Abstract
Novel immunotherapy approaches are transforming the treatment of cancer, yet many patients remain refractory to these agents. One hypothesis is that immunotherapy fails because of a tumor microenvironment that fails to support recruitment of immune cells including CD8+ T cells. Therefore, new approaches designed to initiate a de novo anti-tumor immune response from within the tumor microenvironment are being pursued. Recent evidence has indicated that spontaneous activation of the Stimulator of Interferon Genes (STING) pathway within tumor-resident dendritic cells leads to type I interferon (IFN) production and adaptive immune responses against tumors. This pathway is activated in the presence of cytosolic DNA, that is detected by the sensor cyclic-GMP-AMP synthase (cGAS), and generates cyclic GMP-AMP (cGAMP), which binds and activates STING. As a therapeutic approach, intratumoral injection of STING agonists has demonstrated profound therapeutic effects in multiple mouse tumor models, including melanoma, colon, breast, prostate, and fibrosarcoma. Better characterization of the STING pathway in human tumor recognition, and the development of new pharmacologic approaches to engage this pathway within the tumor microenvironment in patients, are important areas for clinical translation.
Background
The STING pathway
STING (Stimulator of Interferon Genes, also known as TMEM173, MITA, ERIS, and MPYS) is an adapter transmembrane protein that resides in the endoplasmic reticulum (ER). In eukaryotic cells, activation of STING occurs when double stranded DNA gains access to the cytosol. This pathway was originally uncovered in search for a mechanism by which DNA viruses could be sensed by the host immune system. However, STING pathway activation also can occur with certain bacterial and parasitic infections (1), and more recently has been described to occur under conditions when mammalian DNA itself can attain access to the cytosol (2), (3). Cytosolic DNA is detected upon binding to the sensor cyclic-GMP-AMP synthase (cGAS, MB21D1), which catalyzes the synthesis of cyclic GMP-AMP (cGAMP) from guanosine triphosphate (GTP) and adenosine triphosphate (ATP). cGAMP functions as a second messenger that binds and activates STING (4, 5). Upon binding of cGAMP, STING undergoes conformational changes that trigger its trafficking from the ER to the Golgi to perinuclear endosomes (6). Consequently, STING recruits tank-binding kinase 1 (TBK1) and is, in turn, phosphorylated by TBK1, which renders it accessible for the binding of the transcription factor interferon regulatory factor 3 (IRF3) (7). TBK1 then phosphorylates IRF3 which translocates to the nucleus to drive transcription of IFN-β and other genes (8–10) (Fig. 1).
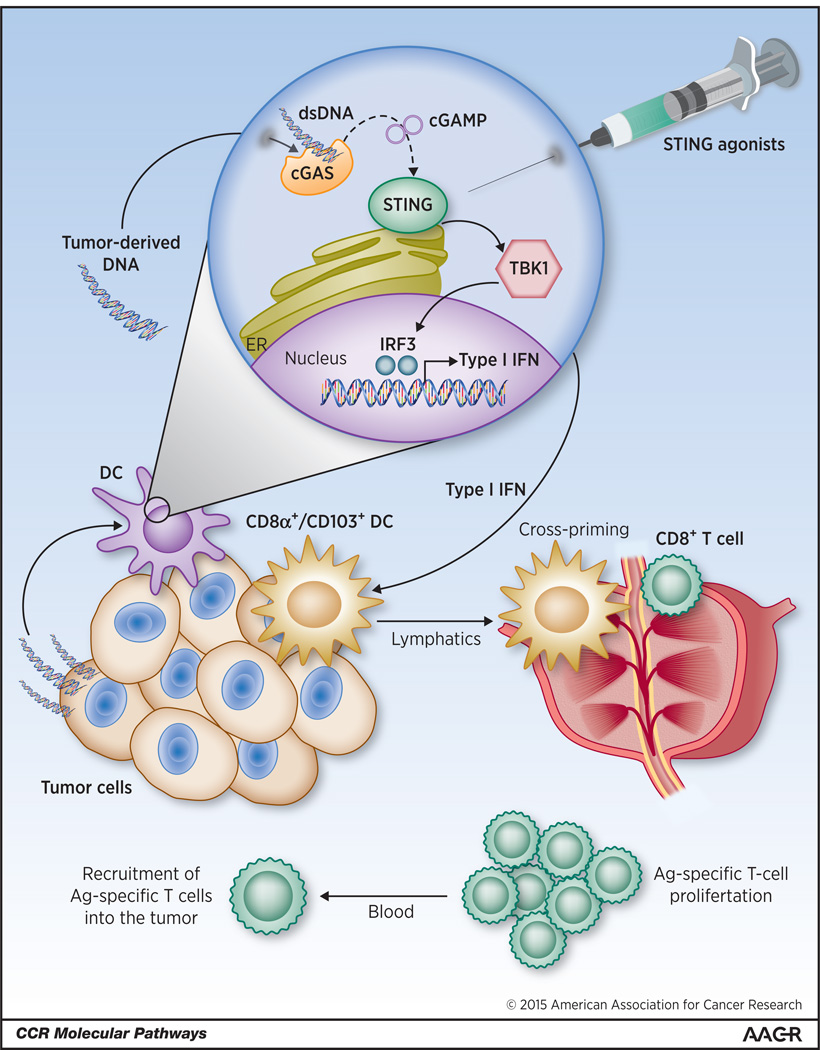
Figure 1.
