Introduction
For nearly half a century, strong associations have been made linking early adventitial activation in disease with endothelial dysfunction [1, 2], and thus challenging the notion that the luminal endothelium is the initial sensor and propagator of cardiovascular disease. Over the past two decades, perturbational studies have established a causal role of adventitial factors in this regard. This review-based update acts as a springboard for a revitalized debate of the adventitia’s role in vascular disease.
From a pathological perspective, the adventitia reportedly plays a deleterious role via production of large amounts of NADPH oxidase (Nox)-derived reactive oxygen species (ROS) in response to vascular injury and disease [3, 4]. A combined adventitial fibroblast and inflammatory cell infiltration facilitated by the vasa vasorum (VV) is expected to synergize and propagate vessel inflammation [5]. In fact, Noxes are highly activated by inflammatory cytokines, hormones, lipids and angiotensin II (AngII) [6]. In the adventitia, Nox-derived ROS is the dominant culprit for intracellular redox signaling cascade activation, either in an autocrine or paracrine manner, leading to subsequent vascular cell activation, proliferation, hypertrophy, and apoptosis and thereby promoting vascular diseases including hypertension and atherosclerosis [7]. Despite these discoveries, studies delving into the role of the adventitia in disease propagation and/or progression remain limited. This gap in knowledge presents a relatively “untapped” opportunity to study vascular disease etiology in a renewed light. This focused mini-review addresses the role of adventitial Nox-derived ROS from a perivascular viewpoint looking inwards to promoting vascular inflammation and disease.
The Adventitia, Adventitial Fibroblasts and ROS: Instigators of Vessel Inflammation
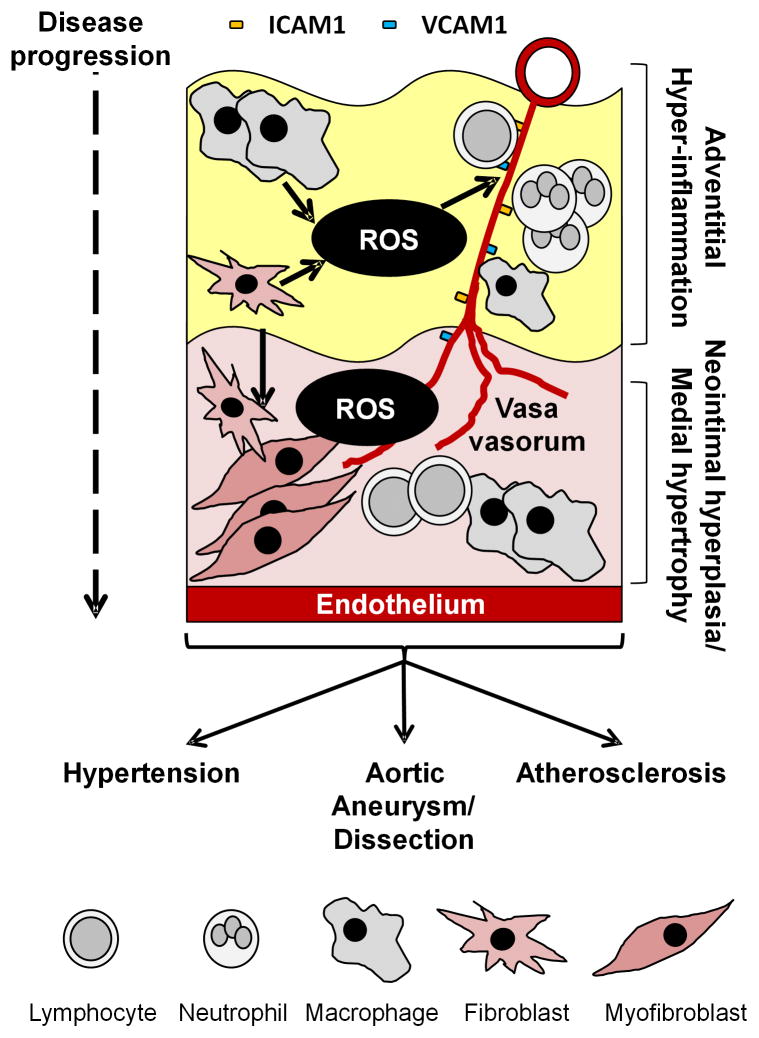
Forming the outermost layer of the vessel wall, the adventitia is emerging as a prominent channel for the progression of vascular remodeling. Within the adventitial milieu, adventitial fibroblasts/myofibroblasts, lymphocytes, stem cell-like vascular and hematopoietic lineage progenitors, and endothelial cells reside [8–10]. To date, the scientific maxim has been that vascular inflammation is initiated at the main luminal endothelial surface and progresses through the media towards the adventitia. However, this rigid hematocentric or “inside-out” view of the vasculature is slowly being debunked by another perspective of the adventitia as an integral tissue of sorts, in its own right, influencing vessel function [11]. Importantly, a plethora of evidence details the direct effects of adventitia-derived ROS on medial growth as well as ROS-dependent vascular wall inflammation in hypertension, aortic aneurysms and atherosclerosis [12]. By extension, the adventitia has profound implications for vessel tone, with adventitial fibroblasts producing substantial amounts of Nox-derived ROS which augment constriction [13, 14]. Additionally, adventitial fibroblast cell-derived cytokines (i.e. IL-1β, IL-6, and MCP-1) are implicated in fibroblast activation and recruitment of inflammatory cells to the adventitial layer which impairs vascular function [15]. Therefore, the directionality of “inside-out” vascular disease hypothesis requires careful reconsideration. Gaining traction within the literature is the notion of an adventitious (from the Latin for “coming from without”) or “outside-in” paradigm, in which the nexus of vascular wall ROS and inflammation propagates in the adventitia and spreads through the media inward toward the intima [16, 17]. Hallmarks of the “outside-in” hypothesis include increased activation and migration of fibroblasts and myofibroblasts, increased VV neovascularization, a relatively high adventitial infiltration of leukocytes, and enhanced adventitia-derived ROS levels [5, 8, 9, 18]. Together, these processes appear to exacerbate attraction and delivery of inflammatory cells to the adventitia and subsequently throughout the vessel wall. Indeed, a complex interaction between vascular cells and different classes of leukocytes (T lymphocytes, neutrophils and monocytes/macrophages) exists and supports the vascular inflammation phenotype [19]. Importantly, adventitial hyperplasia precedes medial hypertrophy and neointimal hyperplasia, and even endothelial dysfunction, in various manifestations of CVD [1, 2, 20, 21]. These findings appear to suggest that the adventitia is an “initial-sensor” and a prominent motivator of vascular phenotype change (see Figure 1).
