Abstract
Mitochondria are responsible for the majority of oxygen consumption in cells, and thus represent a conceptually appealing site for cellular oxygen sensing. Over the past 40 years a number of mechanisms to explain how mitochondria participate in oxygen sensing have been proposed. However, no consensus has been reached regarding how mitochondria could regulate transcriptional and post-translational responses to hypoxia. Nevertheless, a growing body of data continues to implicate a role for increased reactive oxygen species (ROS) signals from the electron transport chain (ETC) in triggering responses to hypoxia in diverse cell types. The present article reviews our progress understanding this field and considers recent advances that provide new insight, helping to lift the fog from this complex topic.
Introduction
Eukaryotic cells rely on the availability of nutrients and molecular oxygen (O2) to meet their metabolic and bioenergetics needs. While single cells or small clusters of cells can sustain adequate cellular O2 uptake from the environment through the process of diffusion alone, more complex organisms require both convective and diffusive transport mechanisms because diffusion by itself is not sufficient to assure reliable O2 delivery. Indeed, multicellular organisms have evolved elaborate systems to assure adequate delivery of molecular oxygen and nutrients to each cell, and they regulate that delivery in accordance with local metabolic needs. Moreover, sudden changes in metabolic demand – arising from changes in activity - dictate the need for dynamic systems that respond rapidly to meet the changing needs. Strong evolutionary pressures drive the refinement of these systems, as a failure to respond to a sudden increase in metabolic need or a change in environment can have lethal consequences. Similarly, a prolonged inability to supply adequate O2 to a respiring cell spells its doom.
Precise regulation of O2 delivery throughout an organism requires feedback control at the molecular, cellular, tissue and organismal levels. Cells face diverse stresses that challenge oxygen delivery and utilization over different spans of time. Accordingly, organisms have acquired complex oxygen sensing systems through evolution, with different time constants that enable them to respond accordingly. Thus, acute decreases in oxygen supply trigger rapid responses, while chronic changes in O2 availability engage slower mechanisms requiring transcriptional activation that need time to implement. Clearly, a response that requires cell proliferation or tissue remodeling is not likely to protect the organism from a hypoxic stress that develops over a period of seconds. On the other hand, translocation of glucose transporters to the plasma membrane to facilitate anaerobic glycolysis would not, by itself, represent an optimal response to chronic oxygen deprivation. In either case, oxygen sensors must be capable of detecting both acute and chronic changes in oxygen availability, to permit the coordinated engagement of responses with short and long time constants1. The present review considers the role of mitochondria as oxygen sensors, it assesses recent advances in this area, and provides a critical analysis of the state of this field with regard to several representative oxygen-sensitive tissues and cells.
Classical theories of mitochondrial O2 sensing mechanisms
Mitochondria are responsible for the lion’s share of oxygen consumption by cells, and thus they represent an appealing site for O2 sensing. The canonical mechanism of mitochondrial O2 sensing is based on bioenergetics and ATP supply. According to that idea, mitochondria become oxygen starved as a cell becomes hypoxic; the resulting bioenergetic deficiency then triggers an alarm and activates the response. However, from a biological perspective such a system represents a poor engineering solution. To understand why, consider that as cells consume O2 an oxygen tension gradient develops between the plasma membrane and the mitochondria. The magnitude of this gradient has been demonstrated to be in the range of 2–4 mmHg based on optical intracellular phosphorescence quenching measurements in whole cells by independent laboratories 2–4. The gradient increases as the rate of O2 consumption rises 3, but the difference in O2 tension between the extracellular space and the mitochondria remains constant as the extracellular O2 tension varies. In other words, at a given rate of O2 consumption, changes in the extracellular O2 tension will produce identical changes at the mitochondria while the difference between the two sites remains constant. For example, if the extracellular O2 tension is 20 and the gradient is 3 mmHg, mitochondrial O2 tension will be 17 mmHg. If the extracellular PO2 increases to 50 mmHg, the mitochondrial O2 tension will increase to 47 and the gradient will remain at 3. Oxygen is consumed at cytochrome oxidase, the terminal complex in the electron transport chain (ETC). The apparent Km of cytochrome oxidase is less than 2 mmHg, which allows the cell to maintain a normal level of oxygen consumption until near-anoxic conditions have been reached5. Hence, mitochondrial ATP production will remain normal until the extracellular O2 tension is close to zero. By this analysis an oxygen sensor that relies on hypoxia to limit mitochondrial ATP production would be excellent for detecting anoxia, but incapable of detecting physiological hypoxia. Indeed, a system that only detects hypoxia when anoxic conditions have been reached will not be useful in preventing that situation from developing.
To address the limitations described above, some investigators have suggested that specialized oxygen sensing systems, such as the glomus cells of the carotid body, express genetic variants of mitochondrial subunits that produce a low-affinity isoform of cytochrome oxidase6. Expression of alternative subunits that shift the Km to a higher level would cause mitochondrial electron flux and oxygen consumption to become O2-limited at more physiological levels of hypoxia7, 8. Oxidative phosphorylation would then become limited by O2 availability in response to small decreases in PO2, producing a signal in the form of decreased ATP concentration. The resulting bioenergetic deficiency would then be transduced by another process that triggers release of neurotransmitters to activate the ventilatory response to hypoxia. In support of that model, it was shown that the rate of fall in carotid body PO2 - after blood flow to a perfused carotid body was halted - tended to slow as tissue oxygen tension decreased. Those results were taken as evidence that an alternative cytochrome oxidase is expressed in the carotid body7, 9. However, other interpretations can also explain those findings. Nevertheless, such a model would dictate that glomus cells in individuals with arterial hypoxemia must exist under continuous bioenergetic stress. If the severity of hypoxia increases, the ATP deficiency would worsen yet the energy demands of the cells for neurotransmitter synthesis, release and reuptake would increase. In any case, although interest in the “low affinity cytochrome oxidase” has persisted 10 no direct evidence of such a system has emerged.
Interestingly, the normal “high affinity” binding of O2 to cytochrome oxidase reflects an “apparent” rather than a “real” enzymatic Km. In that regard, Wilson and colleagues demonstrated that the ability of mitochondria to sustain O2 consumption over a wide range of O2 tensions is achieved not by a low Km, by rather by shifts in the redox status of cytochrome c11, 12. Thus at high PO2, cytochrome c reduction state is low (highly oxidized) and O2 consumption is normal. As the PO2 decreases, cytochrome c reduction state increases, allowing O2 consumption to be preserved even though the abundance of O2 is decreased. Further increases in cytochrome c reduction continue to develop as the O2 tension decreases further, allowing O2 consumption to be sustained until O2 availability becomes critically limiting. This occurs at PO2 levels in the range of 1–3 mmHg (at which point the cytochrome c pool is fully reduced)13–15. Three corollaries arise from this mechanism. First, there is no need to propose the existence of a low affinity cytochrome, as decreases in PO2 over a wide range (100–3 mmHg) are transduced into changes in mitochondrial ETC reduction state in diverse cell types. Second, there is no need to postulate the existence of a persistent bioenergetic crisis in energy supply, as changes in ETC reduction state provide a signal indicative of PO2. Hence, rather than affecting ATP generation, this mechanism transduces changes in PO2 into changes in redox status of the ETC, which could then be used by the cell to activate hypoxia responses.
Mitochondrial ROS as signaling messengers in hypoxia
If decreases in ATP are not responsible for initiating responses to hypoxia, what signals are? One possibility is that mitochondrial reactive oxygen species (ROS) act in triggering the responses to hypoxia. ROS generation by mitochondria was first identified about 50 years ago14, 15. These reactive molecules have long been viewed as damage-inducing agents associated with cell death under pathological conditions such as ischemia-reperfusion injury, UV irradiation, or exposure to hyperoxic conditions16, 17. However, under physiological conditions ROS play important roles in regulating a wide range of cellular responses, including proliferation, differentiation, aging, transcription factor regulation, inflammation, and other regulatory functions18, 19.
The idea that ROS could act as signal transduction messengers in the oxygen sensing response is conceptually attractive. Increases in oxidant generation during hyperoxic ventilation are known to injure the lung through increased ROS generation20–22, while decreases in the generation of extracellular ROS have been reported to occur during hypoxia23. Indeed, studies that began in the 1980’s attempted to link the oxygen-sensitive reactivity of pulmonary artery and ductus arteriosus vascular smooth muscle cells to changes in oxygen radical formation24. Low oxygen levels in vivo are known to cause constriction of pulmonary artery smooth muscle cells (PASMC). High oxygen levels cause constriction of smooth muscle cells in the ductus arteriosus (DA), but cause constriction in systemic artery smooth muscle cells (SASMC). According to the original ROS model developed by Weir, Archer and colleagues, hypoxia should decrease ROS generation in PASMC and in DA23, 25, 26. This attenuation in ROS would then cause a chemically reductive modification of membrane voltage-dependent potassium (Kv) channels. In the case of PASMC, decreases in ROS cause the Kv channels to close, resulting in a decrease in membrane potential that triggers the opening of L-type Ca2+ channels, entry of extracellular Ca2+, and contraction27. In DA cells, increases in oxygen tension would increase ROS generation causing Kv channels to close, thereby leading to membrane depolarization and the associated opening of membrane L-type Ca2+ channels28, 29. Thus, according to that model the opposite effects of ROS on PASMC and DA constriction would arise from differences in their Kv channels, which close in response to low ROS in PASMC and close in response to high ROS in the DA30, 31. Unlike the pulmonary circulation, systemic vascular beds dilate in response to hypoxia. If systemic arterial smooth muscle cells (SASMC) behaved like DA cells, then tissue hypoxia would decrease ROS, causing relaxation via Kv channel opening. However, Michelakis, Archer and coworkers instead proposed that mitochondrial ROS generation occurs in opposite directions in SASMC and PASMC32. Thus, hypoxia would augment mitochondrial ROS generation in SASMC, causing Kv channels to open. By contrast, high systemic tissue PO2 would cause a decrease in mitochondrial ROS generation, which would close Kv channels and cause constriction33, 34. Hence, Kv channel sensitivity to ROS would be similar in PASMC and SASMC but opposite to that in DA, whereas mitochondria in PASMC and DA decrease ROS in response to hypoxia whereas those in SASMC increase ROS generation. This complex set of proposed mechanisms has been the topic of previous reviews33, 35–38, and is summarized in Table 1.
TABLE 1.
O2-dependent ROS regulation of Kv and L-type Ca2+ channel activity in the regulation of tone in PASMC, Ductus Arteriosus and SASMC, as proposed by Archer, Weir, Michelakis and coworkers.
| PASMC | Ductus Arteriosus | SASMC | |||
|---|---|---|---|---|---|
| Hypoxia | High PO2 | Hypoxia | High PO2 | Hypoxia | High PO2 |

