Summary
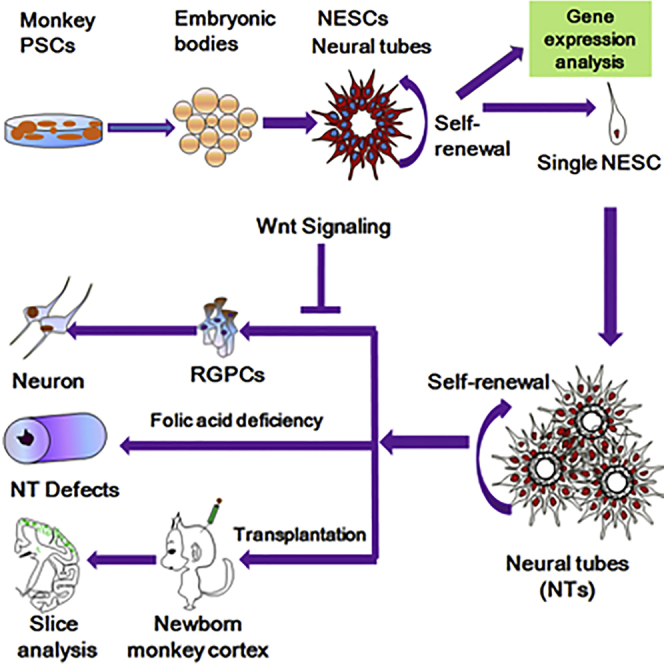
Developing a model of primate neural tube (NT) development is important to promote many NT disorder studies in model organisms. Here, we report a robust and stable system to allow for clonal expansion of single monkey neuroepithelial stem cells (NESCs) to develop into miniature NT-like structures. Single NESCs can produce functional neurons in vitro, survive, and extensively regenerate neuron axons in monkey brain. NT formation and NESC maintenance depend on high metabolism activity and Wnt signaling. NESCs are regionally restricted to a telencephalic fate. Moreover, single NESCs can turn into radial glial progenitors (RGPCs). The transition is accurately regulated by Wnt signaling through regulation of Notch signaling and adhesion molecules. Finally, using the “NESC-TO-NTs” system, we model the functions of folic acid (FA) on NT closure and demonstrate that FA can regulate multiple mechanisms to prevent NT defects. Our system is ideal for studying NT development and diseases.
Graphical Abstract
Highlights
-
•
Long-term cultured neuroepithelial stem cells (NESCs) can be induced from monkey ESCs
-
•
Single NESCs can self-organize into miniature neural tube (NT) structures
-
•
NESCs have high metabolism activity and are restricted to a telencephalic fate
-
•
The “NESC-TO-NTs” system can model and study RPGC transition and NT defect disease
Li, Ji, and colleagues develop a robust and stable system to allow for clonal expansion of single monkey neuroepithelial stem cells (NESCs) to develop into miniature NT-like structures, providing a tractable in vitro platform with which to model and study radial glial progenitor transition, neural tube development, and neural tube defects.
Introduction
Neural tube defects (NTDs) are still poorly understood, especially for human and non-human primates (NHPs) (Wallingford et al., 2013). In rhesus monkeys, at embryonic day 19–20 (E19–20), the neural tube (NT) contains multiple pseudostratified layers of neuroepithelial stem cells (NESCs) (Davignon et al., 1980). Proper cell division, establishment of polarity, and cell movement of NESCs are crucial for NT formation and NT closure (NTC) (Bush et al., 1990). However, abnormal growth of NESCs results in NTDs and, subsequently, defective brain development (Fish et al., 2006). During development, formation of the NT is a time-dependent transient event, difficult to capture, which limits study of it.
After NT formation, the NESC at E12 in mouse undergoes asymmetric divisions to generate one radial glial progenitor cell (RGPC), which exhibits residual neuroepithelial and astroglial properties (Kriegstein and Götz, 2003), and one migratory postmitotic daughter neuron (Kriegstein and Alvarez-Buylla, 2009). Thus, the transition of RGPCs is another fundamental event of brain development. Unfortunately, we still know very little about the RGPC transition process.
Previous reports have demonstrated differentiation of human embryonic stem cells (ESCs) into primitive neural precursor cells (NPCs) or neural rosette cells, which are composed of a myriad of cells along with anterior to posterior cell types (Elkabetz et al., 2008, Koch et al., 2009a, Li et al., 2011). A myriad of cells renders it difficult to study NT development and RGPC transition, even though a few cells maintain clonal expansion. In addition, a human pluripotent stem cells (PSCs)-derived three-dimensional organoid culture system, termed cerebral organoids, was developed to generate various discrete brain regions and could be used to model microcephaly (Lancaster et al., 2013). With the demonstration that the cortex and brain development can be recapitulated in vitro using stem cells (Espuny-Camacho et al., 2013, Lancaster et al., 2013), it is now conceivable that NTC could also be modeled in this way. Although a 3D neural tube system was recently established using embryonic bodies from mouse ESCs (Meinhardt et al., 2014), it is unclear whether the system can be used to model NTC and study NTDs. Furthermore, the system is unable to definitely control NESC self-renewal and differentiation as well as RGPC transition. Therefore, developing a simple culture system, which supports single-ESC-derived NESCs to self-organize into NT-like structures and model the RGPC transition in a stable, controlled, and conserved manner, will be rather advantageous to unveil molecular mechanisms underlying primate NTC and NTDs as well as NESC self-renewal mechanisms.
Results
Rapid Generation of NESCs from Rhesus Monkey ESCs
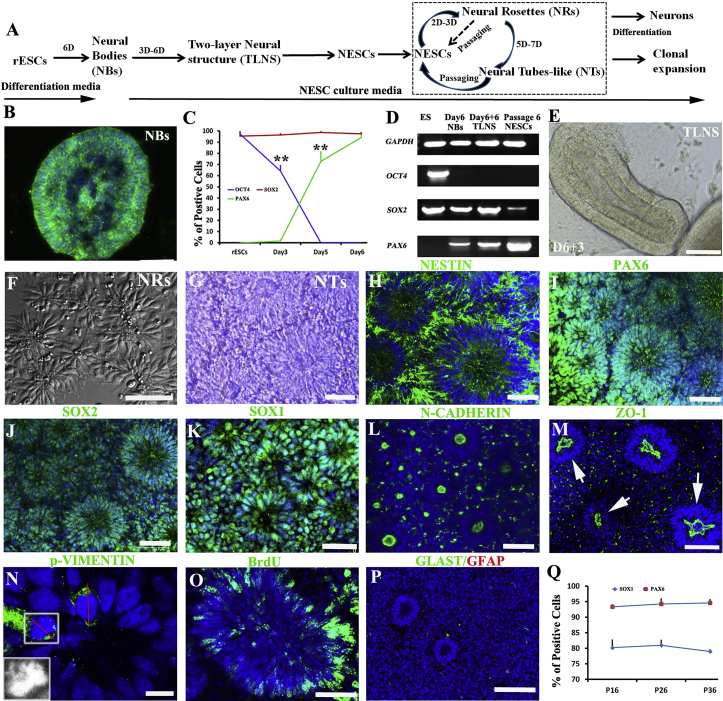
In this study, NESCs were induced from rhesus monkey ESCs (rESCs) using a cocktail containing basic fibroblast growth factor (bFGF) (LaVaute et al., 2009); CHIR99021, a GSK3 inhibitor (Li et al., 2011, Lyashenko et al., 2011); SB431542, a transforming growth factor β (TGF-β) inhibitor (Chambers et al., 2009, Li et al., 2011); compound E, a Notch inhibitor (Li et al., 2011); and LDN193189, an inhibitor of ALK2 and ALK3 (Chambers et al., 2009). rESCs formed embryo bodies (EBs) with typical morphologies at post-differentiation day 2 (pdD2), which quickly formed a neuroepithelial cell layer, referred to as “neural bodies (NBs),” as confirmed by NESTIN staining at pdD5 or pdD6 (Figures 1A, 1B, S1A, and S1B). OCT4, NESTIN, SOX2, and PAX6 staining together with RT-PCR analysis demonstrated that our protocol allowed for rapid and complete downregulation of rESC pluripotency-related genes, with simultaneous induction of NESC-specific genes (Figures 1C, 1D, and S1C–S1E). After pdD6, NBs were cultured on laminin-coated plates in NESC media containing bFGF, LIF, CHIR99021, and SB431542. After 2 to 3 days, typical two-layer neuroectoderm structures were formed (Figure 1E), which were subjected to dissociation and re-plating/passaging. Upon re-plating, polarized structures started to appear at day 2–3 and later on transformed into polarized miniature NT-like structures (Figures 1F and 1G), which can be further passaged for at least 50 passages.