Figure 1.
Schematic representation of the adventitious “outside-in” paradigm in cardiovascular disease. In the “outside-in” model adventitial ROS from multiple cell types propagate a rapidly growing and “leaky” vasa vasorum which provides a gateway for inflammatory cells to enter the media and intima. There leukocytes and myofibroblasts accumulate and cause local SMCs to become hypertrophic or proliferate depending on the disease milieu: hypertension, aortic aneurysm/dissection and atherosclerosis.
Nox: The Nexus of Adventitial Signaling?
Adventitial fibroblast Nox has been implicated in fibroblast function [22], as well as being the major contributor to vascular ROS in CVD [23]. Both superoxide and its metabolite hydrogen peroxide (H2O2), generated from the former by spontaneous dismutation or facilitated by the enzyme superoxide dismutase (SOD), have emerged as important players in vascular pathophysiology due to their signaling roles in a range of cellular processes, including cell proliferation, migration, hypertrophy, and apoptosis [24]. Essentially, Nox-derived ROS remain front runners of adventitial redox signaling as other ROS generating enzymes (e.g. mitochondrial complex I and xanthine oxidases) are yet to be implicated in this response.
The Nox family of enzymes consists of 7 isozymes, namely Nox1-5 and DUOX1-2 [25] with varying expression patterns throughout adventitial resident cells. Early studies in our laboratory demonstrated abundant co-localization of Nox2, p22phox, p47phox and p67phox in aortic vascular adventitia and enrichment of p67phox-dependent Nox activity in cultured adventitial fibroblasts of the rabbit aorta [3]. With regard to the other Nox isoforms and cytosolic subunits, reports illustrate expression of the adventitial fibroblast Nox1 and Nox4 oxidase systems [26, 27]. Notably, Nox4-derived H2O2 is documented to be protective in many vascular and heart disease models [28, 29]. Irrespective of the Nox isoform, fibroblast Nox activity is enhanced by a wide variety of stimuli, including hypoxia, cytokines, hormones, metabolic factors and mechanical injury [6]. As expected, adventitial leukocytes exhibit high Nox2 expression [30] and this increased leukocyte-Nox-ROS is predicted to contribute to an overall increase in local oxidant level following adventitial leukocyte accumulation, a potential result of the chronic sterile inflammasome [31]. In terms of adventitial stem cells, a potential influential role of Nox expression/activation is yet to be explored. That notwithstanding, these findings collectively predict a complex disarray of ROS-mediated signaling from the adventitia in response to various stimuli in vascular disease.
The Vasa Vasorum (VV): The New “Front Door” to Medial Inflammation?
As a result of medial hypertrophy and/or hyperplasia, pO2 levels plummet at the vessel wall core setting in motion hypoxia-dependent and -independent signaling, which include fibroblast and myofibroblast proliferation, increased ROS levels and neovascularization. Consequently, as a microvascular network of vessels luminally lined with endothelial cells and surrounded by smooth muscle cells and pericytes, the VV serves to supply oxygen and nutrients to the walls of large vessels [32]. As a result, these new microvessels are expected to facilitate chemoattraction and delivery of inflammatory cells from the adventitia across the external laminae and into the media eliciting noxious effects [11, 33].
