Abstract
Anesthetic management of patients undergoing pulmonary vein isolation for atrial fibrillation has specific requirements. The feasibility of non-invasive ventilation (NIV) added to deep sedation procedure was evaluated.
Seventy-two patients who underwent ablation procedure were retrospectively revised, performed with (57%) or without (43%) application of NIV (Respironic® latex-free total face mask connected to Garbin ventilator-Linde Inc.) during deep sedation (Midazolam 0.01–0.02 mg/kg, fentanyl 2.5–5 μg/kg and propofol: bolus dose 1–1.5 mg/kg, maintenance 2–4 mg/kg/h).
In the two groups (NIV vs deep sedation), differences were detected in intraprocedural (pH 7.37 ± 0.05 vs 7.32 ± 0.05, p = 0.001; PaO2 117.10 ± 27.25 vs 148.17 ± 45.29, p = 0.004; PaCO2 43.37 ± 6.91 vs 49.33 ± 7.34, p = 0.002) and in percentage variation with respect to basal values (pH −0.52 ± 0.83 vs −1.44 ± 0.87, p = 0.002; PaCO2 7.21 ± 15.55 vs 34.91 ± 25.76, p = 0.001) of arterial blood gas parameters. Two episodes of respiratory complications, treated with application of NIV, were reported in deep sedation procedure. Endotracheal intubation was not necessary in any case. Adverse events related to electrophysiological procedures and recurrence of atrial fibrillation were recorded, respectively, in 36% and 29% of cases.
NIV proved to be feasible in this context and maintained better respiratory homeostasis and better arterial blood gas balance when added to deep sedation.
Keywords: Non-invasive ventilation, Atrial fibrillation, Anesthesiological management, Deep sedation
Introduction
The delicate catheter ablation of atrial fibrillation (AF) involves a relevant risk of major complications although mortality is less than 1 in 2000 [1], [2], [3]. Patients undergoing catheter ablation for AF are required to lie motionless on the procedure table for several hours, and repeated stimuli from ablation are sometimes painful. For these reasons, patients are treated with deep sedation or general anesthesia. The safety of deep sedation in this procedure has already been tested [4], and high-frequency jet ventilation during general anesthesia has also proved to be a safe technique [5].
Aid for anesthetic management in these setting can be non-invasive ventilation (NIV), first used in acute respiratory failure [6], sometimes during sedation [7], subsequently used outside of the intensive care unit [8] and in cardio-surgical procedures [9], [10], [11], [12].
The aim of our study was to evaluate the usefulness of NIV during sedation in catheter ablation of use AF instead of atrial fibrillation compared to deep sedation.
Materials and methods
Study design and patient selection
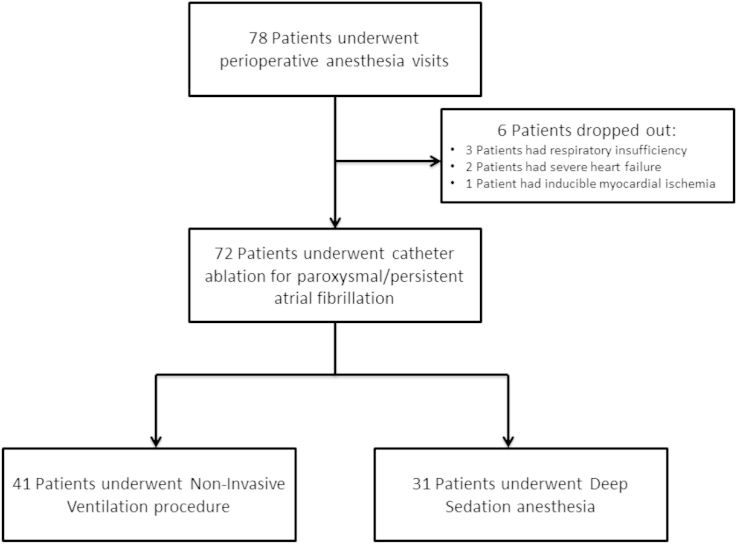
In the study period (24 months), conducted in a single high-volume electrophysiology procedure center, consecutive patients undergoing catheter ablation for paroxysmal/persistent atrial fibrillation [1] were retrospectively revised (Fig. 1).