Working model of the innate immune sensing of tumors leading to spontaneous T cell responses in vivo. In the tumor microenvironment, tumor-derived DNA (likely released by dead cells, or via acquisition of DNA-containing vesicles) can gain access to the cytosol of intratumoral dendritic cells (DCs). Recognition of cytosolic DNA by cyclic GMP-AMP (cGAMP) synthase (cGAS), and generation of cGAMP, leads to the activation of STING (stimulator of interferon genes). This results in the phosphorylation of tank-binding kinase 1 (TBK1) and subsequent activation, which in turn phosphorylates the transcription factor interferon regulatory factor 3 (IRF3). This activates the transcription of type I interferon (IFN) genes. The STING pathway can be also deliberately stimulated by the use of direct STING agonists, when the compounds are therapeutically administered into the tumor microenvironment. In vivo studies using gene-targeted mice demonstrated a crucial role of STING pathway activation, type I IFN production, and its signaling on the BATF3 (basic leucine zipper transcription factor ATF-like 3) lineage of DCs for spontaneous antitumor T cell responses in vivo and recruitment of effector T cells into the tumor microenvironment. dsDNA, double-stranded DNA.
The functional relevance of cGAS to the STING pathway has been demonstrated in cGAS-deficient cells. Production of the cytokines IFN-α and IFN-β, collectively referred to as type I IFNs, is impaired in cGAS−/− macrophages, fibroblasts and dendritic cells that have been transfected with DNA, or infected with DNA viruses including vaccinia virus, HSV-1 or MHV68 (11, 12). In addition, cGAS detects HIV and other retroviruses, since they generate intermediate DNA in their replication cycles (13). Interestingly, it has been demonstrated that cGAMP, as a small molecule second messenger, can be transferred through gap junctions from cGAMP-producing cells to neighboring cells (14), thus comprising a mechanism that enables infected cells to spread innate immune activation to non-infected cells.
Beyond its role in sensing the presence of infectious agents, the STING pathway also is involved with sensing mammalian DNA directly. Pathological accumulation of cytosolic DNA leads to autoimmune diseases such as Aicardi-Goutières syndrome (15) or systemic lupus erythematosus (SLE) (16). This pathological accumulation of cytosolic DNA can be mimicked using DNase II-deficient mice, which are defective in degradation of DNA within lysosomes thereby leading to escape into to the cytosol. Intercrossing of STING-deficient mice with DNase II−/− mice rescues the inflammation-related embryonic lethality normally seen in in these animals (3). These data imply that that activation of the STING pathway is involved in the pathologic consequences of DNA-mediated inflammatory disorders. In further support of this notion, gain-of-function mutations in TMEM173 (the gene encoding STING) have been identified in patients with an inflammatory vascular-pulmonary syndrome, characterized by overproduction of type I IFNs (17).