|

|

|

|

|

|
| ROS Decrease | ROS Increase | ROS Decrease | ROS Increase | ROS Increase | ROS Decrease |

|

|

|

|

|

|
| Kv Closure | Kv Open | Kv Open | Kv Closure | Kv Open | Kv Closure |

|

|

|

|

|

|
| L-type Open | L-type Closure | L-type Closure | L-type Open | L-type Closure | L-type Open |

|

|

|

|

|

|
| Constriction | Relaxation | Relaxation | Constriction | Relaxation | Constriction |
See text for explanation of these responses.
In contrast with the above studies, much recent work reveals that mitochondria in diverse cell types increase, rather than decrease, ROS release to the cytosol during hypoxia39–45. This conclusion is based on work from multiple laboratories using a variety of methods for assessing intracellular ROS signaling44–50. Why should such a disagreement arise? The most likely explanation is the use of different methods to assess ROS generation. The studies by Archer and coworkers relied heavily on lucigenin – a probe that becomes chemiluminescent upon interaction with superoxide anion51, 52. Lucigenin is largely confined to the extracellular space, so their observed decreases in chemiluminescence during hypoxia suggest that extracellular release or generation of superoxide decreases under low oxygen conditions. By contrast, intracellular generation of H2O2 increases during hypoxia, and chemical or genetic sensors of intracellular H2O2 or superoxide signaling reveal small but significant and reversible increases during hypoxia, as described below43, 53–55. So it seems reasonable to conclude that at least some of the controversy regarding whether ROS generation increases or decreases during hypoxia arises because detection probes or measurement systems located in the extracellular space may be insensitive to reactive oxygen molecules generated within the cell. Indeed, studies reveal that ROS generation within cells is regulated differently among subcellular compartments, such that measurements in one compartment can differ markedly from another. As discussed below, this spatial diversity essentially invalidates the idea that “redox status” for an entire cell can be distilled down to a single number.
Redox chemistry and ROS signaling
ROS generation occurs in mitochondria when a single electron is transferred to molecular oxygen from a site along the electron transport chain or from the tricarboxylic acid (TCA) cycle. The result is superoxide, a radical that can be converted rapidly to hydrogen peroxide (H2O2) by the action of superoxide dismutase 56, 57. Superoxide is a moderately reactive molecule that is highly effective in disrupting iron-sulfur clusters associated with certain proteins. For example, superoxide can inactivate aconitase, an iron-sulfur protein that converts citrate to isocitrate. Superoxide can also react with iron in heme groups, as seen in its ability to donate its extra electron to cytochrome c. H2O2 interacts with a different class of cellular targets than superoxide and is perhaps more important as a signaling molecule because it interacts with proteins by oxidizing the thiol groups on cysteine or methionine residues58. The oxidation of cysteines can lead to the generation of inter-molecular or intra-molecular dithiol linkages, which can dramatically alter protein structure and function. H2O2 levels are kept in check by scavenging peroxidases including glutathione peroxidase, peroxiredoxins, and to some extent glutathione itself. Each of these is expressed or regulated in distinct subcellular compartments, allowing independent regulation of redox signaling in each domain. In each case, these systems depend on NADPH for their reducing ability. Catalase also degrades H2O2, but does not require NADPH. Expression of catalase is generally restricted to peroxisomes, its Km for H2O2 is relatively high, and its expression level is low in most cells. The oxidation of protein thiol groups by H2O2 can be reversed by the action of thioredoxins or glutaredoxins, small proteins that seek out oxidized protein thiols and reduce them, using reducing equivalents ultimately derived from NADPH18. Although mitochondrial ROS have the potential to induce cellular damage, for example in states such as ischemia-reperfusion injury 59–64 or other disorders 22, 65, much work indicates that mitochondrial oxidants play important physiological roles in the regulation of a variety of biological processes including the process of O2 sensing66,67, 68.
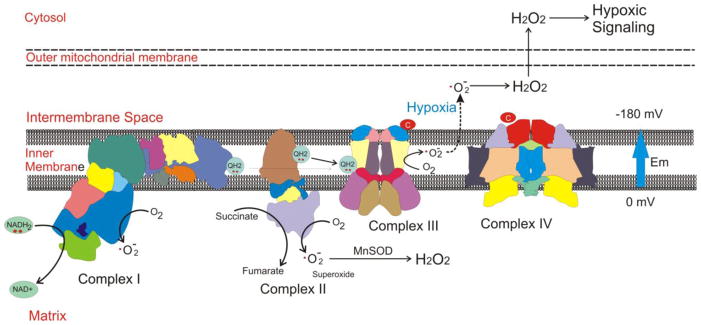
Mitochondria can generate superoxide at complex I 69–72, II 73 or III 74–80, through the escape of electrons from iron-sulfur groups, flavin-containing proteins, or from the free radical ubisemiquinone in the Q cycle of complex III (Figure 1). When generated at a site located on the matrix side of the inner mitochondrial membrane, superoxide is released into the aqueous matrix environment. However, when generated within the inner membrane itself, superoxide is ejected from the membrane into the intermembrane space. This occurs because an electrical field exists within the inner membrane – a consequence of the large transmembrane electrical gradient (−180 mV) and the narrow dimensions of the membrane (~7 nm). The electrical field occurs in a direction that tends to move anions (such as superoxide) toward the intermembrane space, and its extraordinary strength (256,000 volts/cm) provides a powerful incentive driving them out into the intermembrane space18.
Figure 1.
Mitochondrial ETC complexes can generate superoxide from multiple sites. Complexes I and II release ROS primarily to the matrix compartment, where manganese superoxide dismutatase (MnSOD) converts superoxide to hydrogen peroxide (H2O2). Complex III can generate ROS at the Q0 ubiquinone binding site within the inner membrane. Superoxide generated there is ejected into the intermembrane space by the strong electrical field in the membrane (Em) that results from the electrical gradient (−180 mV).
Pharmacological inhibitors acting at specific sites along the ETC can significantly alter ROS generation81, 82. By blocking electron transfer at a particular site, an inhibitor can increase ROS generation from loci proximal to the blockade, but decrease production distal to the site. For example, rotenone, an inhibitor that acts at the downstream end of complex I, increases superoxide production from complex I when NADH is used as a substrate, but decreases ROS generation from complex III by suppressing electron transfer into that complex69. Mitochondrial membrane potential is another important modulator of mitochondrial ROS generation. In cells where oxidative phosphorylation is operating briskly, mitochondrial membrane potential is slightly decreased as a consequence of ATP synthase activity. In that case electron flux along the ETC is increased, which tends to limit the window of time that electrons reside at sites where superoxide could be generated. By contrast, when membrane potential is high the electron flux slows because the free energy necessary for proton extrusion begins to limit electron transfer. Consequently, residence time on flavin moieties and Fe-S groups increases, amplifying the likelihood that electrons will escape to O2. In isolated mitochondria fed succinate (to supply electrons to complex II), high membrane potential can cause complex I function to reverse as electrons from complex II enter in a retrograde direction. This causes ROS generation at a high rate from complex I, an effect that can be abolished by adding rotenone to inhibit electron flux at the distal end of the complex 70.
In the ETC, electrons from complexes I and II are transferred to complex III via ubiquinone, a membrane-associated electron carrier. Two electrons are transferred to ubiquinone at quinone binding sites on complex I and complex II, generating ubiquinol that migrates to a quinol binding site (Qo) at Complex III, where the two electrons are removed sequentially. The first electron is passed to the Rieske iron-sulfur protein (RISP) subunit in Complex III, which subsequently passes the electron to cytochrome c1, to cytochrome c, and finally to cytochrome oxidase. Removal of the first electron from ubiquinol by RISP generates the transient free radical, ubisemiquinone, at the Qo site. Normally, the second electron is rapidly removed by the b cytochromes, thereby returning ubiquinone to the membrane pool. However, if the removal of the unpaired electron from ubisemiquinone is delayed, superoxide may be generated if the electron is instead captured by molecular O2. Thus, the lifetime of the ubisemiquinone at complex III represents a potential mechanism for controlling ROS generation at the Qo site. Interventions that prolong the lifetime of ubisemiquinone lead to a marked increase in superoxide formation at the Qo site. The toxin antimycin A for example prevents the removal of the second electron, thereby causing a large increase in superoxide generation at the Qo site 74.
Superoxide requires a membrane anion channel to cross lipid bilayers, whereas H2O2 can travel through aquaporins. Superoxide can react with iron-sulfur groups, heme moieties, and transition metals associated with proteins, but does not directly oxidize cysteine thiol groups 58. H2O2, by contrast, can attack nucleophilic cysteine residues, making it a powerful and reversible modifier of protein structure and function.
While this review focuses on the role of mitochondrial ROS signals in hypoxia, it is important to remember that other oxidant generating systems, such as the NADPH oxidases (Nox systems) may also participate. For example, in the response to hypoxia in the pulmonary vasculature it has been shown that Nox4 is an important contributor, possibly acting as an amplifying ROS generator 83, 84. Other hypoxia responses may also engage Nox systems 85.
Hypoxia-induced increases in mitochondrial ROS generation
While previous reports had suggested that hypoxia-induced decreases in ROS production were involved in O2 sensing 52, 86, Marshall et al. suggested the opposite: that hypoxia instead induces increases in ROS production 87. Their work using pulmonary artery cell homogenates suggested that superoxide generation increased during hypoxia – an effect that was inhibited by diphenylene iodonium, a flavoptrotein inhibitor of NADPH oxidase but not by the mitochondrial inhibitor myxothiazol, which blocks electron entry into complex III. They concluded that an NADPH oxidase system was responsible, although later studies using mice with genetic deficiency of gp91 (NOX2) effectively contradicted that interpretation88.