As observed in hypertension [34] and atherosclerosis [35], the VV often becomes densely arborized and extends from the adventitial layer into the media. Indeed in atherosclerosis, the nascent VV is considered to be an immature and leaky network, therein promoting vascular inflammation and plaque progression by facilitating extravasation of leukocytes into the medial layer [36]. Moreover, many experimental studies in animal models directly link VV neovascularization with neointimal thickening [37, 38]. Whether neointimal thickening in this setting is the result of direct accumulation of leukocytes in the intima and/or an inflammation-induced smooth muscle migration is still unclear. Interestingly, a niche of adventitial-resident endothelial progenitor cells and multipotent pericytes putatively provide the “building blocks” for neovascularization [9, 39]. However, mechanisms by which these stem cell-like progenitors are mobilized from their adventitial niches and pervade other vessel layers are incompletely understood. And although yet unsubstantiated, a key role of the VV in facilitating migration of these progenitor cells to vascular sites of inflammation and injury is expected. Indeed, as vascular inflammation is known to involve chemotaxis, chemokinesis, and transmigration [33] of adventitial cells, greater consideration of the “outside-in” theory, concordant with a burgeoning VV, is required to fully comprehend neointimal hyperplasia in hypertension and atherosclerosis.
Currently, methods to visualize the VV in vivo are limited to end stage disease using either three-dimensional micro-computed tomography (micro-CT) [40], fluorescence confocal image stacking [38] or multiphoton microscopy [41]. Using micro-CT, Herrmann and colleagues observed increased density of the VV penetrating the tunica adventitia and media of large vessels as early hallmarks of vascular inflammatory disease [20]. As alluded to above, the initiating factor for increasing VV density purportedly involves decreased medial O2 levels in turn eliciting hypoxia-inducible factor-1α (HIF-1α) [34, 42] activation and fibroblast growth factor-2 (FGF2)-dependent VV stabilization [43]. Moreover, Nox-derived ROS play a central role in HIF-1α activation [44]. In this regard, Khatri and colleagues identified transgene over-expression of vascular smooth muscle cell p22phox in mice triggered oxidative stress-induced HIF-1α and enhanced atheroma progression via angiogenic switching [45]. However, while this angiogenic response may appear fortuitous in this context, it is expected to enhance plaque progression via extravasation of leukocytes following endothelial activation. Other reports identify pro-inflammatory cytokines IL-1β and TNFα involvement in VCAM-1 expression via NFkB [46]. Indeed, ROS is known to participate in endothelial adhesion molecule ICAM-1, VCAM-1 and E-selectin expression [47] and compared to wild-type mice, TNFα stimulation does not induce expression of VCAM-1 in coronary microvascular endothelial cells from p47phox null mice, implicating a role of Nox2-derived ROS in this process [48]. Moreover, treatment of endothelial cells with SOD and catalase inhibited ROS-mediated activation of NFkB [49]. Taking these findings together, one might expect a positive feedback loop involving Nox-derived ROS leading to an exaggerated accumulation of leukocytes in the adventitia and outer media. The abovementioned findings are supported by work by Cheng and colleagues who demonstrated that plaque vulnerability and growth correlated with expression of Est2 (a potent transactivator of endothelial pro-angiogenic receptors), and neovascularization [50]. In still another study, Langheinrich and colleagues correlated neovascularization with extravasation of leukocytes and plaque progression [51]. That being said, the existence of a feed-forward interaction between leukocyte-derived and adventitial parenchymal cell-derived ROS propagating VV adhesion molecule expression has not yet been confirmed. Thus, there is still much to be learned regarding adventitial fibroblast ROS, oxidative stress and VV neovascularization cross-talk.
Adventitial Nox-ROS and Vascular Disease
To date, the question of which vessel segment when activated, intima versus adventitia, is the predominant driver of vascular disease remains unanswered. The evidence referenced herein should leave little doubt that the adventitia plays a mounting role in vascular disease progression. That is, the “inside-out” hypothesis falls short of fully addressing the underpinnings for vessel remodeling and neointimal hyperplasia. Nonetheless, we consider it highly likely that both the intima and adventitia play prominent roles.
Hypertension
Hypertension affects 1:3 people in the USA, and, although therapies exist with the aim of reducing blood pressure, e.g. angiotensin converting enzyme (ACE) inhibitors, beta-blockers and diuretics [52], hypertension-induced vascular inflammation remains an intractable problem. In AngII, DOCA-salt and spontaneous hypertensive rat (SHR) models, Nox2-derived ROS remain the main culprits for adventitial redox signaling [53, 54]. Notably, adventitial fibroblast ROS is observed to play an active role in AngII-hypertension associated vessel remodeling by effecting secretion of monocyte chemoattractant protein-1 (MCP-1) and IL-6 [15, 55]. Significantly, adventitial leukocyte accumulation and vessel inflammation are observed in human hypertensive specimens and animal models [56–58] and adventitial macrophage infiltration occurs in conjunction with the development of vascular wall hypertrophy in models of AngII-induced hypertension [17]. Intriguingly, this accumulation is acknowledged to occur in the absence of significant intimal macrophage migration and it remains to be determined whether this macrophage accumulation in hypertension is synergized by “outside-in” infiltration via the VV and resident macrophage progenitor cells [39, 59].
