV on day 1, cisplatin 20 mg/m2 IV from days 1 to 5, and etoposide 100 mg/m2 IV from days 1 to 5), followed by external pelvic radiation at the total dose of 45 Gy over 5 weeks and brachytherapy (dose of 6 Gy in 4 fractions). There were no grade III or IV toxicities during the entire treatment. After a 5-year follow-up, there was no evidence of disease.
3. Discussion
Uterine sex-cord tumors occur most frequently between the fourth and the sixth decades of life (Kunz and Friedrich, 2007, Garuti et al., 2009). Both UTROSCT and ESTSCLE correspond to uterine tumors with sex cord-like elements although they may represent distinct entities (Pradhan and Mohanty, 2013). Histologically, UTROSCT consists predominantly or exclusively by similar ovarian sex-cord tumor elements (Clement and Scully, 1976), and they are characterized by the variety of architectural patterns seen in either granulosa or Sertoli–Leydig ovarian tumors (Czernobilsky, 2008, Pradhan and Mohanty, 2013). On the other hand, ESTSCLE is characterized by sex-cord elements in less than 50% of the tumor area and in lesser degree compared to UTROSCT (Clement and Scully, 1976, Pradhan and Mohanty, 2013).
IHC has been a useful tool in sex-cord differentiation, particularly calretinin, inhibin, CD99, and melan-A (Czernobilsky, 2008, Irving et al., 2006). Some tumors may also have expression of epithelial and smooth muscle markers demonstrating the polyphenotypic profile of these tumors (Czernobilsky, 2008).
Although UTROSCT has been viewed as a variant of endometrial stromal sarcoma, it seems that only ESTSCLE can be considered a truly variant. The t(7;17)(p15;q21) translocation resulting in the fusion gene JAZF1–JJAZ1 that characterizes the endometrial sarcomas is not observed in UTROSCT (Staats et al., 2009).
The correct diagnosis is key to guide optimal management. The prognosis of ESTSCLE is that of endometrial stromal sarcomas, characterized by loco-regional and distant relapses (Czernobilsky, 2008). On the other hand, UTROSCT is considered to be of low malignant potential tumor and most are described as benign (Czernobilsky, 2008, Kunz and Friedrich, 2007, Pradhan and Mohanty, 2013). However, UTROSCT biologic behavior remains to be established, particularly in cases like ours, associated with infiltrative borders, endovascular invasion and extrauterine extension.
Therapy has been based on case reports and data relying on ovarian sex-cord tumor. Surgery with negative margins has been considered the backbone treatment of UTROSCT. Most case reports describe women who underwent hysterectomy with or without bilateral salpingo-oophorectomy as the initial approach. Pelvic lymphadenectomy, omentectomy, parametrectomy, or even uterine cervical resection are rarely described and should be individualized (Franco et al., 2003). Some authors, however, propose conservative surgery as a standard approach (Garuti et al., 2009, Hillard et al., 2004).
The role of adjuvant therapy in UTROSCT is uncertain. One case report described a patient treated with adjuvant pelvic external beam radiotherapy and brachytherapy with no evidence of recurrence at last follow-up (Irving et al., 2006).
Despite the fact that UTROSCT has a favorable biologic behavior, loco-regional relapse may occur especially when simple hysterectomy has been performed. Those patients are submitted to salvage surgery and postoperative external beam radiotherapy (Clement and Scully, 1976, Blinman and Tattersall, 2009, Malfetano and Hussain, 1989). Some case reports describe distant recurrence few years after initial therapy. Generally the sites of metastasis are intra-abdominal and include small bowel, omentum, mesentery, and peritoneum. Subcutaneous tissue, lymph nodes, and bladder involvement are also reported (Malfetano and Hussain, 1989, O'Meara et al., 2009, Biermann et al., 2008). Despite the heterogeneity, salvage treatment included mainly resection of metastatic disease. Some patients had also been treated with postoperative chemotherapy (BEP) or hormonal therapy (tamoxifen followed by anastrozole) (Malfetano and Hussain, 1989, O'Meara et al., 2009, Biermann et al., 2008). Of interest, all patients described had no evidence of disease at the last follow-up.
Our patient underwent radical surgery after an incomplete initial surgical procedure followed by 4 cycles of adjuvant modified BEP, pelvic external beam radiation and brachytherapy due to positive surgical margins and extra-uterine extension. The decision to recommend chemotherapy with BEP was based on ovarian Sertoli–Leydig cell tumors. Adjuvant systemic therapy has been considered in early stage disease with adverse features or in non-organ confined disease (Colombo et al., 2007). Besides, BEP is an active combination regimen for first-line chemotherapy of malignant sex-cord tumors of the ovary (Homesley et al., 1999). Similarly, radiation therapy seems to be effective in sex-cord tumors (Colombo et al., 2007). After a follow-up period of 5 years, the patient has no evidence of recurrence.
