Abstract
Pesticides are a pervasive presence in aquatic ecosystems throughout the world. While pesticides are intended to control fungi, insects, and other pests, their mechanisms of action are often not specific enough to prevent unintended effects, such as on non-target microbial populations. Microorganisms, including algae and cyanobacteria, protozoa, aquatic fungi, and bacteria, form the basis of many food webs and are responsible for crucial aspects of biogeochemical cycling; therefore, the potential for pesticides to alter microbial community structures must be understood to preserve ecosystem services. This review examines studies that focused on direct population-level effects and indirect community-level effects of pesticides on microorganisms. Generally, insecticides, herbicides, and fungicides were found to have adverse direct effects on algal and fungal species. Insecticides and fungicides also had deleterious direct effects in the majority of studies examining protozoa species, although herbicides were found to have inconsistent direct effects on protozoans. Our synthesis revealed mixed or no direct effects on bacterial species among all pesticide categories, with results highly dependent on the target species, chemical, and concentration used in the study. Examination of community-level, indirect effects revealed that all pesticide categories had a tendency to reduce higher trophic levels, thereby diminishing top-down pressures and favoring lower trophic levels. Often, indirect effects exerted greater influence than direct effects. However, few studies have been conducted to specifically address community-level effects of pesticides on microorganisms and further research is necessary to better understand and predict the net effects of pesticides on ecosystem health.
Keywords: herbicide, fungicide, insecticide, bacteria, algae, protozoa, fungi
Introduction
Global pesticide usage is increasing and the presence of pesticides, which include herbicides, fungicides and insecticides, has become pervasive in freshwater and marine ecosystems. Between 2000 and 2007, global pesticide use increased from 2.27 billion to 2.36 billion kg (U. S. Environmental Protection Agency, 2012). In 2001, over five billion kg of chemicals were used in the United States alone (Kiely, Donaldson, and Grube, 2004) and pesticides were detected in over 90% of developed watersheds (defined as any watershed dominated by agricultural, urban or mixed land use) and in over 50% of groundwater wells (Gilliom, 2007).
To safeguard against pesticide contamination, the United States Environmental Protection Agency (USEPA) benchmarks have been established at the “no-effect” level for pesticides, wherein a pesticide concentration below the benchmark would not be expected to have any adverse effects, while a concentration above the benchmark might adversely affect the target organism(s) (Table 1) (Gilliom, 2007; Gilliom et al., 2006; U. S. Environmental Protection Agency, 2012). Based upon these USEPA benchmarks, pesticides exceeded standards for human health in ~10% of agricultural streams, ~7% of urban streams, and ~1% of groundwater tested, while benchmarks for aquatic health were exceeded in 57% of agricultural and 83% of urban streams (Gilliom, 2007; U. S. Environmental Protection Agency, 2004; U. S. Environmental Protection Agency, 2012). Similarly, according to European Union (EU) directives, ~43% of median pesticide levels in surface waters of northeastern Greece (Vryzas et al., 2009) and 12% of ground water tested in northern Spain exceeded regulatory standards (Hildebrandt et al., 2008).
Table 1.
Partial listing of USEPA pesticide benchmarks for human and aquatic health.
| Pesticide Group | Pesticide | Soil Half-Life (days) |
Human (µg L−1)* |
Fish (µg L−1)* | Invertebrates (µg L−1)* |
Nonvascular Plants (µg L−1)* |
Vascular Plants (µg L−1)* |
|---|---|---|---|---|---|---|---|
| Insecticides | carbofuran | 50 | 44 | 1.115 | 0.75 | ||
| chlorpyrifos | 30 | 100 | 0.9 | 0.05 | 140 | ||
| diazinon | 40 | 45 | 0.105 | 3700 | |||
| dimethoate | 7 | 130 | 3100 | 21.5 | 84 | ||
| endosulfan | 50 | 150 | 0.05 | 0.3 | 428 | ||
| fenitrothion | 6 | 860 | 1.15 | ||||
| lindane | 450 | 3960 | 0.85 | 0.5 | |||
| malathion | 1 | 16.5 | 0.295 | 2400 | >9630 | ||
| methoxychlor | 120 | 7.5 | 0.7 | ||||
| permethrine | 40 | 0.395 | 0.0106 | 68 | |||
| Herbicides | 2,4-D | 10 | 12500 | ||||
| atrazine | 60 | 2650 | 360 | <1 | 0.001 | ||
| bentazone | 20 | >50000 | >50000 | 4500 | 5350 | ||
| diuron | 90 | 200 | 80 | 2.4 | 15 | ||
| glyphosate | 47 | 21500 | 26600 | 12100 | 11900 | ||
| linuron | 60 | 1500 | 60 | 13.7 | 2.5 | ||
| paraquat | 1000 | 6000 | 600 | 0.396 | 71 | ||
| simazine | 60 | 3200 | 500 | 2.24 | 140 | ||
| tebuthiuron | 360 | 53000 | 148500 | 50 | 135 | ||
| tri-allate | 82 | 1650 | 600 | 45.5 | 120 | 2400 | |
| Fungicides | captan | 3 | 3300 | 13.1 | 4200 | 320 | >12700 |
| chlorothalonil | 30 | 5.25 | 1.8 | 6.8 | 630 | ||
| dicloran | 140 | 16500 | |||||
| fenpropimorph | 4 | 4950 | |||||
| propiconazole | 30 | 3000 | 425 | 650 | 21 | 4828 | |
| thiophanate methyl | 7 | 4150 | 2700 | 930 | >4700 | ||
| thiram | 15 | 462 | 21 | 105 | 140 | 1600 |
Values are based on USEPA acute toxicity standards.
In addition to understanding how parent pesticide compounds affect watersheds, it is also important to recognize that pesticide intermediates can produce significant effects on the health of aquatic ecosystems. Pesticide intermediates are the breakdown products produced via photolytic, hydrolytic or microbially-induced decay of the parent pesticide compound. Many of these intermediate compounds are not biologically inert and can have magnified or altogether different effects (positive or negative) than the parent compound (Kralj et al., 2007; Yanze-kontchou and Gschwind, 1994; Zeinat et al., 2008).
While most pesticides are designed to target a specific pest or particular pest group, the use of pesticides and their ensuing intermediates can have additional effects on non-target species (Rohr, Kerby, and Sih, 2006). For instance, pesticide exposure has resulted in decreased biodiversity, toxicity to certain algae and diatoms resulting in harmful algal blooms, and alterations in ecosystem food webs, such as increases in heterotrophic activity (Beketov et al., 2013; Benton, Vickery, and Wilson, 2003; Debenest et al., 2010; Malaj et al., 2014; Robinson and Sutherland, 2002). Projections suggest that continued extension of current agricultural practices could produce further harmful effects (Tilman, 1999). Multiple studies have investigated the effects of pesticides on amphibian, arthropod and fish species (Desneux, Decourtye, and Delpuech, 2007; McMahon et al., 2011; Relyea, 2009; Rohr et al., 2003; Rohr and McCoy, 2010; Rohr and Palmer, 2005; Rohr et al., 2006; Rohr et al., 2008; Stark and Banks, 2003); however, relatively little research has addressed the effects of pesticides on microorganisms.
Microorganisms, including algal, bacterial, protozoan, and fungal species, are fundamental components of aquatic ecosystems, providing crucial services such as primary production, decomposition and nutrient cycling. Aquatic microbial communities potentially include a host of waterborne bacterial, protozoan and fungal pathogens. These pathogens may be natural (autochthonous) inhabitants of aquatic ecosystems, e.g. Vibrio species, or they may be allochthonous intruders contributed by human or animal waste. Therefore, any pesticide impact resulting in alteration of microbial communities could result in dramatic changes and damage to an impacted water body, as well as posing a risk to human health. Of the studies on interactions between pesticides and microorganisms, most have examined the role of both naturally occurring and lab-derived microorganisms in the biodegradation of pesticides (Anderson et al., 2002; Cycoń, Wójcik, and Piotrowska-Seget, 2009; De Souza et al., 1998; Levanon, 1993; Seffernick et al., 2007; Wackett et al., 2002; Zeinat et al., 2008). As many microorganisms can breakdown pesticides, using them as a resource, and pesticides can be directly toxic to microorganisms, the effects of pesticides on microorganisms are difficult to predict a priori.
The difficulty in assessing many of the studies investigating the effects of pesticides on microorganisms is that, often, different systems (i.e., lab cultures, microcosms, field studies) are employed along with different target organisms and pesticide concentrations. Of the studies reviewed, some used pesticide concentrations that are ecologically relevant; i.e. levels that could be expected to be found in water bodies as a consequence of stormwater or agricultural runoff from neighboring watersheds. Others use concentrations consistent with direct pesticide applications. Finally, other studies test pesticide concentrations that are not ecologically relevant, and thus are above expected environmental concentrations based on application instructions. Throughout this review, reference will be made to whether pesticide concentrations used were environmentally relevant, meaning that the concentration was at or below what would be expected in a natural water body (based on reports from agencies such as the World Health Organization, USEPA and the US Department of Health and Human Services Agency for Toxic Substances and Disease Registry). In addition to variation in concentrations, studies vary in their endpoints, with some examining direct, population-level effects of a pesticide and others considering direct and indirect effects on microbial communities. As a consequence of a lack of standardization among concentrations, pesticides, organisms, and study complexity, a review of the existing literature seemed more practical than a quantitative meta-analysis.
This review considers studies which have examined the effects of pesticides on populations or communities of microorganisms, including microscopic phototrophs (i.e., algae and cyanobacteria), heterotrophic bacteria, protozoans, and aquatic fungi. Studies were limited to freshwater, estuarine, and marine watersheds, including the underlying sediments, and laboratory culture studies, as these often investigate the effects of pesticides on waterborne microbes. Pesticide effects in unsaturated soils were not reviewed. Trends in pesticide effects for pesticide categories and classes on specific microbial groups are explained, where possible. Where studies have found conflicting results, this review will address potential reasons for these conflicts. Additionally, by examining studies which have focused on direct and indirect effects (see below), this review, will summarize the ecological mechanism(s) by which pesticides act on microbial communities. Ultimately, this review provides a detailed summary of the net pesticide effects observed on microorganisms so that the effects of pesticides on impacted aquatic ecosystems can be better anticipated, as well as highlights the need for continued research, particularly at the community level.
Type and direction of pesticide effects
The effects of pesticides on microbes may be direct (examining only a single species at the population level) or indirect (examining multiple different species and/or trophic levels at the community-level) and either beneficial or adverse (Clements and Rohr, 2009; Verro et al., 2009). Pesticide exposure may prove directly toxic to microbes (an adverse effect) or pesticides may be utilized by microbes, particularly bacterial species, as a nutrient source, facilitating growth and/or survival (a beneficial effect). Additionally, direct effects may be masked by indirect pesticide effects. For example, an initial decrease in a population as a result of a directly toxic pesticide may be ameliorated by an accompanying decrease in predation or competition, masking the initial adverse effect over time (Rohr et al., 2006).
While many direct effects might be easily predicted (i.e., the direct toxicity of an herbicide on an algal species), indirect effects are more common and complex than direct effects and are often more difficult to predict (Relyea, 2009; Rohr and Crumrine, 2005; Rohr, Kerby, and Sih, 2006). Relatively few studies have investigated how indirect effects of pesticides influence microbial survival, despite the frequent observation that such effects are important drivers of microbial community structure (Jousset, 2012; Korajkic, Wanjugi, and Harwood, 2013; Wanjugi and Harwood, 2012). Indirect effects might be either density- or trait-mediated (Rohr, Kerby, and Sih, 2006). Density-mediated indirect effects would be those that result in either the increase or decrease in abundance of a predator, competitor, parasite, or food resource, which subsequently affects the focal organism (Raffel, Martin, and Rohr, 2008; Rohr et al., 2008; Rohr et al., 2008; Rohr et al., 2009). For instance, pesticide application might reduce the abundance of a bacterivorous protozoan species, facilitating the survival of bacterial prey (a beneficial effect for the bacteria). Pesticides might also act to reduce certain microbial populations while leaving others unscathed, which would decrease competition for resources shared by the affected and unaffected populations. In addition to density-mediated indirect effects, pesticides have the capacity to alter traits of organisms, such as behavior, immunity, physiology or morphology, resulting in trait-mediated indirect effects (Rohr and Crumrine, 2005; Rohr et al., 2003; Rohr, Kerby, and Sih, 2006; Rohr and Palmer, 2005; Rohr et al., 2008; Rohr et al., 2009). For example, upon exposure to an herbicide that affects photosynthesis, a facultatively heterotrophic protozoan (capable of photosynthetic or heterotrophic metabolism) might switch from an autotrophic to a heterotrophic mode, causing increased predation on the microbial prey population (an adverse effect) (Debenest et al., 2010; Staley et al., 2014). Pesticides might also stimulate an increase or decrease in anti-parasite or anti-predator behaviors, affecting overall survival (Rohr et al., 2009). Understanding both direct and indirect effects is therefore essential to being able to accurately predict the net impact of pesticide residues on aquatic ecosystems.
Study search criteria
Studies examining the effects of pesticides on microorganisms were selected using the National Institutes of Health PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Web of Science databases. Boolean searches were performed consisting of a pesticide classification and microbial taxa. Pesticide classifications ranged from the general term “pesticide” to more specific pesticide categories (herbicide, insecticide or fungicide), pesticide groups within categories (i.e., organophosphates, carbamates, s-triazines, aromatics, etc.), and, at the most specific, pesticide names (i.e., atrazine, malathion, diazinon, etc.). Terms used for microbial taxa ranged from the general term “microorganism” or “microbe” to more specific microbial taxa (including algae, cyanobacteria, bacteria, protozoa, and fungi). The term “heterotrophic” was also included with bacteria and protozoa, as well as limiting search terms to “aquatic fungi,” to further specify searches. No particular microbial species (i.e., specifically Escherichia coli or Tetrahymena pyriformis) were searched for. Additionally, terms such as “microbial persistence” or “microbial survival” (also repeated with more specific taxa names replacing “microbial”) were also used to remove studies focusing on biodegradation rather than microbial fate from the search results. Also, as there are thousands of pesticides, it was necessary to limit the scope of this review to only those pesticides which were more commonly used, to allow for synthesis of results and avoid an encyclopedic listing of study results. Where only one or two studies could be found utilizing a particular pesticide, those studies were not included unless they represented the sole instance of an experiment utilizing the particular pesticide group.
Statistics
To determine whether types of pesticides generally have beneficial, neutral, or adverse effects on microbes and whether their effects depended upon the microbial taxon, each species in our database received a −1, 0, or 1 if a focal pesticide had a significant adverse effect, no significant effect, or a significant beneficial effect on its survival, respectively. Treating the species as the replicate, we then used a generalized linear model with an ordinal multinomial error distribution to test whether microbial density was influenced significantly by pesticide type (insecticide, herbicide, fungicide), microbial taxon (algae, bacteria, fungi, protozoa), and their interaction. Note that an ordinal multinomial distribution assumes an ordered categorical response variable (−1<0<1) but it does not assume that distances between categories is known or constant. This is appropriate in this case because the distance between a significant adverse effect and a non-significant effect is not the same for every pair of species. We chose not to conduct a traditional meta-analysis based on effect sizes because of how much variation methods and effects there were among these studies. For each taxon by chemical class combination, we also evaluated whether the observed frequency of significant adverse effects, null effects, or significant beneficial effects was greater than expected by chance (with the conservative assumption of an equal probability of falling in any of the three categories) using an observed versus expected Chi-square test. All statistics were conducted in Statistica v 6.0 (Statsoft Inc., Tulsa, OK, USA) with an α of 0.05.
Specific effects of pesticides
Insecticides
The general mode of action of most commercial insecticides is nervous system disruption (Table 2). Organophosphate insecticides (i.e., parathion, diazinon, malathion, chlorpyrifos, etc.) and carbamate insecticides (i.e., carbaryl, carbofuran) hydrolyze acetylcholine and inhibit acetylcholinesterase, resulting in a build-up of the neurotransmitter acetylcholine and eventual death of the organism (Fukuto, 1990). Pyrethroid insecticides and organochlorine insecticides (i.e., DDT, aldrin, dieldrin, endrin) act by binding to γ-aminobutyric acid (GABA) receptors, preventing chloride anions from entering nerve cells (Coats, 1990).
Table 2.