Figure 1.
Rhesus Monkey ESCs Are Rapidly Induced into NESCs
Three independent experiments were repeated for each line (IVF3.2 and IVF3.3).
(A) Schematic representation of the NESC induction and maintenance process.
(B) Embryonic bodies (EBs) were organized into a neuroepithelial layer structure at post-differentiation day 6 (pdD6) by NESTIN staining.
(C) Quantification of SOX2-, OCT4-, and PAX6-positive cells on pdD2, pdD5, and pdD6. Data are expressed as mean ± SD (data from three independent experiments). ∗∗p < 0.01 by Student’s t test.
(D) RT-PCR of differentiated NBs (pdD6 and pdD12) and expandable NESCs (P6).
(E) NBs at pdD6 attached and formed a two-layer structure after being cultured onto laminin-coated plates for 3 additional days in NESC media.
(F) Representative polarized structures of NESCs at low cell density.
(G) Representative NT structures of NESCs at high cell density.
(H–M) Cultured NESCs maintain NT structures expressing NESC markers NESTIN (H), PAX6 (I), SOX2 (J), SOX1 (K), N-CADHERIN (L), and ZO-1 (M).
(N) Mitotic division marker phospho-VIMENTIN staining. The red line indicates symmetrical horizontal division orientation.
(O) BrdU labeling the S-phase cells in an NT.
(P) NESCs were negative for the radial glial progenitor cell markers GFAP and GLAST.
(Q) Quantification of SOX1+ and PAX6+ cells in serial-passaged NESCs.
Data are expressed as mean ± SD (data from three independent experiments). p > 0.05 by Student’s t test. Scale bars: (A and B), 200 μm; (J), 100 μm; (N), 10 μm; others, 50 μm.
These NT-like structures uniformly expressed SOX2, SOX1, NESTIN, and PAX6 and clustered ZO-1 and N-CADHERIN, marking the luminal side (Figures 1H–1M). Using phospho-VIMENTIN to label meta-phase cells, we found most (> 90%, 109 in 120 cells) horizontally dividing cells were located on the apical surface (Figure 1N), whereas BrdU-labeled S-phase nuclei were found to be on the basal surface (Figure 1O), indicative of typical interkinetic nuclear migration (IKNM) found in developing NTs in vivo (Willardsen and Link, 2011). These NESCs were negative for GFAP and GLAST (Figure 1P), two RGPC markers, or TBR2 (Figure S1F), a transcriptional factor expressed by outer or inner sub-ventricular-zone RGPCs and intermediate or basal progenitors in the developing primate cortex (Hansen et al., 2010). Taken together, our novel chemically defined neural induction media and the differentiation protocol allow for rapid and robust conversion of rESCs to self-renewing NESCs and, subsequently, miniature NT-like structures.
Large-Scale Expansion and Stable Conversion of NESCs to Miniature NTs
To our surprise, NESCs in miniature NTs display stable properties as follows. First, rosette or NT structures could be maintained at low or high cell density over passages up to P50 (Figures S1G and S1H). Second, NESCs were routinely passaged at 1:8 to 1:16 every 3 to 4 days, displaying exponential growth over serial passages and resulting in a 2 × 109-fold increase within 2 months without losing obvious proliferative capacity (Figure S1I). Third, NESCs uniformly express stem cell markers after extensive passaging as indicated by fluorescence-activated cell sorting (FCAS) and immunocytochemical analyses (Figures 1Q and S1J–S1L). Cell-cycle profiles of serial-passaged cells are also stable (Figure S1M). Fourth, NESCs could be frozen and thawed without detectable alterations in proliferation or differentiation and retained a stable karyotype for at least 96 population doublings (P33) (Figure S1N).
Single NESCs Self-Organize into NT Structures and Undergo Stable Expansion
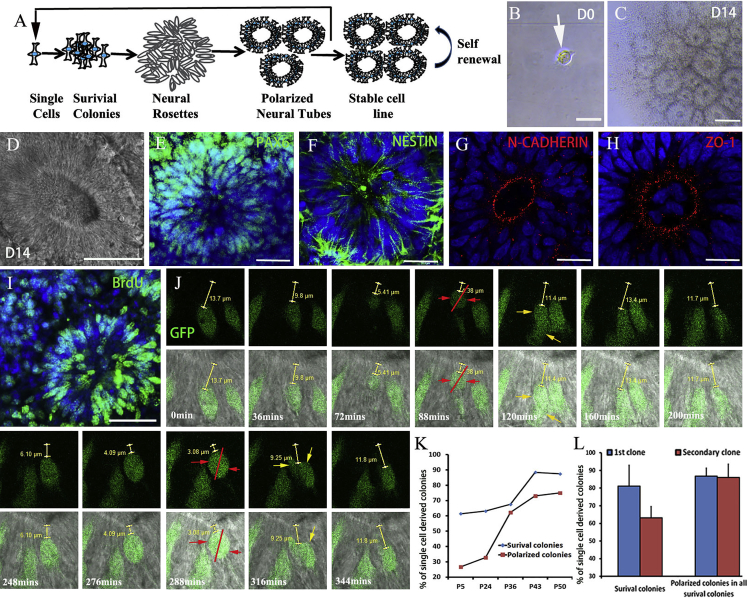
Given that NESCs in the miniature NTs appear to be highly active, we explored whether single NESCs can undergo serial clonal expansion to self-organize into NTs (Figure 2A). One day after seeding, single NESCs (Figure 2B) exhibited stable proliferation with a highly homogeneous morphology (Figure S3). Progressively, 11.8% and 45.46% of the survival colonies began to organize into rosette forms at days 8 to 10, respectively; 69.7% of the survival colonies eventually self-organized NTs with a lumen-expressing PAX6, NESTIN, ZO-1, and N-CADHERIN at day 14 (Figures 2C–2H and S2). Three-dimensional reconstruction confirmed NT miniature structures being composed of multiple layers of neuroepithelial cells (Movie S1). These single-cell-derived NT cells maintained strong proliferation abilities (Figure 2I). Live imaging of GFP-labeled NESCs showed cells displaying IKNM (Willardsen and Link, 2011) and predominantly horizontal (close to apical side) division (Konno et al., 2008) in NTs (Figure 2J; Movie S2). The efficiency of survival cells and polarized colony (NTs) progressively increased over passages (Figure 2K). The single-cell-derived NT colonies maintained long-term self-renewal and formed stable cell lines. Moreover, 63.13% ± 6.5% of clonal cell-line-derived single cells survived and generated secondary colonies, in which 86.09% ± 7.51% of the survival colonies self-organized into NTs at day 14 following seeding (Figure 2L). Our system allows for repetitive and consistent NT morphogenesis from single cells.
Figure 2.
Single NESCs Self-Organize into Miniature NT-like Structures
Three independent experiments were repeated for NESCs from IVF3.2 and IVF3.3.
(A) Schematic representation of single-NESC self-organization into NT structures in the continual colony assays.
(B) A NESC on one well of 96-well plate at day 1.
(C and D) Representative one-cell-derived NT structure at day 14.
(E–H) Single-cell-derived NTs expressed NESC markers PAX6 (E), NESTIN (F), N-CADHERIN (G), and ZO-1 (H).
(I) BrdU incorporation of single-cell-derived NTs.
(J) Live imaging of NESC interkinetic nuclear migration in an NT (also see Movie S2). NESC division predominantly displayed horizontal (close to apical surface) orientation. Red arrows indicate two dividing cells; red lines indicate dividing axis; yellow arrows indicate two divided cells; yellow scales indicate the distance between a lumen and GFP+ cells.
(K) Percentages of single-seeded cells on day 14 that formed NESC-derived survival colonies and NT colonies at sequential passages.
(L) Percentage of inoculated single cells that formed survival colonies (phase 1) and the percentage of survival colonies that formed NT colonies (phase 2) during the continual colony assays.
Data are shown as mean ± SD (data from three independent experiments). No significant difference was found (p > 0.05). Scale bars: (C and I), 100 μm; others, 20 μm.