Type I IFNs and the STING pathway in cancer
Spontaneous T cell responses against tumors in vivo have been observed, both in human cancer patients and in murine models (18). The presence of activated CD8+ T cells in solid tumors correlates with better prognosis in colorectal cancer (19), ovarian cancer (20), breast cancer (21), melanoma (22), gastrointestinal stromal tumors (23), and others. The presence of a T cell response against tumors reflects successful T cell priming by adequate activated antigen presenting cells (APC) in the tumor microenvironment. Taking into account the sterile tumor setting that lacks microbial-derived triggers, activation signals in APCs must come from endogenous adjuvants generated within the tumor (24). Gene expression analysis of human melanoma metastases revealed that tumor infiltration of CD8+ T cells correlates with the expression of genes that are known to be induced by type I IFNs (25). The type I IFN profile has also been shown to predict favorable clinical responses to therapeutic cancer vaccines (26) and to anthracycline-based chemotherapy in patients with breast carcinoma characterized by poor prognosis (27). Infiltration of plasmacytoid dendritic cells (pDCs; a subpopulation of DCs that produces high amounts of type I IFNs) into the skin lesions of vitiligo patients generates type I IFN and thus drives the activation and recruitment of autoimmune T cells (28). Overall, the link between a type I IFN profile and T cell responses suggests that these cytokines might be involved in the generation of an adaptive T cell response against tumor antigens. Indeed, endogenous type I IFN was shown to be required for the prevention of methylcholanthrene-induced and transplantable tumors (29). Mechanistically, animals deficient in the type I IFN signaling pathway, such as deficiency in the Interferon-alpha/beta receptor alpha chain (IFNAR) or in the Signal Transducer and Activator of Transcription 1 (STAT1), showed reduced priming of T cells against tumor-associated antigens. This defect was mapped to the level of the APC compartment, in particular to the basic leucine zipper transcription factor ATF-like 3 (BATF3)-driven lineage of DCs, characterized by expression of CD8α or CD103 in mice (30, 31). Absence of host type I IFN signaling was associated with reduced accumulation of CD8α+ DCs within the tumor. Mixed bone marrow chimeras confirmed that type I IFN signaling in the CD8α+ DC lineage was necessary for maximal T cell priming against tumors in vivo (31). Conditional deletion of the type I IFNR in the CD11c (integrin alpha X chain, a pan-marker of DCs) compartment, also led to poor spontaneous T cell priming against tumors (30). These data suggest that recognition of cancer cells in vivo involves the activation of a pathway within DCs that leads to production of type I IFNs, which in turn drives effective processing of antigens by CD8α+/CD103+ DCs and subsequent presentation of antigenic peptides on major histocompatibility complex (MHC) class I molecules to cytotoxic CD8+ T cells, a process that is known as cross-priming (Fig. 1).
These data provided a clue regarding the innate immune signaling pathways that might be involved in anti-tumor adaptive immune responses, as it must be an innate immune pathway that induces type I IFN production. Mechanistic studies using mouse transplantable tumor models revealed that the role of two of the major sensing pathways, TLR signaling via myeloid differentiation primary response gene 88 (MyD88) and/or TIR-domain-containing adapter-inducing interferon-β (TRIF) (32), and the purinergic receptor P2X, ligand-gated ion channel, 7 (PX72R) signaling due to extracellular ATP binding (33), were dispensable in the generation of spontaneous T cell priming against tumor antigens. However, animals deficient in STING or IRF3 showed a defect in T cell priming and failed to reject immunogenic tumors (34). Ex vivo analysis demonstrated the presence of tumor-derived DNA within the cytosol of tumor-infiltrating DCs, and this correlated with translocation of IRF3 to the nucleus and expression of IFN-β. Therefore, these data strongly suggest that the host STING pathway is the main innate immune sensing pathway for detection of tumors in vivo, and that the activation of this pathway in APCs within the tumor microenvironment drives the subsequent T cell priming against tumor-associated antigens.