The first demonstration that mitochondrial ROS signals control gene transcription in hypoxia came from Chandel et al., who showed that mitochondrial electron transport function is required for stabilization of the HIF-1α transcription factor subunit, through the generation of ROS during hypoxia41, 67. These provocative studies were carried out in hepatoma and osteosarcoma cells and utilized mitochondrial inhibitors, ρ0 cells lacking a functional ETC, and ROS-sensitive fluorescent chemical probes. Those methods led others to question whether the counter-intuitive increases in ROS generation during hypoxia might represent artifacts89. To address those concerns, subsequent studies were carried out using genetically encoded ratiometric redox sensors to assess ROS signaling and genetic techniques to disrupt the mitochondrial electron transport chain. Importantly, other laboratories used complementary methods to test and confirm this hypothesis. These later studies were carried out in primary cells from diverse tissue types as well as in transformed cell lines, with remarkable similarity in responses across cell types55, 90.
Genetic studies implicating complex III in the response to hypoxia were reported by multiple groups, who assessed the role of ROS production from the ETC. Guzy et al. detected increases in cytosolic oxidation during acute hypoxia, using a FRET-based redox sensor, HSP-FRET, and observed a loss of this response in RISP-deficient cells 44. Similarly, Mansfield et al. measured ROS production in embryonic cells from mice with a genetic deletion of cytochrome c45. When cytochrome c is absent, complex III remains fully reduced, which blocks Q cycle operation and prevents ROS generation at the Qo site. As with the RISP-deficient cells, the cytochrome c-null cells lost the ability to generate an oxidant signal in the cytosol during hypoxia, and they also failed to stabilize HIF-1α. However, the same cells retained the ability to stabilize HIF-α in response to prolyl hydroxylase inhibition by DMOG, indicating that the impaired response was specific to the detection of hypoxia. These findings implicate electron flux through complex III as a critical event in the detection of hypoxia in cells.
Evidence for hypoxia-induced increases in ROS generation in the pulmonary circulation came from studies by Waypa et al., showing that hypoxic pulmonary vasoconstriction required electron flux through complex III, and that increases in ROS generation were responsible for eliciting the hypoxic response49, 55. Other studies have led to similar conclusions91–94. If mitochondria release ROS to the cytosol, an oxidant signal in the mitochondrial intermembrane space should accompany the cytosolic oxidant response. Using the thiol redox sensor roGFP targeted to the cytosol, the intermembrane space or the mitochondrial matrix, Waypa et al. compared the basal and hypoxia-induced changes in pulmonary artery smooth muscle cells (PASMC)49. The roGFP sensors are mutants of green fluorescent protein (GFP) that contain adjacent cysteine residues on the outer surface 95. Oxidation in response to H2O2 is mediated by glutaredoxin; the resulting dithiol formation alters the fluorescence properties of the protein and allows it to function as a thiol redox sensor96. Oxidation of the sensor is reversible, allowing it to be calibrated so as to yield an absolute measure of the percent oxidation96, 97. This property allows comparison of different subcellular regions or cell types.
Using PASMCs under normoxic conditions, Waypa et al. observed that cytosolic roGFP was ~20% oxidized, in the intermembrane space it was ~45% oxidized, and in the matrix compartment it was ~70% oxidized. During acute hypoxia, oxidation increased in the cytosol (to ~35%) as well as in the intermembrane space (to ~65%). However, roGFP oxidation decreased in the matrix during hypoxia. Waypa et al. observed similar results in isolated systemic arterial smooth muscle cells49. These findings reveal that hypoxia elicits an oxidative signaling response in the intermembrane space and the cytosol, consistent with the release of superoxide from the inner mitochondrial membrane. Those results also reveal that important differences in redox status can exist among subcellular compartments, with some exhibiting increases and others exhibiting decreases in oxidation during hypoxia. Finally, ROS scavenging in cultured PASMCs during hypoxia abolished the increase in Ca2+ induced by hypoxia in the cytosol, indicating that ROS signals are required for the signal transduction linking hypoxia to the contractile response in these cells 43, 53, 98.
In a subsequent study, Waypa et al. extended these findings by studying PASMC and lungs from adult mice with smooth muscle-specific deletion of the RISP gene55. In PASMC where RISP was deleted using adenoviral Cre recombinase administration, they observed a loss of hypoxia-induced changes in thiol redox in the cytosol and intermembrane space, again implicating complex III in the response to hypoxia. Using tamoxifen to activate Cre recombinase in smooth muscle cells in vivo, they found that the pulmonary vasoconstrictor response to acute hypoxia was lost, as assessed by measurements of right ventricular end-systolic pressure. These findings implicate mitochondrial complex III and ROS signaling in the oxygen sensing response underlying the acute hypoxic pulmonary vasoconstrictor response.
Phylogenetic evidence suggests that post-translational hydroxylation of proteins existed in prokaryotes prior to the evolution of mitochondria. These enzymes are related to modern-day eukaryotic HIF and collagen prolyl hydroxylases, although there is no evidence that the primordial enzymes were involved in hypoxia sensing 99. It is conceivable that the oxygen sensitivity of today’s PHD enzymes represents an evolution of these enzyme systems, arising from mutations that conferred hypoxic responsiveness to some (e.g. HIF PHDs) but not others (e.g. collagen prolyl hydroxylases).
As multiple antioxidant enzymes in cells function to scavenge and degrade ROS in different subcellular compartments, it is fair to ask why these systems allow oxidant signaling to develop or persist. In addressing this it is useful to note that multiple examples of oxidant signaling have been identified in diverse cell types 100, 101. Antioxidant systems help to regulate ROS signaling by limiting the extent of protein oxidation both spatially and temporally 102. In other cases, antioxidant enzymes facilitate ROS signaling by acting as redox relays that transmit oxidant signals from H2O2 to selective protein targets 103. For example, upon oxidation by H2O2, the antioxidant enzyme,peroixiredoxin-2 oxidizes cysteine residues in the transcription factor STAT3 leading to its activation. A similar example is seen in S. cerevisiae, where a glutathione peroxidase (Gpx3) functions as a redox relay that transduces H2O2 signals to oxidatively activate the transcription factor Yap1 104, 105. Such redox relay systems serve to increase the sensitivity to H2O2 signals while conferring greater selectivity toward the intended protein target. Hence, rather than preventing ROS signaling, antioxidant systems both refine and enhance in redox signaling in diverse biological systems.
Further evidence for complex III-derived ROS in O2 sensing
Studies evaluating the role of mitochondrial ROS generation in cellular responses to hypoxia are hindered by the linkage between mitochondrial electron transport and ROS production, oxidative phosphorylation and NAD(H) redox status in the cytosol and mitochondria. In that regard, studies using mitochondrial inhibitors or genetic deletion of components of the electron transport chain may alter ROS generation, but also affect energy production and biochemical processes coupled to NAD+ or NADP+. How can the contribution of these independent effects be mechanistically parsed? One possibility is to identify small molecule inhibitors that interact with mitochondrial proteins in a manner that inhibits ROS generation while preserving normal electron transport and energy production. Such an approach was first described by Jung et al., who screened a library of naturally occurring small molecules in search of compounds capable of suppressing the phenotypic response of endothelial cells to hypoxia106. They identified terpestacin, a small molecule that inhibited the tubular reorganization of cultured endothelial cells during hypoxia and suppressed angiogenesis in subcutaneous tumor xenografts in mice. Using phage display analysis to identify the cellular target of terpestacin binding, that group identified Uqcrb – a small molecular weight subunit of complex III – as the site of action. Subsequent studies revealed that terpestacin suppresses angiogenesis by inhibiting the generation of ROS by complex III during hypoxia. The loss of the ROS signal in turn limits the hypoxia-induced stabilization of HIF-1α, a transcriptional driver of VEGF expression and angiogenesis107. At concentrations that suppress ROS generation, terpestacin did not inhibit cellular respiration or ATP generation. This finding suggests that the loss of O2 sensing in cells or tissues after deletion of RISP or Ndufs2, or after treatment with mitochondrial inhibitors that block electron entry into complex III, are not the result of ATP deficiency or NADH redox changes. The effects of terpestacin were recapitulated by synthetic small molecules that suppress angiogenesis and ROS generation by binding to Uqcrb and inhibiting hypoxia-induced ROS generation 106. In humans, hereditary defects in Uqcrb resulting in early childhood hypoglycemia and lactic acidosis have been reported 108. Cell lines overexpressing the mutant Uqcrb exhibited increased rates of proliferation, evidence of increased mitochondrial ROS generation, and increased pro-angiogenic activities109. Thus, the mutant version of Uqcrb appeared to confer a gain-of-function with respect to ROS generation by the electron transport chain. Treatment of these cells with terpestacin inhibited these effects, indicating that they arose from alterations in complex III function and ROS generation109.
Collectively these studies suggest that the generation of ROS at complex III is regulated in part by Uqcrb. Exactly how this occurs is not clear, but it is possible that alterations in the structure of this subunit – induced by mutations or by interaction with small molecules like terpestacin – change the conformation of the overall complex in a manner that affects the escape of electrons and the formation of superoxide. Further work detailing the structure of the complex in the presence and the absence of terpestacin may shed light on precisely how alterations in the Uqcrb subunit produce this change.