NESCs Stably Produced Highly Enriched Neurons
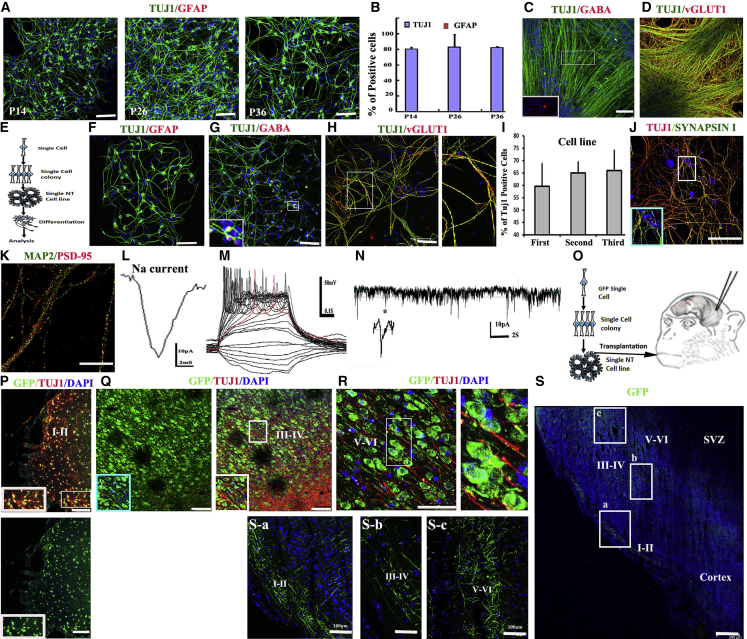
NESCs in vivo have strong abilities to differentiate into neurons without astrocytes (Kriegstein and Alvarez-Buylla, 2009). As expected, over serial passaging, NESCs retained a stable propensity toward neuronal differentiation, whereby more than 80% of differentiated cells become neurons without producing astrocytes (Figures 3A and 3B). Interestingly, the proportion of glutamergic to GABAergic neurons in differentiated neurons is closely associated with the cell density. At high density (9.5 × 103 cells/cm2), over 99% of differentiated neurons are glutamergic with a few (<1%) being GABAergic (Figures 3C and 3D). In contrast, with low density (2.1 × 103 cells/cm2), NESCs-derived GABAergic neurons significantly increased up to 20.09% ± 7.01%. These data suggest that cell-cell communication plays an important role in NESC neuronal differentiation propensity.
Figure 3.
Single NESCs Produced Functional Neurons and Integrate into Newborn Monkey Brains
(A and B) Quantification of differentiated Tuj1+ neurons and GFAP+ astrocytes. Data are represented as mean ± SD (data from three independent experiments) (p > 0.05 by t test).
(C and D) NESCs differentiated into glutamergic (D) and GABAergic (C) neurons at high cell density.
(E–N) Single-NESC-derived stable cell line gave rise to functional neurons. (E) Schematic representation of single NESC function test assays. (F) Single-NESC-derived progenies differentiated into neurons, but not astrocytes. (G and H) Single-NESC-derived progenies differentiated into glutamergic (H) and GABAergic (G) neurons at low cell density. (I) Quantification of differentiated neurons from three different single-cell-derived progenies. Data are shown as mean ± SD (data from three independent experiments). p > 0.05 by t test. (J and K) NESC-derived neurons expressed Synapsin I protein and the post-synapse marker PSD-95 with a punctate pattern. (L and M) A mature neuron exhibited sodium currents and action potentials following current injections. (N) A mature neuron exhibited EPSC.
(O) Schematic representation of single-NESC-derived NT cell transplantation into newborn monkey visual cortex.
(P–R) The injected cells integrated into outer layer (layer I–II) (P), layer III–IV (Q), and layer V–VI (R) of visual cortex and differentiated into neurons.
(S) Injected cells extensively regenerated axon outgrowths, which were found to distribute in the outer layer (I–II) (Sa), deeper layer III–VI (Sb), and layer V–VI (Sc) of cortex.
Scale bars: (K), 50 μm; (S), 400 μm; others, 100 μm.
Individual NESCs Gave Rise to Functional Neurons and Integrated into Newborn Monkey Brains
Differentiation of single-NESC-derived NT cells was spontaneously induced at low cell density to produce functional neurons (Figure 3E). As expected, single NESCs gave rise to glutamergic (76% ± 5.18%) and GABAergic (23.21% ± 3.84%) neurons, not astrocytes (Figures 3F–3H). Quantification of three different single-cell-derived lines showed that they exhibited similar pattern of neuron differentiation (Figure 3I), suggestive of the homogeneity among single NESCs. Furthermore, more than 90% of differentiated neurons were found to form synapse structures expressing Synapsin I pre-synaptic or PSD-95 post-synaptic protein in their axons in a punctate pattern after 32 days or 45 days, respectively, of co-culture with astrocytes (Figures 3J and 3K). Patch clamp recording showed 10 out of the 12 recorded neurons exhibited Na current (Figure 3L) and robust action potential following the current injection (Figure 3M). The recording of functional synapses showed 8 out of the 12 recorded neurons displayed post-synaptic currents (Figure 3N).
To probe the ability of integration into monkey brains for neural repair, single-NESC-derived NT cells labeled by EGFP were injected into the gray matter of visual cortex of two newborn cynomolgus monkeys (postnatal days 24–32) (Figure 3O). After 3 months, the brains were analyzed, and the injected cells were detected on the outermost layer (layer I) (Figure 3P), layer II–III (Figure 3Q), and layer V–IV (Figure 3R) of visual cortex. These cells differentiated into Tuj1+ neurons with typical neuron morphologies (Figures 3P–3R); furthermore, extensive axon outgrowths were found in many sites of cortex and extended from layer I–II into deeper layer of cortex (layer III–VI) (Figures 3Sa–3Sc). Taken together, these data demonstrate that single NESCs behaving as in vivo neuroepithelial cells can produce mature and functional neurons in vitro and in vivo.
NTs Can Be Generated from Monkey iPSC-Derived NESCs in a Similar Manner
To determine whether monkey induced pluripotent stem cells (iPSCs) could also be induced into NESCs in a similar manner and to build the “NESC-TO-NTs” model, one monkey iPSC line was used, and the experiments described above were repeated. As shown in Figure S3, iPSC-derived NESCs were similarly generated, self-organized into NTs, and maintained stable homogeneous, clonogenic, and neurogenic abilities in culture.
NESCs Display Unique Metabolism and Active Wnt Signaling Pathways
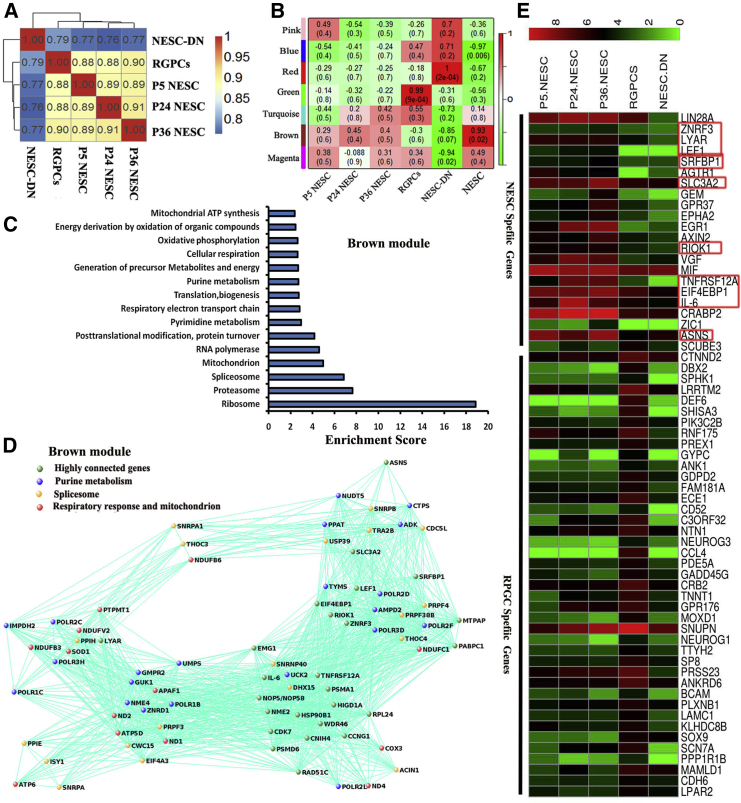
To test whether NESCs have unique gene expression profiles, the global transcriptome of monkey NESCs at various passages (P5, P24, and P36) were analyzed via RNA sequencing (RNA-seq). P36 RGPCs, only cultured for 7 days in NESC culture media removing CHIR99021 (Figures S4A–S4M), and P36 newly differentiated neurons (NESCs-DN) were also analyzed. Sample correlation (Spearman) showed that late-passage NESCs (P36) closely clustered with mid-passage (P24) and early-passage NESCs (P5) than with RGPCs and NESCs-DN (Figure 4A). Total expression genes were clustered using K means, yielding distinct modules (e.g., modules brown, blue, red, and green) (Figure 4B). Using module Eigengene (Langfelder and Horvath, 2008) or module average gene expression levels, we established correlation between modules and cell types. Several cell-type-specific modules were identified (Figure 4B). For example, the brown module is highly specific for NESCs regardless of passage number, whereas genes in the blue module either are not expressed or are expressed at very low levels in NESCs. On the other hand, the red module appears to be neuronal-specific.