Pesticides and mechanism of action.
| Pesticide Category | Groups Included | General Toxic Effect | Specific Site of Action |
|---|---|---|---|
| Herbicide | Glyphosate | Amino Acid Synthesis | EPSP Synthase |
| Sulfonyl Ureas, Imidazolinones | Amino Acid Synthesis | Acetolactate Synthase | |
| Glufosinate | Amino Acid Synthesis | Glutamine Synthetase | |
| Triazines, Anilides, Phenyl Carbamates, Ureas, Biscarbamates, Benzimidazoles, Uracils, Quinones, Hydroxynitriles | Photosynthesis | Hill Reaction of Photosystem II | |
| Bipyridiniums, Heteropentalenes | Photosynthesis (Bleaching) | Photosystem I | |
| Diphenyl ethers, Oxadiazoles, N-phenly imides | Photosynthesis (Bleaching) | Protoporphyrinogen Oxidase | |
| Aryoxyphenoxy propionates, Cyclohexanediones, Chloroacetamide | Lipid Biosynthesis | Acetyl-CoA Carboxylase | |
| Pyridazinones, Fluridone, m-Phenoxybenzamides, 4-Hydroxypyridines | Carotenoid Biosynthesis | Phytoene Desaturase | |
| Aminotriazole | Carotenoid Biosynthesis | Lycopene Cyclase | |
| Dichlormate | Carotenoid Biosynthesis | ζ-Carotene Desaturase | |
| Iosxazolidinones | Carotenoid Biosynthesis | IPP Isomerase and/or Prenyl Transferase | |
| Dinitroanilines, Phosphoric amides, Chlorthaldimethyl, Propyzamide, Cholchicine, Terbutol | Microtubule Biosynthesis | β-Tubulin | |
| Dichlobenil | Cellulose Biosynthesis | Cellulose Synthase | |
| Asulam | Folate Biosynthesis | Dihydropteroate Synthase | |
| Insecticides | |||
| Organophosphates | Carbamates | Nervous System Inhibition | Acetylcholinesterase |
| Organochlorines | Boranes, Chlorinated Acylics, Cyclodienes, Cyclohexanes | Nervous System Inhibition | GABA Receptor |
| Pyrethroids | Nervous System Inhibition | GABA Receptor | |
| Fungicides | Aromatic hydrocarbons, Triazoles | Membrane Biosynthesis | Lipid Biosynthesis |
| Cinnamic acid amide, Morpholine, Triazoles | Membrane Biosynthesis | Sterol Biosynthesis | |
| Hydrochloride | Membrane Biosynthesis | Intracellular Membrane Components | |
| Glucopyranosyl antibiotics, Tetracycline antibiotics | Amino Acid Synthesis | ||
| Phenylpyrroles, Dicarboximides | Signal Transduction | ||
| Pyrimidinamines | Respiration | NADH oxido-reductase Inhibitors | |
| Pyridine carboxamides, Benzamides, Gxathiin carboxamides | Respiration | Succinate-dehydrogenase Inhibitors | |
| 2,6-dinitroanilines, Dinitrophenyl crotonate | Respiration | Oxidative Phosphorylation Uncouplers |
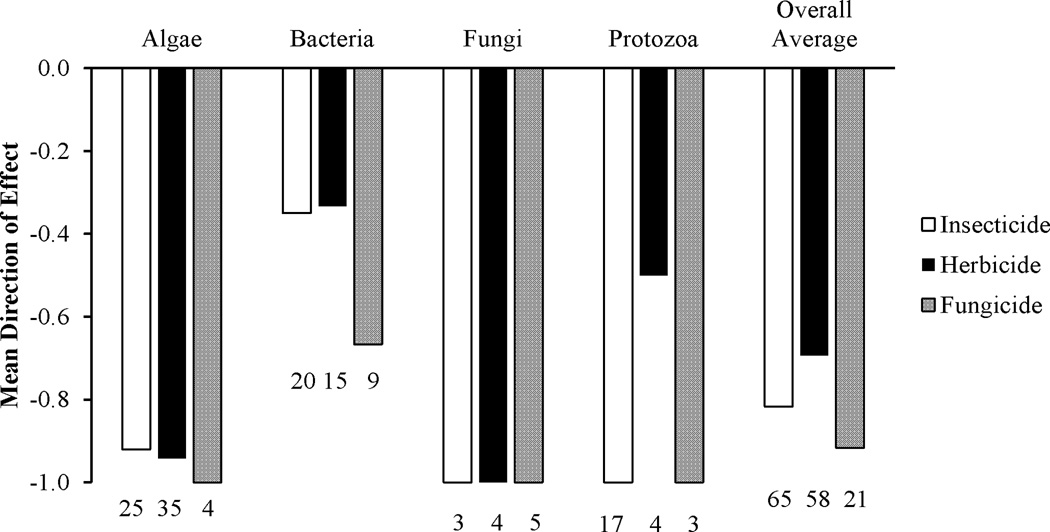
Overall, insecticides caused generally negative effects on algal, bacterial, protozoan, and fungal species (Tables 3 and 4, Figure 1). When considering effects by pesticide (rather than by study as in Table 3, as several studies used more than one pesticide), 92% (of 25 insecticides), 50% (of 20 insecticides), 100% (of 17 insecticides), and 100% (of three insecticides) of insecticides caused direct negative effects on algae (including cyanobacteria), bacteria, protozoa, and aquatic fungi, respectively (Table 4). Eight percent and 35% of insecticides had neutral effects on algae and bacteria, respectively, while 15% of insecticides had direct positive effects on bacterial species (Table 4). While insecticides tend to produce generally negative effects for most microbes, there are some differences in direct effects produced among pesticide groups.
Table 3.
Summary of studies finding direct pesticide effects that were positive, neutral, or negative.
| Direction of Effect | ||||||
|---|---|---|---|---|---|---|
| Taxa | Pesiticide Category | Positive | Neutral | Negative | Mixed | Total Studies |
| Algae | Insecticides | 0 | 1 | 16 | 1 | 18 |
| carbamate | - | - | 4 | - | 4 | |
| organochlorine | - | 1 | 6 | 1 | 8 | |
| organophosphate | - | - | 6 | - | 6 | |
| Herbicides | 0 | 0 | 10 | 2 | 12 | |
| Fungicides | 0 | 0 | 3 | 0 | 3 | |
| Bacteria | Insecticides | 1 | 6 | 5 | 1 | 13 |
| carbamate | - | - | 3 | - | 3 | |
| organochlorine | - | 1 | - | - | 1 | |
| organophosphate | 0 | 5 | 2 | 1 | 8 | |
| pyrethroid | 1 | - | - | - | 1 | |
| Herbicides | 1 | 3 | 3 | 1 | 8 | |
| Fungicides | 0 | 1 | 3 | 1 | 5 | |
| Fungi | Insecticides | 0 | 0 | 3 | 0 | 3 |
| carbamate | - | - | 1 | - | 1 | |
| organophosphate | - | - | 2 | - | 2 | |
| Herbicides | 0 | 0 | 4 | 0 | 4 | |
| Fungicides | 0 | 0 | 4 | 0 | 4 | |
| Protozoa | Insecticides | 0 | 0 | 11 | 0 | 11 |
| carbamate | - | - | 3 | - | 3 | |
| organochlorine | - | - | 3 | - | 3 | |
| organophosphate | - | - | 5 | - | 5 | |
| Herbicides | 0 | 1 | 2 | 0 | 3 | |
| Fungicides | 0 | 0 | 2 | 0 | 2 | |
Table 4.
Summary of pesticides with reported direct effects that were positive, neutral, or negative.
| Direction of Effect | |||||||
|---|---|---|---|---|---|---|---|
| Taxa | Pesticide Category | Positive | Neutral | Negative | Total Chemicals |
χ2a | Pa |
| Algae | Insecticides | 0 | 2 | 23 | 25 | 38.96 | <0.001 |
| carbamate | - | - | 6 | 6 | 12.00 | 0.002 | |
| organochlorine | - | 2 | 11 | 13 | 15.86 | <0.001 | |
| organophosphate | - | - | 6 | 6 | 12.00 | 0.002 | |
| Herbicides | 0 | 2 | 33 | 35 | 58.69 | <0.001 | |
| Fungicides | 0 | 0 | 4 | 4 | 8.00 | 0.018 | |
| Bacteria | Insecticides | 3 | 7 | 10 | 20 | 3.70 | 0.157 |
| carbamate | - | - | 4 | 4 | 8.00 | 0.018 | |
| organochlorine | - | 1 | 1 | - | - | ||
| organophosphate | 2 | 6 | 6 | 14 | 2.29 | 0.319 | |
| pyrethroid | 1 | - | 1 | - | - | ||
| Herbicides | 2 | 6 | 7 | 15 | 2.80 | 0.247 | |
| Fungicides | 1 | 1 | 7 | 9 | 8.00 | 0.018 | |
| Fungi | Insecticides | 0 | 0 | 3 | 3 | 6.00 | 0.050 |
| carbamate | - | - | 1 | 1 | - | - | |
| organophosphate | - | - | 2 | 2 | - | - | |
| Herbicides | 0 | 0 | 4 | 4 | 8.00 | 0.018 | |
| Fungicides | 0 | 0 | 5 | 5 | 10.00 | 0.007 | |
| Protozoa | Insecticides | 0 | 0 | 17 | 17 | 34.00 | <0.001 |
| carbamate | - | - | 3 | 3 | 6.00 | 0.050 | |
| organochlorine | - | - | 6 | 6 | 12.00 | 0.002 | |
| organophosphate | - | - | 8 | 8 | 16.00 | <0.001 | |
| Herbicides | 0 | 2 | 2 | 4 | 2.00 | 0.368 | |
| Fungicides | 0 | 0 | 3 | 3 | 6.00 | 0.050 | |
Results of an observed versus expected Chi-square test assuming an equal expectation for each of the three categories.
Figure 1.
Mean direction of pesticide “direct” effects (+1 for significant positive effects, 0 for null effects, −1 for significant negative effects) for each taxon. Sample sizes appear below each bar.
Organophosphates
Organophosphates, as a class, have adverse direct effects on algal growth; however, of the six studies resulting in direct negative effects, only one was conducted at insecticide concentrations that were environmentally relevant (Table 5). Malathion inhibited chlorophyll production and resulted in death of cyanobacteria at concentrations between 1 – 1000 µg L−1, overlapping with ecologically relevant concentrations of malathion (DeLorenzo, Scott, and Ross, 2001; Torres and O'Flaherty, 1976). The organophosphate diazinon inhibited growth of cyanobacteria at concentrations of 400 000 and 500 000 µg L−1 (Singh, 1973). Although also at concentrations greater than would be expected in the environment, diazinon was toxic to planktonic algae at 10 000 µg L−1 (Butler, Deason, and O'Kelley, 1975). Parathion inhibited reproduction in Chlorella fusca at concentrations of 7860 µg L−1 (Faust et al., 1994). Fenitrothion inhibited growth of both phytoplankton and cyanobacteria at concentrations between 800 and 24 400 µg L−1 (Kent and Currie, 1995). Chlorpyrifos inhibited growth of the marine diatom Minutocellus polymorphus at a concentration of 240 µg L−1 (Walsh et al., 1988).
Table 5.
Studies examining direct effects of pesticides on algae and cyanobacteria.
| a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pesticide Category |
Pesticide Group | Pesticides Used | Pesticide Concentration (µg L−1) |
Ecologically Relevant |
Matrix | Focal Microbes | Direction of Effect |
Reference |
| Insecticide | carbamate | carbaryl | 25000 | N | Lab Culture | planktonic algae | - | (Butler, Deason, et al. 1975) |
| carbamate | earbaryl | 5000 – 14000 | N | Lab Culture | Anabaena flos-aquae, Microcystis flos-aquae, M. aeruginosa, Selenastrum capricornutum, Scenedesmus quadricauda, Sc. obliquus, Chlorella vulgaris, C. pyrenoidosa | - | (Ma, Lu, et al. 2006) | |
| carbamate | carbaryl | 3667 | N | Lab Culture | Scenedesmus quadricuauda, Selenastrum, cupricornutum, Nitzchia sp., Cyclotella Oscillatoria sp., Pseudoanabaena sp., Anabaena inaequalis, Aphanizomenon flos-aquae | - | (Peterson, Boutin et al. 1994) | |
| carbamate | carbaryl | 100–100000 | Y | Lab Culture | Monochrysis lutheri, Dunaliella euchlora, Chlorella sp., Protococcus sp., Phaeodactylem tricornutum | - | (Ukeles 1962) | |
| carbamate | carbofuran | 13000 – 281000 | N | Lab Culture | Anabaena flos-aquae, Microcystis flos-aquae, M. aeruginosa, Selenastrum capricornutum, Scenedesmus quadricauda, Sc. obliquus, Chlorella vulgaris, C. pyrenoidosa | - | (Ma, Lu, et al. 2006) | |
| carbamate | carbofuran | 667 | N | Lab Culture | Scenedesmus quadricuauda, Selenastrum cupricornutum, Nitzchia sp., Cyclorella meneghiana, Microystis aeruginosa, Oscillatoria sp., Pseudoanabaena sp., Anabaena inaequalis, Aphanizomenon flos-aquae | - | (Peterson, Boutin et al. 1994) | |
| organochlorine | aldrin | 100000 | N | Lab Culture | freshwater algae | - | (Clegg and Koevenig 1974) | |
| organochlorine | benzene hexachloride | > 30000 | N | Lab Culture | Cylindrospermum sp., Aulosira fertilissima, Plectobena boryanum | - | (Singh 1973) | |
| organochlorine | DDT | 3.6 – 36 | Y | Unknown | Selenastum capricornutum | - | (Lee, Fang et al. 1976) | |
| organochlorine | DDT | 200–600 | N | Lab Culture | Monochrysis lutheri, Dunaliella euchlora, Chlorella sp., Protococcus sp., Phaeodactylum tricornutum | - | (Ukeles 1962) | |
| organochlorine | dieldrin | 100000 | N | Unknown | freshwater algae | - | (Clegg and Koevenig 1974) | |
| organochlorine | endosulfan | 41500 and 28500 | N | Lab Culture | Chlorella vulgaris, Anabaena doliolum | - | (Mohapatra and Mohanty 1992) | |
| organochlorine | endrin | 100000 | N | Lab Culture | freshwater algae | - | (Clegg and Koevenig 1974) | |
| organochiorine | endrin | 500000 | N | Lab Culture | Cylindrospermum sp., Aulosira fertilissima, Plecronema boryanum | Neutral | (Singh 1973) | |
| organochlorine | lindane | 4220 | N | Lab Culture | Chlorella fusca | - | (Faust, Altenburger et al. 1994) | |
| organochlorine | lindane | > 100000 | N | Lab Culture | Cylindrospermum sp,. Aulosira fertilissima, Plectonema boryanum | - | (Singh 1973) | |
| organochlorine | lindane | 1000 – 9000 | N | Lab Culture | Monochrysis lutheri, Dunaliella euchlora, Chlorella sp., Protococcus sp., Phaeodactylum tricornutum | - | (Ukeles 1962) | |
| organochlorine | methoxychlor | 100 | Y | Lab Culture | Chlorella pyrenoidosa | - | (Kricher, Urey et al. 1975) | |
| organochlorine | methoxychlor | 10 | Y | Lab Culture | planktonic algae | Neutral | (Butler. Deason, et al. 1975) | |
| organochlorine | mirex | 100 | Y | Lab Culture | Chlorella pyrenidosa | - | (Kricher, Urey et al. 1975) | |
| organophosphate | chlorpyrifos | 240 | N | Lab Culture | Minutocellus polymorphus | - | (Walsh, McLaughlin et al. 1988) | |
| organophosphate | diazinon | 400000–500000 | N | Lab Culture | Cylindrospermum sp., Aulosira fertilissima, Plectonema boryanum | - | (Singh 1973) | |
| organophosphate | diazinon | 10000 | N | Lab Culture | planktonic algae | - | (Butler, Deason, et al. 1975) | |
| organophosphate | fenitrothion | 800–24400 | N | Lab Culture | freshwater phytoplnakton | - | (Kent and Currie 1995) | |
| organophosphate | malathion | 1 – 1000 | Y | Lab Culture | Chlorella vulgaris, Chlorococcum hypnosporum, Oscillatoria lutea, Tribonema, Stigeoclonium tenue, Vaucheria geminata | - | (Torres and O'Flaherty 1976) | |
| organophosphate | parathion | 7860 | N | Lab Culture | Chlorella fusca | - | (Faust, Altenburger et al. 1994) | |
| b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Herbicide | s-triazine | atrazine | 0.12 – 5.8 | Y | Marine Mesocosms | Thalassiosira puntigera, T. rotula, Nitzschia pungens, Skeletonema costatum, Phaeocystis globosa | - | (Bester, Huhnerfuss et al. 1995) |
| phenoxy | 2,4-D | 4000 | N | Lab Culture | planktonic algae | - | (Butler, Deason, et al. 1975) | |
| s-triazine | atrazine | 1000 | N | Lab Culture | planktonic algae | - | (Butler, Deason, et al. 1975) | |
| bipyrimidine | diquat | 19 – 395 | Y | Lab Culture | Anabaena flos-aquae, Microcystis aeruginosa, Selenastrum capricornutum, Chlorella vulgaris | - | (Campbell, Bartell et al. 2000) | |
| s-triazine | atrazine | 90 – 3000 | Y | Lab Culture | Selenastrum capricornutum, Chlorella vulgaris, Chlamydomonas reinhardi, Scenedesmus quadricauda, Microcystis sp., Anabaena flosque | - | (Fairchild, Ruessler, et al. 2009) | |
| chloroacetanilides | metazachlor | 58 | Y | Lab Culture | Chlorella fusca | - | (Faust, Altenburger et al. 1994) | |
| glyphosate | glyphosate | 377000 | N | Lab Culture | Chlorella fusca | - | (Faust, Altenburger et al. 1994) | |
| phenoxy | 2,4-D | 88900 | N | Lab Culture | Chlorella fusca | - | (Faust, Altenburger et al. 1994) | |
| s-triazine | simazine | 73 | Y | Lab Culture | Chlorella fusca | - | (Faust, Altenburger et al. 1994) | |
| subtituted urea | bentazon | 42500 | N | Lab Culture | Chlorella fusca | - | (Faust, Altenburger et al. 1994) | |
| subtituted urea | chlorotoluron | 23 | Y | Lab Culture | Chlorella fusca | - | (Faust, Altenburger et al. 1994) | |
| subtituted urea | methabenzthiazuron | 44 | Y | Lab Culture | Chlorella fusca | - | (Faust, Altenburger et al. 1994) | |
| thiocarbamates | tri-allate | 3870 | N | Lab Culture | Chlorella fusca | - | (Faust, Altenburger et al. 1994) | |