Studies of terpestacin suggest that complex III controls cellular O2 sensing responses by generating ROS signals during hypoxia, and rule out the possible contributions caused by altered mitochondrial ATP generation. However, terpestacin treatment does appear to alter mitochondrial membrane potential (ΔΨ m)106. As Δ ;Ψm is critical for the generation of ROS as well as for transport of proteins, ions and small molecules across the inner membrane, it is important to understand if and how that effect could be altering the O2 sensing response. Important new insight into this question comes from recent studies by Orr et al., who used high throughput chemical screening to identify compounds capable of selectively limiting superoxide production by complex III 110. The compounds identified in the initial screen were then subjected to further screening to eliminate those that were unselective for the Qo site of complex III, or that produced any impairment of bioenergetic function or Δ ;Ψm. This analysis yielded three compounds that selectively limited superoxide and H2O2 production at the Qo site of complex III without affecting normal electron flux or bioenergetics capacity. Those investigators then assessed the ability of the three compounds to inhibit the stabilization of HIF-1α in hypoxia. Using HEK-293 cells, they found that the inhibitors significantly but incompletely attenuated HIF-1α induction110. The site(s) where these compounds bind is not yet clear and the mechanism by which superoxide generation is inhibited is not known. While it is possible they interact with Uqcrb in a way that mimics the effect of terpestacin on ROS generation, it is also possible that they act at completely different sites. In either case, further studies analyzing the structure of the purified complex in the presence and absence of these interacting molecules is likely to provide important insight into how they alter ROS generation. As pointed out by the authors, it seems likely that the binding of these compounds to complex III leads to a structural shift that limits the ability of ubisemiquinone to transfer its electron to O2. One way to achieve this would be to shorten the lifetime of the semiquinone at the Qo pocket, by increasing the rate at which the semiquinone’s electron is transferred to the b cytochromes. Such an effect would not alter flux of electrons through the complex, but would lessen the probability of electron escape to O2.
Carotid body O2 sensors and the role of mitochondria ROS
Although multiple models have been proposed to explain the mechanism of O2 sensing in the carotid body, no consensus has been reached regarding the underlying mechanisms. The small tissue mass comprising the carotid body has long precluded biochemical analysis, while the diverse mix of cells has complicated the characterization of the transcriptome in the glomus cells. Nevertheless, emerging data points to the role of mitochondria in the O2 sensitivity of those cells, through a mechanism influenced by ROS signaling. Complex I is comprised of approximately 44 subunits, and crystallization studies of a simpler version of this complex in Thermus thermophiles reveal a structure containing 64 transmembrane helices and 9 iron-sulfur clusters 111. One portion of the “L-shaped” complex resides within the inner membrane, while the other region containing the quinone binding site in proximity to the N2 Fe-S cluster extends out into the matrix compartment. Structural characteristics suggest that redox-driven conformational changes involved in electron transfer drive the translocation of 4 protons across the membrane with each cycle. Mutations in genes encoding complex I subunits have been linked to a number of neurodegenerative and cardiomyopathic diseases 112, 113. Some genetic mutations partially disrupt the function of complex I while essentially preserving its assembly, whereas other deletions prevent assembly of intact complex I. When the structure and function of complex I are disrupted, NADH oxidation and proper electron transfer/proton extrusion may become impaired, thereby inhibiting mitochondrial oxidative phosphorylation. Other genetic alterations of ETC subunits can expose FMN or Fe-S groups, increasing the generation of ROS while preserving the ability to oxidize NADH. Changes in ROS generation by the complex can potentially create oxidant stress, and may thereby disrupt oxidant-mediated signaling in the organelle or the cell.
In a recent study, Fernandez-Aguera and coworkers used conditional deletion of the Ndufs2 subunit of complex I to study the mechanisms of O2 transduction in catecholaminergic cells, including those of the carotid body114. In adult mice, expression of Cre recombinase under the control of the tyrosine hydroxylase promoter led to the cell-specific deletion of Ndufs2, a nuclear-encoded gene. Loss of Ndufs2 abolished the systemic hypoxic ventilatory response without disrupting the ventilatory response to hypercapnia, indicating that complex I function was required for hypoxic sensitivity. Despite loss of ETC function, the morphology of the glomus cells was preserved, as was cellular ATP concentration. This was surprising, because glomus cells have long been considered to have high oxygen consumption rates and to be dependent on oxidative phosphorylation. In cells lacking Ndufs2 the activity of complex I was essentially abolished, indicating that these cells can survive normally on glycolytic ATP generation alone 114. The authors did show that mitochondria isolated from Ndufs2 null cells can respire normally on succinate, and that succinate levels were increased in the intact cells. However, it is unlikely that intact cells can sustain significant mitochondrial respiration in the absence of complex I function, as the generation of succinate via oxidation of 2-oxoglutarate requires a supply of NAD+, which cannot be regenerated from NADH in the absence of complex I activity.
Electrophysiological measurements in the Ndufs2-deficient glomus cells revealed that secretory responses to high K+ or zero glucose were normal, whereas the response to hypoxia was absent. Likewise, increases in cytosolic Ca2+ in response to hypoxia were essentially abolished while the responses to K+ or zero glucose were preserved114. Perforated patch clamp studies suggested that hypoxia decreases voltage-dependent and background K+ currents in normal glomus cells, and that this response is abrogated in the Ndufs2-deficient cells. To explore the mechanism underlying this effect, they added low concentrations of N-acetyl cysteine (NAC), a thiol reductant, to the recording pipette. In the Ndufs2-deficient cells, this restored the membrane resistance to levels seen in wild type cells, although curiously it did not restore hypoxic sensitivity. By contrast, dialysis of wild type cells with H2O2 at low micromolar concentrations produced an increase in input resistance that mimicked the hypoxic response. That finding suggests that hypoxia triggers an increase in oxidative stress that mediates the closure of K+ channels, leading to Ca2+ influx and secretion of neurotransmitters. Based on the high input resistance in the knockout cells during normoxia – a condition reversed by NAC - the data suggested that disruption of complex I function caused by loss of Ndufs2 leads to a constitutive increases in ROS generation that mimics the hypoxic response in normal cells. Collectively these findings implicate ROS generation from mitochondria in triggering the inhibition of membrane K+ channels of glomus cells during hypoxia.
These interesting findings nevertheless raise new questions. Is complex I normally the source of ROS generation during hypoxia in glomus cells? In the study by Fernandez-Aguera et al. the loss of Ndufs2 blocked complex I activity and appeared to cause an increase in basal normoxic ROS generation that abolished responses to hypoxia. But loss of complex I function also abolishes electron flux into complex III, which in turn will prevent ROS generation from that site. Rotenone, a drug that inhibits the transfer of electrons at the distal end of complex I, has been shown to effectively block hypoxic sensitivity in the carotid body 115. Therefore, additional studies are needed to establish whether the normal source of ROS generation during hypoxia is complex I or complex III. A second question relates to the biophysics of ROS signaling in response to hypoxia. By virtue of its localization in the mitochondrial inner membrane, superoxide generated at complex III is ejected into the intermembrane space, where dismutation into H2O2 would allow it to reach the cytosol to participate in signaling. By contrast, one arm of complex I containing FMN and Fe-S groups extends from the membrane into the mitochondrial matrix. If superoxide is generated at those site(s) during hypoxia, it would be released into the matrix compartment instead. While it is possible that ROS generated in the matrix can leak to the cytosol, the rapid response to hypoxia elicited in the carotid body would suggest that the kinetics of the signaling system must be rapid and concise. Future studies utilizing complementary knockout models that produce different phenotypes in modifying the electron transport chain are likely to help in resolving these issues.
While the above studies provide support for the role of mitochondrial ROS in carotid body hypoxic chemosensitivity, a recent study of the olfactory receptor, Olfr78, challenges that model116. In that study, Chang et al. demonstrated that glomus cells express this olfactory receptor, and that homozygous deletion in knockout mice produced a selective loss of the ventilatory response to 10% O2 without affecting the response to 5% CO2. While measuring carotid sinus nerve activity, they found no alteration in normoxic spike frequency whereas the increased firing rate during hypoxia was abrogated in the knockouts compared to control mice. The Olfr78 receptor is sensitive is known to respond to acetate and propionate, but their studies revealed that it also responds to lactate in a dose-dependent manner, and at physiological concentrations. Collectively these findings suggest that hypoxia triggers an increase in ventilation by causing an increase in lactate signaling through the Olfr78 receptor in glomus cells. This model involves mitochondria, which normally metabolize pyruvate through the electron transport chain. As proposed, during hypoxia oxygen availability at cytochrome oxidase would begin to limit cytochrome oxidase activity, shifting the flux of pyruvate toward lactate dehydrogenase and the formation of lactic acid. In vivo, the glomus cells would presumably respond to endogenous lactate generated in the carotid body, as well as to lactate produced elsewhere in the body and transported to the glomus cells in the circulation. While intriguing, these provocative findings nevertheless raise important questions. First, why would mitochondrial oxidative phosphorylation become O2-limited at relatively mild levels of arterial hypoxiemia, given that lung ventilation begins to increase below an arterial PO2 of ~60 mmHg? Second, the model 116 would lead one to predict that loss of Ndufs2 – reported by Fernandez-Aguera et al. 114–should have caused a maximal increase in lung ventilation and carotid sinus nerve activity because the loss of complex I activity would redirect the entire pyruvate flux toward lactate. However, that response was not observed in their study. Finally, studies of isolated superfused carotid body preparations continue to show hypoxic responsiveness despite experimental conditions that should limit the ability to accumulate lactate. In any case, the findings of Chang et al. will need to be reconciled with the results of Fernandez-Aguera et al. through future studies.