Our laboratory discovered AngII induces p67phox-dependent adventitial ROS in the mouse [60] and this was recently corroborated by the Cowley laboratory, who showed that p67phox knockout rats display significantly lower mean arterial pressures in a salt-sensitive hypertension model compared to wild-type rats [61]. Moreover, Nox1/2 inhibition, using the non-selective VasoPharm compounds, inhibited endothelial dysfunction in SHR rat aortas, with an evident decrease in adventitial ROS and potential Nox2 expression [62]. Therefore, as adventitial Nox2 is activated in hypertension models, we hypothesized Nox2-derived ROS plays a paracrine role to induce medial hypertrophy [63]. Importantly, our group recently expanded on this notion by discovering that extracellular feed-forward ROS signaling promotes SMC hypertrophy via aquaporin 1 and Nox1 [64]. Similarly, AngII-induced vessel hypertrophy was reportedly attenuated with adventitial catalase gene transfer [65]. In aggregate, these studies implicate Nox2 as a potential therapeutic target for inhibiting adventitial fibroblast activation, medial hypertrophy and hypertension.
Aortic aneurysm/dissection
Aortic aneurysms (AAs) are a chronic vascular degenerative disease characterized by the deterioration of the aortic wall architecture leading to progressive segmental dilation and lethal risk of aortic rupture [66]. To date, limited therapeutic strategies exist for combatting AA outside of surgical repair, and much of the disease etiology is unknown. A large proportion of AA is characterized by an inflammatory response within the aortic wall which includes dramatic adventitial remodelling [67]. As with hypertension, AA pathogenesis commences with macrophage recruitment and accumulation in the adventitia; however it differs in ensuing migration and redistribution throughout the media [68]. Moreover, reports identify that other leukocytes accumulate and populate the adventitia, including neutrophils and lymphocytes [69]. Therefore, the adventitia appears to act as an efficient ‘gateway’ for leukocyte infiltration. The lethality of AA stems from acute rupture (aortic dissection) allowing luminal blood to permeate the media. In an acute model of aortic dissection, adventitial CXCL1/G-CSF expression following dissection triggered local neutrophil recruitment which exaggerated the disease [70]. This finding alone is expected to stimulate a line of inquiry into a plausible role of neutrophils in exacerbated ROS generation and dissection of the vessel wall. Furthermore, Nox2 was recently identified as a central culprit for aortic dissection in an AngII-infused ApoE-null mouse model [71]. Fan and colleagues elegantly demonstrate that increased endothelial Nox2 expression and ROS promote cyclophillin-A secretion and SMC-derived reactive species, leading to vascular inflammation and dissection. While evidence of adventitial VV permeating the medial layer is clear in AA [72], whether VV Nox2 or intimal Nox2 is the culpable endothelial source requires further inquiry. A caveat to these findings is the knowledge that global Nox2 deficiency exacerbated AngII-induced AAs [73] via increased macrophage IL-1β and MMP activity. Conversely, Meng and colleagues recently demonstrated regulatory T-lymphocytes (T-regs) prevent AngII-induced abdominal aneurysms in an ApoE-null mouse model [74]. In particular, T-regs were able to decrease aortic expression of MCP-1, IL-6 and ICAM-1, as well as reduce MMP-2 and MMP-9 expression. In fact, redox mechanisms are linked with expression of MCP-1 and ICAM-1, and activation of MMPs. Therefore, a T-reg-mediated dampening of immune response is likely to involve suppression of Nox/ROS. Further studies are thus warranted to fully appreciate the role of the adventitial VV as well as the specific classes of leukocytes which ostensibly dictate AA disease progression.
Atherosclerosis
Atherosclerosis is a multifactorial, multicellular vascular inflammatory disease. Current therapeutic strategies involve statins (lipid lowering agents) and dietary control, in addition to surgical stenting. However, modulators of vascular inflammation are conspicuously absent. Not surprisingly, therefore, increased VV vascularity is a hallmark of the disease. At this juncture, it should be clear that an attendant leukocyte infiltration would promote atheromatous plaque progression [75, 76]. In animal models, endothelial activation portends chronic inflammation and plaque progression [77] and the role of Nox2 in endothelial cell activation and dysfunction is well defined [6]. Therefore, an unrestrained neovascularization and endothelial activation in atherosclerosis is consistent with global Nox2 deficiency reducing early plaque burden and atherosclerosis [78]. That said, cell-specific Nox2 knockouts would be useful in addressing the relative and temporal contribution of endothelial dysfunction versus sterile inflammasome response in the disease [79]. The role of adventitial fibroblasts in atherosclerosis has been documented, where they are described as early activators in the disease [80] and migrate through the vessel wall as differentiated myofibroblasts. Finally, a cross-talk between adventitial cells (including progenitor cells) and SMCs in the propagation of plaques is expected to be the basis of many intriguing studies in coming years.