4. Conclusion
The case report describes a patient with UTROSCT, an uncommon tumor with variable biologic behavior, treated with radical surgery after an incomplete initial surgical procedure, followed by adjuvant modified BEP and pelvic external beam radiotherapy and brachytherapy, with complete remission for a period of five years. As UTROSCT are rare tumors, there are no prospective studies to delineate the optimal management. Therefore, aside from surgery with the goal of obtaining negative margins, the extension of surgery as well as the decision of adjuvant therapy must be individualized based on the extension of disease and pathologic features.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgment
The authors would like to thank Phillip Scheinberg, MD for being the text revisor.
Contributor Information
Jéssica Ribeiro Gomes, Email: jribeirog@gmail.com.
Filomena M. Carvalho, Email: filomena@usp.br.
Maurício Abrão, Email: msabrao@attglobal.net.
Fernando Cotait Maluf, Email: maluffc@uol.com.br.
References
- Biermann K., Heukamp L.C., Büttner R., Zhou H. Uterine tumor resembling an ovarian sex cord tumor associated with metastasis. Int. J. Gynecol. Pathol. 2008;27(1):58–60. doi: 10.1097/pgp.0b013e318057faf5. [DOI] [PubMed] [Google Scholar]
- Blinman P., Tattersall M.H. A case of uterine tumour resembling ovarian sex cord tumour responding to second-line, single agent anastrazole. Intern. Med. J. 2009;39(9):617–619. doi: 10.1111/j.1445-5994.2009.01998.x. [DOI] [PubMed] [Google Scholar]
- Clement P.B., Scully R.E. Uterine tumors resembling ovarian sex-cord tumors. A clinicopathologic analysis of fourteen cases. Am. J. Clin. Pathol. 1976;66(3):512–525. doi: 10.1093/ajcp/66.3.512. [DOI] [PubMed] [Google Scholar]
- Colombo N., Parma G., Zanagnolo V., Insinga A. Management of ovarian stromal cell tumors. J. Clin. Oncol. 2007;25(20):2944–2951. doi: 10.1200/JCO.2007.11.1005. (10) [DOI] [PubMed] [Google Scholar]
- Czernobilsky B. Uterine tumors resembling ovarian sex cord tumors: an update. Int. J. Gynecol. Pathol. 2008;27(2):229–235. doi: 10.1097/PGP.0b013e3181569a21. [DOI] [PubMed] [Google Scholar]
- Franco S., Andrade M.J., Silva T.S. Uterine tumor resembling ovarian sex-cord tumor. Acta Med. Port. 2003;16(5):365–367. [PubMed] [Google Scholar]
- Garuti G., Gonfiantini C., Mirra M. Uterine tumor resembling ovarian sex cord tumors treated by resectoscopic surgery. J. Minim. Invasive Gynecol. 2009;16(2):236–240. doi: 10.1016/j.jmig.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Hillard J.B., Malpica A., Ramirez P.T. Conservative management of a uterine tumor resembling an ovarian sex cord-stromal tumor. Gynecol. Oncol. 2004;92(1):347–352. doi: 10.1016/j.ygyno.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Homesley H.D., Bundy B.N., Hurteau J.A., Roth L.M. Bleomycin, etoposide, and cisplatin combination therapy of ovarian granulosa cell tumors and other stromal malignancies: a Gynecologic Oncology Group study. Gynecol. Oncol. 1999;2(2):131–137. doi: 10.1006/gyno.1998.5304. [DOI] [PubMed] [Google Scholar]
- Irving J.A., Carinelli S., Prat J. Uterine tumors resembling ovarian sex cord tumors are polyphenotypic neoplasms with true sex cord differentiation. Mod. Pathol. 2006;19(1):17–24. doi: 10.1038/modpathol.3800475. [DOI] [PubMed] [Google Scholar]
- Kunz J., Friedrich T. Uterine tumor resembling ovarian sex cord tumor: case report and review of literature. Praxis (Bern 1994) 2007;96(31–32):1177–1181. doi: 10.1024/1661-8157.96.31.1177. (2) [DOI] [PubMed] [Google Scholar]
- Malfetano J.H., Hussain M. A uterine tumor that resembled ovarian sex-cord tumors: a low-grade sarcoma. Obstet. Gynecol. 1989;74(3 Pt 2):489–491. [PubMed] [Google Scholar]
- O'Meara A.C., Giger O.T., Kurrer M., Schaer G. Case report: recurrence of a uterine tumor resembling ovarian sex-cord tumor. Gynecol. Oncol. 2009;114(1):140–142. doi: 10.1016/j.ygyno.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Pradhan D., Mohanty S.K. Uterine tumors resembling ovarian sex cord tumors. Arch. Pathol. Lab. Med. 2013;137:1832–1836. doi: 10.5858/arpa.2012-0634-RS. [DOI] [PubMed] [Google Scholar]
- Staats P.N., Garcia J.J., Dias-Santagata D.C. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) lack the JAZF1–JJAZ1 translocation frequently seen in endometrial stromal tumors. Am. J. Surg. Pathol. 2009;33(8):1206–1212. doi: 10.1097/PAS.0b013e3181a7b9cf. [DOI] [PubMed] [Google Scholar]