Abstract
Background
Pilomatrix carcinomas are rare, frequently occurring in older male patients. We report a case of vulvar pilomatrix carcinoma in a 30-year-old woman, the second known reported case occurring on the external genitalia.
Case
A 30-year-old female originally presented at an outside institution for the management of an asymptomatic vulvar mass that was biopsied and read as invasive squamous cell carcinoma. Pathology review at our institution reclassified the vulvar mass as a low-grade pilomatrix carcinoma. The patient underwent radical hemivulvectomy without an inguinal–femoral groin node dissection. She has remained without evidence of disease recurrence for more than 5 years since her diagnosis.
Conclusion
Pilomatrix carcinoma can be confused for an invasive squamous cell carcinoma. Due to its low risk of metastases, a less radical surgical approach can be taken. Consideration of this unusual malignancy is important in the determination of appropriate management.
Keywords: Pilomatrix carcinoma, Squamous cell carcinoma, Vulva, Surgical management, Malignant pilomatrixoma, Shadow cells
Highlights
-
•
We present a rare case of vulvar pilomatrix carcinoma in a 30 year old female.
-
•
Pilomatrix carcinoma can be confused for an invasive squamous-cell carcinoma.
-
•
Due to its low risk of metastases, a less radical surgical approach can be taken.
1. Introduction
Pilomatrix carcinoma, a malignant variant of pilomatrixoma, was first described in 1980 by Lopansri and Mihm (Lopansri and Mihm, 1980). Frequently located in the head and neck region, it is a rare, low-grade tumor that often has a male-predominant ratio and occurs in older patients (Cornejo and Deng, 2013). Here, we report a case of pilomatrix carcinoma occurring on the vulva of a 30-year old female, the second known reported case occurring on the external genitalia. Due to its low risk of metastasis and the indolent nature of these tumors, an accurate pathologic diagnosis aids in treatment decisions and ensures that the appropriate surgical procedure is performed.
2. Case
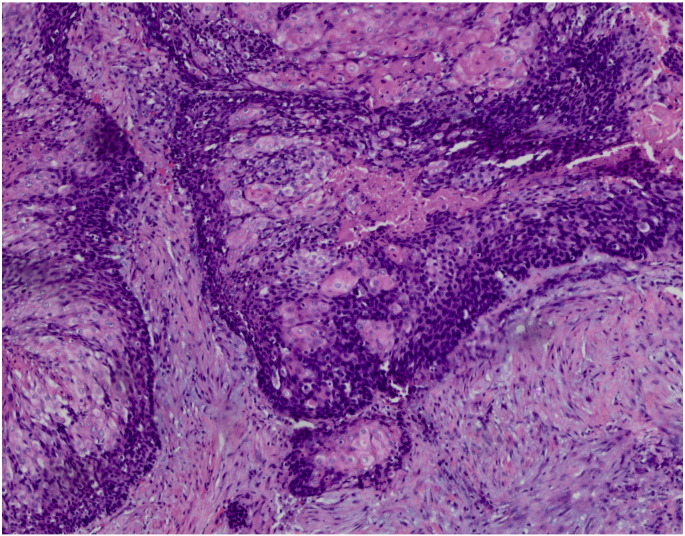
A 30-year old woman presented to an outside institution with an asymptomatic 3 cm irregular mass in the right labia majora. The mass was hard, fixed and nontender. The patient underwent excision of the vulvar mass. Final pathology was read as an invasive keratinizing, moderately to poorly differentiated squamous cell carcinoma with perineural and vascular invasion noted. Based upon this pathology, a radical hemivulvectomy and right inguinal femoral groin node dissection was proposed. The patient presented to our institution for a second opinion. Pathology review performed by a certified dermatopathologist revealed an epithelial neoplasm characterized by the presence of islands of epithelial cells with large central areas of necrosis and parakeratosis (Fig. 1). The periphery of the lobules showed small basaloid cells with some degree of maturation and low mitotic activity (Fig. 2). Nuclei were slightly enlarged with vesicular chromatin pattern but no significant nucleoli. The stroma was desmoplastic and contained a prominent inflammatory reaction with giant cells secondary to the presence of pilar type keratin. None of the sections showed evidence of surface or squamous epithelium. In some of the sections, focal perineural extension of the tumor was present, with large nerve bundles noted. However, unequivocal vascular invasion was not identified on sections examined. Immunostains for p16 were not performed. The pattern of growth and extensive pilo-matriceal differentiation supported the diagnosis of a skin appendage cell neoplasm, consistent with a low grade pilomatrix carcinoma of the vulva. A CT scan of the abdomen and pelvis was performed, showing no evidence of metastatic disease.