Mitochondrial O2 sensing and regulation of Hypoxia-Inducible Factors (HIF)
Gene expression in hypoxia is regulated primarily by Hypoxia-Inducible Factors, HIF-1 and HIF-2. HIFs can potentially regulate many hundreds of genes, with important roles in normal physiology as well as in diseases such as cancer. HIF-1 induces expression of enzymes involved in glycolysis, glucose uptake, vascular mitogens, and genes involved in regulation of vascular tone, and metabolism. HIF-1 is required during embryonic development, and homozygous genetic deletion causes embryonic lethality 117–119.
HIF-1 and HIF-2 are critical for regulation of oxygen homeostasis at all stages of life, in both health and disease.120–122 HIF functions as a heterodimer comprised of α and β subunits 123. Under normoxic conditions HIF activity is low, but it becomes increasingly activated as oxygen levels drop below 5% O2 124. The regulation of HIF in accordance with oxygenation arises from the O2-dependent degradation of the alpha subunit. Both the α and β subunits are continuously transcribed and translated, but under normoxic conditions the α subunit is rapidly degraded. During hypoxia, degradation of the β is progressively inhibited, allowing the protein to accumulate, dimerize, and activate transcription. Proteasomal degradation of the α subunit is regulated by post-translational modification of a region of the protein known as the oxygen-dependent degradation domain (ODD) 125. Degradation is initiated by hydroxylation at proline residues in the ODD by 2-oxoglutarate-dependent hydroxylases126, 127. These HIF prolyl hydroxylases (PHDs) incorporate a free Fe2+ atom, and require 2-oxoglutarate and O2 as substrates. HIF prolyl hydroxylases are related to but distinct from the 2-oxoglutarate-dependent hydroxylases involved in collagen processing or demethylation. Although three HIF prolyl hydroxylases have been implicated in HIF regulation, PHD2 is the only one involved in the O2-dependent regulation of the α subunit 128. Genetic loss of PHD2 in mice disrupts the O2-dependent regulation of HIF-α and causes embryonic lethality 129. Hydroxylation of HIF-α enables its interaction with von Hippel Lindau protein (VHL). VHL functions as the E3 ubiquitin ligase that targets the protein for proteasomal degradation 130, 130, 131. Loss of VHL is leads to unregulated activation of HIF 132.
If ROS are released from the mitochondria to regulate HIF, they must cross the intermembrane space to reach the cytosol and the nucleus. To test that idea, Sabharwal et al. expressed peroxiredoxin-5 (Prdx5), a scavenger of H2O2, in the intermembrane space (IMS) to intercept and degrade these ROS signals before they reach the cytosol 133. Prdx5 is normally expressed in the matrix compartment but not in the IMS. To target the protein to the IMS, they used the murine presequence that directs SMAC/Diablo to the IMS. Using viral vectors to express the protein in PASMCs, they confirmed that it attenuated the hypoxia-induced increase in oxidant signaling in the cytosolic and IMS compartments. Moreover, IMS-Prdx5 abrogated the hypoxia-induced increase in cytosolic Ca2+ in these cells. Finally, it produced a dose-dependent decrease in HIF-1α stabilization during hypoxia, along with the activation of HIF-dependent gene expression. Importantly, IMS-Prdx5 did not interfere with the mitochondrial ETC or oxygen consumption in the cells 133. These findings provide alternative evidence that mitochondrial ROS signals are critical for the regulation of HIF-1α stabilization in hypoxia.
How are PHDs regulated by ROS? These hydroxylases require O2 as a substrate for degradation of HIF-α, so perhaps prolyl hydroxylase itself functions as an O2 sensor by becoming limited by oxygen availability in hypoxia. If so, then PHD2 should exhibit a Km for O2 that is compatible with its decreased activity during hypoxia 134. Studies with recombinant PHD protein demonstrate an inherently low affinity for O2, consistent with that idea. However, recombinant proteins produced in E. coli lack post-translational modifications that can affect function in mammalian cells. Hence, in vitro assays may not recapitulate the function of the enzyme under biologically relevant conditions. However, it is clear that PHDs are inactive during anoxia, so at some level these enzymes must be capable of sensing a lack of O2.
HIF transcriptional activity is also regulated by the HIF asparaginyl hydroxylase (Factor Inhibiting HIF, FIH), a member of the 2-oxoglutarate- and O2-dependent hydroxylase family. FIH hydroxylates HIF at a conserved asparagine residue near its carboxy terminus 135, 136. In normoxia, asparagine hydroxylation by FIH disrupts the interaction between HIF and the p300 transcriptional co-activator, preventing transcription of the target gene. By contrast, during hypoxia FIH activity decreases, allowing transcriptional activation of HIF-dependent genes. This mechanism acts largely as a safety switch to prevent accidental activation of hypoxia-regulated genes. FIH has been suggested to act as an oxygen sensor for the same reasons described above for PHDs 137. However, identical concerns arise regarding the applicability of in vitro measurements with recombinant proteins to recapitulate the in vivo response.
Nevertheless, hydroxylation of FIH in cells has been reported to be more sensitive to exogenous oxidants than is PHD, whereas PHD is more sensitive to hypoxia 138, 139. These observations are not inconsistent with the ROS theory of hypoxia sensing per se, as the concentrations and locations of ROS signaling during hypoxia are difficult to replicate when exogenous oxidants are applied to cells. Indeed, different human cell lines exhibit differences in HIF prolyl hydroxylation at a single level of hypoxia, underscoring the complexity of regulation in these systems 139.
Studies utilizing genetic deletion of mitochondrial electron transport complex subunits have been used to demonstrate the role of these complexes in ROS signaling. However, a secondary effect of disabling the ETC is that oxygen consumption is abolished. When this happens, intracellular PO2 rises because the O2 gradient between extracellular and intracellular oxygen tension declines. Some investigators have argued that this rise in cellular O2 tension, rather than the loss of ROS signaling, is responsible for the associated decrease in HIF activation after ETC inhibition 140. However, based on direct and indirect measurements, the magnitude of the intracellular gradient of oxygen tension is only 2–4 mmHg 2–4, 141. Hence, inhibition of oxygen consumption would only cause a small rise in intracellular PO2. Moreover, recent studies have described the ability of site-specific inhibitors of mitochondrial complex III to inhibit ROS generation and prevent hypoxic stabilization of HIF-1α. Those inhibitors do not suppress electron transfer or mitochondrial oxygen consumption, indicating that abrogation of complex III ROS generation is itself sufficient to inhibit hypoxia activation of HIF 110. Further evidence that mitochondrial ROS, rather than a decrease in oxygen consumption, is responsible for initiating hypoxic responses comes from the studies of terpestacin, and of antioxidants targeted to the intermembrane space of mitochondria 106, 133. Those studies show an attenuation of hypoxic responsiveness without an effect on O2 consumption. Collectively these results suggest that loss of oxygen consumption and a corresponding rise in cellular oxygen levels is not responsible for the results observed in the studies of mitochondrial inhibition.
How might mitochondrial ROS signals lead to the inhibition of PHD and FIH? One possibility is that the hydroxylases are post-translationally modified by redox signals. PHD2 is known to interact with other proteins 142, so it is conceivable that ROS signals could modify these protein-protein interactions and thereby affect PHD2 activity. It is also possible that ROS could modify PHD and FIH functions, either by oxidizing cysteine residues or by attacking the coordinated iron atom. 138 In the former case H2O2 would be a likely candidate that could act either directly by oxidizing a thiol group or indirectly by oxidizing an antioxidant enzyme in a redox relay system 103. Oxidation of the iron atom would also inactivate the enzyme, through either H2O2 or superoxide attack.
If ROS regulate HIF activation in hypoxia, then mitochondria-targeted antioxidants should abolish that response. MitoQ, a mitochondria-targeted antioxidant compound has such an effect 143, although questions about the effect of this compound on mitochondrial oxygen consumption have arisen.
Regulation of lifespan by hypoxia-induced mitochondrial ROS