Concluding remarks and personal perspective
A chronic increase in adventitial activation and Nox-derived ROS has emerged as a catalyst for vascular hypertrophy and hyperplasia in vascular disease. In this regard, our perspective as vascular biologists requires an alternate and complementary view of the typical hematocentric paradigm associated with disease initiation and progression. Instead, a greater focus on the adventitia from a perivascular perspective is required to fully investigate the relative contribution of adventitial fibroblasts, vascular stem cell-like progenitors and inflammatory cells to disease progression. Inevitably, this vantage point is expected to illuminate our appreciation the adventitial VV as a prominent gateway responsible for overall vascular inflammation. In closing, surprisingly few studies have emerged over the past 5 years in Hypertension, Circulation or Circulation Research which explore the adventitia in disease aside from key discoveries documenting pools of adventitial stem cell progenitors. Thus, further investigation of the role of the adventitia will greatly improve our knowledge of vascular disease progression and, importantly, accelerate basic research to better understand the sequence of events in vascular remodeling. From a pre-clinical perspective, inhibitory agents targeting specific Nox enzymes are expected to permit dissection of the precise roles of Nox-derived ROS and form the basis for new drug therapies treating vascular inflammation.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grant, P01HL103455-04, R01HL112914 (to Dr P.J. Pagano) and by the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interests.
References
- 1.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 2.Li XD, Chen J, Ruan CC, Zhu DL, Gao PJ. Vascular endothelial growth factor-induced osteopontin expression mediates vascular inflammation and neointima formation via Flt-1 in adventitial fibroblasts. Arterioscler Thromb Vasc Biol. 2012;32:2250–2258. doi: 10.1161/ATVBAHA.112.255216. [DOI] [PubMed] [Google Scholar]
- 3.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci U S A. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovasc Res. 2007;75:679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Chan EC, Datla SR, Dilley R, Hickey H, Drummond GR, Dusting GJ. Adventitial application of the NADPH oxidase inhibitor apocynin in vivo reduces neointima formation and endothelial dysfunction in rabbits. Cardiovasc Res. 2007;75:710–718. doi: 10.1016/j.cardiores.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y, Xu Q. Adventitial biology: differentiation and function. Arterioscler Thromb Vasc Biol. 2011;31:1523–1529. doi: 10.1161/ATVBAHA.110.221176. [DOI] [PubMed] [Google Scholar]
- 9.Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–1539. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Psaltis PJ, Harbuzariu A, Delacroix S, Witt TA, Holroyd EW, Spoon DB, Hoffman SJ, Pan S, Kleppe LS, Mueske CS, Gulati R, Sandhu GS, Simari RD. Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation. 2012;125:592–603. doi: 10.1161/CIRCULATIONAHA.111.059360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. NADPH oxidases in vascular pathology. Antioxid Redox Signal. 2014;20:2794–2814. doi: 10.1089/ars.2013.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cascino T, Csanyi G, Al Ghouleh I, Montezano AC, Touyz RM, Haurani MJ, Pagano PJ. Adventitia-derived hydrogen peroxide impairs relaxation of the rat carotid artery via smooth muscle cell p38 mitogen-activated protein kinase. Antioxid Redox Signal. 2011;15:1507–1515. doi: 10.1089/ars.2010.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 15.Tieu BC, Ju X, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Brasier AR, Tilton RG. Aortic adventitial fibroblasts participate in angiotensin-induced vascular wall inflammation and remodeling. J Vasc Res. 2011;48:261–272. doi: 10.1159/000320358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tellides G, Pober JS. Inflammatory and immune responses in the arterial media. Circ Res. 2015;116:312–322. doi: 10.1161/CIRCRESAHA.116.301312. [DOI] [PubMed] [Google Scholar]
- 17.Csanyi G, Taylor WR, Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med. 2009;47:1254–1266. doi: 10.1016/j.freeradbiomed.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An SJ, Liu P, Shao TM, Wang ZJ, Lu HG, Jiao Z, Li X, Fu JQ. Characterization and functions of vascular adventitial fibroblast subpopulations. Cell Physiol Biochem. 2015;35:1137–1150. doi: 10.1159/000373939. [DOI] [PubMed] [Google Scholar]