| glyphosate | glyphosate | 8900 – 89000 | N | Freshwater Mesocosms | periphyton | - | (Goldsborough and Brown 1988) | |
| c | ||||||||
|---|---|---|---|---|---|---|---|---|
| bypyrimidine | paraquat | 10000 | N | Freshwater Mesocosms | freshwater cyanobacteria, chlorophytes, chrysophytes | - | (Kosinski 1984) | |
| s-triazine | atrazine | 10000 | N | Freshwater Mesocosms | freshwater cyanobacteria, chlorophytes, chrysophytes | - | (Kosinski 1984) | |
| s-triazine | atrazine | 130 – 620 nM | Y | Lab Culture | Navicula sp. and Nephroselmis pyriformis | - | (Magnusson, Heimann et al. 2008) | |
| substituted urea | diuron | 16 – 33 nM | Y | Lab Culture | Navicula sp. and Nephroselmis pyriformis | - | (Magnusson, Heimann et al. 2008) | |
| aldehyde | acrolein | 1000 | N | Lab Culture | Scenedesmus quadricuauda, Selenastrum cupricornutum, Nitzchia sp., Cyclotella meneghiana, Microystis aeruginosa, Oscillatoria sp., Pseudoanabaena sp., Anabaena inaequalis, Aphanizomenon flos-aquae | - | (Peterson, Boutin et al. 1994) | |
| bipyrimidine | diquat | 733 | N | Lab Culture | Scenedesmus quadricuauda, Selenastrum cupricornutum, Nitzchia sp., Cyclotella meneghiana, Microystis aeruginosa, Oscillatoria sp., Pseudoanabaena sp., Anabaena inaequalis, Aphanizomenon flos-aquae | - | (Peterson, Boutin et al. 1994) | |
| glyphosate | glyphosate | 2848 | N | Lab Culture | Scenedesmus quadricuauda, Selenastrum cupricornutum, Nitzchia sp., Cyclotella meneghiana, Microystis aeruginosa, Oscillatoria sp., Pseudoanabaena sp., Anabaena inaequalis, Aphanizomenon flos-aquae | - | (Peterson, Boutin et al. 1994) | |
| phenoxy | 2,4-D | 2917 | N | Lab Culture | Scenedesmus quadricuauda, Selenastrum cupricornutum, Nitzchia sp., Cyclotella meneghiana, Microystis aeruginosa, Oscillatoria sp., Pseudoanabaena sp., Anabaena inaequalis, Aphanizomenon flos-aquae | Neutral | (Peterson, Boutin et al. 1994) | |
| pyridine | picloram | 1760 | N | Lab Culture | Scenedesmus quadricuauda, Selenastrum cupricornutum, Nitzchia sp., Cyclotella meneghiana, Microystis aeruginosa, Oscillatoria sp., Pseudoanabaena sp., Anabaena inaequalis, Aphanizomenon flos-aquae | Neutral | (Peterson, Boutin et al. 1994) | |
| s-triazine | atrazine | 2667 | N | Lab Culture | Scenedesmus quadricuauda, Selenastrum cupricornutum, Nitzchia sp., Cyclotella meneghiana, Microystis aeruginosa, Oscillatoria sp., Pseudoanabaena sp., Anabaena inaequalis, Aphanizomenon flos-aquae | - | (Peterson, Boutin et al. 1994) | |
| s-triazine | simazine | 2667 | N | Lab Culture | Scenedesmus quadricuauda, Selenastrum cupricornutum, Nitzchia sp., Cyclotella meneghiana, Microystis aeruginosa, Oscillatoria sp., Pseudoanabaena sp., Anabaena inaequalis, Aphanizomenon flos-aquae | - | (Peterson, Boutin et al. 1994) | |
| substituted urea | tebuthiuron | 5900 | N | Lab Culture | Scenedesmus quadricuauda, Selenastrum cupricornutum, Nitzchia sp., Cyclotella meneghiana, Microystis aeruginosa, Oscillatoria sp., Pseudoanabaena sp., Anabaena inaequalis, Aphanizomenon flos-aquae | - | (Peterson, Boutin et al. 1994) | |
| phenoxy | 2,4-D | 1 – 100000 | Y | Lab Culture | Chlorella vulgaris, Chlorococcum hypnosporum, Oscillatoria lutea, Tribonema, Stigeoclonium tenue, Vaucheria geminata | Neutral | (Torres and O'Flaherty 1976) | |
| s-triazine | atrazine | 1 – 1000 | Y | Lab Culture | Chlorella vulgaris, Chlorococcum hypnosporum, Oscillatoria lutea, Tribonema, Stigeoclonium tenue, Vaucheria geminata | - | (Torres and O'Flaherty 1976) | |
| s-triazine | simazine | 1 – 1000 | Y | Lab Culture | Chlorella vulgaris, Chlorococcum hypnosporum, Oscillatoria lutea, Tribonema, Stigeoclonium tenue, Vaucheria geminata | - | (Torres and O'Flaherty 1976) | |
| substituted urea | diuron | 0.02 – 400 | Y | Lab Culture | Monochrysis lutheri, Dunaliella euchlora, Chlorella sp., Protococcus sp., Phaeodactylum tricornutum | - | (Ukeles 1962) | |
| substituted urea | fenuron | 0.02 – 400 | Y | Lab Culture | Monochrysis lutheri, Dunaliella euchlora, Chlorella sp., Protococcus sp., Phaeodactylum tricornutum | - | (Ukeles 1962) | |
| substituted urea | monuron | 0.02 – 400 | Y | Lab Culture | Monochrysis lutheri, Dunaliella euchlora, Chlorella sp., Protococcus sp., Phaeodactylum tricornutum | - | (Ukeles 1962) | |
| substituted urea | neburon | 0.02 – 400 | Y | Lab Culture | Monochrysis lutheri, Dunaliella euchlora, Chlorella sp., Protococcus sp., Phaeodactylum tricornutum | - | (Ukeles 1962) | |
| s-triazine | atrazine | 20 | Y | Lab Culture | Minutocellus polymorphus | - | (Walsh, McLaughlin et al. 1988) | |
| Fungicide | imidazole | prochloraz | 24 | Y | Lab Culture | Chlorella fusca | - | (Faust, Altenburger et al. 1994) |
| organomercury | 1 | Y | Lab Culture | marine diatoms and phytoplankton | - | (Harriss, white et al. 1970) | ||
| triazine | anilazine | 1390 | Y | Lab Culture | Chlorellas fusca | - | (Faust, Altenburger et al. 1994) | |
| triazole | propiconazole | 83 | Y | Lab Culture | Scenedesmus quadricuauda, Selenastrum cupricornutum, Nitzchia sp., Cyclotella meneghiana, Microystis aeruginosa, Oscillatoria sp., Pseudoanabaena sp., Anabaena inaequalis, Aphanizomenon flos-aquae | - | (Peterson, Boutin et al. 1994) | |
Organophosphate insecticides also produced direct negative effects on protozoa (Table 6), and were frequently toxic to protozoa at concentrations lower than were toxic to algae; however, only one of five studies reviewed used pesticide concentrations not exceeding environmentally relevant concentrations. Fenitrothion and chlorpyrifos inhibited the growth of Tetrahymena pyriformis (at 500– 2500 µg L−1 and 1000–10 0000 µg L−1, respectively) and, at higher concentrations of fenitrothion (5000–10 000 µg L−1), cell death also resulted (Lal, Saxena, and Lal, 1987). Organophosphates were generally toxic to the ciliate Colypidium campulum at much higher concentrations than would be expected in the environment, exceeding 10 000 µg L−1 (Dive, Leclerc, and Persoone, 1980). Parathion was found to inhibit survival of Euglena gracilis at a concentration of 1200 µg L−1 (Moore, 1970). Malathion was lethal to ciliates (at concentrations between 1000–30 000 µg L−1) and inhibited growth of Euglena gracilis (at a concentration of 7250 µg L−1) (Moore, 1970; Weber, Shea, and Berry, 1982). Another study found that malathion significantly reduced swimming velocity of E. gracilis at concentrations ranging from 6000–50 000 µg L−1 (Azizullah, Richter, and Hader, 2011).
Table 6.
Studies examining direct effects of pesticides on protozoa.
| Pesticide Category |
Pesticide Croup | Pesticides Used |
Pesticide Concentration (µg L−1) |
Ecologically Relevant |
Matrix | Focal Microbes | Direction of Effect |
Reference |
|---|---|---|---|---|---|---|---|---|
| Insecticide | carbamate | carbaryl | > 10000 | N | Lab Culture | Colpidium campulum | - | (Dive, Leclerc et al 1980) |
| carbamate | carbaryl | 1000 – 100000 | Y | Estuarine Mesocosms | ciliate protozoa | - | (Weber, Shea et al. 1982) | |
| carbamate | carbofuran | 9000–50000 | N | Lab Culture | Euglena gracilis | - | (Azizullah, Richter et al. 2011) | |
| organochlorine | aldrin | > 10000 | N | Lab Culture | Colpidium campulum | - | (Dive, Leclerc et al. 1980) | |
| organochlorine | DDT | 10000–100000 | N | Lab Culture | Tetrahymena pyriformis | - | (Lal, Saxena et al. 1987) | |
| organochlorine | dieldrin | > 10000 | N | Lab Culture | Colpidium campulum | - | (Dive, Leclerc et al. 1980) | |
| organochlorine | lindane | > 10000 | N | Lab Culture | Colpidium campulum | - | (Dive, Leclerc et al. 1980) | |
| organochlorine | methoxychlor | > 10000 | N | Lab Culture | Colpidium campulum | - | (Dive, Leclerc et al. 1980) | |
| organochlorine | mirex | 0.9 | Y | Lab Culture | Tetrahymena pyriformis | - | (Cooley, Keltner et al. 2007) | |
| organophosphate | chlorpyrifos | 100 – 2500 | Y | Lab Culture | Tetrahymena pyriformis | - | (Lal, Saxena et al. 1987) | |
| organophosphate | dimethoate | > 10000 | N | Lab Culture | Colpidium campulum | - | (Dive, Leclerc et al. 1980) | |
| organophosphate | fenitrothion | 500 – 10000 | Y | Lab Culture | Tetrahymena pyriformis | - | (Lal, Saxena et al. 1987) | |
| organophosphate | malathion | 6000 – 50000 | N | Lab Culture | Euglena gracilis | - | (Azizullah, Richter et al. 2011) | |
| organophosphate | malathion | > 10000 | N | Lab Culture | Colpidium campulum | - | (Dive, Leclerc et al. 1980) | |
| organophosphate | malathion | 7250 | N | Lab Culture | Euglena gracilis | - | (Moore 1970) | |
| organophosphate | malathion | 1000 – 30000 | N | Estuarine Mesocosms | ciliate protozoa | - | (Weber, Shea et al. 1982) | |
| organophosphate | parathion | 1200 | N | Lab Culture | Euglena gracilis | - | (Moore 1970) | |
| Herbicide | bipyrimidine | diquat | 2940 | N | Lab Culture | Euglena gracilis, Ochromonas danica, Navicula pelliculosa, Cryptomonas ovata | - | (Campbell, Bartell et al. 2000) |
| phenoxy | 2,4-D | > 10000 | N | Lab Culture | Colpidium campulum | Neutral | (Dive, Leclerc et al. 1980) | |
| substituted urea | chlorotoluron | 298500 | N | Lab Culture | Tetrahymena pyriformis | - | (Nelieu Bonnemoy, et al., 2010 | |
| substituted urea | fenuron | > 10000 | N | Lab Culture | Colpidium campulum | Neutral | (Dive, Leclerc et al. 1980) | |
| Fungicide | carbamate | nabam | 50 – 10000 | N | Lab Culture | Euglena gracilis | - | (Moore 1970) |
| carbamate | thiram | 300 | N | Lab Culture | Colpidium campulum | - | (Dive, Leclerc et al. 1980) | |
| carbamate | vapam | 1000 – 10000 | N | Lab Culture | Euglena gracilis | - | (Moore 1970) | |
Organophosphate insecticides have also induced negative effects on aquatic fungi (Table 7). Diazinon, at concentrations ranging between 0.05 and 50 µg L−1, promoted sporulation of Heliscella stellata (Flores et al., 2014). Although above environmentally relevant concentrations, dimethoate (at 2500 µg L−1) increased fungal mycelia density, zoosporangia concentration, and the number of sexual and asexual organs in the species Achyla racemosa, Dictyuchus monosporus, Saprolegnia ferax, Thraustotheca clavata, and Allomyces arbuscula (Khallil and Omar, 1993). However, in the same study, dimethoate concentrations ranging from 5000 to 75 000 µg L−1, completely inhibited mycelial growth of all five species.
Table 7.
Studies examining direct effects of pesticides on aquatic fungi.
| Pesticide Category |
Pesticide Croup | Pesticides Used | Pesticide Concentration (µg L−1) |
Ecologically Relevant |
Matrix | Focal Microbes | Direction of Effect |
Reference |
|---|---|---|---|---|---|---|---|---|
| Insecticide | carbamate | carbaryl | 2500 | Y | Lab Culture | Batrachochytrium dendrobatidis | - | (Hanlon and Paris, 2012) |
| organophosphate | diazinon | 0.05 – 50 | Y | Lab Culture | Heliscella stellata, Lunaspora curvula, Sigmoid type-2 aquatic fungi | - | (Flores, Banjac et al., 2014) | |
| organophosphate | dimethoate | 2500 – 75000 | N | Lab Culture | Achyla racemosa, Dictyuchus monosporus, Saprolegnia ferax, Thraustotheca clavata, and Allomyces arbuscula | - | (Khallil and Omar, 1993) | |
| Herbicide | bipyrimidine | diquat | 2 – 10 | Y | Lab Culture | Helicoon elegans, Pseudoaergerita matsushimae, Hormiactus ontariensis, Beverwykella pulmonaria and Sigmoid type-2 fungi | - | (Fronda and Kendrick 1995) |
| glyphosate | glyphosate | 50000 – 500000 | N | Lab Culture | Annulatascus velatisporus, Composporium antennatum, Helicosporium griseum, and Massarina sp. | - | (Tsui Hyde et al. 2001) | |
| glyphosate | glyphosate | 500 | Y | Lab Culture | Batrachochytrium dendrobatidis | - | (Hanlon and Paris, 2012) | |
| s-triazine | atrazine | 0.0106 – 106 | Y | Lab Culture | Batrachochytrium dendrobatidis | - | (McMahon, Romansic, et al., 2013) | |
| Fungicide | aromatic | chlorothalonil | 0.0176 – 1.76 and 32–176 | Y | Lab Culture | Batrachochytrium dendrobatidis | - | (McMahon, Romansic et al., 2013) |
| aromatic | chlorothalonil | > 260 | Y | Lab Culture | Cryptococcus flavescens, Trichoderma hamatum, Fusarium sporotrichioides, Mucor hiemalas, Pythium spp., Helicoon richonis, Helicodendran tubulosum | - | (Dijksterhius, van Doorn, et al., 2011 | |
| imidazole | imazalil | 0.1–100 | Y | Lab Culture | Heliscella stellata, Lunaspora curvula, Sigmoid type-2 aquatic fungi | - | (Flores, Banjac et al., 2014) | |
| imidazole | imazalil | 10000 – 210000 | N | Lab Culture | Cryptococcus flavescens, Trichoderma hamatum, Fusarium sporotrichioides, Mucor hiemalas, Pythium spp., Helicoon richonis, Helicodendran tubulosum | - | (Dijksterhius van Doorn, et al., 2012 | |
| phosphanoglycine | thiophanate methyl | 1500 | N | Lab Culture | Batrachochytrium dendrobatidis | - | (Hanlon and Paris, 2012) | |
The direct effects of organophosphate insecticides on bacteria have been less consistent than direct effects on other taxa (Table 3), although neutral effects were most commonly observed at environmentally relevant concentrations (Table 8). For example, diazinon was not found to effect the abundance of rumen bacteria in lab cultures at concentrations ranging from 0 to 500 000 µg L−1 (Williams et al., 1963). Similarly, diazinon, at concentrations as high as 0.005 µg L−1, did not inhibit the growth of E. coli or Klebsiella pneumoniae (Higgins and Hohn, 2008). Malathion was also not directly toxic to E. coli or Bacillus subtilis in in vitro tests (Kerszman, 1993), and no effects on growth rate or survival of E. coli, Enterococcus faecalis, E. coli O157:H7, or Salmonella enterica Typhimurium were observed in simplified in vitro microcosms using environmentally relevant concentrations of 101 and 202 µg L−1 (Staley et al., 2012).
Table 8.