Figure 4.
NESCs Display Unique Metabolism and Active Wnt Signaling Pathways
(A) Sample correlation (Spearman) of RNA sequencing analysis of the early-, medial-, late-passage NESCs (P5, P24, and P36), RGPCs, and NESCs-ND (differentiated neurons).
(B) K-mean clustering detected modules’ relative expression.
(C) Representative GO function terms of NESCs specific to the brown module.
(D) Brown module (NESC-specific) hub-gene network.
(E) Some specific genes expressed at higher levels in NESCs or RGPCs, respectively. Red boxes represent these genes that appeared in the brown module hub-gene network in (D).
The cell-type-specific modules were subjected to Gene Ontology (GO) analyses (Figures 4C and S5). As expected, GO term enrichment showed NESCs have higher expression of genes related to RNA splicing, energy metabolism, mitochondrion, and purine and pyrimidine metabolism (Figures 4C and S5A). Hub gene analyses of the brown (NESC-specific) module revealed highly connected genes and further confirmed important roles of splicing, mitochondria function, and purine and pyrimidine metabolism for NESCs (Figure 4D). In contrast, neurons express genes relative to axon guidance, neurotrophin signaling pathways, and endocytosis (Figure S5B). In addition, ASNS, LYAR, and ZNRF3, which are hub genes in the brown module (Figure 4D), have recently been shown to be related to microcephaly or NTDs (Hao et al., 2012, Ruzzo et al., 2013, Wang et al., 2012), consistent with the notion that these three factors control NESC development. Moreover, other hub genes such as LEF1 and ZNRF3 (Hao et al., 2012) are critical components of the Wnt signaling pathway. Their presence in the brown module hub-gene network suggests that Wnt signaling is involved in regulating NESC biology, consistent with the result from CHIR99021 dropout experiments (Figures 5 and S4).
Figure 5.
The Mechanisms Controlling “NESC-TO-NTs” Self-Organization and Radial Glial Progenitor Cell Transition
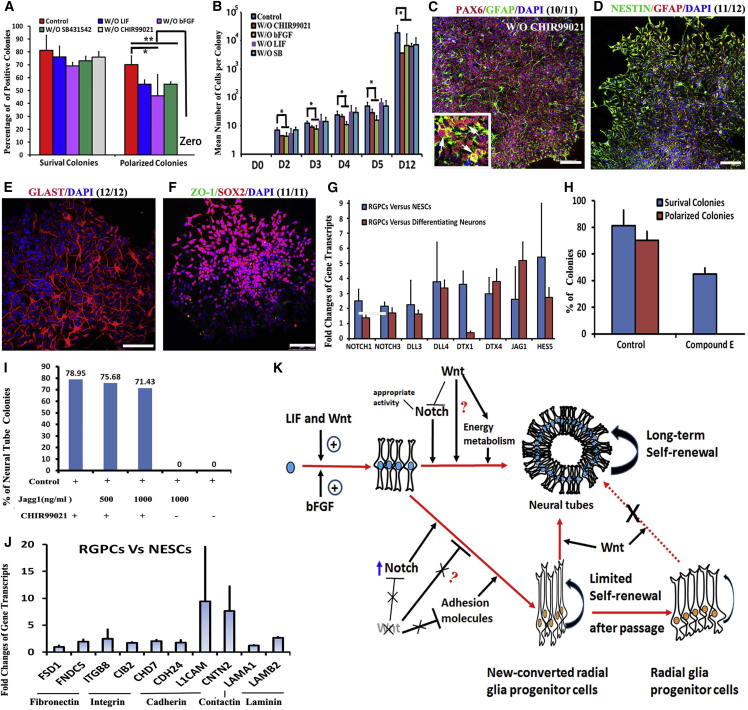
(A) Comparison of survival colonies and polarized colonies numbers (as noted by ZO-1 staining) versus the number of seeded single cells on day 14 in five different culture conditions.
(B) The proliferation difference of single cells cultured in five different culture conditions. Data are expressed as mean ± SD (data from three independent experiments) in (A) and (B). ∗p < 0.05; ∗∗p < 0.01 by Student’s t test.
(C–F) Wnt signaling inactivation results in the loss of NT formation and subsequently promotes RGPC transition. Transited cells expressed RGPC markers PAX6 and GFAP (C), NESTIN and GFAP (D), GLAST (E), and SOX2 (F), but not ZO-1 (F).
(G) Fold change of expressions of genes related to Notch signaling in RGPCs versus NESCs and NESCs-DN.
(H) The inhibition of Notch signaling completely abolished NT formation.
(I) Quantification of NT colonies when single NESCs were cultured in different conditions. Jagg1 is a ligand of Notch signaling.
(J) Fold change of expressions of cell adhesion molecules.
(K) Working model of the “NESC-TO-NTs” self-organization and RGPC transition.
Scale bars, 100 μm. Data in (G)–(J) are from three independent experiments.
To identify genes unique to NESCs, RGPCs cultured for only 7 days in media without CHIR99021 were chosen as control to narrow down the candidate gene list. These RGPCs represented newly formed RGPCs as they just began to express GFAP and GLAST but still could be converted back into NESCs upon addition of CHIR99021 (Figures S4A–S4K). Therefore, the transcriptome of these RGPCs is only slightly different from that of NESCs (Figure 4A). NESCs uniquely expressed some genes that have been demonstrated to be involved in NT development, such as Wnt signaling genes LEF1, ZIC1 (Merzdorf and Sive, 2006), and AXIN2 (Bowman et al., 2013), and other NT development genes: LIN28A (Balzer et al., 2010), IL-6 (Islam et al., 2009), and ASNS (Ruzzo et al., 2013) (Figure 4E). These data indicate these genes may play critical roles in NESC maintenance.
NESCs Are Regionally Restricted to a Telencephalic Fate
To further understand whether NESCs are regionally specified by their pattern of gene expression, we used a list of region-specific genes from a published database (Batista-Brito et al., 2008, Elkabetz et al., 2008, Long et al., 2009, Mariani et al., 2012, Zhang et al., 2013). On the basis of the expression levels of these genes, we concluded that the overall expression pattern of the NESCs at different passages (P5, P24, and P36) was typical of forebrain dorsal and ventral features, but not with midbrain, hindbrain, or neural crest features (Figure S6A).
Differentiation assays showed that NESCs gave rise to telencephalic neurons, including dorsal-I-layer to dorsal-VI-layer cortical glutamatergic neurons and ventral GABAergic neurons, but not other neuron subtypes containing N-methyl-D-aspartate receptor (NMDA), adrenergic or serotonergic neurons, and midbrain dopamine and hindbrain neurons (Figure S6B). The differentiation potentials of cortical neurons for NESCs were next evaluated. NESCs underwent TBR2+PAX6− intermediate progenitors and gave rise to TBR1+ cortical neurons in SDM media (Figures S6C and S6D). Further characterizations showed that these cortical neurons at least included BRN2+ and CALRETININ+GABA− upper-layer neurons as well as CTIP2+ and FOXP2+ deep-layer neurons (Figures S6E–S6H). In contrast, NESCs were unable to differentiate into adrenergic, serotonergic, and midbrain dopamine neurons (Figure S6I). Furthermore, NESCs failed to produce dopamine neurons in the dopamine differentiation media (Kriks et al., 2011) or motoneurons in the motoneuron differentiation media (Figure S6J) (Hu and Zhang, 2009). NESCs are regionally restricted to a telencephalic fate and have the ability to give rise to cortical neurons.