A protective role for the type I IFN and the STING pathways has also been reported in various additional in vivo tumor models. Melanoma and lymphoma cell lines expressing OVA peptide (B16.OVA and EL4.OVA, respectively) generated an adaptive immune response against tumor-associated antigens after cryoablation treatment in an IFNAR-dependent manner (35). This study also demonstrated that the CD11c+ subset is the main source of type I IFNs after sensing DNA released by dying cells. The molecular mechanism governing this effect involved activation of the STING/TBK1/IRF3 pathway. Interestingly, STING deficiency in DCs also impaired the generation of anti-nuclear antibodies in an inducible model of SLE. These data imply that the generation of an anti-tumor response by dying cells and the generation of autoimmunity share the same molecular mechanism of DNA sensing. Expression of type I IFNs was also detected to be induced in a model of glioma generated using a sleeping beauty transposon system (36). In this model, CD11b+ (integrin alpha M chain) brain-infiltrating leukocytes were the main type I IFN producers in a partially STING-dependent manner. Thus, mouse survival and production of type I IFNs were reduced in glioma-bearing mice having a non-functional mutation (I199N) in STING (37). Two independent studies have demonstrated the protective role of STING in an inducible colon cancer model using azoxymethane/dextran sodium sulfate (AOM/DSS) (38, 39). STING-deficient hosts were more susceptible to colitis and displayed markedly increased tumor formation with accelerated kinetics. One of these studies showed that this protective role of the STING pathway must be explained by the activation of inflammatory wound repair initiating cytokines and the suppression of growth inhibitory IL-22 binding protein (IL-22BP) by IL-18 (38). The other study found increased levels of the proinflammatory cytokines IL-6 and keratinocyte chemoattractant (KC) in STING-deficient mice, due to the impaired regulation of the NF-kB and STAT3-signaling pathways (39). Thus, STING-dependent innate immunity appears to control tumorigenesis in this model. However, contrary to the protective role of STING and the above models, it has been also shown that STING−/− animals are resistant to 7,12-dimethylbenz[a]anthracene (DMBA)-induced skin cancer (40). In this model, STING activation by DNA leaked from the nucleus of carcinogen-damaged cells in the dermis led to cytokine production and recruitment of infiltrating phagocytes that, in turn, drove inflammatory processes, thereby promoting tumor development. Tumor development was ablated in STING-deficient mice, indicating that activation of this pathway is a necessary component of inflammation-induced carcinogenesis in some settings.
Clinical-Translational Advances
Development of STING agonists as a cancer therapeutic
The discovery that STING is a crucial component of the innate immune sensing of tumors has generated two main clinical implications. First, as a type I IFN signature is linked to tumor T cell infiltration, and since this phenotype correlates with better outcomes, it is plausible that STING activation in the tumor microenvironment and subsequent production of type I IFNs could be used as a prognostic/predictive biomarker. Second, induction of STING activation or direct release of STING-derived cytokines in the tumor microenvironment might have immunotherapeutic potential in the clinic. One reasonable strategy is to deliberately activate host STING in the tumor microenvironment, in order to activate efficient cross-priming of tumor specific antigens to CD8+ T cells and facilitate the trafficking of effector T cells by inducing the production of key chemokines. This rationale has motivated the development of direct agonists of STING as a potential cancer therapeutic (Fig. 1).
Interestingly, a previous anti-cancer drug that had been in development has recently been discovered to be an agonist of mouse STING. Flavone acetic acid (FAA) showed substantial activity against murine colon tumors (41) through a novel mechanism of hemorrhagic necrosis. These encouraging data led to clinical translation, with this new class of agents being described as vascular disrupting agents. However this agent failed in a Phase I clinical trial and showed no activity in rat tumor models (42), raising the question of possible species-specificity. In an attempt to obtain similar drugs that produced tumor hemorrhagic necrosis, the molecular structure of FAA was modified, generating several compounds; 5,6-dimethyllxanthenone-4-acetic acid (DMXAA) was the compound with the highest potency and it also showed activity against a rat mammary carcinoma (43). Similar to FAA, DMXAA showed anti-tumor activity in different mouse models (44). However, this agent also failed in the clinic when combined with chemotherapy in a Phase III trial in non-small cell lung cancer (45). It is noteworthy that the molecular target of DMXAA was not known at that time, which hampered further development. Interestingly, recent structure-function studies of mouse and human STING demonstrated that DMXAA is a direct ligand for mouse STING (46–49), but not for human STING. This difference likely explains the lack of clinical activity of this compound in humans. These observations re-ignited enthusiasm for developing agonists of human STING that might recapitulate the potent anti-tumor activity observed with mouse STING agonists in vivo.