Studies examining direct effects of pesticides on bacteria.
| a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pesticide Category |
Pesticide Group | Pesticides Used | Pesticide Concentration (µg L−1) |
Ecologically Relevant |
Matrix | Focal Microbes | Direction of Effect |
Reference |
| Insecticide | carbamate | carbaryl | 9400000 | N | Lab Culture | E. coli O157:H7, Salmonella typhimurium, Sal. enteritidis, Shigella sonnei, S. flexneri, Listeria moncytogenes | − | (Guan, Blank et al. 2001) |
| carbamate | carbaryl | 5000 | N | Lab Culture | Vibrio phosphoreum | − | (Somasundaram, Coats et al. 1990) | |
| carbamate | carbaryl | 100000 | N | Estuarine Mesocosms | sediment bacteria | − | (Weber and Rosenberg, 1984) | |
| carbamate | carbofuran | 20500 | Y | Lab Culture | Vibrio phosphoreum | − | (Somasundaram, Coats et al. 1990) | |
| organochlorine | lindane | 500–500000 | N | Rumen Fluid Microcosms | rumen bacteria | Neutral | (Williams, Robbins et al, 1963) | |
| organophosphate | chlorpyrifos | 6600000 | N | Lab Culture | E. coli O157:H7, Salmonella typhimurium, Sal. enteritidis, Shigella sonnei, S. flexneri, Listeria monocytogenes | + | (Guan, Blank et al. 2001) | |
| organophosphate | chlorpyrifos | 46300 | N | Lab Culture | Vibrio phosphoreum | − | (Somasundaram, Coats et al. 1990) | |
| organophosphate | diazinon | 14600000 | N | Lab Culture | E. coli O157:H7, Salmonella typhimurium, Sal. enteritidis, Shigella sonnei, S. flexneri, Listeria monocytogenes | − | (Guan, Blank et al. 2001) | |
| organophosphate | diazinon | 0.005 | Y | Lab Culture | Eshcerichia coli, Klebsiella pneumoniae | Neutral | (Higgins and Hohn 2008) | |
| organophosphate | diazinon | 10300 | N | Lab Culture | Vibrio phosphoreum | − | (Somasundaram, Coats et al. 1990) | |
| organophosphate | diazinon | 500 – 500000 | Y | Rumen Fluid Microcosms | rumen bacteria | Neutral | (Williams, Robbins et al. 1963) | |
| organophosphate | dimethoate | 14800000 | N | Lab Culture | E. coli O157:H7, Salmonella typhimurium, Sal. enteritidis, Shigella sonnei, S. flexneri, Listeria moncytogenes | − | (Guan, Blank et al. 2001) | |
| organophosphate | dimethoate | 500 – 500000 | N | Rumen Fluid Microcosms | rumen bacteria | Neutral | (Williams, Robbins et al. 1963) | |
| organophosphate | malathion | Unknown | Unknown | Escherichia coli, Bacillus subtilis | Neutral | (Kerszman 1993) | ||
| organophosphate | malathion | 500 | N | Sludge Chamber Microcosms | heterotrophic bacteria | − | (Pai, Wang et al. 2009) | |
| organophosphate | malathion | 101, 202 | Y | Freshwater Mesocosms | Escherichia coli. Enterococcus faecalis | Neutral | (Staley, Rohr et al. 2010) | |
| organophosphate | malathion | 101, 202 | Y | Freshwater Mesocosms | Eschericia coli, E. coli O157:H7, Salmonella typhimurium, Enterococcus faecalis | Neutral | (Staley, Senkbeil et al. 2012) | |
| organophosphate | parathion | 8500 | N | Lab Culture | Vibrio phosphoreum | − | (Somasundaram, Coats et al. 1990) | |
| pyrethroid | permethrin | 1000000 | N | Lab Culture | E. coli O157:H7, Salmonella typhimurium, Sal. enteritidis, Shigella sonnei, S. flexneri, Listeria moncytogenes | + | (Guan, Blank et al. 2001) | |
| b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Herbicide | bipyrimidine | diquat | 25000–50000 | N | Lab Culture | Erwinia carotovora, Pseudomonas fluorescens, Bacillus sp. | Neutral | (Breazeale and Camper 1972) |
| bipyrimidine | paraquat | 25000–50000 | N | Lab Culture | Erwinia carotovora, Pseudomonas fluorescens, Bacillus sp. | Neutral | (Breazeale and Camper 1972) | |
| bipyrimidine | paraquat | 20000000 | N | Lab Culture | E. coli O157:H7, Salmonella typhimurium, Sal. enteritidis, Shigella sonnei, S. flexneri, Listeria moncytogenes | - | (Guan, Blank et al. 2001) | |
| glyphosate | glyphosate | 13400000 | N | Lab Culture | E. coli O157:H7, Salmonella typhimurium, Sal. enteritidis, Shigella sonnei, S. flexneri, Listeria moncytogenes | - | (Guan, Blank et al. 2001) | |
| glyphosate | glyphosate | 500 | Y | Sludge Chamber Microcosms | heterotrophic bacteria | - | (Pai, Wang et al. 2009) | |
| phenoxy | 2,4-D | 31400000 | N | Lab Culture | E. coli O157:H7, Salmonella typhimurium, Sal. enteritidis, Shigella sonnei, S. flexneri, Listeria moncytogenes | - | (Guan, Blank et al. 2001) | |
| phenoxy | 2,4-D | 5 and 10mM | Lab Culture | Escherichia coli, Klebsiella pneumoniae | - | (Higgins and Hohn 2008) | ||
| phenoxy | 2,4-D | 100700 | N | Lab Culture | Vibrio phosphoreum | - | (Somasundaram, Coats et al. 1990) | |
| s-triazine | atrazine | 25000 – 50000 | N | Lab Culture | Erwinia carotovora, Pseudomonas fluorescens, Bacillus sp. | Neutral | (Breazeale and Camper 1972) | |
| s-triazine | atrazine | 0.25 – 22.5 | Y | Lab Culture | Escherichia coli, Klebsiella pneumoniae | Neutral | (Higgins and Hohn 2008) | |
| s-triazine | atrazine | 1 – 100 | Y | Lab Culture | Escherichia coli, Enterococcus faecalis | + | (Koutsotoli, Dimou et al. 2005) | |
| s-triazine | atrazine | 102, 204 | Y | Freshwater Microcosms | Escherichia coli, E. coli O157:H7, Salmonella typhimurium, Enterococcus faecalis | Neutral | (Staley, Senkbeil et al. 2012) | |
| s-triazine | siniazine | 0.000025 – 0.0025 | Y | Lab Culture | Escherichia coli, Klebsiella pneumoniae | Neutral | (Higgins and Hohn 2008) | |
| substituted urea | chlorotoluron | 68200 | N | Lab Culture | Vibrio fisherii | - | (Nelieu Bonnemoy, et al., 2010 | |
| substituted urea | linuron | 22600000 | N | Lab Culture | E. coli O157:H7, Salmonella typhimurium, Sal. enteritidis, Shigella sonnei, S. flexneri, Listeria moncytogenes | + | (Guan, Blank et al. 2001) | |
| Fungicide | aromatic | chlorothalonil | 20000000 | N | Lab Culture | E. coli O157:H7, Salmonella typhimurium, Sal. enteritidis, Shigella sonnei, S. flexneri, Listeria moncytogenes | + | (Guan, Blank et al. 2001) |
| aromatic | chlorothalonil | 169, 338 | Y | Freshwater Microcosms | Eschericia coli, E. coli O157:H7, Salmonella typhimurium, Enterococcus faecalis | Neutral | (Staley, Senkbeil et al. 2012) | |
| aromatic | dicloran | 0.14 | Y | Unknown | Salmonella YG1041 | - | (de Oliviera, Sakagami et al. 2009) | |
| carbamate | thiram | 13400000 | N | Lab Culture | E. coli O157:H7, Salmonella typhimurium, Sal. enteritidis, Shigella sonnei, S. flexneri, Listeria moncytogenes | - | (Guan, Blank et al. 2001) | |
| carbamate | thiram | 3000 – 70000 | N | Freshwater Mesocosms | sediment bacteria | - | (Milenkovski, Baath et al. 2010) | |
| dicarboximide | captan | 3000 – 70000 | N | Freshwater Mesocosms | sediment bacteria | - | (Milenkovski, Baath et al. 2010) | |
| hydrochloride | acriflavine | 1000 | N | Lab Culture | Staphylococcus aureus | - | (Kawai and Yamagishi 2009) | |
| morpholine | fenpropimorph | 3000 – 70000 | N | Freshwater Mesocosms | sediment bacteria | - | (Milenkovski, Baath et al. 2010) | |
| triazole | propiconazole | 3000 – 70000 | N | Freshwater Mesocosms | sediment bacteria | - | (Milenkovski, Baath et al. 2010) | |
However, when environmentally realistic concentrations of organophosphates were exceeded, negative and positive effects were observed (Tables 3 and 8), although in most cases this was likely due to other confounding factors or marked differences in toxicity assessment. At concentrations of 3 300 000 to 9 900 000 µg L−1, well above environmentally relevant concentrations, chlorpyrifos increased abundance of L. monocytogenes, Salmonella sp., Shigella sp., and E. coli O157:H7 100–1000-fold over a 96-hour period in lab cultures (Guan et al., 2001). However, in the same study, diazinon(at 14 600 000 µg L−1) had toxic effects on Listeria monocytogenes, Salmonella sp., Shigella sp., and Escherichia coli O157:H7 after 24 h in lab cultures, decreasing concentrations from approximately 102 CFU ml−1 to undetectable levels. However, the addition of diazinon also greatly reduced the solution pH to 3.70, which would have contributed to decreases in bacterial densities (Guan et al., 2001). Additionally, malathion inhibited bacterial growth in sludge at concentrations of 500 µg L−1, although this concentration is also higher than would be expected in the environment (Pai et al., 2009). One study of note also found toxic effects of chlorpyrifos (46 300 µg L−1), diazinon (10 300 µg L−1), and parathion (8500 µg L−1) on Vibrio phosphoreum (Somasundaram et al., 1990); however, in addition to noting that these concentrations were all above environmental expectation, the method of assessing toxicity, based upon bioluminescence, was also markedly different from the other study reviewed here and may have be the result of a sublethal effect.
Overall, the direct effects of organophosphate insecticides are negative for algal, protozoan and fungal species, both at and above environmentally relevant concentrations; however, at concentrations that would be expected within the environment, organophosphate insecticides have not produced any direct effects on bacterial species. At elevated organophsphate concentrations, direct effects are influenced by the specific chemical being utilized, the concentration applied and the target bacteria, as both negative and positive effects have been reported (Table 8). In several studies confounding variables (i.e., low pH) and differences in toxicity assessment might better explain non-neutral effects.
When more complex systems are explored and indirect effects are possible, i.e. when diminished predation by protozoa increases bacterial survival, organophosphate insecticides seemed to be generally beneficial for lower trophic levels. The application of chlorpyrifos to a freshwater pond system was associated with cyanobacterial blooms, which was hypothesized to be a possible result of the removal of top-down pressures associated with predation and/or a bottom-up effect by increasing phosphorous concentrations (Butcher, Boyer, and Fowle, 1977). Chlorpyrifos removed predation by herbivorous crustaceans, resulting in increased phytoplankton, cyanobacteria and periphyton growth (Hurlbert, Mulla, and Wilson, 1972). In mesocosms designed to simulate a tidal creek, chlorpyrifos applied at 10 µg L−1 increased heterotrophic bacterial abundance, while decreasing heterotrophic ciliate and flagellate (protozoan) abundance (DeLorenzo, Scott, and Ross, 1999). Similarly, parathion at 50 000 µg L−1 tended to increase abundance of heterotrophic bacteria in water samples collected from a natural lake (Lopez et al., 2006). However, another study revealed no significant effects of the pesticide on natural protozoan, bacterial, or fungal species in microcosms, even when concentrations exceeded water quality criteria three-fold (~12 000 µg L−1) (Pratt, Bowers, and Balczon, 1993). Similarly, malathion at 101 and 202 µg L−1 did not have any significant effect on E. coli or Enterococcus spp. in the sediment of complex freshwater mesocosms (Staley, Rohr, and Harwood, 2010). While none of these studies were designed to specifically isolate indirect effects of organophosphates, the algal and bacterial populations tended to increase in community settings. Generally, organophosphates seem to remove top-down pressures, allowing algal blooms or increased bacterial abundance, suggesting that indirect effects may be more pronounced than the negative direct effects on many populations of lower trophic levels.
Carbamates
Carbamate insecticides have similarly been found to have adverse direct effects on algae, protozoan, and fungal species, both at and above environmentally relevant concentrations (Tables 5–7). At environmentally relevant concentrations, carbaryl had an EC50 ranging from 5000–14 000 µg L−1 for green algae and cyanobacteria (Ma et al., 2006). Carbaryl inhibited carbon uptake in diatoms and green algae at concentrations ranging from 100–100 000 µg L−1 (Ukeles, 1962), and, at concentrations exceeding environmental relevance (25 000 µg L−1) was toxic to planktonic algae (Butler, Deason, and O'Kelley, 1975). Carbaryl and carbofuran exhibited direct toxicity to phytoplankton species at 3667 and 667 µg L−1, respectively (Peterson et al., 1994), and carbofuran had an environmentally relevant EC50 ranging from 13 000 to 281 000 µg L−1 for certain species of green algae and cyanobacteria (Ma et al., 2006). Protozoan species were also adversely affected, with carbofuran application reducing cell growth of E. gracilis after 72 hours of exposure, at a concentration of 50 000 µg L−1, although this concentration is higher than would be expected in the environment (Azizullah, Richter, and Hader, 2011). Toxic effects of carbaryl on protozoa when applied at a concentration of up to 10 000 µg L−1 have also been observed (Weber, Shea, and Berry, 1982). At concentrations exceeding 10 000 µg L−1, carbaryl was also toxic to protozoan Colypidium campulum, although these concentrations are not environmentally relevant. The aquatic fungal species, Batrachochytrium dendrobatidis, a pathogen of amphibians, had significantly decreased zoosporangia densities when exposed to 2500 µg L−1 of carbaryl (Hanlon and Parris, 2012).
Bacterial studies examining the direct effects of carbamate insecticides also produced adverse effects, regardless of whether or not insecticide concentrations exceeded environmental expectations (Table 8). Carbaryl had a half maximal effective concentration (EC50) of 5000 µg L−1 on V. phosphoreum, reducing optical density by 50%, while metabolites of carbaryl had an EC50 of 3700 µg L−1; carbofuran had an EC50 of 20 500 µg L−1 (Somasundaram et al., 1990). Carbaryl concentrations at 9 400 000 µg L−1 were also toxic to L. monocytogenes, Salmonella sp., Shigella sp., and E. coli O157:H7 after 24 h in lab cultures (Guan et al., 2001). Weber and Rosenberg (1984) found that bacterial diversity was diminished at a carbaryl concentration of 100 000 µg L−1 in model estuarine systems.
When the experimental design allowed observation of indirect effects of carbamate on target species by the chemicals’ effects on factors such as competition or predation, carbamates have resulted in positive effects for some species and trophic levels. In mixed cultures, growth of Ent. faecalis was inhibited by a carabaryl concentration of 5000 µg L−1, while Staphylococcus aureus and Sal. enterica experienced increased growth rates at the same concentration (Guthrie, Anugwelem, and Davis, 1981). In aquatic mesocosms designed to simulate pond communities, carbaryl application resulted in a decrease in zooplankton abundance when applied over a range of 100–20 000 µg L−1, while microbial and phytoplankton concentrations increased (Downing et al., 2008). Affected ecosystems in this study showed signs of recovery after 40 days, but changes in zooplankton population diversity were noted (Downing et al., 2008).
Organochlorines
Direct effects of organochlorine insecticides have been mostly negative; although, where neutral effects were observed, this was likely the result of reduced chemical concentrations or parameters (i.e., ATP production or survival) that were not measured in all studies investigating the same chemical (Table 5). Dichlorodiphenyltrichloroethane (DDT), at concentrations both in the environmentally relevant range, 3.6–36 µg L−1, and above, 200–600 µg L−1, inhibited photosynthesis in marine algae (Lee, Fang, and Freed, 1976; Ukeles, 1962). Mirex and methoxychlor, at environmentally relevant concentrations of 100 µg L−1, also inhibited growth of Chlorella pyrenoidosa (Kricher, Urey, and Hawes, 1975), although methoxychlor at 10 µg L−1 had no effect on certain species of green algae and cyanobacteria, likely as a result of the reduced concentration (Butler, Deason, and O'Kelley, 1975). All other studies reviewed utilized organochlorine concentrations exceeding environmentally relevant concentrations. Benzene hexachloride was toxic to cyanobacteria at concentrations exceeding 30 000 µg L−1 (Singh, 1973). Similarly, lindane had adverse effects on algal species with concentrations ranging from 1000–9000 µg L−1 (Faust et al., 1994; Ukeles, 1962). Endosulfan was toxic to cyanobacteria (Anabaena doliolum) and green algae (Chlorella vulgaris) at concentrations >28 500 and 41 500 µg L−1, respectively (Mohapatra and Mohanty, 1992). Aldrin, dieldrin, and endrin, at concentrations of 100 000 µg L−1, lowered ATP production, but did not result in reduced algal abundance (Clegg and Koevenig, 1974). Endrin at 500 000 µg L−1 had no effect on cyanobacterial species (Singh, 1973), although this study measured only survival and not ATP production.
Organochlorine insecticides also have adverse direct effects on protozoan species at and above environmentally relevant concentrations (Table 6). Mirex (0.9 µg L−1) and DDT (10 000–100 000 µg L−1) produced detrimental effects on T. pyriformis cell growth (Cooley, Keltner, and Forester, 2007; Lal, Saxena, and Lal, 1987). Endosulfan, dieldrin, endrin, aldrin, lindane and methoxychlor were toxic to Colpidium campylum at concentrations > 10 000 µg L−1 (Dive, Leclerc, and Persoone, 1980). Limited research has been conducted on the direct effects of organochlorine insecticides on bacteria. A single study of the effects of organochlorines on bacteria found no direct effect of lindane at concentrations ranging from 500–500 000 µg L−1 on rumen bacteria (Table 8) (Williams et al., 1963).
Studies on organochlorines where indirect effects were possible are limited. In a study utilizing mesocosms to simulate a tidal creek, endosulfan, at concentrations of 1 and 10 µg L−1 resulted in decreased heterotrophic bacterial abundance and increased phototroph abundance, although many cyanobacterial species became undetectable (DeLorenzo, Scott, and Ross, 1999). In another study of community level effects, endosulfan (at 1 µg L−1) increased phototrophic carbon assimilation, although diatom, dinoflagellate, algal species were significantly reduced (Downing et al., 2004). Aldin and lindane, at concentrations of 50 000 µg L−1, resulted in increased heterotrophic bacterial abundance in samples taken from a natural lake (Lopez et al., 2006). In samples isolated from a tropical estuary system, bacterial abundance was reduced by endosulfan, DDT, and lindane at concentrations ranging from 2 to 2000 µg L−1, with bacteria in the water column experiencing greater detrimental effects than those in the sediment (Rajendran, Rajendran, and Venugopalan, 1990). In streams treated with 124 µg L−1 of methoxychlor, conidia production of aquatic hyphomycetes increased by up to 3–5 times (Suberkropp and Wallace, 1992). Given the differences in observed effects, it is difficult to draw conclusions regarding the community-level effects of organochlorines. Unlike carbamate and organophosphate insecticides, these studies have reported decreases in both heterotrophic and phototrophic populations, although which populations are affected may be dependent upon the chemical and concentration used, as well as the system being studied (i.e., lake, creek, estuary).