HIF has been shown to extend the replicative lifespan in mammalian cells 144, 145. In C. elegans, the HIF homolog EGL-9 has also been shown to extend lifespan 146, although the mechanisms underlying this response are not fully clear. As hypoxia triggers EGL-9 signaling, this suggests that mitochondria-dependent O2 sensing could contribute to lifespan extending effects that could be independent of HIF. To test this, Schieber and Chandel exposed either larvae or adult C. elegans to transient hypoxia and observed a significant increase in lifespan147. Using RNAi feeding to alter gene expression in intestinal cells, they suppressed the Target of Rapamycin (TOR) homolog ET-363 and found that the hypoxia-induced lifespan extension was abolished. RHEB-1 is an upstream regulator of TORC1 signaling, and RNAi suppression of TORC1 expression similarly abolished the response to hypoxia, as did suppression of RAPTOR/DAF-15, a scaffold protein partner of TORC1. These findings indicated that HIF-independent TORC1 signaling mediates the increase in lifespan triggered by transient hypoxia in C. elegans. In further studies they found that suppression of ELT-2 – an intestine-specific GATA-type erythroid-like-2 transcription factor – also abolished the lifespan response to hypoxia. One gene regulated by ELT-2 is GSTO-1, an intestinal omega-class glutathione-S-transferase and putative antioxidant protein. Expression of GSTO-1 during hypoxia was inhibited by RNAi against TORC1 or RHEB-1, while suppression of GSTO-1 abolished the lifetime extension effect of hypoxia. How do these cells sense a decrease in O2 and link this to TOR signaling to effect metabolic adaptations and increase lifespan? The answer was suggested from long-lived mitochondrial mutant worms that generate increased basal levels of ROS and activate HIF-1-dependent increases in lifespan 148. To test whether ROS signals were required for the TORC1-dependent lifespan extension after transient hypoxia, worms were treated with the antioxidant BHA, which attenuated the longevity response to hypoxia. Finally, addition of the ROS-generating compound paraquat was sufficient to confer a lifespan extension that was blocked by RNAi against the TORC1 components RHEB-1 and TOR, or the intestinal transcription factor ELT-2. Collectively these results indicate that hypoxia-induced ROS signaling activates a TOR-dependent upregulation of the ELT-2 transcriptome, leading to upregulated expression of antioxidant proteins including GSTO-1, resulting in lifespan extension.
Summary
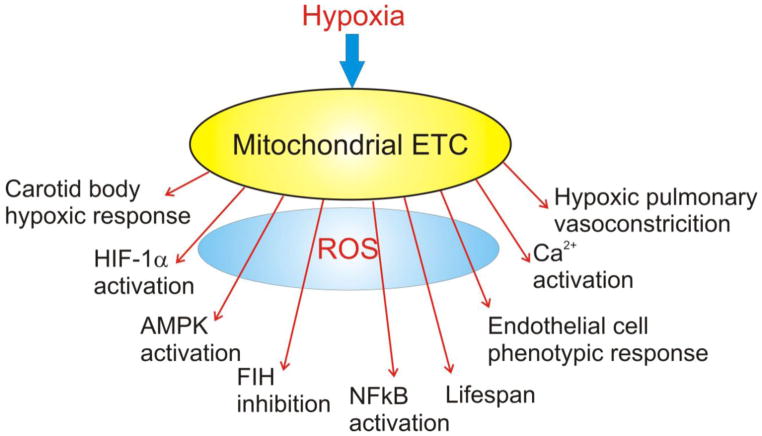
Cellular O2 sensing is an important biological process, and mitochondria have been implicated in the activation of diverse cellular responses to hypoxia (Figure 2). Mitochondria signal the onset of hypoxia through the generation of ROS signals by the ETC. When released to the IMS, these signals can escape to the cytosol where they participate in thiol redox signaling involved in the activation of transcription factors and the initiation of post-translational responses. A wide range of biological processes have been linked to hypoxia-induced release of ROS signals including carotid body O2 chemotransduction, hypoxic pulmonary vasoconstriction, HIF-1α activation, lifespan regulation, proliferation, differentiation and inflammatory processes. While controversies in the field still exist, emerging work is shedding new light on the mechanisms of mitochondrial O2 sensing, which is important in both health and disease.
Figure 2.
Mitochondria regulate diverse responses to hypoxia through the release of ROS signals to the cytosol.
Acknowledgments
NIH Grants HL35440 and HL122062
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schumacker PT. Cellular and molecular mechanisms of O2 sensing. In: Swensen ER, Bärtsch P, editors. High altitude: Human adaptation to hypoxia. 1. New York: Springer; 2014. pp. 1–22. [Google Scholar]
- 2.Koo YE, Cao Y, Kopelman R, Koo SM, Brasuel M, Philbert MA. Real-time measurements of dissolved oxygen inside live cells by organically modified silicate fluorescent nanosensors. Anal Chem. 2004 May 1;76(9):2498–505. doi: 10.1021/ac035493f. [DOI] [PubMed] [Google Scholar]
- 3.Mik EG, Stap J, Sinaasappel M, Beek JF, Aten JA, van Leeuwen TG, Ince C. Mitochondrial PO2 measured by delayed fluorescence of endogenous protoporphyrin IX. Nat Methods. 2006 Nov;3(11):939–45. doi: 10.1038/nmeth940. [DOI] [PubMed] [Google Scholar]
- 4.Hogan MC. Phosphorescence quenching method for measurement of intracellular Po2 in isolated skeletal muscle fibers. J Appl Physiol. 1999;86(2):720–4. doi: 10.1152/jappl.1999.86.2.720. [DOI] [PubMed] [Google Scholar]
- 5.Chandel NS, Budinger GRS, Choe SH, Schumacker PT. Cellular respiration during hypoxia: Role of cytochrome oxidase as the oxygen sensor in hepatocytes. J Biol Chem. 1997;272(1):111–2. doi: 10.1074/jbc.272.30.18808. [DOI] [PubMed] [Google Scholar]
- 6.Mills E, Jobsis FF. Mitochondrial respiratory chain of carotid body and chemoreceptor response to changes in oxygen tension. J Neurophysiol. 1972 Jul;35(4):405–28. doi: 10.1152/jn.1972.35.4.405. [DOI] [PubMed] [Google Scholar]
- 7.Buerk DG, Nair PK, Whalen WJ. Two-cytochrome metabolic model for carotid body PtiO2 and chemosensitivity changes after hemorrhage. J Appl Physiol. 1989 Jul;67(1):60–8. doi: 10.1152/jappl.1989.67.1.60. [DOI] [PubMed] [Google Scholar]
- 8.Nair PK, Buerk DG, Whalen WJ, Schubert RW. Two cytochrome oxygen consumption model and mechanism for carotid body chemoreception. Adv Exp Med Biol. 1986;200:293–300. doi: 10.1007/978-1-4684-5188-7_36. [DOI] [PubMed] [Google Scholar]
- 9.Buerk DG, Nair PK, Whalen WJ. Evidence for second metabolic pathway for O2 from PtiO2 measurements in denervated cat carotid body. J Appl Physiol. 1989 Oct;67(4):1578–84. doi: 10.1152/jappl.1989.67.4.1578. [DOI] [PubMed] [Google Scholar]
- 10.Buckler KJ, Turner PJ. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J Physiol. 2013 Jul 15;591(Pt 14):3549–63. doi: 10.1113/jphysiol.2013.257741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson DF, Rumsey WL, Green TJ, Vanderkooi JM. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J Biol Chem. 1988;263:2712–8. [PubMed] [Google Scholar]
- 12.Wilson DF, Mokashi A, Chugh D, Vinogradov S, Osanai S, Lahiri S. The primary oxygen sensor of the cat carotid body is cytochrome a3 of the mitochondrial respiratory chain. FEBS Lett. 1994 Sep 12;351(3):370–4. doi: 10.1016/0014-5793(94)00887-6. [DOI] [PubMed] [Google Scholar]
- 13.Rumsey WL, Schlosser C, Nuutinen EM, Robiolio M, Wilson DF. Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J Biol Chem. 1990;265:15392–9. [PubMed] [Google Scholar]
- 14.Jensen PK. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-tranport particles. Biochim Biophys Acta. 1966;122:157–66. doi: 10.1016/0926-6593(66)90057-9. [DOI] [PubMed] [Google Scholar]
- 15.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128(3):617–30. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL, McCord JM, Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987;107:526–45. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 17.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;3:159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 18.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat Rev Cancer. 2014 Oct 24;14(11):709–21. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012 Oct 26;48(2):158–67. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berkelhamer SK, Kim GA, Radder JE, Wedgwood S, Czech L, Steinhorn RH, Schumacker PT. Developmental differences in hyperoxia-induced oxidative stress and cellular responses in the murine lung. Free Radic Biol Med. 2013 Mar 14; doi: 10.1016/j.freeradbiomed.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrow KN, Lee KJ, Perez M, Schriewer JM, Wedgwood S, Lakshminrusimha S, Smith CL, Steinhorn RH, Schumacker PT. Brief hyperoxia increases mitochondrial oxidation and increases phosphodiesterase 5 activity in fetal pulmonary artery smooth muscle cells. Antioxid Redox Signal. 2012 Aug 1;17(3):460–70. doi: 10.1089/ars.2011.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256(21):10986–92. [PubMed] [Google Scholar]
- 23.Michelakis ED, Archer SL, Weir EK. Acute hypoxic pulmonary vasoconstriction: a model of oxygen sensing. Physiol Res. 1995;44(6):361–7. [PubMed] [Google Scholar]
- 24.Archer SL, Peterson D, Nelson DP, DeMaster EG, Kelly B, Eaton JW, Weir EK. Oxygen radicals and antioxidant enzymes alter pulmonary vascular reactivity in the rat lung. J Appl Physiol. 1989;66:102–11. doi: 10.1152/jappl.1989.66.1.102. [DOI] [PubMed] [Google Scholar]
- 25.Weir EK, Archer SL. The mechanism of acute hypoxic pulmonary vasoconstriction: a tale of two channels. FASEB J. 1995;9:183–9. doi: 10.1096/fasebj.9.2.7781921. [DOI] [PubMed] [Google Scholar]
- 26.Reeve HL, Weir EK, Nelson DP, Peterson DA, Archer SL. Opposing effects of oxidants and antioxidants on K+ channel activity and tone in rat vascular tissue. Exp Physiol. 1995 Sep;80(5):825–34. doi: 10.1113/expphysiol.1995.sp003890. [DOI] [PubMed] [Google Scholar]
- 27.Weir EK, Reeve HL, Peterson DA, Michelakis ED, Nelson DP, Archer SL. Pulmonary vasoconstriction, oxygen sensing, and the role of ion channels - Thomas A. Neff Lecture. Chest. 1998 Jul;114(1):17S–22S. doi: 10.1378/chest.114.1_supplement.17s-a. [DOI] [PubMed] [Google Scholar]
- 28.Reeve HL, Tolarova S, Nelson DP, Archer S, Weir EK. Redox control of oxygen sensing in the rabbit ductus arteriosus. J Physiol (Lond ) 2001 May 15;533(1):253–61. doi: 10.1111/j.1469-7793.2001.0253b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michelakis ED, Rebeyka I, Wu X, Nsair A, Thebaud B, Hashimoto K, Dyck JR, Haromy A, Harry G, Barr A, Archer SL. O2 sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res. 2002 Sep 20;91(6):478–86. doi: 10.1161/01.res.0000035057.63303.d1. [DOI] [PubMed] [Google Scholar]
- 30.Weir EK, Archer SL. The role of redox changes in oxygen sensing. Respir Physiol Neurobiol. 2010 Dec 31;174(3):182–91. doi: 10.1016/j.resp.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Archer S, Michelakis E. The mechanism(s) of hypoxic pulmonary vasoconstriction: potassium channels, redox O(2) sensors, and controversies. News Physiol Sci. 2002 Aug;17:131–7. doi: 10.1152/nips.01388.2002. [DOI] [PubMed] [Google Scholar]
- 32.Michelakis ED, Hampl V, Nsair A, Wu XC, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res. 2002 Jun 28;90(12):1307–15. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- 33.Bonnet S, Archer SL. Potassium channel diversity in the pulmonary arteries and pulmonary veins: implications for regulation of the pulmonary vasculature in health and during pulmonary hypertension. Pharmacol Ther. 2007 Jul;115(1):56–69. doi: 10.1016/j.pharmthera.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Mechanisms of disease - Acute oxygen-sensing mechanisms. N Engl J Med. 2005 Dec 10;353(19):2042–55. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunham-Snary KJ, Hong ZG, Xiong PY, Del Paggio JC, Herr JE, Johri AM, Archer SL. A mitochondrial redox oxygen sensor in the pulmonary vasculature and ductus arteriosus. Pflugers Arch. 2015 Sep 23; doi: 10.1007/s00424-015-1736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 37.Moudgil R, Michelakis ED, Archer SL. The role of k+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation, and apoptosis: implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension. Microcirculation. 2006 Dec;13(8):615–32. doi: 10.1080/10739680600930222. [DOI] [PubMed] [Google Scholar]
- 38.Archer SL, Michelakis ED, Thebaud B, Bonnet S, Moudgil R, Wu XC, Weir EK. A central role for oxygen-sensitive K+ channels and mitochondria in the specialized oxygen-sensing system. Novartis Found Symp. 2006;272:157–71. [PubMed] [Google Scholar]