The NESC identity is different from several published primitive ESC-derived NSCs (Koch et al., 2009a, 2009b) or neural rosette genes (Li et al., 2011), which express a specific set of anterior-posterior neural genes, such as Hoxb2, Nurr1, En-1, Lmx1b, Pax2, and Pitx3, and have the ability to produce midbrain or hindbrain neurons in specific conditions. RNA-seq data further showed NESCs displayed negative or low expression of published neural rosette genes, including DACH1, PLZF, LMO3, NR2F1, DMRT3, FLAM70A, MMRN1, and PLAGL1 as well as LHX2, RFX4, ARX, and ASCL1, which are also expressed in FGF2- and EGF-expanded NSCs (Figure S6K) (Elkabetz et al., 2008, Reinhardt et al., 2013), indicating that these genes are unnecessary for maintaining NESCs. Our NESCs with NT formation are different from published neural rosette cells or primitive NSCs.
Wnt Signaling Was Required for the “NESC-TO-NTs” Self-Organization
To ask whether Wnt signaling is required for the “NESC-TO-NTs” self-organization, the transitions from single NESC to NT structures were analyzed in different culture conditions using dropout experiments. The removal of leukemia inhibitory factor (LIF) alone from the growth media, but not of bFGF or SB431542, resulted in decreased NT formation and secondary NT formation by either decreasing proliferation or promoting differentiation (p < 0.05) (Figures 5A and 5B). In contrast, CHIR99021 depletion completely inhibited NT formation (Figures 5A–5E and S4A–S4H). Further studies showed that no stable cell line beyond passage 5 was eventually obtained in the deficient media (n = 7), whereas proliferating cell lines were generated from all examined colonies (n = 3) in the other three conditions (e.g., without LIF, without SB431542, and without bFGF). These data suggested Wnt signaling is required for the “NESC-TO-NT” conversion.
Single NESCs Can Undergo RGPC Transition in Absence of Wnt Signaling
To reveal whether NESCs can turn into RGPCs, CHIR99021 alone was removed from NESCs culture media in single-cell cultures. Interestingly, all progenies of single NESCs were progressively converted into GFAP+ cells, which expressed RGPC markers but were no longer capable of NT formation (Figures 5C–5F and S4A–S4H). The early addition of CHIR99021 followed by late withdrawal or early depletion followed by late addition of CHIR99021 showed that GSK3β inhibition was a key to prevent RGPC genesis (Figures S4H–S4K). Although newly formed RGPCs can go back to the NESC state upon CHIR99021 treatment, once passaged, RGPCs lost the ability to convert back to NESCs and differentiate into neurons when LIF, bFGF, and SB431542 were removed from culture media (Figure S4I). Furthermore, addition of XAV939, a Wnt inhibitor, also completely inhibited NT formation and converted NESCs into RGPCs (Figure S4L). These data suggest that Wnt acts to maintain NESC identity via inhibiting transition of RGPCs.
“NESC-TO-RGPC” transition was reported to be regulated by Notch signaling (Gaiano et al., 2000). Interestingly, the genes of Notch signaling are highly expressed in RGPCs compared to in NESCs and NESCs-DN (Figure 5G), which is suggestive of inhibition ability of Wnt signaling to Notch signaling. Elimination of baseline Notch activity, via application of a Notch inhibitor compound E, completely inhibited NT formation (Figure 5H) and promoted neurogenesis (data not shown), whereas the use of a Notch ligand Jagg1 decreased NT formation even in the presence of CHIR99021 (Figure 5I), suggesting that the appropriate level of Notch signaling activity is a key to the delicate balance between NESC maintenance and RGPC transition. However, Jagg1 addition was unable to rescue the NT deficiency caused by CHIR99021 withdrawal (Figure 5I), indicating that Wnt signaling regulated NT formation via regulating other pathways besides Notch signaling. Cell adhesion molecules (CAMs) have been demonstrated to play important roles in neural stem cell (NSC)/NPC self-renewal, differentiation, and neuronal migration (Bian, 2013). Interestingly, we found that expression levels of CAMs, including Fibronectin, Integrin, Cadherin, L1CAM, and CNTN2 (Contactin-2), significantly increased during the transition of NESCs to RGPCs (Figure 4J). The working model of Wnt signaling for NT and RPGC transition is summarized in Figure 5K.
“NESC-TO-NTs” Conversion Could Model NTDs Caused by Folic Acid Deficiency
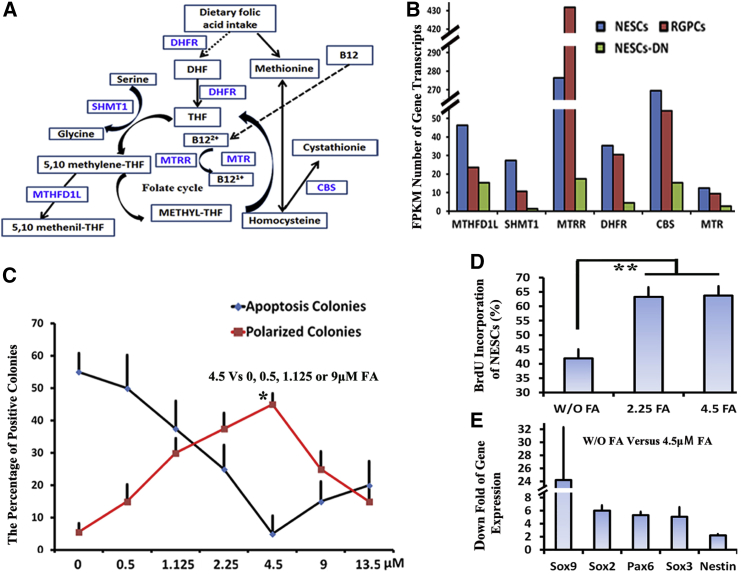
Despite the strong clinical link between folate and NTDs, the biochemical mechanisms through which folic acid (FA) acts during NT development remain undefined (Momb et al., 2013). To determine whether the “NESC-TO-NTs” conversion in our system has a similar dependence on FA as it does in vivo during NTC, single-cell clonal assays were performed in growth media with and without FA. Mutation or knockouts of a number of key enzymes involved in folate metabolism have been reported to result in NTDs (Figure 6A) (Beaudin et al., 2012, Momb et al., 2013, Padmanabhan et al., 2013). Actually, these key enzymes of folate metabolism were highly expressed in NESCs versus in NESCs-DN or RGPCs (Figure 6B). Furthermore, efficiency of NT formation was closely associated with FA additions (Figure 6C). The increases of FA concentration significantly inhibited NESC apoptosis and promoted NT self-organization when FA concentration was less than 4.5 μM; however, once above 4.5 μM, NESC apoptosis and NT self-organization were negatively correlated with FA concentration. BrdU incorporation showed that NESC proliferation was significantly inhibited in the absence of FA (Figure 6D). FA deficiency resulted in a significant decrease of stem cell marker expressions (Figure 6E), but not of RPGC and differentiated neuron markers (Figure S7A), indicating that FA deficiency changes stem cell identity not through promoting NESC differentiation. These findings are consistent with previous reports showing that folate deficiency has been established as a risk factor of NTDs (Beaudin et al., 2012, Boyles et al., 2011), whereas FA fortification of the food supply has been temporally associated with declines in the prevalence of NTDs (Botto et al., 2006).
Figure 6.
“NESC-TO-NTs” Conversion Models NTDs Caused by Folic Acid Deficiency
(A and B) Folate metabolism pathway. Blue names (A) indicate key enzymes involved in folate metabolism whose expression levels are higher in NESCs versus RGPCs or NESCs-DN (B).
(C) NT formation was closely correlated with FA concentrations.
(D) BrdU incorporation assays of NESCs in 2.5 μM FA (2.5FA), 4.5 μM FA (4.5FA), and the media without FA (W/O-NESCs).
(E) Down folds of stem cell markers when NESCs were cultured in the 2.5FA or 4.5FA relative to W/O-NESCs.
Data are showed as mean ± SD in (C), (D), and (E) (data from three independent experiments). ∗p < 0.05, and ∗∗p < 0.01 by Student’s t test between each. Data in (B) are from two independent experiments.