Fig. 1.
Islands of epithelial cells with large central areas of necrosis and parakeratosis present in desmoplastic stroma. The star represents the shadow cells and the arrow shows the periphery of the lobules with the basaloid cells. Hematoxylin and eosin stain, × 4 magnification.
Fig. 2.
Periphery of the lobules with small basaloid cells with some degree of maturation, low mitotic activity, slightly enlarged nuclei and vesicular chromatin. Hematoxylin and eosin stain, 10 × magnification.
The patient was taken to the operating room where a right radical hemivulvectomy was performed. Exam under anesthesia revealed an indurated area measuring 2 cm in the region where the mass was previously excised. The indurated area was re-excised with 1 cm clear margins. Final pathology revealed no residual tumor. Histologic changes were consistent with dermal and subcutaneous scarring and foreign body giant cell reaction to suture. Eight years later, the patient remains without evidence of recurrent disease.
3. Discussion
Pilomatrix carcinoma, a malignant variant of benign pilomatrixoma, is extremely rare, with just over 100 cases reported in the English literature (Cornejo and Deng, 2013). Although these tumors may arise in children and young adults, most cases occur in older people with an average age of 52 and are three times more common in males (Cornejo and Deng, 2013). Most of the lesions are located in the head and posterior neck region but can also occur in the trunk and extremities (Hardisson et al., 2001). The average size of these tumors is 4 cm, ranging from 0.5 to 20 cm (Hardisson et al., 2001). Benign pilomatrixomas, however, are common tumors found in younger female individuals. Although they are also found in the head and neck area, they are slower growing and usually measure 1 to 3 cm in diameter (Sau et al., 1993).
Diagnosis of pilomatrix carcinoma is made on an assemblage of histological features to distinguish from benign pilomatrixoma. Benign pilomatrixoma is often a sharply circumscribed tumor, made up of uniform basaloid cells that are arranged at the periphery of the dermis (Hardisson et al., 2001). These cells show small and uniform nuclei and scanty pale cytoplasm (Sau et al., 1993). At the center of tumor, there are widespread organized regions of keratinization, squamous differentiation, and formation of shadow cells (Sau et al., 1993). Unlike its benign counterpart, features of pilomatrix carcinoma include sheets and islands of basaloid cells arranged in irregular nests throughout the tumor. They display cytologic atypia with enlarged pleomorphic nuclei, desmoplastic stromal reaction, ulceration and areas of necrosis (De Galvez-Aranda et al., 2002). Asymmetric infiltrative expansion into subcutaneous tissue or muscle can be seen, as well as invasion into vascular or perineural structures (De Galvez-Aranda et al., 2002).
The differential diagnoses of pilomatrix carcinoma include proliferating pilomatrixoma, aggressive pilomatrixoma, basal cell carcinoma with matrical differentiation, and squamous cell carcinoma. Proliferating pilomatrixomas are relatively large well-circumscribed neoplasms consisting of mostly basaloid cells, with increased mitotic activity (Cornejo and Deng, 2013, Gazic et al., 2011). However, there is no vascular or perineural involvement or infiltration. Aggressive pilomatrixoma is similar to pilomatrix carcinoma as it exhibits basaloid cells with significant cytological atypia, an increased mitotic activity, and a permeating growth pattern; however, there are more perineural or vascular invasion, atypical mitoses and nuclear atypia in pilomatrix carcinoma (Hardisson et al., 2001). Basal cell carcinoma with matrical differentiation is distinguished microscopically by peripheral nuclear palisading, retraction clefts between the tumor nodule and the stroma, and a surrounding desmoplastic stroma (Hardisson et al., 2001, Sau et al., 1993). Squamous cell carcinoma may comprise of basaloid cells with cytologic atypia, mitoses and necrosis but lacks shadow cells. They also may display infiltrative growth pattern and invasion into perineural or vascular structures (Cornejo and Deng, 2013).