- 19.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrmann J, Lerman LO, Rodriguez-Porcel M, Holmes DR, Jr, Richardson DM, Ritman EL, Lerman A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–766. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 21.Arribas SM, Gonzalez C, Graham D, Dominiczak AF, McGrath JC. Cellular changes induced by chronic nitric oxide inhibition in intact rat basilar arteries revealed by confocal microscopy. J Hypertens. 1997;15:1685–1693. doi: 10.1097/00004872-199715120-00073. [DOI] [PubMed] [Google Scholar]
- 22.Xu F, Liu Y, Shi L, Liu W, Zhang L, Cai H, Qi J, Cui Y, Wang W, Hu Y. NADPH oxidase p47phox siRNA attenuates adventitial fibroblasts proliferation and migration in apoE(−/−) mouse. J Transl Med. 2015;13:38. doi: 10.1186/s12967-015-0407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Niculescu R, Wang D, Patel S, Davenpeck KL, Zalewski A. Increased NAD(P)H oxidase and reactive oxygen species in coronary arteries after balloon injury. Arterioscler Thromb Vasc Biol. 2001;21:739–745. doi: 10.1161/01.atv.21.5.739. [DOI] [PubMed] [Google Scholar]
- 24.Brown DI, Griendling KK. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ Res. 2015;116:531–549. doi: 10.1161/CIRCRESAHA.116.303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol. 2014;9:119–145. doi: 10.1146/annurev-pathol-012513-104651. [DOI] [PubMed] [Google Scholar]
- 26.Haurani MJ, Cifuentes ME, Shepard AD, Pagano PJ. Nox4 oxidase overexpression specifically decreases endogenous Nox4 mRNA and inhibits angiotensin II-induced adventitial myofibroblast migration. Hypertension. 2008;52:143–149. doi: 10.1161/HYPERTENSIONAHA.107.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittal M, Roth M, Konig P, et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 28.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 29.Smyrnias I, Zhang X, Zhang M, Murray TV, Brandes RP, Schroder K, Brewer AC, Shah AM. Nicotinamide adenine dinucleotide phosphate oxidase-4-dependent upregulation of nuclear factor erythroid-derived 2-like 2 protects the heart during chronic pressure overload. Hypertension. 2015;65:547–553. doi: 10.1161/HYPERTENSIONAHA.114.04208. [DOI] [PubMed] [Google Scholar]
- 30.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 31.Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, Kopf M. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14:1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- 32.Versari D, Gossl M, Mannheim D, Daghini E, Galili O, Napoli C, Lerman LO, Lerman A. Hypertension and hypercholesterolemia differentially affect the function and structure of pig carotid artery. Hypertension. 2007;50:1063–1068. doi: 10.1161/HYPERTENSIONAHA.107.093260. [DOI] [PubMed] [Google Scholar]
- 33.Rocha SF, Schiller M, Jing D, Li H, Butz S, Vestweber D, Biljes D, Drexler HC, Nieminen-Kelha M, Vajkoczy P, Adams S, Benedito R, Adams RH. Esm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circ Res. 2014;115:581–590. doi: 10.1161/CIRCRESAHA.115.304718. [DOI] [PubMed] [Google Scholar]
- 34.Kuwahara F, Kai H, Tokuda K, Shibata R, Kusaba K, Tahara N, Niiyama H, Nagata T, Imaizumi T. Hypoxia-inducible factor-1alpha/vascular endothelial growth factor pathway for adventitial vasa vasorum formation in hypertensive rat aorta. Hypertension. 2002;39:46–50. doi: 10.1161/hy1201.097200. [DOI] [PubMed] [Google Scholar]
- 35.Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 36.Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovasc Res. 2007;75:649–658. doi: 10.1016/j.cardiores.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khurana R, Zhuang Z, Bhardwaj S, Murakami M, De Muinck E, Yla-Herttuala S, Ferrara N, Martin JF, Zachary I, Simons M. Angiogenesis-dependent and independent phases of intimal hyperplasia. Circulation. 2004;110:2436–2443. doi: 10.1161/01.CIR.0000145138.25577.F1. [DOI] [PubMed] [Google Scholar]
- 38.Drinane M, Mollmark J, Zagorchev L, Moodie K, Sun B, Hall A, Shipman S, Morganelli P, Simons M, Mulligan-Kehoe MJ. The antiangiogenic activity of rPAI-1(23) inhibits vasa vasorum and growth of atherosclerotic plaque. Circ Res. 2009;104:337–345. doi: 10.1161/CIRCRESAHA.108.184622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Psaltis PJ, Simari RD. Vascular Wall Progenitor Cells in Health and Disease. Circ Res. 2015;116:1392–1412. doi: 10.1161/CIRCRESAHA.116.305368. [DOI] [PubMed] [Google Scholar]
- 40.Wilson SH, Herrmann J, Lerman LO, Holmes DR, Jr, Napoli C, Ritman EL, Lerman A. Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation. 2002;105:415–418. doi: 10.1161/hc0402.104119. [DOI] [PubMed] [Google Scholar]
- 41.Rademakers T, Douma K, Hackeng TM, Post MJ, Sluimer JC, Daemen MJ, Biessen EA, Heeneman S, van Zandvoort MA. Plaque-associated vasa vasorum in aged apolipoprotein E-deficient mice exhibit proatherogenic functional features in vivo. Arterioscler Thromb Vasc Biol. 2013;33:249–256. doi: 10.1161/ATVBAHA.112.300087. [DOI] [PubMed] [Google Scholar]