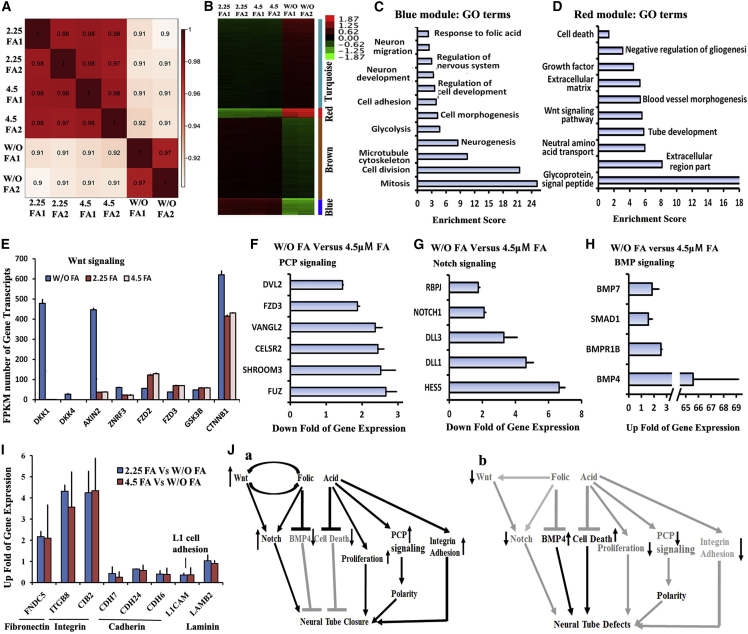
To understand the mechanisms of how FA prevents NTDs, the gene expression profiles from NESCs cultured in media with FA (FA-NESCs) or without FA (W/O-NESCs) were analyzed. Cluster analysis showed that gene expression profiles are significantly different between FA-NESCs and W/O-NESCs (Figure 7A). Differential expression analysis showed that 5,346 genes display significant difference in the two cultures (Table S2). These differential expression genes were clustered using K means, yielding four distinct modules (modules turquoise, red, brown, and blue) (Figure 7B). Using module Eigengene (Langfelder and Horvath, 2008) or module average gene expression levels, correlations between modules and cell types were established. Turquoise and red modules are highly or moderately specific for W/O-NESCs, whereas genes in the brown and blue modules are highly or moderately relative to FA-NESCs, respectively. As expected, GO term enrichment of the cell-type-specific modules showed FA presence most significantly promotes expression of genes related to mitosis, cell division, microtubule cytoskeleton, neurogenesis, cell morphogenesis, cell adhesion, neuron migration, and neuron development (Figure 7C; Table S2), which were key for NTC as reported (Copp and Greene, 2013, Copp et al., 2013). Second, FA presence affects expression of genes involved in energy metabolism, chromosome, double-strand break repair, and mitochondrion (Figure S7B; Table S2). In contrast, FA deficiency most significantly induces expression of glycoprotein, the Wnt signaling pathway, growth factor, tube development, and extracellular matrix (Figure 7D; Table S2). FA deprivation affects cell organelles relative to cell death and apoptosis (the Golgi apparatus and endoplasmic reticulum), protein transport, and kinase (Figure S7C; Table S2).
Figure 7.
Folic Acid Can Regulate Multiple Signaling Pathways to Prevent NTDs
(A) Sample cluster of RNA-seq of NESCs cultured in the without FA media or the 2.25 or 4.5 μM FA media.
(B) K-mean clustering detected modules’ relative expression of differential genes between FA-NESCs and W/O-NESCs.
(C) Representative blue-module-specific GO function terms for FA-NESCs.
(D) Representative red-module-specific GO function terms for W/O-NESCs.
(E–I) The change of Wnt signaling (E), PCP signaling (F), and Notch signaling (G) pathways. (H) The change of BMP signaling. (I) The up fold of expression of cell adhesion molecules in FA-NESCs.
(J) The working model of FA inducing NT closure and FA deficiency inducing NTDs.
Data in (E)–(I) are from two independent experiments.
Interestingly, we also noted that FA deficiency significantly inhibited the Wnt signaling activity by upregulating expression of Wnt signaling inhibitors, including DKK1, AXIN2, and ZNRF3 (Figure 7E). PCP, Notch, and BMP signaling have been reported to be key for NTC, and their abnormal expressions result in NTDs (Copp and Greene, 2013, Copp et al., 2013). Consistent with these reports, PCP and Notch signaling were significantly downregulated in W/O-NESCs, whereas BMP signaling was upregulated (Figures 7F–7H). We also found that FA maintains normal expression of integrin CAMs, which was involved in NT development (Fournier-Thibault et al., 2009), but not for CADHERIN, LICAM, and LAMININ (Figure 7I). In addition, we found many genes whose expression levels were upregulated for at least 10 folds by FA induction (Figure S7D; Table S2). In contrast, large number of genes, such as NOTUM, DKK1, mml-let-7g, and IGFBP7, were specific for W/O-NESCs, indicating that they are negatively regulated by FA (Figure S7E; Table S2).
Together, these results show that FA promotes NTC by regulating multiple mechanisms, such as activating Wnt, Notch, PCP, and integrin CAM pathways; promoting cell proliferation; and inhibiting BMP4 pathway and cell death (Figure 7J). Thus, the “NESC-TO-NTs” system could recapitulate the FA mechanisms for NTC in vivo and can be used to identify multiple novel candidate genes or pathways involved in NTDs.
Discussion
In summary, a simple culture system was successfully developed to generate expandable single NESCs derived from monkey ESCs in vitro. A large number of experiments were used to demonstrate that the system is suitable for studying NTC and related disorders. These cells were found to (1) be polarized neuroepithelial cells; (2) self-organize into miniature NT structures at a cellular level; (3) have robust expansion ability; (4) display IKNM; (5) produce functional neurons and integrate into monkey brains; (6) be dependent on FA for NT formation; (7) express many NT genes, such as LIN28A, ASNS, IL-6, LYAR, and ZNRF3, but not for RPGC markers, including GFAP, GLAST, and TBR2; and (8) turn into RGPCs once deprived of Wnt signaling. These characteristics show these cells behave similarly as in vivo NT NESCs.
The ability to reproducibly generate NT structures and clonal expansion from single NESCs, as demonstrated in this study, offers several advantages for studying brain development and disease: (1) stability—our NESCs faithfully self-renew. Their cell-cycling parameters, differentiation potentials, gene expression profiles, and NT formation process remain stable for over 50 passes. (2) Single cell function—individual NESCs self-organize into NTs, complete RGPC transition, and give rise to functional neurons. These special properties render the power to reveal how highly complex neural developmental processes organize at a cellular level. (3) Clonal growth—the clonal expansion feature of single NESCs will allow for targeted gene editing facilitated by TALEN (Liu et al., 2014) or CRISPR-Cas9 technologies (Niu et al., 2014). Acquisition of mutant or genetically corrected clones of cells is ideal for disease modeling or mutation corrections using NESCs.
The NT formation and “NESC-to-RGPCs” transition at the onset of neurogenesis are two fundamental events in the early developing brain (Götz and Huttner, 2005). However, the developmental regulations are unclear due to being hard to definitely control the two stages. In this study, the two stages could be definitely regulated at a cellular level. Although Wnt signaling has been demonstrated to play an important role in NSC/NPC formation and function throughout developmental time (Bowman et al., 2013), the effects of Wnt signaling on the two processes are still obscure. Our data clearly showed that both processes are regulated by Wnt signaling activity. Using the system, we found novel insights about the underlying mechanisms of NT formation and RGPC transition. A very interesting discovery is that Notch signaling has an opposite function for the two fates. The specific functions of Notch signaling are related to degree of its activity (Figure 5O). Additionally, previous studies showed L1CAM and CNTN2 are important for neuronal migration, axonal growth, guidance and fasciculation, neuronal survival, and synaptic plasticity (Tonosaki et al., 2014). In the study, L1CAM and CNTN2 were shown to be fundamental to RGPC transition and formation. Thus, the system provides a new platform to study and screen mechanisms of NTC and RGPC transition.