The discovery of cyclic-dinucleotides (CDNs), bacterial second messengers with a variety of physiological effects (50), as natural ligands of STING (8, 51), combined with the identification of cGAMP as a key cyclic dinucleotide in metazoa (5), provided a framework for pursuing STING-activating therapeutics. Of note, CDNs had been used as effective vaccine adjuvants even before their role as STING ligands was discovered (52). However, the therapeutic anti-tumor effect of CDNs has only recently been tested. Intraperitoneal injection of cyclic-GMP (cGMP) has been shown to inhibit the growth of pre-established 4T1 breast tumors (53). In this study, the bacterial-derived canonical cGMP, which contains two 3’-5’ linkages, was used. This molecule may not be suitable for clinical development, since single nucleotide polymorphisms in the human STING (hSTING) gene have been shown to affect responsiveness to canonical CDNs (54, 55). Non-canonical cGAMP, generated by the activity of mammalian cGAS, contains a single 3’-5’ and a single 2’-5’phosphodiester bond, and activates all hSTING variants (14, 54–56). Rational modifications of CDNs led to synthetic dithio mixed-linkage CDNs that were tested in vitro and in vivo for their capacity to activate all hSTING variants in addition to mSTING (57). The lead molecule ML RR-S2 CDA showed several features that improved both stability and lipophilicity, promoting significantly increased STING signaling as compared with endogenous and pathogen-derived CDNs. Similarly to DMXXA, intratumoral injection of ML RR-S2 CDA into pre-established B16 melanoma tumors caused complete tumor elimination in most of treated mice, induced lasting systemic antigen-specific CD8+ T cell immunity. Around 50% of treated animals were free of tumors and survived more than 150 days after intratumoral injection. Furthermore, they were completely protected against a second tumor re-challenge. Similar results were seen in the 4T-1 breast cancer and MC26 colon cancer models.
These preclinical studies suggest that intratumoral injection of ML RR-S2 CDA is necessary to achieve a maximal therapeutic effect. While this may limit the application of this compound to the treatment of directly accessible tumors, it has been shown that local treatment of one tumor induces systemic immunity that effectively induces regression of distant tumors. Thus, an abscopal effect may facilitate systemic anti-tumor activity. These principles are similar to those involved with the therapeutic activity of the oncolytic virus T-VEC for patients with melanoma (58), or the TLR9 agonist CpG along with local low-dose radiation therapy in patients with non-Hodgkin’s lymphoma (59). Both of these approaches induce regression of non-treated tumors upon intratumoral application to a single lesion.
Additional strategies to bring type I IFNs to the tumor microenvironment
Due to the benefit of type I IFN induction in innate immune activation in the tumor microenvironment, alternative approaches also have been investigated to transform the tumor microenvironment favorably for T cell-mediated regression. These include intratumoral injection of TLR ligands (60), introduction of tumor necrosis factor (TNF) ligand superfamily member 14 (LIGHT) (61), and injection of oncolytic viruses (62). In addition, strategies to directly deliver type I IFN into the tumor microenvironment using tumor-targeting mAbs coupled to IFN-β have been investigated (63). The therapeutic effect of low doses of type I IFNs to the tumor microenvironment was shown to be T cell-dependent and mediated through type I IFN signaling on host DCs. However, high doses of intratumoral type I IFN might have mainly an anti-angiogenic effect, which was mediated through the IFNAR on endothelial cells (64, 65). Finally, directed radiation to the tumor site also induces type I IFN production, thereby augmenting T cell priming (66). Mechanistically, the induction of type I IFNs by local radiation also appears to depend on the host STING pathway (67), and cGAMP treatment of tumors potentiates the therapeutic effect of radiation by enhancing tumor-specific CD8+ T cell functions.
Conclusions
The STING pathway of cytosolic DNA sensing is an important innate immune sensing mechanism, driving type I IFN production in the tumor context. Intratumoral STING agonists hold promise as a cancer therapeutic. Numerous questions remain unanswered, and a deeper biologic understanding of the pathway is still needed. First, the mechanism by which DNA derived from tumor cells gains access to host APCs is not yet known. Second, the activation of STING, and its functional consequences, in different cell subsets within the tumor microenvironment need to be addressed. Third, as radiation induces type I IFN by activation of the STING pathway, the role of STING in the efficacy of other cancer therapeutics, including chemotherapy and kinase inhibitors, also should be explored. Finally, there is little known about the negative and positive regulators of the STING pathway that could be relevant for the cancer context. A deeper understanding of feedback mechanisms will facilitate continued development of alternative strategies to favorably regulate the STING pathway as a therapeutic.