Fig. 1.
Patients flow diagram of the study.
All patients provided informed consent to participate in the study. Exclusion criteria for the procedure were left ejection fraction less than 40%, moderate-to-severe mitral valve disease, severe heart failure (New York Heart Association functional class III or IV), expected surgery for structural heart disease, secondary AF (due to cardiac surgery, infection or hyperthyroidism), severe COPD with concomitant respiratory insufficiency (PaO2 < 60 mmHg), presence of inducible myocardial ischemia or other acute illness underway, or contraindications to anesthesia. Complications and recurrence of AF occurring within 48 h of the electrophysiological procedure were also examined.
Anesthesiological management
Perioperative anesthesia visits and sedation procedures were performed according to SIAARTI (Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva) guidelines [13], [14], including pulmonary function tests in the anesthetic assessment. Routine monitoring (electrocardiography, pulse oximetry, invasive arterial pressure, seriate arterial blood gas analysis and apnea monitor) were performed. Sedation and NIV were performed after trans-septal puncture in order to rule out left-sided emboli through neurologic assessment.
Midazolam 0.01–0.02 mg/kg IV was administered immediately before monitoring, fentanyl (2.5–5 μg/kg) was administered as analgesic and sedation was performed by propofol infusion (bolus dose 1–1.5 mg/kg, maintenance 2–4 mg/kg/h). The infusion rate was carefully titrated to achieve a target sedation level of a Ramsay sedation scale [15] of 2–3. Mean arterial pressure was maintained above 75 mmHg during the entire procedure.
During deep sedation, the patients were breathing spontaneously and were given supplemental oxygen (FiO2 80–100%) to maintain the oxygen saturation level above 92%.
In patients undergoing NIV ventilation the procedure was performed with a Respironic® latex-free total face mask connected to Garbin ventilator (Linde Inc., Herrsching, Germany) in Spontaneous/Temporized mode applying incorporated algorithms to improve patient-ventilator synchrony by adjusting to changing breathing patterns and dynamic leaks. I-PAP, E-PAP and respiratory rate were modified according to the clinical response, tolerance of the patient to obtaining exhaled tidal volume of 6–8 ml/kg; FiO2 requirement was 40% or less to maintain oxygen saturation above 92%. The ventilator settings were adjusted on the basis of pulse oximetry and serial measurement of arterial blood gases.
Sedation was halted when the mechanical ventilation was discontinued or at the end of the catheter ablation procedure. At the end of the procedure, patients were transferred, according to hospital protocol, to the intensive care unit for monitoring.
Catheter ablation
Oral anticoagulation with stable international normalized ratio (INR) superior to 2.0 was ensured for at least 3 weeks before ablation. Transesophageal echocardiography was performed within 24 h before the procedure, to rule out the presence of left atrial thrombi. Local anesthesia (lidocaine 1%) was administered subcutaneously at the femoral venous access site. After septal puncture of interatrial septum, intravenous heparin was administered according to institutional standards. The ablation procedure was guided by electroanatomical mapping (NavX, St. Jude Medical Inc., Minneapolis, MN, USA or CARTO-3 mapping system, Biosense Webster, Diamond Bar, CA, USA) with image integration using computer tomography or magnetic resonance reconstruction of the left atrium. Catheter ablation procedure was performed using radiofrequency energy or cryoablation balloon ablation catheter (Arctic Front, Medtronic, Minneapolis, MN, USA). The electroanatomic mapping systems and the energy sources used during the procedure were selected by the electrophysiology team in relation to clinical characteristics of the patient.