- 39.Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem. 1998;273(19):11619–24. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- 40.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–8. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 41.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial Complex III stabilize HIF-1-alpha during hypoxia: A mechanism of O2 sensing. J Biol Chem. 2000 May 31;275:25130–8. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 42.Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of Oxidants in NF-kappaB Activation and TNF-alpha Gene Transcription Induced by Hypoxia and Endotoxin. J Immunol. 2000 Jul 15;165(2):1013–21. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 43.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002 Oct 18;91(8):719–26. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 44.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005 Jun;1(6):401–8. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005 Jun;1(6):393–9. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearlstein DP, Ali MH, Mungai PT, Hynes KL, Gewertz BL, Schumacker PT. Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia. Arterioscler Thromb Vasc Biol. 2002;22(4):566–73. doi: 10.1161/01.atv.0000012262.76205.6a. [DOI] [PubMed] [Google Scholar]
- 47.Guzy RD, Mack MM, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and gene transcription in yeast. Antioxid Redox Signal. 2007 Sep;9(9):1317–28. doi: 10.1089/ars.2007.1708. [DOI] [PubMed] [Google Scholar]
- 48.Lebuffe G, Schumacker PT, Shao ZH, Anderson T, Iwase H, Vanden Hoek TL. ROS and NO trigger early preconditioning: relationship to mitochondrial KATP channel. Am J Physiol Heart Circ Physiol. 2003;284(1):H299–H308. doi: 10.1152/ajpheart.00706.2002. [DOI] [PubMed] [Google Scholar]
- 49.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia Triggers Subcellular Compartmental Redox Signaling in Vascular Smooth Muscle Cells. Circ Res. 2010 Feb 2;106(3):526–35. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao JP, Zhou ZG, Hu HL, Guo Z, Wang T, Zhen GH, Zhang ZX. The relationships among reactive oxygen species, hypoxia-inducible factor 1alpha and cell proliferation in rat pulmonary arterial smooth muscle cells under hypoxia. Sheng Li Xue Bao. 2007 Jun 25;59(3):319–24. [PubMed] [Google Scholar]
- 51.Archer SL, Nelson DP, Weir EK. Simultaneous measurement of O2 radicals and pulmonary vascular reactivity in rat lung. J Appl Physiol (1985 ) 1989 Nov;67(5):1903–11. doi: 10.1152/jappl.1989.67.5.1903. [DOI] [PubMed] [Google Scholar]
- 52.Archer SL, Nelson DP, Weir EK. Detection of activated O2 species in vitro and in rat lungs by chemiluminescence. J Appl Physiol (1985 ) 1989 Nov;67(5):1912–21. doi: 10.1152/jappl.1989.67.5.1912. [DOI] [PubMed] [Google Scholar]
- 53.Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006 Oct 27;99(9):970–8. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- 54.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res. 2010 Feb 19;106(3):526–35. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, Schumacker PT. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med. 2013 Feb 15;187(4):424–32. doi: 10.1164/rccm.201207-1294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, Nystrom T, Belousov V, Schumacker PT, Winterbourn CC. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011 Apr 6;13(4):361–6. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009 Jan 1;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brigelius-Flohe R, Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal. 2011 Oct 15;15(8):2335–81. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem. 1993 Sep 5;268(25):18532–41. [PubMed] [Google Scholar]
- 60.Becker LB, Vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999 Dec;277(6 Pt 2):H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 61.Di Lisa F, Menabo R, Canton M, Petronilli V. The role of mitochondria in the salvage and the injury of the ischemic myocardium. Biochim Biophys Acta. 1998 Aug 10;1366(1–2):69–78. doi: 10.1016/s0005-2728(98)00121-2. [DOI] [PubMed] [Google Scholar]
- 62.Ferrari R. The role of mitochondria in ischemic heart disease. J Cardiovasc Pharmacol. 1996;28( Suppl 1):S1–10. doi: 10.1097/00005344-199600003-00002. [DOI] [PubMed] [Google Scholar]
- 63.Turrens JF, Beconi M, Barilla J, Chavez UB, McCord JM. Mitochondrial generation of oxygen radicals during reoxygenation of ischemic tissues. Free Radical Res Commun. 1991;12–13(Pt 2):681–9. doi: 10.3109/10715769109145847. [DOI] [PubMed] [Google Scholar]
- 64.Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29:2571–83. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- 65.Shimada H, Hirai K, Simamura E, Pan J. Mitochondrial NADH-quinone oxidoreductase of the outer membrane is responsible for paraquat cytotoxicity in rat livers. Arch Biochem Biophys. 1998 Mar 1;351(1):75–81. doi: 10.1006/abbi.1997.0557. [DOI] [PubMed] [Google Scholar]
- 66.Boveris A, Cadenas E. Mitochondrial production of hydrogen peroxide regulation by nitric oxide and the role of ubisemiquinone. IUBMB Life. 2000;50(4–5):245–50. doi: 10.1080/713803732. [DOI] [PubMed] [Google Scholar]
- 67.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA. 1998;95:11715–20. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chandel NS, Schumacker PT. Cellular oxygen sensing by mitochondria: old questions, new insight. J Appl Physiol. 2000 May;88(5):1880–9. doi: 10.1152/jappl.2000.88.5.1880. [DOI] [PubMed] [Google Scholar]
- 69.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–7. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Votyakova TV, Reynolds IJ. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001 Oct;79(2):266–77. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 71.Genova ML, Ventura B, Giuliano G, Bovina C, Formiggini G, Castelli GP, Lenaz G. The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron-sulfur cluster N2. FEBS Lett. 2001 Sep 21;505(3):364–8. doi: 10.1016/s0014-5793(01)02850-2. [DOI] [PubMed] [Google Scholar]
- 72.Li Y, Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun. 1998 Dec 18;253(2):295–9. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- 73.Misra HP, Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972;247:188–92. [PubMed] [Google Scholar]
- 74.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–14. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 75.Garcia-Ruiz C, Colell A, Morales A, Kaplowitz N, Fernandez-Checa JC. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: studies with isolated mitochondria and rat hepatocytes. Molecular Pharmacology. 1995 Nov;48(5):825–34. [PubMed] [Google Scholar]
- 76.Kwong LK, Sohal RS. Substrate and site specificity of hydrogen peroxide generation in mouse mitochondria. Arch Biochem Biophys. 1998;350:118–26. doi: 10.1006/abbi.1997.0489. [DOI] [PubMed] [Google Scholar]
- 77.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997 Apr 25;272(17):11369–77. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 78.Quillet-Mary A, Jaffrezou JP, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J Biol Chem. 1997 Aug 22;272(34):21388–95. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- 79.Gille L, Nohl H. The ubiquinol/bc1 redox couple regulates mitochondrial oxygen radical formation. Arch Biochem Biophys. 2001 Apr 1;388(1):34–8. doi: 10.1006/abbi.2000.2257. [DOI] [PubMed] [Google Scholar]
- 80.Zhang L, Yu LD, Yu CA. Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. J Biol Chem. 1998 Dec 18;273(51):33972–6. doi: 10.1074/jbc.273.51.33972. [DOI] [PubMed] [Google Scholar]
- 81.Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–12. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orr AL, Ashok D, Sarantos MR, Shi T, Hughes RE, Brand MD. Inhibitors of ROS production by the ubiquinone-binding site of mitochondrial complex I identified by chemical screening. Free Radic Biol Med. 2013 Dec;65:1047–59. doi: 10.1016/j.freeradbiomed.2013.08.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fuchs B, Sommer N, Dietrich A, Schermuly RT, Ghofrani HA, Grimminger F, Seeger W, Gudermann T, Weissmann N. Redox signaling and reactive oxygen species in hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol. 2010 Dec 31;174(3):282–91. doi: 10.1016/j.resp.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 84.Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, Hanze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal. 2008 Oct;10(10):1687–98. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- 85.Thannickal VJ. Oxygen in the evolution of complex life and the price we pay. Am J Respir Cell Mol Biol. 2009 May;40(5):507–10. doi: 10.1165/rcmb.2008-0360PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paky A, Michael JR, Burke-Wolin TM, Wolin MS, Gurtner GH. Endogenous production of superoxide by rabbit lungs: effects of hypoxia or metabolic inhibitors. J Appl Physiol. 1993 Jun;74(6):2868–74. doi: 10.1152/jappl.1993.74.6.2868. [DOI] [PubMed] [Google Scholar]
- 87.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Resp Cell Molec Biol. 1996;15:633–44. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- 88.Archer SL, Reeve HL, Michelakis E, Puttagunta L, Waite R, Nelson DP, Dinauer MC, Weir EK. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc Natl Acad Sci U S A. 1999;96:7944–9. doi: 10.1073/pnas.96.14.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vaux EC, Metzen E, Yeates KM, Ratcliffe PJ. Regulation of hypoxia-inducible factor is preserved in the absence of a functioning mitochondrial respiratory chain. Blood. 2001 Jul 15;98(2):296–302. doi: 10.1182/blood.v98.2.296. [DOI] [PubMed] [Google Scholar]