- 42.Sano M, Sasaki T, Hirakawa S, Sakabe J, Ogawa M, Baba S, Zaima N, Tanaka H, Inuzuka K, Yamamoto N, Setou M, Sato K, Konno H, Unno N. Lymphangiogenesis and angiogenesis in abdominal aortic aneurysm. PLoS One. 2014;9:e89830. doi: 10.1371/journal.pone.0089830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mollmark JI, Park AJ, Kim J, Wang TZ, Katzenell S, Shipman SL, Zagorchev LG, Simons M, Mulligan-Kehoe MJ. Fibroblast growth factor-2 is required for vasa vasorum plexus stability in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2012;32:2644–2651. doi: 10.1161/ATVBAHA.112.252544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsushima S, Kuroda J, Ago T, Zhai P, Ikeda Y, Oka S, Fong GH, Tian R, Sadoshima J. Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia-inducible factor-1alpha and upregulation of peroxisome proliferator-activated receptor-alpha. Circ Res. 2013;112:1135–1149. doi: 10.1161/CIRCRESAHA.111.300171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation. 2004;109:520–525. doi: 10.1161/01.CIR.0000109698.70638.2B. [DOI] [PubMed] [Google Scholar]
- 46.Kim HJ, Park KG, Yoo EK, Kim YH, Kim YN, Kim HS, Kim HT, Park JY, Lee KU, Jang WG, Kim JG, Kim BW, Lee IK. Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Antioxid Redox Signal. 2007;9:301–307. doi: 10.1089/ars.2006.1456. [DOI] [PubMed] [Google Scholar]
- 47.Yan S, Zhang X, Zheng H, Hu D, Zhang Y, Guan Q, Liu L, Ding Q, Li Y. Clematichinenoside inhibits VCAM-1 and ICAM-1 expression in TNF-alpha-treated endothelial cells via NADPH oxidase-dependent IkappaB kinase/NF-kappaB pathway. Free Radic Biol Med. 2015;78:190–201. doi: 10.1016/j.freeradbiomed.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Teng L, Fan LM, Meijles D, Li JM. Divergent effects of p47(phox) phosphorylation at S303-4 or S379 on tumor necrosis factor-alpha signaling via TRAF4 and MAPK in endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:1488–1496. doi: 10.1161/ATVBAHA.112.247775. [DOI] [PubMed] [Google Scholar]
- 49.Fan C, Li Q, Ross D, Engelhardt JF. Tyrosine phosphorylation of I kappa B alpha activates NF kappa B through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J Biol Chem. 2003;278:2072–2080. doi: 10.1074/jbc.M206718200. [DOI] [PubMed] [Google Scholar]
- 50.Cheng C, Tempel D, Den Dekker WK, et al. Ets2 determines the inflammatory state of endothelial cells in advanced atherosclerotic lesions. Circ Res. 2011;109:382–395. doi: 10.1161/CIRCRESAHA.111.243444. [DOI] [PubMed] [Google Scholar]
- 51.Langheinrich AC, Michniewicz A, Sedding DG, Walker G, Beighley PE, Rau WS, Bohle RM, Ritman EL. Correlation of vasa vasorum neovascularization and plaque progression in aortas of apolipoprotein E(−/−)/low-density lipoprotein(−/−) double knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:347–352. doi: 10.1161/01.ATV.0000196565.38679.6d. [DOI] [PubMed] [Google Scholar]
- 52.Moran AE, Odden MC, Thanataveerat A, Tzong KY, Rasmussen PW, Guzman D, Williams L, Bibbins-Domingo K, Coxson PG, Goldman L. Cost-effectiveness of hypertension therapy according to 2014 guidelines. N Engl J Med. 2015;372:447–455. doi: 10.1056/NEJMsa1406751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen F, Barman S, Yu Y, Haigh S, Wang Y, Dou H, Bagi Z, Han W, Su Y, Fulton DJ. Caveolin-1 is a negative regulator of NADPH oxidase-derived reactive oxygen species. Free Radic Biol Med. 2014;73:201–213. doi: 10.1016/j.freeradbiomed.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montezano AC, Touyz RM. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid Redox Signal. 2014;20:164–182. doi: 10.1089/ars.2013.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension. 2010;55:99–108. doi: 10.1161/HYPERTENSIONAHA.109.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan CT, Moore JP, Budzyn K, Guida E, Diep H, Vinh A, Jones ES, Widdop RE, Armitage JA, Sakkal S, Ricardo SD, Sobey CG, Drummond GR. Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice. Hypertension. 2012;60:1207–1212. doi: 10.1161/HYPERTENSIONAHA.112.201251. [DOI] [PubMed] [Google Scholar]
- 58.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 59.Psaltis PJ, Puranik AS, Spoon DB, Chue CD, Hoffman SJ, Witt TA, Delacroix S, Kleppe LS, Mueske CS, Pan S, Gulati R, Simari RD. Characterization of a resident population of adventitial macrophage progenitor cells in postnatal vasculature. Circ Res. 2014;115:364–375. doi: 10.1161/CIRCRESAHA.115.303299. [DOI] [PubMed] [Google Scholar]
- 60.Cifuentes ME, Rey FE, Carretero OA, Pagano PJ. Upregulation of p67(phox) and gp91(phox) in aortas from angiotensin II-infused mice. Am J Physiol Heart Circ Physiol. 2000;279:H2234–2240. doi: 10.1152/ajpheart.2000.279.5.H2234. [DOI] [PubMed] [Google Scholar]