Statistical analysis
The normal distribution for continuous variables was assessed by Kolmogorov–Smirnov test. Categorical variables were represented as percentages, continuous variables as mean ± standard deviation. Comparisons between groups were performed by unpaired samples two-sided t-test or chi-square test, with continuity correction, where appropriate. Intra-procedural changes of arterial blood gas variables were expressed as percentage variation of their basal values. A p-value less than 0.05 was considered statistically significant.
Results
During the study period 72 electrophysiology procedures carried out in deep sedation were evaluated; 41 (57%) procedures were performed with NIV (NIV group) and the other 31 (43%) with-out NIV (deep sedation group). The clinical characteristics of the patients and the procedural details are shown in Table 1.
Table 1.
Clinical characteristics of patients.
| Characteristic | All patients n = 72 |
Non-invasive ventilation n = 41 |
Deep sedation n = 31 |
p-Value |
|---|---|---|---|---|
| Age (years) | 62 ± 10 | 62 ± 10 | 62 ± 10 | 0.854 |
| Male gender, n (%) | 58 (81) | 33 (81) | 25 (79) | 0.856 |
| Hypertension, n (%) | 36 (50) | 20 (49) | 16 (52) | 1.000 |
| Diabetes, n (%) | 9 (13) | 5 (12) | 4 (14) | 0.868 |
| Smoke exposure, n (%) | 32 (44) | 18 (44) | 14 (45) | 0.906 |
| Current smoke exposure, n (%) | 14 (31) | 7 (17) | 7 (22) | 0.102 |
| ASA I, n (%) | 2 (3) | 2 (5) | 0 (0) | 0.632 |
| ASA II, n (%) | 70 (97) | 39 (95) | 31 (100) | 0.632 |
| NYHA functional class I, n (%) | 38 (53) | 23 (56) | 15 (48) | 0.492 |
| NYHA functional class II, n (%) | 34 (47) | 18 (44) | 16 (52) | 0.492 |
| Weight (Kg) | 82 ± 15 | 81 ± 16 | 83 ± 15 | 0.569 |
| Height (cm) | 171 ± 22 | 173 ± 19 | 169 ± 24 | 0.696 |
| Body mass index | 27 ± 4 | 27 ± 4 | 28 ± 4 | 0.261 |
| Left ventricular ejection fraction, n (%) | 57 ± 8 | 56 ± 9 | 59 ± 6 | 0.237 |
| FVC (liters) | 4.33 ± 1.24 | 4.36 ± 1.29 | 4.27 ± 1.16 | 0.767 |
| FEV1 (liters) | 3.18 ± 0.88 | 3.14 ± 0.88 | 3.25 ± 0.91 | 0.628 |
| Time of catheter ablation procedure, min | 262 ± 43 | 261 ± 42 | 268 ± 47 | 0.516 |
| Radiofrequency energy ablation catheter, n (%) | 59 (82) | 34 (83) | 25 (81) | 1.000 |
| Cryoablation balloon catheter, n (%) | 13 (18) | 7 (17) | 6 (19) | 1.000 |