NTDs are severe congenital malformations affecting 1 in every 1,000 pregnancies. Despite the strong clinical link between folate and NTDs, a lack of evidence related to FA pathway mutants and NTD risk pathways indicates the need for novel approaches to elucidate how FA affects NTC. Our present findings show the growth and gene expression characteristics of NESCs are similar to that of NT neuroepithelial cells. Using this system, we demonstrated that FA can regulate multiple mechanisms to prevent NTDs. The relationships among these pathways in NTDs may be uncovered by the system. In addition, we also noted many novel genes regulated by FA. Some novel mechanisms in NTDs might be uncovered by studying their functions. Because of their similarity to humans, NHPs are important models for studying human disease and developing therapeutic strategies. Recently, using TALEN- or CRISPR/Cas9-mediated gene targeting in one-cell embryos, we successfully generated gene mutant rhesus and cynomolgus monkeys (Liu et al., 2014, Niu et al., 2014). If we can identify some key genes for NT development by using the system, using gene editing to produce NTD monkeys with specific genetic mutations will be important to uncover the mechanisms controlling this disorder. Therefore, the system may provide a tractable platform with which to screen small molecules for therapeutic/preventive potential related to NTDs and to help gain new important insights into these disorders.
Experimental Procedures
Induction and Expansion of Neuroepithelial Stem Cells
IVF3.2 and IVF3.3 rESCs and monkey fibroblast-derived iPS line 1.1 were cultured on X-ray-inactivated CF-1 mouse embryonic φμbroblasts (MEFs) in ESCs growth media (DMEM/F12 [1:1] [Invitrogen] containing 15% KSR [Invitrogen] and 5 ng/mL bFGF [Millipore]) (Chen et al., 2015, Li et al., 2005, Sun et al., 2011).
ESCs or iPS1.1 were digested with Collagenase IV (Gibco), and neural induction was induced by switching from ESC growth media to differentiation media in suspension culture (Advance DMEM/F12 [1:1] [Invitrogen]: Neurobasal media [Invitrogen] [1:1 mixture] supplemented with 1 × N2 [Invitrogen], 1 × B27 [Invitrogen], 10 ng/ml bFGF [Millipore], 3 μM CHIR99021 [Cellagen Technology], 5 μM SB431542 [Cellagen Technology], 0.2 μM compound E, and 0.1 μM LDN193189 [Cellagen Technology]). After 6 days, EBs were transferred to 5 μg/ml laminin (Gibco)-coated plates for attachment culture, and the media were switched to NESCs culture media (Neurobasal media, including B27, N2, and NEAA [Sigma], 1% Glutmax [Sigma], 3 μM CHIR99021, 5 μM SB431542, 10 ng/ml bFGF, and 1,000 U/ml hLIF [Millipore]). To encourage cell propagation, 0.025% trypsin was used to digest NESCs when passaging. NESCs were routinely passaged to 1:8 to 1:16 ratios every 3 to 4 days. For NT formation, NESCs were continually cultured 8 to 10 days before passaging.
NESC Differentiation
For spontaneous differentiation, NESCs were cultured on plates coated with laminin (5 μg/ml) and gelatin (0.05%) in differentiation media (SDM) (Neurobasal supplemented with N2, B27, NEAA, and Glutmax). On day 6, 10 ng/ml BDNF (R&D Systems) and 10 ng/ml GDNF (R&D Systems) were added to the media to induce terminal maturation of neurons. For GABAergic neuron differentiation, 10 ng/ml SHH (R&D Systems) was added to the differentiation media for the first 4 days. On day 5, 10 ng/ml BDNF and 10 ng/ml GDNF were added to the media to induce terminal maturation of neurons. For density differentiation experiments, the concentrations of high cell density or low cell density were 9.5 × 103 cells/cm2 or 2.1 × 103 cells/cm2, respectively.
NESCs Modeling Neural Tube Defects Caused by Folic Acid Deficiency
Clonal assays of single NESCs were used as a model to mimic NTDs caused by FA deficiency. After colonies had been cultured in NESC media for 7 days, the media was changed to FA-free NESC media, which constituted RPIM 1640 media, N2, B27, NEAA, 1% Glutmax, 3 μM CHIR99021, 5 μM SB431542, 10 ng/ml bFGF, and 1,000 U/ml hLIF supplemented with different concentrations of FA (Sigma) until day 14. The FA concentrations were 0 μM, 1.125 μM, 2.25 μM, 4.5 μM, 9 μM, and 13.5 μM, respectively. On day 14, apoptosis and NT colonies were quantified as the number of dead colonies or with ZO-1 staining, respectively.
Author Contributions
T.L. conceived the idea for this project, designed and conducted experiments, optimized NESCs differentiation and culture conditions, analyzed data, and wrote the manuscript with Y.E.S. and W.J. X.H. and J.R. help to edit the manuscript. X.Z., B.L., Z.A., Z.X., X.Q., Y.C., Y.L., and Y.N. performed experiments and analyzed data under the supervision of T.L. and W.J.; K.Z. analyzed RNA sequencing data.
Acknowledgments
This work was funded by grants from the Chinese Ministry of Science and Technology 973 program (2012CBA01307), the Key Technologies Research and Development Program of China (2014BAI03B01), the National Natural Science Foundation of China (31360231 and 31271599), and Yunnan Basic Research Projects (2014FC004).
Published: November 12, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, seven figures, two tables, and two movies and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.10.007.
Contributor Information
Weizhi Ji, Email: wji@kbimed.com.
Tianqing Li, Email: litq@kbimed.com.
Accession Numbers
The accession number for the RNA sequencing data reported in this paper is GEO: GSE73892.
Supplemental Information
The GO terms are related to Figure 7 and Figure S6.
Self-organized neural tubes by single NESCs were fixed and stained with DAPI (blue) on day 14. A single-cell-derived neural tube was scanned using a Leica TCS SP8 confocal laser scanning system. Ten neural tubes were scanned.
Live imaging of retrovirus GFP infected NESCs revealed movement of nuclei along apical and basal surfaces. Arrows mark two NESCs, in particular, with clear IKNM. Horizontal divisions (0–30°, spindle orientation) were close to the apical side in NTs. Time is shown in hr:min.
References
- Balzer E., Heine C., Jiang Q., Lee V.M., Moss E.G. LIN28 alters cell fate succession and acts independently of the let-7 microRNA during neurogliogenesis in vitro. Development. 2010;137:891–900. doi: 10.1242/dev.042895. [DOI] [PubMed] [Google Scholar]
- Batista-Brito R., Machold R., Klein C., Fishell G. Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb. Cortex. 2008;18:2306–2317. doi: 10.1093/cercor/bhm258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin A.E., Abarinov E.V., Malysheva O., Perry C.A., Caudill M., Stover P.J. Dietary folate, but not choline, modifies neural tube defect risk in Shmt1 knockout mice. Am. J. Clin. Nutr. 2012;95:109–114. doi: 10.3945/ajcn.111.020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S. Cell adhesion molecules in neural stem cell and stem cell-based therapy for neural disorders. In: Bonfanti L., editor. Neural Stem Cells: New Perspectives. InTech; 2013. pp. 349–380. [Google Scholar]
- Botto L.D., Lisi A., Bower C., Canfield M.A., Dattani N., De Vigan C., De Walle H., Erickson D.J., Halliday J., Irgens L.M. Trends of selected malformations in relation to folic acid recommendations and fortification: an international assessment. Birth Defects Res. A Clin. Mol. Teratol. 2006;76:693–705. doi: 10.1002/bdra.20307. [DOI] [PubMed] [Google Scholar]
- Bowman A.N., van Amerongen R., Palmer T.D., Nusse R. Lineage tracing with Axin2 reveals distinct developmental and adult populations of Wnt/β-catenin-responsive neural stem cells. Proc. Natl. Acad. Sci. USA. 2013;110:7324–7329. doi: 10.1073/pnas.1305411110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles A.L., Ballard J.L., Gorman E.B., McConnaughey D.R., Cabrera R.M., Wilcox A.J., Lie R.T., Finnell R.H. Association between inhibited binding of folic acid to folate receptor α in maternal serum and folate-related birth defects in Norway. Hum. Reprod. 2011;26:2232–2238. doi: 10.1093/humrep/der144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K.T., Lynch F.J., DeNittis A.S., Steinberg A.B., Lee H.Y., Nagele R.G. Neural tube formation in the mouse: a morphometric and computerized three-dimensional reconstruction study of the relationship between apical constriction of neuroepithelial cells and the shape of the neuroepithelium. Anat. Embryol. (Berl.) 1990;181:49–58. doi: 10.1007/BF00189727. [DOI] [PubMed] [Google Scholar]
- Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Niu Y., Li Y., Ai Z., Kang Y., Shi H., Xiang Z., Yang Z., Tan T., Si W. Generation of Cynomolgus Monkey Chimeric Fetuses using Embryonic Stem Cells. Cell Stem Cell. 2015;17:116–124. doi: 10.1016/j.stem.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Copp A.J., Greene N.D. Neural tube defects--disorders of neurulation and related embryonic processes. Wiley Interdiscip. Rev. Dev. Biol. 2013;2:213–227. doi: 10.1002/wdev.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp A.J., Stanier P., Greene N.D.E. Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 2013;12:799–810. doi: 10.1016/S1474-4422(13)70110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon R.W., Parker R.M., Hendrickx A.G. Staging of the early embryonic brain in the baboon (Papio cynocephalus) and rhesus monkey (macaca mulatta) Anat. Embryol. (Berl.) 1980;159:317–334. doi: 10.1007/BF00317654. [DOI] [PubMed] [Google Scholar]
- Elkabetz Y., Panagiotakos G., Al Shamy G., Socci N.D., Tabar V., Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espuny-Camacho I., Michelsen K.A., Gall D., Linaro D., Hasche A., Bonnefont J., Bali C., Orduz D., Bilheu A., Herpoel A. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Fish J.L., Kosodo Y., Enard W., Pääbo S., Huttner W.B. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc. Natl. Acad. Sci. USA. 2006;103:10438–10443. doi: 10.1073/pnas.0604066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier-Thibault C., Blavet C., Jarov A., Bajanca F., Thorsteinsdóttir S., Duband J.L. Sonic hedgehog regulates integrin activity, cadherin contacts, and cell polarity to orchestrate neural tube morphogenesis. J. Neurosci. 2009;29:12506–12520. doi: 10.1523/JNEUROSCI.2003-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N., Nye J.S., Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Götz M., Huttner W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Hansen D.V., Lui J.H., Parker P.R.L., Kriegstein A.R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Hao H.-X., Xie Y., Zhang Y., Charlat O., Oster E., Avello M., Lei H., Mickanin C., Liu D., Ruffner H. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- Hu B.-Y., Zhang S.-C. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc. 2009;4:1295–1304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam O., Gong X., Rose-John S., Heese K. Interleukin-6 and neural stem cells: more than gliogenesis. Mol. Biol. Cell. 2009;20:188–199. doi: 10.1091/mbc.E08-05-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P., Opitz T., Steinbeck J.A., Ladewig J., Brüstle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc. Natl. Acad. Sci. USA. 2009;106:3225–3230. doi: 10.1073/pnas.0808387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno D., Shioi G., Shitamukai A., Mori A., Kiyonari H., Miyata T., Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Kriegstein A.R., Götz M. Radial glia diversity: a matter of cell fate. Glia. 2003;43:37–43. doi: 10.1002/glia.10250. [DOI] [PubMed] [Google Scholar]
- Kriegstein A., Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S., Shim J.-W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Renner M., Martin C.-A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P., Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVaute T.M., Yoo Y.D., Pankratz M.T., Weick J.P., Gerstner J.R., Zhang S.-C. Regulation of neural specification from human embryonic stem cells by BMP and FGF. Stem Cells. 2009;27:1741–1749. doi: 10.1002/stem.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Wang S., Xie Y., Lu Y., Zhang X., Wang L., Yang S., Wolf D., Zhou Q., Ji W. Homologous feeder cells support undifferentiated growth and pluripotency in monkey embryonic stem cells. Stem Cells. 2005;23:1192–1199. doi: 10.1634/stemcells.2004-0286. [DOI] [PubMed] [Google Scholar]
- Li W., Sun W., Zhang Y., Wei W., Ambasudhan R., Xia P., Talantova M., Lin T., Kim J., Wang X. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc. Natl. Acad. Sci. USA. 2011;108:8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Chen Y., Niu Y., Zhang K., Kang Y., Ge W., Liu X., Zhao E., Wang C., Lin S. TALEN-mediated gene mutagenesis in rhesus and cynomolgus monkeys. Cell Stem Cell. 2014;14:323–328. doi: 10.1016/j.stem.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.E., Cobos I., Potter G.B., Rubenstein J.L.R. Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb. Cortex. 2009;19(Suppl 1):i96–i106. doi: 10.1093/cercor/bhp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyashenko N., Winter M., Migliorini D., Biechele T., Moon R.T., Hartmann C. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat. Cell Biol. 2011;13:753–761. doi: 10.1038/ncb2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J., Simonini M.V., Palejev D., Tomasini L., Coppola G., Szekely A.M., Horvath T.L., Vaccarino F.M. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA. 2012;109:12770–12775. doi: 10.1073/pnas.1202944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt A., Eberle D., Tazaki A., Ranga A., Niesche M., Wilsch-Bräuninger M., Stec A., Schackert G., Lutolf M., Tanaka E.M. 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Reports. 2014;3:987–999. doi: 10.1016/j.stemcr.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzdorf C.S., Sive H.L. The zic1 gene is an activator of Wnt signaling. Int. J. Dev. Biol. 2006;50:611–617. doi: 10.1387/ijdb.052110cm. [DOI] [PubMed] [Google Scholar]
- Momb J., Lewandowski J.P., Bryant J.D., Fitch R., Surman D.R., Vokes S.A., Appling D.R. Deletion of Mthfd1l causes embryonic lethality and neural tube and craniofacial defects in mice. Proc. Natl. Acad. Sci. USA. 2013;110:549–554. doi: 10.1073/pnas.1211199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y., Shen B., Cui Y., Chen Y., Wang J., Wang L., Kang Y., Zhao X., Si W., Li W. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156:836–843. doi: 10.1016/j.cell.2014.01.027. [DOI] [PubMed] [Google Scholar]
- Padmanabhan N., Jia D., Geary-Joo C., Wu X., Ferguson-Smith A.C., Fung E., Bieda M.C., Snyder F.F., Gravel R.A., Cross J.C., Watson E.D. Mutation in folate metabolism causes epigenetic instability and transgenerational effects on development. Cell. 2013;155:81–93. doi: 10.1016/j.cell.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt P., Glatza M., Hemmer K., Tsytsyura Y., Thiel C.S., Höing S., Moritz S., Parga J.A., Wagner L., Bruder J.M. Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PLoS ONE. 2013;8:e59252. doi: 10.1371/journal.pone.0059252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzo E.K., Capo-Chichi J.M., Ben-Zeev B., Chitayat D., Mao H., Pappas A.L., Hitomi Y., Lu Y.F., Yao X., Hamdan F.F. Deficiency of asparagine synthetase causes congenital microcephaly and a progressive form of encephalopathy. Neuron. 2013;80:429–441. doi: 10.1016/j.neuron.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Wei Q., Zhang Y., He X., Ji W., Su B. MicroRNA profiling of rhesus macaque embryonic stem cells. BMC Genomics. 2011;12:276. doi: 10.1186/1471-2164-12-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonosaki M., Itoh K., Umekage M., Kishimoto T., Yaoi T., Lemmon V.P., Fushiki S. L1cam is crucial for cell locomotion and terminal translocation of the Soma in radial migration during murine corticogenesis. PLoS ONE. 2014;9:e86186. doi: 10.1371/journal.pone.0086186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J.B., Niswander L.A., Shaw G.M., Finnell R.H. The continuing challenge of understanding, preventing, and treating neural tube defects. Science. 2013;339:1222002. doi: 10.1126/science.1222002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Fulkerson C.M., Malek R., Ghassemifar S., Snyder P.W., Mendrysa S.M. Mutations in Lyar and p53 are synergistically lethal in female mice. Birth Defects Res. A Clin. Mol. Teratol. 2012;94:729–737. doi: 10.1002/bdra.23048. [DOI] [PubMed] [Google Scholar]
- Willardsen M.I., Link B.A. Cell biological regulation of division fate in vertebrate neuroepithelial cells. Dev. Dyn. 2011;240:1865–1879. doi: 10.1002/dvdy.22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Pak C., Han Y., Ahlenius H., Zhang Z., Chanda S., Marro S., Patzke C., Acuna C., Covy J. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The GO terms are related to Figure 7 and Figure S6.
Self-organized neural tubes by single NESCs were fixed and stained with DAPI (blue) on day 14. A single-cell-derived neural tube was scanned using a Leica TCS SP8 confocal laser scanning system. Ten neural tubes were scanned.
Live imaging of retrovirus GFP infected NESCs revealed movement of nuclei along apical and basal surfaces. Arrows mark two NESCs, in particular, with clear IKNM. Horizontal divisions (0–30°, spindle orientation) were close to the apical side in NTs. Time is shown in hr:min.