Pyrethroids
Pyrethroid insecticides have received little attention in comparison to organophosphate, carbamate, and organochlorine insecticides (Tables 3 and 4). Guan, Blank, et al. (2001) found that a commercially available solution of pyrethroid insecticides caused a 100–1000-fold increase in the abundance of L. monocytogenes, Salmonella spp., Shigella spp., and E. coli O157:H7 after 96 hours. However, the concentrations they applied, 500 000 to 1 500 000 µg L−1, far exceed what would be expected in the environment. At the community level, pyrethroids indirectly facilitated bacterial survival by reducing predation by Daphnia magna at environmentally relevant concentrations ranging from 0–3 µg L−1 (Foit, Chatzinotas, and Liess, 2010).
Benzamides
Currently, there is limited research on the effect of benzamides on microorganisms. In watersheds treated with the insecticide dimlin at 0.03 kg ha−1, the biodiversity of aquatic fungi increased with no observed direct effects on conidia production or fungal densities (Dubey, Stephenson, and Edwards, 1995).
Insecticide summary
With the exception of pyrethroid and benzamide insecticides, for which research is limited, all other insecticide groups tend to have direct negative effects on algal, protozoan and fungal species. A χ2 test revealed significantly more negative direct effects of insecticides for both algal and protozoan taxa (P < 0.001 for both), while the quantity of direct negative effects for fungal taxa was nearly significant, but should be considered with caution because of a low sample size for fungi (P = 0.05, Table 4). Direct effects of insecticides on bacteria have generally been negative, with the exception of the organophosphate group, where several studies have shown no effect of organophosphates at environmentally relevant concentrations. Whether organophosphate insecticides will produce a negative or no effect on bacteria seems to be dependent upon the target bacterial species, organic content in the culture media, and the specific chemical in use. A χ2 test revealed that there was no significant tendency toward positive, neutral, or negative direct effects of insecticides on bacterial species (P = 0.157, Table 4). At the community level, however, insecticides tend to have favorable impacts on the lower trophic levels, generally algal and bacterial taxa, by reducing top-down pressures from protozoan or zooplankton predation. Additionally, algal and bacterial species have shorter generation times than taxa at higher trophic levels and thus probably recover sooner from any direct effects.
Bioconcentration of insecticides
In addition to studies reporting direct and indirect effects (positive or negative) of insecticides on microorganisms, several studies have revealed that various algal, protozoan, and bacterial species can bioconcentrate certain organophosphate and organochlorine insecticides, with no beneficial or adverse effects to those species. For example, 1000 µg L−1 of parathion was concentrated 100-fold over a seven day period by the green algae Scenedesmus obliquus, the cyanobacterium Anacystis nidulans, and protozoan species E. gracilis, Paramecium bursaris, and P. multimicronucleatum (Gregory, Reed, and Priester, 1969). Additionally, parathion was found to be concentrated 1000-fold by T. pyriformis (Shalesh and Anil, 2007). Similar effects have been observed for organochlorine insecticides. DDT was bioconcentrated 1000-fold by S. obliquus, A nidulans, T. pyriformis, E. gracilis, P. bursaria, and P. multimicronucleatum (Gregory, Reed, and Priester, 1969). Endosulfan was concentrated over 1,000-fold in cyanobacteria (Rao and Lal, 1987), and mirex was concentrated by 1000-fold in marine algae (Hollister, Walsh, and Forester, 1975). Aldrin, dieldrin and endrin were also concentrated by algal species (Vance and Drummond, 1969), and dieldrin was concentrated 1000-fold by T. pyriformis (Bhatnagar, Kumar, and Lal, 1988).
While none of these studies reported any positive or negative direct effects on the microbes, the potential for community-level effects in environmental settings as a consequence of bioconcentration should be considered. Several studies (listed above) observed community-level effects of insecticides, generally concluding that the effect of reducing higher predatory trophic levels indirectly benefits algal and bacterial populations. These results, at least in part, may be attributed to bioconcentration and resulting biomagnification of insecticides by organisms belonging to predatory guilds. Additionally, while microorganisms are the subject of this review, it should be noted that pesticides can also have toxic effects on higher-order aquatic organisms, such as amphibians, arthropods, and fish (Rico et al., 2011; Rohr et al., 2008; Yu et al., 2013). Therefore, while there may be no observed direct effects of pesticides on microorganisms, the potential for concentration and biomagnification must be taken into account when considering how pesticide applications affect aquatic ecosystems, and ultimately human health.
Herbicides
Herbicides comprise a vast array of differing biochemical groups and, as such, encompass a variety of modes of action (Table 2). Herbicides can act by blocking amino acid synthesis through inhibition of 5-enolpyruvl-shikimate-3-phosphate synthase (EPSP synthase), acetolactate synthase, or glutamine synthetase (Duke, 1990). Carotenoid, lipid, microtubule, cellulose, or folate synthase may also be inhibited (Duke, 1990). The most prominent mode of action of herbicides is via inhibition of photosynthesis. One mechanism by which photosynthesis may be compromised is through bleaching, where pesticides act in concert with photosystem I to produce free radicals (Duke, 1990). The majority of herbicides, including triazines, anilides, ureas, phenyl and carbamates (Table 2), act by blocking electron transport through disruption of the Hill Reaction in photosystem II (Duke, 1990).
As might be expected, herbicides have almost exclusively negative direct effects on algal species, as well as protozoa and fungi (Table 3, Figure 1). However, direct effects of herbicides on bacteria tend to vary depending on the group of herbicide used (Table 3, Figure 1). When considering effects by pesticide rather than by study, (as many studies used more than one pesticide), 94% of herbicides (of 35 herbicides) had negative direct effects, while six percent have produced no effects on algal and cyanobacterial species (Table 4). Fifty percent (of four herbicides) had negative effects on protozoa, while the other 50% had no direct effects (Table 4). One hundred percent of herbicides (of four herbicides) had direct negative effects on aquatic fungi (Table 4). Positive direct effects of herbicides on bacterial species were reported for 13% of herbicides (of 15 reviewed here), negative effects were reported for 47% of herbicides, and no effects were reported for 40% (Table 4).
Direct effects of herbicides on algal species have generally been negative, both at and about environmentally relevant concentrations (Table 5). At environmentally relevant concentrations, the bipyridinium herbicide diquat was generally more toxic to cyanobacteria and golden algae (EC50 of 22–46 µg L−1) than green algae (EC50 of 19–395 µg L−1) (Campbell, Bartell, and Shaw, 2000). Inhibition of carbon uptake by 99–100% was also observed in diatom and cyanobacteria species when exposed to diquat at 700 µg L−1 (Peterson et al., 1994). The aldehyde herbicide acrolein, as well as the substituted urea herbicide tebuthiuron (at 1000 µg L−1 and 5900 µg L−1, respectively), were also inhibited carbon uptake by nine algal species, although these concentrations exceeded environmental relevance (Peterson et al., 1994). In excess of what would be expected in the environment (10 000 µg L−1), paraquat significantly reduced cyanobacterial respiration (Kosinski, 1984).
Phenylurea herbicides also have toxic effects on algal species (Table 5), with diuron being the most toxic of the group, followed by monuron, neburon, and fenuron (Ukeles, 1962). Diuron produced lethal effects on marine algae at a concentration of 4 µg L−1 (Ukeles, 1962), and C. fusca reproduction was inhibited by chlortoluron at 23 µg L−1 (Faust et al., 1994). Diuron also had an EC50 between 16 and 33 nM for estuarine microalgae (Magnusson, Heimann, and Negri, 2008). Peterson, Boutin, et al. (1994) found that the glycine derivative glyphosate had an EC50 of 2848 µg L−1 (exceeding environmentally expected concentrations) for cyanobacterial and green algal species. Glyphosate also had an EC50 ranging from 8900–89 000 µg L−1 (also exceeding environmental relevance) for different freshwater periphyton communities and significantly reduced carbon uptake in diatoms and cyanobacteria (Goldsborough and Brown, 1988). The acetanilide herbicide metachlor also inhibited carbon uptake in green algae, and both metachlor (at 3000 µg L−1) and the imidazolinone herbicide imazethapyr (at 67 µg L−1) reduced carbon uptake in cyanobacteria (Peterson et al., 1994).
Multiple triazine herbicides were tested at and above expected environmental concentrations and all resulted in decreased carbon uptake in green algae, diatoms and cyanobacteria (Peterson et al., 1994). Simazine disrupted reproduction of C. fusca at a concentration of 73 µg L−1, respectively (Ukeles, 1962). Low concentrations of atrazine, i.e. 0.12–5.8 µg L−1, resulted in increased amino acid production, lower pH, and lower chlorophyll production in marine phytoplankton (Bester et al., 1995). Additionally, and within the range of environmental relevance, atrazine had an EC50 ranging from 90–3000 µg L−1 for certain species of algae (Fairchild, Ruessler, and Carlson, 2009). Atrazine also had an EC50 ranging from 120–630 nM for two species of estuarine microalgae (Magnusson, Heimann, and Negri, 2008). Atrazine was also toxic to green algae and cyanobacteria at 1000 µg L−1 (Butler, Deason, and O'Kelley, 1975).
Although most herbicides produce negative direct effects on algal species, the effects of specific chemicals and the concentrations required to achieve toxicity can be particularly species dependent. For example, Butler, Deason, et al. (1975) found that 2,4-D (at 4000 µg L−1) was toxic to planktonic algae, but that no inhibitory effect was observed at lower concentrations. Similarly, in a study of nine algal species, while a majority of herbicides examined did have adverse direct effects (as stated above), no inhibition of algal growth was observed with exposure to 2,4-D at a lower concentration of 2917 µg L−1(Peterson et al., 1994). Additionally, no inhibition of chlorophyll production was noted with 2,4-D at concentrations ranging from 1–100 000 µg L−1 (Torres and O'Flaherty, 1976), although it should be noted that the measurement of chlorophyll production measured in this study differed from measurements of growth in the previous two studies. Lastly, picloram (1760 µg L−1), while found to inhibit growth of most cyanobacterial species, actually promoted growth of four algal species tested (Peterson et al., 1994); while this supports the idea of species-specific differences regarding herbicide effects, it should also be noted that this is the only study reviewed here to test this chemical.
In contrast to algal species, fewer studies have examined the direct effects of herbicides on heterotrophic bacterial and protozoan species, although direct effects were negative for all but triazine herbicide, both at and above environmentally relevant concentrations(Tables 6 and 8). At environmentally relevant concentrations, coliform growth was reduced when exposed to 2,4,-D at concentrations of 5–10 mM (Higgins and Hohn, 2008). Additionally, although exceeding environmentally relevant concentrations, another study found a significant reduction in bacterial growth rates when 25 000–50 000 µg L−1 of diquat or paraquat were present (Breazeale and Camper, 1972). Glyphosate, at a concentration of 500 µg L−1, reduced the growth of heterotrophic bacteria (Pai et al., 2009). Although potentially a result of indirect effects, the bipyridinium herbicide diquat, at concentrations ranging from 300–30 000 µg L−1, decreased the abundance of freshwater algal and bacterial species and protozoan species richness (Melendez et al., 1993). At concentrations exceeding environmental relevance, phenoxyalkane herbicides 2,4-D and 2,4,5-T had EC50 values for V. fisherii of 100 700 and 51 700 µg L−1, respectively, while their metabolites had much lower EC50 values of 5000 and 1800 µg L−1, respectively. These results suggest that metabolites of these herbicides are more toxic than their parent compounds (Somasundaram et al., 1990). Diquat had an EC50 value of 2940 µg L−1 for E. gracilis (Campbell, Bartell, and Shaw, 2000). Phenlyurea herbicides diuron and chlortoluron also had EC50 values of 68 200 and 298 500 µg L−1, respectively, for V. fisherii and 10 700 and 8200 µg L−1, respectively for T. pyriformis (Nelieu et al., 2010). Linuron exposures of 11 300 000 and 33 900 000 µg L−1 for 96h caused a 100–1000-fold increase in the abundance of E. coli O157:H7, L. monocytogenes, Salmonella sp., and Shigella sp.; however, glyphosate and paraquat resulted in significant decreases of the same bacterial species after one hour (Guan et al., 2001).
In contrast to most other herbicide groups, triazine herbicides have tended to produce neutral effects on bacterial and protozoan species (Tables 6 and 8). However, Higgins found no effect of atrazine (0.25 – 22.5µg L−1) or simazine (0.000025 – 0.0025 µg L−1) on coliform growth (Higgins and Hohn, 2008). Similarly, Breazeale and Camper (1972) also found no significant effect of atrazine on bacterial growth at 25 000 and 50 000µg L−1. At concentrations of 102 and 204 µg L−1, atrazine had no effect on E. coli, Ent. faecalis, Sal. enterica Typhimurium, or E. coli O157:H7 growth or survival in simplified microcosms (Staley et al., 2012). The lack of effects of both atrazine and its metabolite deethylatrazine on both bacterial and protozoan abundance have also been reported (DeLorenzo, Scott, and Ross, 1999). A singular study found atrazine concentrations ranging from 0.1–100 µg L−1 allowed for the growth E. coli and Ent. faecalis populations; however, in this study, no pesticide-free control was used for comparison (Koutsotoli et al., 2005).
Herbicides have also had negative effects on aquatic fungi at and above environmentally relevant concentrations (Table 7). Glyphosate at 500 µg L−1 significantly decreased zoosporangia concentration and zoospore production of the pathogenic fungi B. dendrobatidis (Hanlon and Parris, 2012). Atrazine also reduced growth of B. dendrobatidis (0.0106–106 µg L−1) both in culture and on infected tadpoles (McMahon, Romansic, and Rohr, 2013; Rohr et al., 2013). The bipyridilium herbicide Diquat (at concentrations between 0.2–10 µg L−1), inhibited the growth of Helicoon elegans, Pseudoaergerita matsushimae, Hormiactus ontariensis, and Beverwykella pulmonaria to varying degrees among the species (Fronda and Kendrick, 1985). Although exceeding environmentally relevant concentrations, glyphosate (at 50 000–500 000 µg L−1) inhibited biomass production in Annulatascus velatisporus, Composporium antennatum, Helicosporium griseum, and Massarina sp., though the degree of inhibition varied among these species (Tsui, Hyde, and Hodgkiss, 2001).
Community-level effects of herbicides
Atrazine is one of the most widely used pesticides in studies examining community level effects. Multiple studies have been conducted detailing the effects of atrazine exposure on phytoplankton, revealing that negative effects are dependent on atrazine concentration, length of exposure and species of phytoplankton (Huber, 1993; Solomon et al., 1996). In contrast, several studies have shown that periphyton growth can be indirectly stimulated with ecologically relevant concentrations of atrazine (Brock, Lahr, and Brink, 2000; Herman, Kaushik, and Solomon, 1986; Pratt et al., 1988; Rohr, Halstead, and Raffel, 2012; Rohr et al., 2008; Staley, Rohr, and Harwood, 2011). Atrazine toxicity has also been demonstrated toward macrophytes, benthic invertebrates, zooplankton, fish and amphibian species (Rohr and McCoy, 2010; Solomon et al., 1996). Atrazine application can therefore result in profound shifts across multiple trophic levels, resulting in a myriad of potential indirect effects on algae, bacteria and protozoa, depending on the complexity of the system.
In several studies, atrazine exposure has had an indirect effect mediated via a reduction in competition among phototrophs. Atrazine affected experimental pond communities at concentrations as low as 1–5 µg L−1 and as high as 500 µg L−1, reducing green algae and flagellate abundance while increasing cryptomonads and golden algae (deNoyelles, Kettle, and Sinn, 1982). Another study by Hamala and Kolliq (1985) found that a concentration of 100 µg L−1 of atrazine reduced cryptomonads and diatoms but increased cyanobacteria (Hamala and Kolliq, 1985). Additionally, studies have observed algal-mediated indirect effects of atrazine, often resulting in the increase of bacterial and protozoan abundance. In the sediments of complex freshwater mesocosms, the addition of atrazine at 102 or 204 µg L−1 increased abundance of E. coli and Enterococcus spp. (Staley, Rohr, and Harwood, 2010) compared to controls. Further, in simplified microcosms where algal populations were allowed to establish, atrazine application at 102 µg L−1 resulted in decreases in both phytoplankton and E. coli abundance in the water column, but increases in E. coli abundance in the sediments (Staley, Rohr, and Harwood, 2011). A reduction in chlorophyll coupled with increases in cyanobacteria and bacterial abundance was reported with atrazine concentrations ranging from 40–160 µg L−1, although bacterial abundance decreased over 45 hours (DeLorenzo et al., 1999). In the same study, atrazine had no significant effect on the abundance of small ciliates, although large ciliates and small flagellates increased, while large flagellate abundance declined (DeLorenzo et al., 1999). At concentrations of 20 and 200 µg L−1, green and golden algae decreased, while increasing the relative abundance of diatoms and heterotrophic protozoa (Downing et al., 2004).
Further, atrazine tends to stimulate heterotrophic microbial activity in community settings. In water samples collected from a natural lake, an increase in heterotrophic bacterial abundance with an atrazine concentration of 50 000 µg L−1 was observed (Lopez et al., 2006). Similarly, Hamala and Kolliq (1985) found that a concentration of 100 µg L−1 increased heterotrophic activity (Hamala and Kolliq, 1985). Stimulation of heterotrophic bacteria was also observed for atrazine concentrations ranging from 3–100 µg L−1, although at concentrations ranging from 100–300 µg L−1, primary production was practically eliminated in laboratory experiments (Pratt et al., 1997). In a study utilizing freshwater microcosms to assess the impact of atrazine (at 102 µg L−1) on competition and predation, no effect was observed on autochthonous protozoan predators; however, inoculated strains of Ent. faecalis and E. coli O157:H7 remained significantly elevated, likely a result of atrazine reducing autochthonous (naturally present) competitors (Staley et al., 2014).