Acknowledgments
L. Corrales was supported by a postdoctoral fellowship from the Cancer Research Institute. Some work performed that led to this review was supported by R01CA181160 from the NIH to T.F. Gajewski.
L. Corrales and T.F. Gajewski are listed as co-inventors on a patent-pending application related to the use of STING agonist as cancer treatment, which is owned by the University of Chicago.
Footnotes
Disclosure of Potential Conflicts of Interest
No other potential conflicts of interest were disclosed.
References
- 1.Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends Immunol. 2014;35:88–93. doi: 10.1016/j.it.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Rongvaux A, Jackson R, Harman CC, Li T, West AP, de Zoete MR, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci U S A. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Cai X, Wu J, Cong Q, Chen X, Li T, et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347:aaa2630. doi: 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]
- 8.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdette DL, Vance RE. STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol. 2013;14:19–26. doi: 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature. 2013;503:530–544. doi: 10.1038/nature12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M, et al. Mutations in the gene encoding the 3'–5' DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat Genet. 2006;38:917–920. doi: 10.1038/ng1845. [DOI] [PubMed] [Google Scholar]
- 16.Lee-Kirsch MA, Gong M, Chowdhury D, Senenko L, Engel K, Lee YA, et al. Mutations in the gene encoding the 3'–5' DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat Genet. 2007;39:1065–1067. doi: 10.1038/ng2091. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Montealegre Sanchez GA, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371:507–518. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 19.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 21.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 22.Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30:2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 23.Rusakiewicz S, Semeraro M, Sarabi M, Desbois M, Locher C, Mendez R, et al. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 2013;73:3499–3510. doi: 10.1158/0008-5472.CAN-13-0371. [DOI] [PubMed] [Google Scholar]
- 24.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–474. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 25.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulloa-Montoya F, Louahed J, Dizier B, Gruselle O, Spiessens B, Lehmann FF, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol. 2013;31:2388–2395. doi: 10.1200/JCO.2012.44.3762. [DOI] [PubMed] [Google Scholar]
- 27.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 28.Bertolotti A, Boniface K, Vergier B, Mossalayi D, Taieb A, Ezzedine K, et al. Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res. 2014;27:398–407. doi: 10.1111/pcmr.12219. [DOI] [PubMed] [Google Scholar]
- 29.Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 30.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 33.Lister MF, Sharkey J, Sawatzky DA, Hodgkiss JP, Davidson DJ, Rossi AG, et al. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 2007;4:5. doi: 10.1186/1476-9255-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klarquist J, Hennies CM, Lehn MA, Reboulet RA, Feau S, Janssen EM. STING-mediated DNA sensing promotes antitumor and autoimmune responses to dying cells. J Immunol. 2014;193:6124–6134. doi: 10.4049/jimmunol.1401869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohkuri T, Ghosh A, Kosaka A, Zhu J, Ikeura M, David M, et al. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol Res. 2014;2:1199–1208. doi: 10.1158/2326-6066.CIR-14-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun. 2011;79:688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn J, Konno H, Barber GN. Diverse roles of STING-dependent signaling on the development of cancer. Oncogene. 2015 Feb 2; doi: 10.1038/onc.2014.457. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Q, Man SM, Gurung P, Liu Z, Vogel P, Lamkanfi M, et al. Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J Immunol. 2014;193:4779–4782. doi: 10.4049/jimmunol.1402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn J, Xia T, Konno H, Konno K, Ruiz P, Barber GN. Inflammation-driven carcinogenesis is mediated through STING. Nat Commun. 2014;5:5166. doi: 10.1038/ncomms6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plowman J, Narayanan VL, Dykes D, Szarvasi E, Briet P, Yoder OC, et al. Flavone acetic acid: a novel agent with preclinical antitumor activity against colon adenocarcinoma 38 in mice. Cancer Treat Rep. 1986;70:631–635. [PubMed] [Google Scholar]