- 61.Evans LC, Ryan RP, Broadway E, Skelton MM, Kurth T, Cowley AW., Jr Null mutation of the nicotinamide adenine dinucleotide phosphate-oxidase subunit p67phox protects the Dahl-S rat from salt-induced reductions in medullary blood flow and glomerular filtration rate. Hypertension. 2015;65:561–568. doi: 10.1161/HYPERTENSIONAHA.114.04468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, Neff C, Shah AM, Wingler K, Schmidt HH. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension. 2010;56:490–497. doi: 10.1161/HYPERTENSIONAHA.109.149187. [DOI] [PubMed] [Google Scholar]
- 63.Liu J, Ormsby A, Oja-Tebbe N, Pagano PJ. Gene transfer of NAD(P)H oxidase inhibitor to the vascular adventitia attenuates medial smooth muscle hypertrophy. Circ Res. 2004;95:587–594. doi: 10.1161/01.RES.0000142317.88591.e6. [DOI] [PubMed] [Google Scholar]
- 64.Al Ghouleh I, Frazziano G, Rodriguez AI, Csanyi G, Maniar S, St Croix CM, Kelley EE, Egana LA, Song GJ, Bisello A, Lee YJ, Pagano PJ. Aquaporin 1, Nox1, and Ask1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc Res. 2013;97:134–142. doi: 10.1093/cvr/cvs295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu CF, Zhang J, Shen K, Gao PJ, Wang HY, Jin X, Meng C, Fang NY. Adventitial gene transfer of catalase attenuates angiotensin II-induced vascular remodeling. Mol Med Rep. 2015;11:2608–2614. doi: 10.3892/mmr.2014.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamanouchi D, Morgan S, Kato K, Lengfeld J, Zhang F, Liu B. Effects of caspase inhibitor on angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:702–707. doi: 10.1161/ATVBAHA.109.200527. [DOI] [PubMed] [Google Scholar]
- 67.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 68.Hans CP, Koenig SN, Huang N, Cheng J, Beceiro S, Guggilam A, Kuivaniemi H, Partida-Sanchez S, Garg V. Inhibition of Notch1 signaling reduces abdominal aortic aneurysm in mice by attenuating macrophage-mediated inflammation. Arterioscler Thromb Vasc Biol. 2012;32:3012–3023. doi: 10.1161/ATVBAHA.112.254219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eliason JL, Hannawa KK, Ailawadi G, Sinha I, Ford JW, Deogracias MP, Roelofs KJ, Woodrum DT, Ennis TL, Henke PK, Stanley JC, Thompson RW, Upchurch GR., Jr Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation. 2005;112:232–240. doi: 10.1161/CIRCULATIONAHA.104.517391. [DOI] [PubMed] [Google Scholar]
- 70.Anzai A, Shimoda M, Endo J, et al. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ Res. 2015;116:612–623. doi: 10.1161/CIRCRESAHA.116.304918. [DOI] [PubMed] [Google Scholar]
- 71.Fan LM, Douglas G, Bendall JK, McNeill E, Crabtree MJ, Hale AB, Mai A, Li JM, McAteer MA, Schneider JE, Choudhury RP, Channon KM. Endothelial cell-specific reactive oxygen species production increases susceptibility to aortic dissection. Circulation. 2014;129:2661–2672. doi: 10.1161/CIRCULATIONAHA.113.005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka H, Zaima N, Sasaki T, et al. Adventitial vasa vasorum arteriosclerosis in abdominal aortic aneurysm. PLoS One. 2013;8:e57398. doi: 10.1371/journal.pone.0057398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kigawa Y, Miyazaki T, Lei XF, Nakamachi T, Oguchi T, Kim-Kaneyama JR, Taniyama M, Tsunawaki S, Shioda S, Miyazaki A. NADPH oxidase deficiency exacerbates angiotensin II-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2014;34:2413–2420. doi: 10.1161/ATVBAHA.114.303086. [DOI] [PubMed] [Google Scholar]
- 74.Meng X, Yang J, Zhang K, An G, Kong J, Jiang F, Zhang Y, Zhang C. Regulatory T cells prevent angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E knockout mice. Hypertension. 2014;64:875–882. doi: 10.1161/HYPERTENSIONAHA.114.03950. [DOI] [PubMed] [Google Scholar]
- 75.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 76.Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, Biedermann BC. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 2004;110:2843–2850. doi: 10.1161/01.CIR.0000146787.16297.E8. [DOI] [PubMed] [Google Scholar]
- 77.Fotis L, Agrogiannis G, Vlachos IS, Pantopoulou A, Margoni A, Kostaki M, Verikokos C, Tzivras D, Mikhailidis DP, Perrea D. Intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 at the early stages of atherosclerosis in a rat model. In Vivo. 2012;26:243–250. [PubMed] [Google Scholar]
- 78.Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 79.Douglas G, Bendall JK, Crabtree MJ, Tatham AL, Carter EE, Hale AB, Channon KM. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE(−)/(−) mice. Cardiovasc Res. 2012;94:20–29. doi: 10.1093/cvr/cvs026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu F, Ji J, Li L, Chen R, Hu WC. Adventitial fibroblasts are activated in the early stages of atherosclerosis in the apolipoprotein E knockout mouse. Biochem Biophys Res Commun. 2007;352:681–688. doi: 10.1016/j.bbrc.2006.11.073. [DOI] [PubMed] [Google Scholar]