Community-level effects on biofilm algae and bacteria have also been studied using the substituted urea herbicide diuron. At environmentally relevant concentrations ranging from 0.07 to 7 µg L−1, diuron reduced diatom abundance and photosynthetic efficiency (Ricart et al., 2009). Unlike many of the community-level studies using atrazine, however, diuron also reduced bacterial abundance. By contrast, another study using slightly higher concentrations of diuron (10 µg L−1) did find an increase in bacterial abundance, productivity and diversity; the mechanism for this was hypothesized as being due to phototrophic lysis and an increase in the amount of organic matter released as a result of diuron exposure (Pesce et al., 2006).
Herbicide summary
The studies reviewed here have demonstrated population-level direct effects of herbicides on algal and fungal taxa that were significantly negative (P < 0.001 and P = 0.018, respectively; Table 4). Again, we encourage interpreting the fungal results with caution because of low sample sizes in this group. However, the community-level indirect effects of herbicides, particularly atrazine, make predictions of the effects of herbicides difficult in natural environments. While direct effects of herbicides on individual algal species are almost exclusively negative, the indirect effects, mediated via a reduction of competition, tend to favor certain phototrophic taxa at the community level (i.e., decreasing diatoms, but increasing cyanobacteria). In contrast to algal and fungal taxa, we found no significant tendency toward positive, neutral, or negative direct effects of herbicides on bacterial or protozoal species (P = 0.247 and 0.368, respectively; Table 4). Although the majority of studies assessing direct effects of herbicides on bacterial and protozoan populations have found negative effects (with the exception of triazine herbicides), at the community level, a decline in phototrophs has generally been associated with an increase in heterotrophic bacterial and protozoan populations (Pesce et al., 2006; Pratt et al., 1997). As is the case with insecticides, these studies suggest that indirect positive effects of herbicides, particularly on heterotrophic taxa, may outweigh direct negative effects. Given the relative paucity of community-level studies compared to those examining direct effects, further research involving multiple trophic levels is needed to be able to make accurate predictions of how herbicides will affect impacted aquatic environments.
Fungicides
Fungicides, like herbicides, have a variety of different potential modes of action (Table 2), with many tending to function as general biocides (Maltby, C., and Van den Brink, 2009). Aromatic hydrocarbon fungicides, triazoles, and hydrochlorines are generally deleterious to membrane synthesis, acting to block either lipid or sterol biosynthesis or inhibiting the biosynthesis of intracellular membrane components (Yang et al., 2011). Fungicides (e.g., pyrimidinamines, pyridine caboxamindes, benzamides; Table 2) may also act by diminishing cellular respiration, either by disrupting oxidative phosphorylation or specifically targeting NADH oxido-reductase or succinate-dehydrogenase (Yang et al., 2011). Further, phenylpyrrole and dicarboximide fungicides act by blocking signal transduction (Yang et al., 2011).
Relative to herbicides and insecticides, few studies have investigated the direct effects of fungicides on algal species; however, at environmentally relevant concentrations, the direct effects have been consistently negative (Table 5, Figure 1). A triazole derivative of propiconazole (83 µg L−1) inhibited carbon uptake by as much as 31% in the green algae S. quadricauda and cyanobacterium Microsystis (Peterson et al., 1994). Harris, White, et al. (1970) found that organomercury fungicides at concentrations of less than 1 µg L−1 reduced growth and photosynthesis in phytoplankton and diatom species. The triazine fungicide anilazine (1390 µg L−1) and the imidazole fungicide prochloraz (24 µg L−1) were also toxic to C. fusca (Faust et al., 1994).
Direct negative effects of fungicides on heterotrophic protozoan species have also been consistently negative (Figure 1), although these studies utilized fungicide concentrations exceeding environmentally relevant concentrations (Table 6). Fungicides have been found to be harmful to ciliate protozoa, specifically C. campulum (Dive, Leclerc, and Persoone, 1980). At concentrations ranging from 50–10 000 µg L−1, thiocarbamate fungicides nabam and vapam were found to inhibit growth of the flagellate protozoan E. gracilis (Moore, 1970). While the studies listed all provide evidence of direct negative effects of fungicides on algal and protozoan species, it should be noted that these studies represent only a small variety of fungicides on a limited sampling of algal and protozoan species.
Relatively few studies have investigated the effects of fungicides on aquatic fungal species; however, those studies which have been conducted have, unsurprisingly, found negative effects (Table 7, Figure 1). The imidazole fungicide, imazalil, reduced the sporulation of Heliscella stellata, Lunalospora curvula, and other sigmoid type-2 aquatic fungi at concentrations ranging between 0.1–100 µg L−1 (Flores et al., 2014). Imazalil also had a EC100 on aquatic fungi at concentrations ranging from 10 000 to 210 000 µg L−1 (Dijksterhuis et al., 2011). The fungicide chlorothalonil (at 0.0176–1.76 and 32–176 µg L−1) reduced the growth of Batrachochytrium dendrobatidis in culture and on infected tadpoles (McMahon, Romansic, and Rohr, 2013). Chlorothalonil was also toxic to aquatic fungi with an EC100 at concentrations exceeding 260 µg L−1 (Dijksterhuis et al., 2011). Also, although exceeding environmentally relevant concentrations, the phosphanoglycine fungicide, thiophanate methyl, significantly decreased the zoosporangia number of Batrachochytrium dendrobatidis at 1500 µg L−1 (Hanlon and Parris, 2012).
Similar to herbicides, the direct effects of fungicides on bacterial species tend to be negative, but can be highly variable among specific pesticides and dependent on the concentration used (Table 8). Acriflavine had dose-dependent bactericidal effects on S. aureus with concentrations as low as 1000 µg L−1 (Kawai and Yamagishi, 2009). At concentrations which exceed environmentally relevant concentrations, captan, thiram, fenpropimorph and propiconazole, with EC50 values ranging from 3000–70 000 µg L−1, produced toxic effects, measured via a decline in leucine incorporation and denitrification of a bacteria community in a constructed wetland (Milenkovski et al., 2010). Thiram (13 400 000 µg L−1 in lab cultures) also significantly reduced Salmonella spp., Shigella spp., L. monocytogenes, and E. coli O157:H7 densities relative to controls, although this result was likely due to the accompanying shift in pH to 9.21 (Guan et al., 2001). In the same study, the abundance of E. coli O157:H7, Salmonella sp., Shigella sp., and L. monocytogenes increased by 100–1000-fold when exposed to commercially available chlorothalonil at concentrations of 10 000 and 30 000 µg/L over 96 hours (Guan et al., 2001). However, at environmentally relevant concentrations, chlorothalonil at 170 or 340 µg L−1 had no effect on abundance or growth rate of E. coli, Ent. faecalis, E. coli O157:H7, or Sal. enterica Typhimurium in simplified microcosms (Staley et al., 2012). That these studies found conflicting effects of chlorothalonil is likely because of the use of different matrices (lab cultures v. microcosms) and highly different concentrations of chlorothalonil. Lastly, dichloran, at a concentration of 0.14 µg L−1, had almost no effects on Sal. enterica Typhimurium, contributing less than 0.1% to mutagenic activity (de Oliviera et al., 2009). While the direct effects of fungicides on bacterial species tend to be negative, these results seem to be highly dependent on the specific fungicide and concentration used; further, the relative lack of studies makes any generalization of the effects of fungicides on bacterial species cautionable.
Community-level effects of fungicides
Community-level effects of fungicides have not been extensively investigated. In the sediments of complex freshwater mesocosms, chlorothalonil had no significant effect on sediment E. coli or Enterococcus spp. levels at concentrations of 170 or 340 µg L−1 (Staley, Rohr, and Harwood, 2010). However, in a study by Downing, DeLorenzo et al., 20 µg L−1 of chlorothalonil was found to stimulate heterotrophic bacterial activity, increase diatoms and chlorophytes, and reduce golden algae and heterotrophic protozoa (Downing et al., 2004). Similarly, captan, applied at 50 000 µg L−1, increased heterotrophic bacterial abundance in a lake ecosystem (Lopez et al., 2006). In freshwater microcosms, 170 µg L−1 chlorothalonil reduced autochthonous protozoan densities as effectively as cycloheximide (a known inhibitor of protein synthesis in eukaryotes), providing an indirect positive effect on bacterial densities (Staley et al., 2014). Utilizing complex (i.e., containing multiple trophic levels) freshwater mesocosms, chlorothalonil (at concentrations of 164 and 328 µg L−1) was found to have ecosystem-level effects, increasing mortality to zooplankton and algal species (as well as amphibians and gastropods), while decreasing decomposition and water clarity and elevating dissolved oxygen and primary productivity (McMahon et al., 2012). Also utilizing complex freshwater mesocosms, chlorothalonil (at 164 µg L−1) increased chlorophyll a production in phytoplankton and periphyton, and decreased leaf litter decomposition and the abundance of herbivorous species (Halstead et al., 2014). Based upon these studies in community settings, fungicides have a more pronounced effect on higher trophic levels, thereby mediating increases in algal and bacterial abundance, which is similar to the effects of many insecticides. An additional possibility is that certain bacterial strains are capable of metabolizing fungicides or utilizing organic material released from dying fungal species. While no studies reviewed here have specifically examined that possibility, additional nutrients from either source could facilitate bacterial proliferation.
Fungicide summary
While the number of studies considering the effects fungicides on microorganisms has been relatively few, most fungicides seem to exhibit generally biocidal effects. A χ2 test revealed a significantly greater occurrence of negative direct effects for algal, bacterial, and fungal taxa (P = 0.018, 0.018, and 0.007, respectively) and a nearly significant number for protozoal taxa (P = 0.05; although 100% of studies reviewed here found negative direct effects). Similar to other pesticide categories, however, the indirect, community-level effects of fungicides seem to target higher trophic levels, resulting in increases of lower trophic levels, such as algal and bacterial species.
Pesticide Summary
Based upon the studies reviewed, the direct effects of all pesticide types produced, on average, negative effects for algal, protozoal, fungal and bacterial species (Figures 1, 2; all averages are negative). Importantly, there was a significant pesticide type-by-taxon interaction (χ21=7.11, P=0.007), indicating that the effect of a given pesticide type was dependent on the taxon examined (Figure 2). This is not surprising given that pesticides are designed to target particular taxa, such as herbicides being designed to target photosynthetic organisms. This interaction seemed to be most strongly driven by the fact that all three pesticide types seemed to have similar adverse effects on algae and fungi, but protozoans were much more strongly affected by insecticides and fungicides than herbicides (Figures 1, 2). Interestingly, there was also a significant main effect of pesticide type (χ21=6.04, P=0.048) because, on average, fungicides seemed to be most deadly to microbes and herbicides least deadly to microbes (Figures 1, 2). There was also a significant main effect of taxon (χ21=16.31, P<0.001) because, on average, fungi and algae seemed to be most sensitive to pesticides and bacteria were least sensitive to pesticides (Figures 1, 2).
Figure 2.
Interaction between pesticide type (insecticide, herbicide, fungicide) and taxon (algae, bacteria, protozoa, fungi) on microbial densities (+1 for significant increases in densities, 0 for null effects, −1 for significant decreases in densities).
While, on average, direct effects of all pesticide categories on all microbial taxa are negative, these direct negative effects tend to be offset when studies investigated community-level, indirect effects. In community settings, both insecticides and fungicides had a tendency to reduce heterotrophic predators, resulting in increases in the abundance of lower trophic levels, particularly algal and bacterial species. Community-level effects of herbicides have been mostly limited to the specific herbicide atrazine (although with several exceptions) making generalization of the indirect effects of herbicides more difficult. However, the studies available do reveal a tendency for the reduction of phototrophs, increasing the abundance of heterotrophic taxa, mostly bacterial and protozoan species. Additionally, increases in bacterial abundance in the presence of any pesticide might also result from increased nutrient sources, either from direct metabolism of pesticides or from released organic material from species experiencing lethal or lytic effects.
Ecological relevance and areas for further research
Based upon the studies presented in this review and the ubiquity of pesticides in water bodies in the United States, European Union, and throughout the world, it is likely that most aquatic ecosystems are in some way affected by the presence of pesticides. Understanding these effects is important to safeguard both aquatic and human health. Multiple studies have revealed the capacity of microbes to concentrate pesticides, serving as vectors for pesticide delivery to higher trophic levels (Cooley, Keltner, and Forester, 2007; Gregory, Reed, and Priester, 1969; Jo et al., 2011; Lal, Saxena, and Lal, 1987). Furthermore, studies have revealed that direct effects of pesticides on one trophic level may indirectly affect the abundance of species at higher or lower trophic levels (Foit, Chatzinotas, and Liess, 2010; Relyea, 2009; Staley, Rohr, and Harwood, 2011).
Understanding the ecological effects of pesticides and potentially different responses among bacterial and protozoan species may also have implications for microbial regulatory criteria and human health. Presently, microbial water quality is assessed by quantifying the abundance of fecal indicator bacteria (FIB; i.e., E. coli and enterococci) in contaminated water bodies (U. S. Environmental Protection Agency, 2002). Epidemiological studies have shown that elevated concentrations of FIB have been correlated with increased risk of contracting gastrointestinal disease (Colford et al., 2007; Wade et al., 2008; Wade et al., 2003). However, different survival patterns among FIB (E. coli and enterococci) have been observed (Korajkic et al., 2013). Further, not all microbial, protozoan or viral pathogens exhibit the same persistence in secondary environments as do FIB (Jenkins et al., 2011; Korajkic, Wanjugi, and Harwood, 2013; Staley et al., 2014; Wanjugi and Harwood, 2012). As pesticides might have different effects on different bacterial and protozoan species, it is increasingly important to understand whether FIB are affected identically to the pathogens they are used to predict or whether the ubiquity of pesticides in contaminated water bodies is confounding regulatory measures.
Of the studies reviewed, predicting pesticide effects on bacterial species seems to be the most problematic. The general trend of direct negative effects on algae, protozoa, and aquatic fungi has emerged for many pesticides. However, the direct effects of pesticides on bacterial species are less easily generalized. For any given pesticide, studies have tended to show contrasting or null effects depending on the bacterial species and concentration and specific pesticide examined, the duration of exposure, the presence of sediments and humic materials, and environmental factors (e.g. pH, DO). Given the broad diversity of physiological strategies utilized by bacteria, it is perhaps not surprising that we were unable to draw any general conclusions regarding how different classes of pesticides affect bacteria.
However, understanding direct effects of pesticides on any microorganism may be less important than understanding the indirect effects that occur when microbial populations interact in an ecosystem. In aggregate, the studies reviewed here show that a particular direct effect of a given pesticide observed in a simplified study may not manifest in a more complex system that includes various taxa and trophic levels. In many cases, a direct negative effect on a target species has been found to be offset at the community level, suggesting that indirect effects have a strong influence on the net effect (direct + indirect effects) of a pesticide. However, despite the importance of indirect effects, few studies have examined indirect and/or net effects of pesticides at the community level (DeLorenzo et al., 1999; DeLorenzo, Scott, and Ross, 1999; Downing et al., 2008; Downing et al., 2004; Lopez et al., 2006; Staley, Rohr, and Harwood, 2010; Staley, Rohr, and Harwood, 2011; Staley et al., 2014). Nevertheless, the handful of studies that have examined the effects of pesticides on microbial communities suggest that there is trophic downgrading, a pattern that seems to be occurring globally as a result of various stressors(Estes et al., 2011). The more rapid recovery of lower trophic levels coupled with the greater sensitivity of higher trophic levels to many pesticides, such as insecticides and fungicides, seems to be driving this trophic downgrading.
As pesticide use intensifies, the impact of pesticide residues on aquatic ecosystems is likely to increase. Given the importance of microbial activities in these environments, the ability to accurately predict the effects of pesticides is essential to safeguarding ecosystems. Further, understanding how pesticide residues influence the fate of both pathogenic and regulatory-associated microbes, particularly bacteria, is important for the protection of human health. Despite the urgent need to understand how pesticides influence different trophic levels and impact the ecosystem as whole, most studies have focused on direct effects, utilizing in vitro studies. To better understand and more accurately predict the impact of pesticides in real-world aquatic environments, more systematic research is needed at the community-level impacts with a focus on determining the net effects (direct and indirect effects) of pesticides on microorganisms.
Acknowledgments
The authors would also like to gratefully acknowledge the excellent constructive comments of three anonymous reviewers who were selected by the Editor. These comments were helpful in improving the review.
Declaration of interest
The authors’ affiliation is as shown on the cover page. Preparation of the review was funded by grants from the National Science Foundation (EF-1241889 to J.R.R.), the National Institutes of Health (R01GM109499 to J.R.R.), the US Department of Agriculture (NRI 2009-35102-0543 to J.R.R. and V.J.H), and the US Environmental Protection Agency grant (CAREER 83518801 to J.R.R.). The author’s affiliation is as shown on the cover page. The authors have sole responsibility for the writing and content of the paper. None of the authors of this review have been involved in legal proceedings, appeared before regulatory bodies or consulted with commercial entities with regard to the products discussed in this review.