- 90.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011 Sep;31(17):3531–45. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gillespie MN, Pastukh VM, Ruchko MV. Controlled DNA “damage” and repair in hypoxic signaling. Respir Physiol Neurobiol. 2010 Dec 31;174(3):244–51. doi: 10.1016/j.resp.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang QS, Zheng YM, Dong L, Ho YS, Guo Z, Wang YX. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med. 2007 Mar 1;42(5):642–53. doi: 10.1016/j.freeradbiomed.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rathore R, Zheng YM, Li XQ, Wang QS, Liu QH, Ginnan R, Singer HA, Ho YS, Wang YX. Mitochondrial ROS-PKCepsilon signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochem Biophys Res Commun. 2006 Dec 22;351(3):784–90. doi: 10.1016/j.bbrc.2006.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang YX, Zheng YM. Role of ROS signaling in differential hypoxic Ca2+ and contractile responses in pulmonary and systemic vascular smooth muscle cells. Respir Physiol Neurobiol. 2010 Dec 31;174(3):192–200. doi: 10.1016/j.resp.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Remington SJ. Fluorescent proteins: maturation, photochemistry and photophysics. Curr Opin Struct Biol. 2006 Dec;16(6):714–21. doi: 10.1016/j.sbi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 96.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem. 2004 Mar 26;279(13):13044–53. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 97.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004 May 21;279(21):22284–93. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 98.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001 Jun 22;88(12):1259–66. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 99.Scotti JS, Leung IK, Ge W, Bentley MA, Paps J, Kramer HB, Lee J, Aik W, Choi H, Paulsen SM, Bowman LA, Loik ND, Horita S, Ho CH, Kershaw NJ, Tang CM, Claridge TD, Preston GM, McDonough MA, Schofield CJ. Human oxygen sensing may have origins in prokaryotic elongation factor Tu prolyl-hydroxylation. Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):13331–6. doi: 10.1073/pnas.1409916111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu HJ, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005 Nov 11;97(10):967–74. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 101.Finkel T. Signal transduction by mitochondrial oxidants. J Biol Chem. 2012 Feb 10;287(7):4434–40. doi: 10.1074/jbc.R111.271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bae YS, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells. 2011 Dec;32(6):491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rhee SG, Woo HA, Kil IS, Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem. 2012 Feb 10;287(7):4403–10. doi: 10.1074/jbc.R111.283432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002 Nov 15;111(4):471–81. doi: 10.1016/s0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 105.Azevedo D, Tacnet F, Delaunay A, Rodrigues-Pousada C, Toledano MB. Two redox centers within Yap1 for H2O2 and thiol-reactive chemicals signaling. Free Radic Biol Med. 2003 Oct 15;35(8):889–900. doi: 10.1016/s0891-5849(03)00434-9. [DOI] [PubMed] [Google Scholar]
- 106.Jung HJ, Shim JS, Lee J, Song YM, Park KC, Choi SH, Kim ND, Yoon JH, Mungai PT, Schumacker PT, Kwon HJ. Terpestacin inhibits tumor angiogenesis by targeting UQCRB of mitochondrial complex III and suppressing hypoxia-induced reactive oxygen species production and cellular oxygen sensing. J Biol Chem. 2010 Apr 9;285(15):11584–95. doi: 10.1074/jbc.M109.087809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cho YS, Jung HJ, Seok SH, Payumo AY, Chen JK, Kwon HJ. Functional inhibition of UQCRB suppresses angiogenesis in zebrafish. Biochem Biophys Res Commun. 2013 Apr 19;433(4):396–400. doi: 10.1016/j.bbrc.2013.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haut S, Brivet M, Touati G, Rustin P, Lebon S, Garcia-Cazorla A, Saudubray JM, Boutron A, Legrand A, Slama A. A deletion in the human QP-C gene causes a complex III deficiency resulting in hypoglycaemia and lactic acidosis. Hum Genet. 2003 Jul;113(2):118–22. doi: 10.1007/s00439-003-0946-0. [DOI] [PubMed] [Google Scholar]
- 109.Chang J, Jung HJ, Jeong SH, Kim HK, Han J, Kwon HJ. A mutation in the mitochondrial protein UQCRB promotes angiogenesis through the generation of mitochondrial reactive oxygen species. Biochem Biophys Res Commun. 2014 Dec 12;455(3–4):290–7. doi: 10.1016/j.bbrc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 110.Orr AL, Vargas L, Turk CN, Baaten JE, Matzen JT, Dardov VJ, Attle SJ, Li J, Quackenbush DC, Goncalves RL, Perevoshchikova IV, Petrassi HM, Meeusen SL, Ainscow EK, Brand MD. Suppressors of superoxide production from mitochondrial complex III. Nat Chem Biol. 2015 Nov;11(11):834–6. doi: 10.1038/nchembio.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baradaran R, Berrisford JM, Minhas GS, Sazanov LA. Crystal structure of the entire respiratory complex I. Nature. 2013 Feb 28;494(7438):443–8. doi: 10.1038/nature11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Russell LK, Finck BN, Kelly DP. Mouse models of mitochondrial dysfunction and heart failure. J Mol Cell Cardiol. 2005;38(1):81–91. doi: 10.1016/j.yjmcc.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 113.Schapira AH. Human complex I defects in neurodegenerative diseases. Biochim Biophys Acta. 1998 May 6;1364(2):261–70. doi: 10.1016/s0005-2728(98)00032-2. [DOI] [PubMed] [Google Scholar]
- 114.Fernandez-Aguera MC, Gao L, Gonzalez-Rodriguez P, Pintado CO, rias-Mayenco I, Garcia-Flores P, Garcia-Perganeda A, Pascual A, Ortega-Saenz P, Lopez-Barneo J. Oxygen Sensing by Arterial Chemoreceptors Depends on Mitochondrial Complex I Signaling. Cell Metab. 2015 Nov 3;22(5):825–37. doi: 10.1016/j.cmet.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 115.Ortega-Saenz P, Pardal R, Fernandez MG, Lopez-Barneo J. Rotenone selectively occludes sensitivity to hypoxia in rat carotid body glomus cells. J Physiol (Lond ) 2003 May 1;548(3):789–800. doi: 10.1113/jphysiol.2003.039693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chang AJ, Ortega FE, Riegler J, Madison DV, Krasnow MA. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature. 2015 Nov 12;527(7577):240–4. doi: 10.1038/nature15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia- inducible factor 1 alpha. Genes Dev. 1998 Jan 15;12(2):149–62. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997 Mar 27;386(6623):403–7. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 119.Maltepe E, Simon MC. Oxygen, genes, and development: An analysis of the role of hypoxic gene regulation during murine vascular development. J Mol Med. 1998 May;76(6):391–401. doi: 10.1007/s001090050231. [DOI] [PubMed] [Google Scholar]
- 120.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011 Jan 15;183(2):152–6. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010 May;2(3):336–61. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 122.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010 Feb 4;29(5):625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang GL, Jiang B-H, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix- PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996 Oct;271(4 Pt 1):C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 125.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998 Jul 7;95(14):7987–92. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001 Apr 20;292(5516):464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 127.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001 Apr 20;292(5516):468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 128.Semenza GL. HIF-1, O2, and the 3 PHDs: How animal cells signal hypoxia to the nucleus. Cell. 2001 Oct 5;107(1):1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 129.Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein. Mol Cell Biol. 2006 Nov;26(22):8336–46. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 131.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshet E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998 Jul 30;394(6692):485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 132.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008 May 23;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 133.Sabharwal SS, Waypa GB, Marks JD, Schumacker PT. Peroxiredoxin-5 targeted to the mitochondrial intermembrane space attenuates hypoxia-induced reactive oxygen species signalling. Biochem J. 2013 Dec 15;456(3):337–46. doi: 10.1042/BJ20130740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mole DR, Maxwell PH, Pugh CW, Ratcliffe PJ. Regulation of HIF by the von Hippel-Lindau tumour suppressor: Implications for cellular oxygen sensing. IUBMB Life. 2001;52(1–2):43–7. doi: 10.1080/15216540252774757. [DOI] [PubMed] [Google Scholar]
- 135.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain: A hypoxic switch. Science. 2002 Feb 1;295(5556):858–61. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 136.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002 Jun 15;16(12):1466–71. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tarhonskaya H, Hardy AP, Howe EA, Loik ND, Kramer HB, McCullagh JS, Schofield CJ, Flashman E. Kinetic Investigations of the Role of Factor Inhibiting Hypoxia-inducible Factor (FIH) as an Oxygen Sensor. J Biol Chem. 2015 Aug 7;290(32):19726–42. doi: 10.1074/jbc.M115.653014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Masson N, Singleton RS, Sekirnik R, Trudgian DC, Ambrose LJ, Miranda MX, Tian YM, Kessler BM, Schofield CJ, Ratcliffe PJ. The FIH hydroxylase is a cellular peroxide sensor that modulates HIF transcriptional activity. EMBO Rep. 2012;13(3):251–7. doi: 10.1038/embor.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tian YM, Yeoh KK, Lee MK, Eriksson T, Kessler BM, Kramer HB, Edelmann MJ, Willam C, Pugh CW, Schofield CJ, Ratcliffe PJ. Differential sensitivity of hypoxia inducible factor hydroxylation sites to hypoxia and hydroxylase inhibitors. J Biol Chem. 2011 Apr 15;286(15):13041–51. doi: 10.1074/jbc.M110.211110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: Effect on HIF1alpha. Science. 2003 Dec 12;302(5652):1975–8. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 141.Wilson DF. Contribution of diffusion to the oxygen dependence of energy metabolism in cells. Experienta. 1990;46:1160–2. doi: 10.1007/BF01936927. [DOI] [PubMed] [Google Scholar]
- 142.Song D, Li LS, Heaton-Johnson KJ, Arsenault PR, Master SR, Lee FS. Prolyl hydroxylase domain protein 2 (PHD2) binds a Pro-Xaa-Leu-Glu motif, linking it to the heat shock protein 90 pathway. J Biol Chem. 2013 Apr 5;288(14):9662–74. doi: 10.1074/jbc.M112.440552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sanjuan-Pla A, Cervera AM, Apostolova N, Garcia-Bou R, Victor VM, Murphy MP, McCreath KJ. A targeted antioxidant reveals the importance of mitochondrial reactive oxygen species in the hypoxic signaling of HIF-1alpha. FEBS Lett. 2005 May 9;579(12):2669–74. doi: 10.1016/j.febslet.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 144.Bell EL, Klimova TA, Eisenbart J, Schumacker PT, Chandel NS. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol. 2007 Aug;27(16):5737–45. doi: 10.1128/MCB.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Welford SM, Bedogni B, Gradin K, Poellinger L, Broome PM, Giaccia AJ. HIF1alpha delays premature senescence through the activation of MIF. Genes Dev. 2006 Dec 15;20(24):3366–71. doi: 10.1101/gad.1471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mehta R, Steinkraus KA, Sutphin GL, Ramos FJ, Shamieh LS, Huh A, Davis C, Chandler-Brown D, Kaeberlein M. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science. 2009 May 29;324(5931):1196–8. doi: 10.1126/science.1173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schieber M, Chandel NS. TOR signaling couples oxygen sensing to lifespan in C. elegans. Cell Rep. 2014 Oct 9;9(1):9–15. doi: 10.1016/j.celrep.2014.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010 Dec 7;20(23):2131–6. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]