Pilomatrix carcinomas are locally aggressive and can recur up to 50–60% of cases with simple excision (Hardisson et al., 2001, Sau et al., 1993). However, distant metastases involving the lung, brain, lymph nodes, bone, skin, and retroperitoneum are uncommon, occurring in 10% of cases (Cornejo and Deng, 2013). The preferred treatment for pilomatrix carcinoma is wide local excision with margins between 5 to 30 mm (Cornejo and Deng, 2013, Hardisson et al., 2001). Other promising treatments include Mohs micrographic surgery, which can attain a more exact margin control, and electron-beam radiation therapy, which may be appropriate for patients in whom surgery cannot be performed, and has been successful in a few cases (Cornejo and Deng, 2013, Melancon et al., 2011).
It is important to make the diagnosis of a pilomatrix carcinoma involving the vulva pre-operatively as this changes the surgical approach from a radical procedure including inguinal–femoral groin node dissection with potential long term morbidity, such as lymphedema, to a less radical procedure that provides adequate local control. In summary, this case is the second reported case in the published English literature of a pilomatrix carcinoma occurring on the external genitalia; the other occurred in an 85-year old woman with a clitoral lesion (Gazic et al., 2011). Consideration of this rare tumor should be considered when evaluating a young woman with a solitary lesion found to be a vulvar malignancy. Younger women are usually found to have HPV related dysplasia and cancer, which is usually multifocal and rarely an isolated lesion. As pilomatrix carcinoma of the vulva is rare, it is critical to recognize the appropriate diagnosis in order to facilitate that the appropriate, less radical surgical approach is performed minimizing potential long term morbidity.
Conflict of interest
The authors declare that there are no conflicts of interest.
Contributor Information
Mihae Song, Email: Mihae.song@gmail.com.
Marina Chekmareva, Email: chekmam1@rwjms.rutgers.edu.
Gloria Bachmann, Email: bachmaga@rwjms.rutgers.edu.
Darlene Gibbon, Email: gibbonda@cinj.rutgers.edu.
References
- Cornejo K.M., Deng A. Pilomatrix carcinoma: a case report and review of the literature. Am. J. Dermatopathol. 2013;35:389–394. doi: 10.1097/DAD.0b013e318274b7da. [DOI] [PubMed] [Google Scholar]
- De Galvez-Aranda M.V., Herrera-Ceballos E., Sanchez-Sanchez P., Bosch-Garcia R.J., Matilla-Vicente A. Pilomatrix carcinoma with lymph node and pulmonary metastasis: report of a case arising on the knee. Am. J. Dermatopathol. 2002;24:139–143. doi: 10.1097/00000372-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Gazic B., Sramek-Zatler S., Repse-Fokter A., Pizem J. Pilomatrix carcinoma of the clitoris. Int. J. Surg. Pathol. 2011;19:827–830. doi: 10.1177/1066896910397882. [DOI] [PubMed] [Google Scholar]
- Hardisson D., Linares M.D., Cuevas-Santos J., Contreras F. Pilomatrix carcinoma: a clinicopathologic study of six cases and review of the literature. Am. J. Dermatopathol. 2001;23:394–401. doi: 10.1097/00000372-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Lopansri S., Mihm M.C., Jr. Pilomatrix carcinoma or calcifying epitheliocarcinoma of Malherbe: a case report and review of literature. Cancer. 1980;45:2368–2373. doi: 10.1002/1097-0142(19800501)45:9<2368::aid-cncr2820450922>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Melancon J.M., Tom W.L., Lee R.A., Jackson M., Jiang S.I. Management of pilomatrix carcinoma: a case report of successful treatment with Mohs micrographic surgery and review of the literature. Dermatol. Surg. 2011;37:1798–1805. doi: 10.1111/j.1524-4725.2011.02170.x. [DOI] [PubMed] [Google Scholar]
- Sau P., Lupton G.P., Graham J.H. Pilomatrix carcinoma. Cancer. 1993;71:2491–2498. doi: 10.1002/1097-0142(19930415)71:8<2491::aid-cncr2820710811>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]