References
- 1.Anderson KL, Wheeler KA, Robinson JB, Tuovinen OH. Atrazine mineralization potential in two wetlands. Water Research. 2002;36:4785–4794. doi: 10.1016/s0043-1354(02)00209-9. [DOI] [PubMed] [Google Scholar]
- 2.Azizullah A, Richter P, Hader D-P. Comparative toxicity of the pesticides carbofuran and malathion to the freshwater flagellate Euglena gracilis. Ecotoxicology. 2011;20:1442–1454. doi: 10.1007/s10646-011-0701-6. [DOI] [PubMed] [Google Scholar]
- 3.Beketov MA, Kefford BJ, Schäfer RB, Liess M. Pesticides reduce regional biodiversity of stream invertebrates. Proc Natl Acad Sci USA. 2013;110:11039–11043. doi: 10.1073/pnas.1305618110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benton TG, Vickery JA, Wilson JD. Farmland biodiversity: is habitat heterogeneity the key? Trends in Ecology & Evolution. 2003;18:182–188. [Google Scholar]
- 5.Bester K, Huhnerfuss H, Brockmann U, Rick HJ. Biological effects of triazine herbicide contamination on marine phytoplankton. Environmental Contamination and Toxicology. 1995;29:277–283. [Google Scholar]
- 6.Bhatnagar P, Kumar S, Lal R. Uptake and bioconcentration of dieldrin, dimethoate, and permethrin by Tetrahymena pyriformis. Water, Air, and Soil Pollution. 1988;40:345–349. [Google Scholar]
- 7.Breazeale FW, Camper ND. Effect of selected herbicides on bacterial growth rates. Applied Microbiology. 1972;23:431–432. doi: 10.1128/am.23.2.431-432.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brock TCM, Lahr J, Brink PJvd. Alterra-Rapport 88. The Netherlands: Alterra, Green World Research, Alterra, Wageningen; 2000. Ecological risks of pesticides in freshwater ecosystems; Part 1: herbicides. ed.^eds. [Google Scholar]
- 9.Butcher JE, Boyer MG, Fowle CD. Some changes in pond chemistry and photosynthetic activity following treatment with increasing concentrations of chlorpyrifos. Bull Environ Contam Toxicol. 1977;17:752–758. doi: 10.1007/BF01685965. [DOI] [PubMed] [Google Scholar]
- 10.Butler GL, Deason TR, O'Kelley JC. The effect of atrazine, 2,4-D, methoxychlor, carbaryl, and diazinon on the growth of planktonic algae. British Phycological Journal. 1975;10:371–376. [Google Scholar]
- 11.Campbell KR, Bartell SM, Shaw JL. Characterizing aquatic ecological risks from pesticides using a diquat dibromide case study. II. Approaches using quotients and distributions. Environ Toxicol Chem. 2000;19:760–774. [Google Scholar]
- 12.Clegg TJ, Koevenig JL. The effect of four chlorinated hydrocarbon pesticides and one organophosphate pesticide on ATP levels in three species of photosynthetic freshwater algae. Bot Gaz. 1974;135:369–372. [Google Scholar]
- 13.Clements WH, Rohr JR. Community responses to contaminants: Using basic ecological principles to predict ecotoxicological effects. Environ Toxicol Chem. 2009;28:1789–1800. doi: 10.1897/09-140.1. [DOI] [PubMed] [Google Scholar]
- 14.Coats JR. Mechanisms of toxic action and structure-activity relationships for organochlorine and synthetic pyrethroid insecticides. Environ Health Perspect. 1990;87:255–262. doi: 10.1289/ehp.9087255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colford JM, Wade TJ, Schiff KC, Wright CC, Griffith JF, Sanduh SK, Burns S, Sobsey M, Lovelace G, Wesiberg SB. Water quality indicators and the risk of illness at beaches with nonpoint sources of fecal contamination. Epidemology. 2007;18:27–35. doi: 10.1097/01.ede.0000249425.32990.b9. [DOI] [PubMed] [Google Scholar]
- 16.Cooley NR, Keltner JM, Forester J. Mirex and Aroclor 1254: Effect on and accumulation by Tetrahymena pyriformis strain W. Journal of Eukaryotic Microbiology. 2007;19:636–638. doi: 10.1111/j.1550-7408.1972.tb03547.x. [DOI] [PubMed] [Google Scholar]
- 17.Cycoń M, Wójcik M, Piotrowska-Seget Z. Biodegradation of the organophosphorus insecticide diazinon by Serratia sp. and Pseudomonas sp. and their use in bioremediation of contaminated soil. Chemosphere. 2009;76:494–501. doi: 10.1016/j.chemosphere.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 18.de Oliviera DP, Sakagami M, Warren S, Kummrow F, de Umbuzeiro GA. Evaluation of dichloran's contribution to the mutagenic activity of cristais river, Brazil, water samples. Environ Toxicol Chem. 2009;28:1881–1884. doi: 10.1897/09-103.1. [DOI] [PubMed] [Google Scholar]
- 19.De Souza ML, Newcombe D, Alvey S, Crowley DE, Hay A, Sadowsky MJ, Wackett LP. Molecular basis of a bacterial consortium: interspecies catabolism of atrazine. Applied and Environmental Microbiology. 1998;64:178–184. doi: 10.1128/aem.64.1.178-184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debenest T, Silvestre J, Coste M, Pinelli E. Effects of pesticides on freshwater diatoms. Rev Environ Contam Toxicol. 2010;203:87–103. doi: 10.1007/978-1-4419-1352-4_2. [DOI] [PubMed] [Google Scholar]
- 21.DeLorenzo ME, Lauth J, Pennington PL, Scott GI, Ross PE. Atrazine effects on the microbial food web in tidal creek mesocosms. Aquatic Toxicology. 1999;46:241–251. [Google Scholar]
- 22.DeLorenzo ME, Scott GI, Ross PE. Effects of the agricultural pesticides atrazine, deethylatrazine, endosulfan, and chlorpyrifos on an estuarine microbial food web. Environmental Toxicology and Chemistry. 1999;18:2824–2835. [Google Scholar]
- 23.DeLorenzo ME, Scott GI, Ross PE. Toxicity of pesticides to aquatic microorganisms: A review. Environmental Toxicology and Chemistry. 2001;20:84–98. doi: 10.1897/1551-5028(2001)020<0084:toptam>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.deNoyelles F, Kettle WD, Sinn DE. The response of plankton communities in experimental ponds to atrazine, the most heavily used pesticide in the United States. Ecology. 1982;63:1285–1293. [Google Scholar]
- 25.Desneux N, Decourtye A, Delpuech J-M. The sublethal effects of pesticides on beneficial arthropods. Annual Review of Entomology. 2007;52:81–106. doi: 10.1146/annurev.ento.52.110405.091440. [DOI] [PubMed] [Google Scholar]
- 26.Dijksterhuis J, Doorn Tv, Samson R, Postma J. Effects of seven fungicides on non-target aquatic fungi. Water Air Soil Poll. 2011;222:421–425. doi: 10.1007/s11270-011-0836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dive D, Leclerc H, Persoone G. Pesticide toxicity on the ciliate protozoan Colpidium campylum: possible consequences of the effect of pesticides in the aquatic environment. Ecotoxicology and Environmental Safety. 1980;4:129–133. doi: 10.1016/0147-6513(80)90014-7. [DOI] [PubMed] [Google Scholar]
- 28.Downing AL, DeVanna KM, Rubeck-Schurtz CN, Tuhela L, Grunkmeyer H. Community and ecosystem responses to a pulsed pesticide disturbance in freshwater ecosystems. Ecotoxicology. 2008;17:539–548. doi: 10.1007/s10646-008-0211-3. [DOI] [PubMed] [Google Scholar]
- 29.Downing HF, DeLorenzo ME, Fulton MH, Scott GI, Madden CJ, Kucklick JR. Effects of the agricultural pesticides atrazine, chlorothalonil, and endosulfan on south Florida microbial assemblages. Ecotoxicology. 2004;13:245–260. doi: 10.1023/b:ectx.0000023569.46544.9f. [DOI] [PubMed] [Google Scholar]
- 30.Dubey T, Stephenson SL, Edwards PJ. 10th Central Hardwood Forest Conference. West Virginia: United States Department of Agriculture: Forest Service; 1995. Dimlin effects on leaf-decomposing aquatic fungi on the Fernow Experimental Forest, West Virginiaed. ^eds. [Google Scholar]
- 31.Duke SO. Overview of herbicide mechanisms of action. Environmental Health Perspectives. 1990;87:263–271. doi: 10.1289/ehp.9087263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soulé ME, Virtanen R, Wardle DA. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 33.Fairchild JF, Ruessler DS, Carlson AR. Comparative sensitivity of five species of macrophytes and six species of algae to atrazine, metribuzin, alachlor, and metolachlor. Environ Toxicol Chem. 2009;17:1830–1834. [Google Scholar]
- 34.Faust M, Altenburger R, Boedeker W, Grimme LH. Algal toxicity of binary combinations of pesticides. Bull Environ Contam Toxicol. 1994;53:134–141. doi: 10.1007/BF00205150. [DOI] [PubMed] [Google Scholar]
- 35.Flores L, Banjac Z, Farre M, Larranaga A, Mas-Marti E, Munoz I, Barcelo D, Elosegi A. Effects of a fungicide (imazalil) and an insecticide (diazinon) on stream fungi and invertebrates associated with litter breakdown. Science of the Total Environment. 2014:532–541. doi: 10.1016/j.scitotenv.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 36.Foit K, Chatzinotas A, Liess M. Short-term disturbance of a grazer has long-term effects on bacterial communities-relevance of trophic interactions for recovery from pesticide effects. Aquatic Toxicology. 2010;99:205–211. doi: 10.1016/j.aquatox.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Fronda A, Kendrick B. Uptake of diquat by four aero-aquatic hyphomycetes. Environ Pollut (Series A) 1985;37:229–244. [Google Scholar]
- 38.Fukuto TR. Mechanism of action of organophosphorous and carbamate insecticides. Environmental Health Perspectives. 1990;87:245–254. doi: 10.1289/ehp.9087245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilliom RJ. Pesticides in the U.S. streams and groundwater. Environ Sci Technol. 2007;41:3408–3414. doi: 10.1021/es072531u. [DOI] [PubMed] [Google Scholar]
- 40.Gilliom RJ, Barbash JE, Crawford CG, Hamilton PA, Martin JD, Nakagaki N, Nowell LH, Scott JC, Stackelberg PE, Thelin GP, Wolock DM. Circular. Vol. 1291. Reston, VA: U.S. Geological Survey; 2006. The quality of our nation's water - Pesticides in the nation's streams and ground water, 1992–2001. [Google Scholar]
- 41.Goldsborough LG, Brown DJ. Effect of glyphosate (Roundup® formulation) on periphytic algal photosynthesis. Bull Environ Contam Toxicol. 1988;41:253–260. doi: 10.1007/BF01705439. [DOI] [PubMed] [Google Scholar]
- 42.Gregory WW, Reed JK, Priester LE. Accumulation of parathion and DDT by some algae and protozoa. Journal of Eukaryotic Microbiology. 1969;16:69–71. doi: 10.1111/j.1550-7408.1969.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 43.Guan TY, Blank G, Ismond A, Van Acker R. Fate of foodborne bacterial pathogens in pesticide products. Journal of Science of Food and Agriculture. 2001;81:503–512. [Google Scholar]
- 44.Guthrie RK, Anugwelem UA, Davis EM. Responses of bacteria to the presence of carbaryl in water. II. Pure culture v. mixed culture response. Journal of the American Water Resources Association. 1981;17:1005–1007. [Google Scholar]
- 45.Halstead NT, McMahon TA, Johnson SA, Raffel TR, Romansic JM, Crumrine PW, Rohr JR. Community ecology theory predicts the effects of agrochemical mixtures on aquatic biodiversity and ecosystem properties. Ecol Lett. 2014;17:932–941. doi: 10.1111/ele.12295. [DOI] [PubMed] [Google Scholar]
- 46.Hamala JA, Kolliq HP. The effects of atrazine on periphyton communities in controlled laboratory ecosystems. Chemosphere. 1985;14:1391–1408. [Google Scholar]
- 47.Hanlon SM, Parris MJ. The impact of pesticides on the pathogen Batrachochytrium dendrobatidis independent of potential hosts. Arch Environ Contam Toxicol. 2012;63:137–143. doi: 10.1007/s00244-011-9744-1. [DOI] [PubMed] [Google Scholar]
- 48.Herman D, Kaushik NK, Solomon KR. Impact of atrazine on periphyton in fresh-water enclosures and some ecological consequences. Can J Fish Aquat Sci. 1986;43:1917–1925. [Google Scholar]
- 49.Higgins J, Hohn C. Effects of prevalent freshwater chemical contaminants on in vitro growth of Escherichia coli and Klebsiella pneumoniae. Environmental Pollution. 2008;152:259–266. doi: 10.1016/j.envpol.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 50.Hildebrandt A, Guillamon M, Lacorte S, Tauler R, Barcelo D. Impact of pesticides used in agriculture and vineyards to surface and groundwater quality (North Spain) Water Res. 2008;42:3315–3326. doi: 10.1016/j.watres.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Hollister TA, Walsh GE, Forester J. Mirex and marine unicellular algae: Accumulation, population growth and oxygen evolution. Bull Environ Contam Toxicol. 1975;14:753–759. doi: 10.1007/BF01685254. [DOI] [PubMed] [Google Scholar]
- 52.Huber W. Ecotoxicological relevance of atrazine in aquatic systems. Environ Toxicol Chem. 1993;12:1865–1881. [Google Scholar]
- 53.Hurlbert SH, Mulla MS, Wilson HR. Effects of an organophosphorous insecticide on the phytoplankton, zooplankton, and insect populations in fresh-water ponds. Ecol Monogr. 1972;42:269–299. [Google Scholar]
- 54.Jenkins MB, Fisher DS, Endale DM, Adams P. Comparative die-off of Escherichia coli O157:H7 and fecal indicator bacteria in pond water. Environ Sci Technol. 2011;45:1853–1858. doi: 10.1021/es1032019. [DOI] [PubMed] [Google Scholar]
- 55.Jo Q, Kim SK, Lee CS, Lee PY. Live algae as a vector candidate for hydrophobic polychlorinated biphenyls translocation to bivalve filter feeders for laboratory toxicity test. J Environ Biol. 2011;32:787–791. [PubMed] [Google Scholar]
- 56.Jousset A. Ecological and evolutive implications of bacterial defense against predators. Environ Microbiol. 2012;14:1830–1843. doi: 10.1111/j.1462-2920.2011.02627.x. [DOI] [PubMed] [Google Scholar]
- 57.Kawai M, Yamagishi J-I. Mechanisms of action of acriflavine: electron microscopic study of cell wall changes induced in Staphylococcs aureus by acriflavine. Microbiology and Immunology. 2009;53:481–486. doi: 10.1111/j.1348-0421.2009.00151.x. [DOI] [PubMed] [Google Scholar]
- 58.Kent RA, Currie D. Predicting algal sensitivity to a pesticide stress. Environ Toxicol Chem. 1995;10:209–216. [Google Scholar]
- 59.Kerszman G. Of bacteria and men - toxicity of 30 MEIC chemicals to bacteria and humans. Atla-Alternatives to Laboratory Animals. 1993;21:233–238. [Google Scholar]
- 60.Khallil AMA, Omar SA. Influence of the insecticide Dimethoate on some metabolic activities of five zoosporic fungi. Journal of Basic Microbiology. 1993;33:405–411. [Google Scholar]
- 61.Kiely T, Donaldson D, Grube A. EPA-733-R-04-001. Washington D. C.: U. S. Environmental Protection Agency; 2004. Pesticide industry sales and usage: 2000 and 2001 market estimates. [Google Scholar]
- 62.Korajkic A, McMinn BR, Harwood VJ, Shanks OC, Fout GS, Ashbolt NJ. Differential decay of enterococci and Escherichia coli originating from two fecal pollution sources. Appl Environ Microbiol. 2013;79:2488–2492. doi: 10.1128/AEM.03781-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korajkic A, Wanjugi P, Harwood VJ. Indigenous microbiota and habitat influence Escherichia coli survival more than sunlight in simulated aquatic habitats. Appl Environ Microbiol. 2013;79:5329–5337. doi: 10.1128/AEM.01362-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosinski RJ. The effects of terrestrial herbicides on the community structure of stream periphyton. Environ Pollut A. 1984;36:165–189. [Google Scholar]
- 65.Koutsotoli AD, Dimou DS, Alamanos YP, Maipa VE. Inductive effects of environmental concentration of atrazine on Escherichia coli and Enterococcus faecalis. Folia Microbiol (Praha) 2005;50:283–287. doi: 10.1007/BF02931407. [DOI] [PubMed] [Google Scholar]
- 66.Kralj MB, U C, Franko M, Trebse P. Comparison of photocatalysis and photolysis of malathion, isomalathion, malaoxon, and commerical malathion - products and toxicity studies. Water Res. 2007;27:4504–4514. doi: 10.1016/j.watres.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Kricher JC, Urey JC, Hawes ML. The effects of mirex and methoxychlor on the growth and productivity of Chlorella pyrenoidosa. Bull Environ Contam Toxicol. 1975;14:617–620. doi: 10.1007/BF01683381. [DOI] [PubMed] [Google Scholar]
- 68.Lal S, Saxena DM, Lal R. Uptake, metabolism and effects of DDT, fenitrothion and chlorpyrifos on Tetrahymena pyriformis. Pesticide Science. 1987;21:181–191. [Google Scholar]
- 69.Lee SS, Fang SC, Freed VH. Effect of DDT on photosynthesis of Selenastrum capricornutum. Pestic Biochem Physiol. 1976;6:46–51. [Google Scholar]
- 70.Levanon D. Roles of Fungi and Bacteria in the Mineralization of the Pesticides Atrazine, Alachlor, Malathion and Carbofuran in Soil. Soil Biology & Biochemistry. 1993;25:1097–1105. [Google Scholar]
- 71.Lopez L, Pozo C, Rodelas B, Calvo C, Gonzalez-Lopez J. Influence of pesticides and herbicides presence on phosphatase activity and selected bacterial microbiota of a natural lake system. Ecotoxicology. 2006;15:487–493. doi: 10.1007/s10646-006-0084-2. [DOI] [PubMed] [Google Scholar]