- 42.Cummings J, Smyth JF. Flavone 8-acetic acid: our current understanding of its mechanism of action in solid tumours. Cancer Chemother Pharmacol. 1989;24:269–272. doi: 10.1007/BF00304756. [DOI] [PubMed] [Google Scholar]
- 43.Liu JJ, Ching LM, Goldthorpe M, Sutherland R, Baguley BC, Kirker JA, et al. Antitumour action of 5,6-dimethylxanthenone-4-acetic acid in rats bearing chemically induced primary mammary tumours. Cancer Chemother Pharmacol. 2007;59:661–669. doi: 10.1007/s00280-006-0321-7. [DOI] [PubMed] [Google Scholar]
- 44.Baguley BC, Ching LM. Immunomodulatory actions of xanthenone anticancer agents. BioDrugs. 1997;8:119–127. doi: 10.2165/00063030-199708020-00005. [DOI] [PubMed] [Google Scholar]
- 45.Lara PN, Jr, Douillard JY, Nakagawa K, von Pawel J, McKeage MJ, Albert I, et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:2965–2971. doi: 10.1200/JCO.2011.35.0660. [DOI] [PubMed] [Google Scholar]
- 46.Conlon J, Burdette DL, Sharma S, Bhat N, Thompson M, Jiang Z, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol. 2013;190:5216–5225. doi: 10.4049/jimmunol.1300097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao P, Ascano M, Zillinger T, Wang W, Dai P, Serganov AA, et al. Structure-function analysis of STING activation by c[G(2',5')pA(3',5')p] and targeting by antiviral DMXAA. Cell. 2013;154:748–762. doi: 10.1016/j.cell.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S, Li L, Maliga Z, Yin Q, Wu H, Mitchison TJ. Anticancer flavonoids are mouse-selective STING agonists. ACS Chem Biol. 2013;8:1396–1401. doi: 10.1021/cb400264n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prantner D, Perkins DJ, Lai W, Williams MS, Sharma S, Fitzgerald KA, et al. 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J Biol Chem. 2012;287:39776–39788. doi: 10.1074/jbc.M112.382986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McWhirter SM, Barbalat R, Monroe KM, Fontana MF, Hyodo M, Joncker NT, et al. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J Exp Med. 2009;206:1899–1911. doi: 10.1084/jem.20082874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karaolis DK, Means TK, Yang D, Takahashi M, Yoshimura T, Muraille E, et al. Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol. 2007;178:2171–2181. doi: 10.4049/jimmunol.178.4.2171. [DOI] [PubMed] [Google Scholar]
- 53.Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, et al. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res. 2014;2:901–910. doi: 10.1158/2326-6066.CIR-13-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diner EJ, Burdette DL, Wilson SC, Monroe KM, Kellenberger CA, Hyodo M, et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 2013;3:1355–1361. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao P, Ascano M, Wu Y, Barchet W, Gaffney BL, Zillinger T, et al. Cyclic [G(20,50)pA(30,50)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11:1018–1030. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goins WF, Huang S, Cohen JB, Glorioso JC. Engineering HSV-1 vectors for gene therapy. Methods Mol Biol. 2014;1144:63–79. doi: 10.1007/978-1-4939-0428-0_5. [DOI] [PubMed] [Google Scholar]
- 59.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grauer OM, Molling JW, Bennink E, Toonen LW, Sutmuller RP, Nierkens S, et al. TLR ligands in the local treatment of established intracerebral murine gliomas. J Immunol. 2008;181:6720–6729. doi: 10.4049/jimmunol.181.10.6720. [DOI] [PubMed] [Google Scholar]
- 61.Zou W, Zheng H, He TC, Chang J, Fu YX, Fan W. LIGHT delivery to tumors by mesenchymal stem cells mobilizes an effective antitumor immune response. Cancer Res. 2012;72:2980–2989. doi: 10.1158/0008-5472.CAN-11-4216. [DOI] [PubMed] [Google Scholar]
- 62.Miyamoto S, Inoue H, Nakamura T, Yamada M, Sakamoto C, Urata Y, et al. Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res. 2012;72:2609–2621. doi: 10.1158/0008-5472.CAN-11-3185. [DOI] [PubMed] [Google Scholar]
- 63.Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, et al. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gajewski TF, Corrales L. New perspectives on type I IFNs in cancer. Cytokine Growth Factor Rev. 2015;26:175–178. doi: 10.1016/j.cytogfr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spaapen RM, Leung MY, Fuertes MB, Kline JP, Zhang L, Zheng Y, et al. Therapeutic activity of high-dose intratumoral IFN-beta requires direct effect on the tumor vasculature. J Immunol. 2014;193:4254–4260. doi: 10.4049/jimmunol.1401109. [DOI] [PubMed] [Google Scholar]
- 66.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]