- 72.Ma J, Lu N, Qin W, Xu R, Wang Y, Chen X. Differential responses of eight cyanobacterial and green algal species to carbamate insecticides. Ecotoxicology and Environmental Safety. 2006;63:268–274. doi: 10.1016/j.ecoenv.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Magnusson M, Heimann K, Negri AP. Comparative effects of herbicides on photosynthesis and growth of tropical estuarine microalgae. Marine Pollution Bulletin. 2008;56:1545–1552. doi: 10.1016/j.marpolbul.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 74.Malaj E, von der Ohe PC, Grote M, Kühne R, Mondy CP, Usseglio-Polatera P, Brack W, Schäfer RB. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc Natl Acad Sci USA. 2014;111:9549–9554. doi: 10.1073/pnas.1321082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maltby L, C BT, Van den Brink PJ. Fungicide risk assessment for aquatic ecosystems: importance of interspecific variation, toxic mode of action, and exposure regime. Environ Sci Technol. 2009;43:7556–7563. doi: 10.1021/es901461c. [DOI] [PubMed] [Google Scholar]
- 76.McMahon TA, Halstead NT, Johnson S, Raffel TR, Romansic JM, Crumrine PW, Boughton RK, Martin LB, Rohr JR. The fungicide chlorothalonil is nonlinerarly associated with corticosterone levels, immunity, and mortality in amphibians. Environ Health Perspect. 2011;119:1098–1103. doi: 10.1289/ehp.1002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McMahon TA, Halstead NT, Johnson S, Raffel TR, Romansic JM, Crumrine PW, Rohr JR. Fungicide-induced declines of freshwater biodiversity modify ecosystem functions and services. Ecol Lett. 2012;15:714–722. doi: 10.1111/j.1461-0248.2012.01790.x. [DOI] [PubMed] [Google Scholar]
- 78.McMahon TA, Romansic JM, Rohr JR. Nonmonotonic and monotonic effects of pesticides on the pathogenic fungus Batrachochytrium dendrobatidis in culture and on tadpoles. Environ Sci Technol. 2013;16:7958–7964. doi: 10.1021/es401725s. [DOI] [PubMed] [Google Scholar]
- 79.Melendez AL, Kepner RL, Balczon JM, Pratt JR. Effects of diquat on freshwater microbial communities. Arch Environ Contam Toxicol. 1993;25:95–101. [Google Scholar]
- 80.Milenkovski S, Baath E, Lindgren PE, Berglund O. Toxicity of fungicides to natural bacterial communities in wetland water and sediment measured using leucine incorporation and potential denitrification. Ecotoxicology. 2010;19:285–294. doi: 10.1007/s10646-009-0411-5. [DOI] [PubMed] [Google Scholar]
- 81.Mohapatra PK, Mohanty RC. Growth pattern changes of Chlorella vulgaris and Anabaena doliolum due to toxicity of dimethoate and endosulfan. Bull Environ Contam Toxicol. 1992;49:576–581. doi: 10.1007/BF00196301. [DOI] [PubMed] [Google Scholar]
- 82.Moore RB. Effects of pesticides on growth and survival of Euglena gracilis Z. Bull Environ Contam Toxicol. 1970;8:81–83. doi: 10.1007/BF01558316. [DOI] [PubMed] [Google Scholar]
- 83.Nelieu S, Bonnemoy F, Bonnet JL, Lefeuvre L, Baudiffier D, Heydorff M, Quemeneur A, Azam D, Dcrot PH, Lagadic L, Bohatier J, Einhorn J. Ecotoxicological effects of diruon and chlorotoluron nitrate-induced photodegradation products monospecific and aquatic mescosm-integrated studies. Environmental Toxicology and Chemistry. 2010;29:2644–2652. doi: 10.1002/etc.341. [DOI] [PubMed] [Google Scholar]
- 84.Pai TY, Wang SC, Lin CY, Liao WC, Chu HH, Lin TS, Liu CC, Lin SW. Two types of organophosphate pesticides and their combined effects on heterotrophic growth rates in activated sludge process. Journal of Chemical Technology and Biotechnology. 2009;84:1773–1779. [Google Scholar]
- 85.Pesce S, Fajon C, Bardot C, Bonnemoy F, Protelli C, Bohatier J. Effects of phenylurea herbicide diuron on natural riverine microbial communities in an experimental study. Aquatic Toxicology. 2006;78:303–314. doi: 10.1016/j.aquatox.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 86.Peterson HG, Boutin C, Martin PA, Freemark KE, Ruecker NJ, Moody MJ. Aquatic phytotoxicity of 23 pesticides applied at expected environmental concentrations. Aquatic Toxicology. 1994;28:275–292. [Google Scholar]
- 87.Pratt JR, Bowers NJ, Balczon JM. A microcosm using naturally derived microbial communities: comparative ecotoxicology. In: Wayne GL, Hughes JS, Lewis MA, editors. Environmental Toxicoloy and Risk Assessment, Stp 1179. Philadelphia PA: American Society for Testing and Materials; 1993. pp. 178–191. ^eds. [Google Scholar]
- 88.Pratt JR, Bowers NJ, Niederlehner BR, Cairns J. Effects of atrazine on freshwater microbial communities. Archives of Environmental Contamination and Toxicology. 1988;17:449–457. doi: 10.1007/BF01055510. [DOI] [PubMed] [Google Scholar]
- 89.Pratt JR, Melendez AE, Barreiro R, Bowers NJ. Predicting the ecological effects of herbicides. Ecological Applications. 1997;7:1117–1124. [Google Scholar]
- 90.Raffel TR, Martin LB, Rohr JR. Parasites as predators: unifying natural enemy ecology. Trends in Ecology & Evolution. 2008;23:610–618. doi: 10.1016/j.tree.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 91.Rajendran R, Rajendran N, Venugopalan VK. Effect of organochloride pesticides on the bacterial population of a tropical estuary. Microbios Lett. 1990;44:57–63. [Google Scholar]
- 92.Rao VVSN, Lal R. Uptake and metabolism of insecticides by blue-green algae Anabaena and Aulosira fertilissima. Microbios Lett. 1987;36:143–147. [Google Scholar]
- 93.Relyea RA. A cocktail of contaminants: how mixtures of pesticides at low concentrations affect aquatic communities. Oecologia. 2009;159:363–376. doi: 10.1007/s00442-008-1213-9. [DOI] [PubMed] [Google Scholar]
- 94.Ricart M, Barcelo D, Geiszinger A, Guasch H, Alda MLd, Romani AM, Vidal G, Villagrasa M, Sabater S. Effects of low concentrations of the phenylurea herbicide diuron on biofilm algae and bacteria. Chemosphere. 2009;76:1392–1401. doi: 10.1016/j.chemosphere.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 95.Rico A, Waichman AV, Geber-Correa R, van den Brink PJ. Effects of malathion and carbendazim on Amazonia freshwater organisms: comparison of tropical and temperate species sensitivity distributions. Ecotoxicology. 2011;20:625–634. doi: 10.1007/s10646-011-0601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Robinson RA, Sutherland WJ. Post-war changes in arable farming and biodiversity in Great Britain. Journal of Applied Ecology. 2002;39:157–176. [Google Scholar]
- 97.Rohr JR, Crumrine PW. Effects of an herbicide and an insecticide on pond community structure and processes. Ecological Applications. 2005;15:1135–1147. [Google Scholar]
- 98.Rohr JR, Elskus AA, Shepherd BS, Crowley PH, McCarthy TM, Niedzwiecki JH, Sager T, Sih A, Palmer BD. Lethal and sublethal effects of atrazine, carbaryl, endosulfan, and octylphenol on the streamside salamander (Ambystoma barbouri) Environ Toxicol Chem. 2003;22:2385–2392. doi: 10.1897/02-528. [DOI] [PubMed] [Google Scholar]
- 99.Rohr JR, Halstead NT, Raffel TR. The herbicide atrazine, algae, and snail populations. Environ Toxicol Chem. 2012;31:973–974. doi: 10.1002/etc.1796. [DOI] [PubMed] [Google Scholar]
- 100.Rohr JR, Kerby JL, Sih A. Community ecology as a framework for predicting contaminant effects. Trends in Ecology & Evolution. 2006;21:606–613. doi: 10.1016/j.tree.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 101.Rohr JR, McCoy KA. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environ Health Perspect. 2010;118:20–32. doi: 10.1289/ehp.0901164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rohr JR, Palmer BD. Aquatic herbicide exposure increases salamander desiccation risk eight months later in a terrestrial environment. Environ Toxicol Chem. 2005;24:1253–1258. doi: 10.1897/04-448r.1. [DOI] [PubMed] [Google Scholar]
- 103.Rohr JR, Raffel TR, Halstead NT, McMahon TA, Johnson SA, Boughton RK, Martin LB. Early-life exposure to a herbicide has enduring effects on pathogen-induced mortality. Proceedings of the Royal Society B: Biological Sciences. 2013;280 doi: 10.1098/rspb.2013.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rohr JR, Raffel TR, Sessions SK, Hudson PJ. Understanding the net effects of pesticides on amphibian trematode infections. Ecol Appl. 2008;18:1743–1753. doi: 10.1890/07-1429.1. [DOI] [PubMed] [Google Scholar]
- 105.Rohr JR, Sager T, Sesterhenn TM, Palmer BD. Exposure, postexposure, and density-mediated effects of atrazine on amphibians: Breaking down net effects into their parts. Environ Health Perspectives. 2006;114:46–50. doi: 10.1289/ehp.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rohr JR, Schotthoefer AM, Raffel TR, Carrick HJ, Halstead N, Hoverman JT, Johnson CM, Johnson LB, Lieske C, Piwoni MD, Schoff PK, Beasley VR. Agrochemicals increase trematode infections in a declining amphibian species. Nature. 2008;455:1235–1239. doi: 10.1038/nature07281. [DOI] [PubMed] [Google Scholar]
- 107.Rohr JR, Swan A, Raffel TR, Hudson PJ. Parasites, info-disruption, and the ecology of fear. Oecologia. 2009;159:447–454. doi: 10.1007/s00442-008-1208-6. [DOI] [PubMed] [Google Scholar]
- 108.Seffernick JL, Aleem A, Osborne JP, Johnson G, Sadowsky MJ, Wackett LP. Hydroxyatrazine N-ethylaminohydrolase (AtzB): an amidohydrolase superfamily enzyme catalyzing deamination and dechlorination. J Bacteriol. 2007;189:6989–6997. doi: 10.1128/JB.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shalesh S, Anil P. Effect of parathion on aquatic ciliate protozoan, Tetrahymena pyriformis in river Kali at district Etah (U.P.) Journal of Ecophysiology & Occupational Health. 2007;7:125–127. [Google Scholar]
- 110.Singh PK. Effect of pesticides on blue-green algae. Arch Microbiol. 1973;89:317–320. doi: 10.1007/BF00408898. [DOI] [PubMed] [Google Scholar]
- 111.Solomon KR, Baker DB, Richards RP, Dixon KR, Kaline SJ, Point TWL, Kendall RJ, Weisskopf CP, Giddings JM, Giesy JP, Lenwood W, Hall J, Williams WM. Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem. 1996;15:31–76. doi: 10.1002/etc.2050. [DOI] [PubMed] [Google Scholar]
- 112.Somasundaram L, Coats JR, Racke KD, Stahr HM. Application of the Microtox system to assess the toxicity of pesticides and their hydrolysis metabolites. Bull Environ Contam Toxicol. 1990;44:254–259. doi: 10.1007/BF01700144. [DOI] [PubMed] [Google Scholar]
- 113.Staley ZR, Rohr JR, Harwood VJ. The effect of agrochemicals on indicator bacteria densities in outdoor mesocosms. Environ Microbiol. 2010;12:3150–3158. doi: 10.1111/j.1462-2920.2010.02287.x. [DOI] [PubMed] [Google Scholar]
- 114.Staley ZR, Rohr JR, Harwood VJ. A test of direct and indirect effects of agrochemicals on the survival of fecal indicator bacteria. Appl Environ Microbiol. 2011;77:8765–8774. doi: 10.1128/AEM.06044-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Staley ZR, Rohr JR, Senkbeil JK, Harwood VJ. Agrochemicals indirectly increase survival of E. coli O157:H7 and indicator bacteria by reducing ecosystem services. Ecological Applications. 2014;24:1945–1953. doi: 10.1890/13-1242.1. [DOI] [PubMed] [Google Scholar]
- 116.Staley ZR, Senkbeil JK, Rohr JR, Harwood VJ. Lack of direct effects of agrochemicals on zoonotic pathogens and fecal indicator bacteria. Appl Environ Microbiol. 2012;78:8146–8150. doi: 10.1128/AEM.01815-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stark JD, Banks JE. Population-level effects of pesticides and other toxicatns on arthropods. Annual Review of Entomology. 2003;48:505–519. doi: 10.1146/annurev.ento.48.091801.112621. [DOI] [PubMed] [Google Scholar]
- 118.Suberkropp K, Wallace JB. Aquatic hyphomycetes in insecticide-treated and untreated streams. J N Am Benthol Soc. 1992;11:165–171. [Google Scholar]
- 119.Tilman D. Global environmental impacts of agricultural expansion: the need for sustainable and efficient practices. Proc Natl Acad Sci USA. 1999;96:5995–6000. doi: 10.1073/pnas.96.11.5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Torres AMR, O'Flaherty LM. Influence of pesticides on Chlorella, Chlorococcum, Stigeoclonium (Chlorophyceae), Tribonema, Vaucheria (Xanthophyceae) and Oscillatoria (Cyanophyceae) Phycologia. 1976;15:25–36. [Google Scholar]
- 121.Tsui CKM, Hyde KD, Hodgkiss IJ. Effects of glyphosate on lignicolous freshwater fungi of Hong Kong. Sydowia. 2001;53:167–174. [Google Scholar]
- 122.U. S. Environmental Protection Agency. Method 1600: enterococci in water by membrane filtration using membrane-enterococcus indoxy-B-D-glucoside agar (mEI) EPA 821-R-02-022. 2002 [Google Scholar]
- 123.U. S. Environmental Protection Agency. Office of Prevention, Pesticides, and Toxic Substances. Office of Pesticide Programs; Washington D. C.: 2004. Jan 23, Overview of the Ecological Risk Assessment Process in the Office of Pesticide Programs. 2004. [Google Scholar]
- 124.U. S. Environmental Protection Agency. 2006–2007 Pesticide Market Estimates: Usage. 2012 [Google Scholar]
- 125.U. S. Environmental Protection Agency. Human Health Benchmarks for Pesticides. EPA-822-F-12-001. 2012 [Google Scholar]
- 126.Ukeles R. Growth of pure cultures of marine phytoplankton in the presence of toxicants. Appl Microbiol. 1962;10:532–537. doi: 10.1128/am.10.6.532-537.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vance BD, Drummond W. Biological concentration of pesticides by algae. J Phycol. 1969;61:360–362. [Google Scholar]
- 128.Verro R, Finzio A, Otto S, Vighi M. Predicting pesticide environmental risk in intensive agricultural areas. I: Screening level risk assessment of individual chemicals in surface waters. Environ Sci Technol. 2009;43:522–529. doi: 10.1021/es801855f. [DOI] [PubMed] [Google Scholar]
- 129.Vryzas Z, Vassiliou G, Alexoudis C, Papdopoulou-Mourkidou E. Spatial and temporal distribution of pesticide residues in surface waters in northeastern Greece. Water Res. 2009;43:1–10. doi: 10.1016/j.watres.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 130.Wackett LP, Sadowsky MJ, Martinez B, Shapir N. Biodegradation of atrazine and related s-triazine compounds: from enzymes to field studies. Applied Microbiology and Biotechnology. 2002;58:39–45. doi: 10.1007/s00253-001-0862-y. [DOI] [PubMed] [Google Scholar]
- 131.Wade TJ, Calderon RL, Brenner KP, Sams E, Beach M, Haugland R, Wymer L, Dufour AP. High sensitivity of children to swimming-associated gastrointestinal illness: results using a rapid assay of recreational water quality. Epidemiology. 2008;19:375–383. doi: 10.1097/EDE.0b013e318169cc87. [DOI] [PubMed] [Google Scholar]
- 132.Wade TJ, Pai N, Eisenberg JN, Colford JM. Do U.S. Environmental Protection Agency water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environ Health Perspect. 2003;111:1102–1109. doi: 10.1289/ehp.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Walsh GE, McLaughlin LL, Yoder MJ, Moody PH, Lores EH, Forester J, Wessinger-Duvall PB. Minutocellus polyorphus: A new marine diatom for use in algal toxicity tests. Environ Toxicol Chem. 1988;7:925–929. [Google Scholar]
- 134.Wanjugi P, Harwood VJ. The influence of predation and competition on the survival of commensal and pathogenic fecal bacteria in aquatic habitats. Environ Microbiol. 2012;28:517–526. doi: 10.1111/j.1462-2920.2012.02877.x. [DOI] [PubMed] [Google Scholar]
- 135.Weber FH, Shea TB, Berry ES. Toxicity of certain insecticides to protozoa. Bull Environ Contam Toxicol. 1982;28:628–631. doi: 10.1007/BF01605596. [DOI] [PubMed] [Google Scholar]
- 136.Williams PP, Robbins JD, Gutierrez J, Davis RE. Rumen bacterial and protozoal responses to insecticide substrates. Appl Microbiol. 1963;11:517–522. doi: 10.1128/am.11.6.517-522.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang C, Hamel C, Vujanovic V, Gan Y. Fungicide: Modes of action and possible impact on nontarget microorganisms. ISRN Ecology. 2011 2011. [Google Scholar]
- 138.Yanze-kontchou C, Gschwind N. Mineralization of the herbicide atrazine as a carbon source by a Pseudomonas strain. Appl Environ Microbiol. 1994;60:4297–4302. doi: 10.1128/aem.60.12.4297-4302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu S, Wages MR, Cai Q, Maul JD, Cobb GP. Lethal and sublethal effects of three insecticides on two developmental stages of Xenopus laevis and comparison with other amphibians. Environ Toxicol Chem. 2013;32:2056–2064. doi: 10.1002/etc.2280. [DOI] [PubMed] [Google Scholar]
- 140.Zeinat KM, Nashwa AH, Fetyan A, Mohamed AI, Sherif EN. Biodegradation and detoxification of malathion by Bacillus thuringiensis MOS-5. Aust J Basic Appl Sci. 2008;2:724–732. [Google Scholar]