| Time of anesthesia, min | 162 ± 51 | 164 ± 47 | 171 ± 56 | 0.754 |
| Time of NIV, min | N/A | 156 ± 44 | N/A | N/A |
| Basal pH | 7.42 ± 0.03 | 7.41 ± 0.01 | 7.42 ± 0.01 | 0.216 |
| Basal PaO2 (mmHg) | 85.04 ± 10.53 | 82.89 ± 11.81 | 87.69 ± 12.97 | 0.150 |
| Basal PaCO2 (mmHg) | 39.15 ± 4.38 | 40.50 ± 0.85 | 36.88 ± 0.84 | 0.008 |
| Basal HCO3− (mmol/L) | 25.32 ± 0.25 | 25.65 ± 0.31 | 24.68 ± 0.43 | 0.072 |
| Intraprocedural pH | 7.35 ± 0.06 | 7.37 ± 0.05 | 7.32 ± 0.05 | 0.001 |
| Intraprocedural PaO2 (mmHg) | 128.57 ± 37.81 | 117.10 ± 27.25 | 148.17 ± 45.29 | 0.004 |
| Intraprocedural PaCO2 (mmHg) | 45.57 ± 7.59 | 43.37 ± 6.91 | 49.33 ± 7.34 | 0.002 |
| Intraprocedural HCO3− (mmol/L) | 24.62 ± 1.92 | 24.71 ± 2.12 | 24.48 ± 1.53 | 0.622 |
| Δ pH (basal to intraprocedural,% change) | −0.84 ± 0.94 | −0.52 ± 0.83 | −1.44 ± 0.87 | 0.002 |
| Δ PaO2 (basal to intraprocedural,% change) | 48.01 ± 45.60 | 37.14 ± 30.95 | 68.29 ± 60.94 | 0.079 |
| Δ PaCO2 (basal to intraprocedural,% change) | 16.80 ± 23.60 | 7.21 ± 15.55 | 34.91 ± 25.76 | 0.001 |
| Δ HCO3− (basal to intraprocedural,% change) | −2.76 ± 8.35 | −3.08 ± 9.76 | 2.17 ± 4.87 | 0.684 |
| Procedural complication, n (%) | 26/72 (36) | 13/41 (32) | 13/31 (42) | 0.518 |
| Major procedural complication*, n (%) | 5/26 (19) | 2/13 (15) | 3/13 (23) | 1.000 |
| Recurrence of AF occurring within 48 h, n (%) | 21/72 (29) | 11/41 (27) | 10/31 (32) | 0.810 |
ASA: American Society of Anesthesiologists physical status classification system, FEV1: forced expiratory volume in the 1st second, FVC: Forced Vital Capacity, NYHA: New York Heart Association. * = Major procedural complications are those that required intervention, or led to long-term disability or prolonged hospitalization. Bold values have been used to indicate statistically significant variables.
NIV arm of this study has been described separately elsewhere [16].
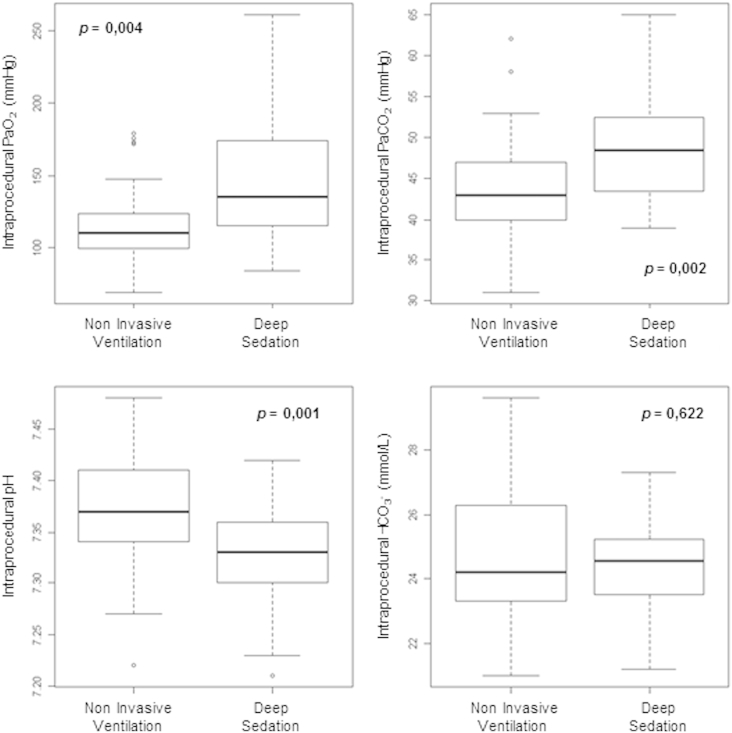
The two groups of patients showed no differences in these fields, but between the two groups, a significant variation was present in the basal PaCO2 (p = 0.008) – with values in the physiological range – and in intra-procedural blood gas parameters (pH p = 0.001, PaO2 p = 0.004, PaCO2 p = 0.002 respectively (Fig. 2)).
Fig. 2.
Intra-procedural differences in arterial blood gas variables.
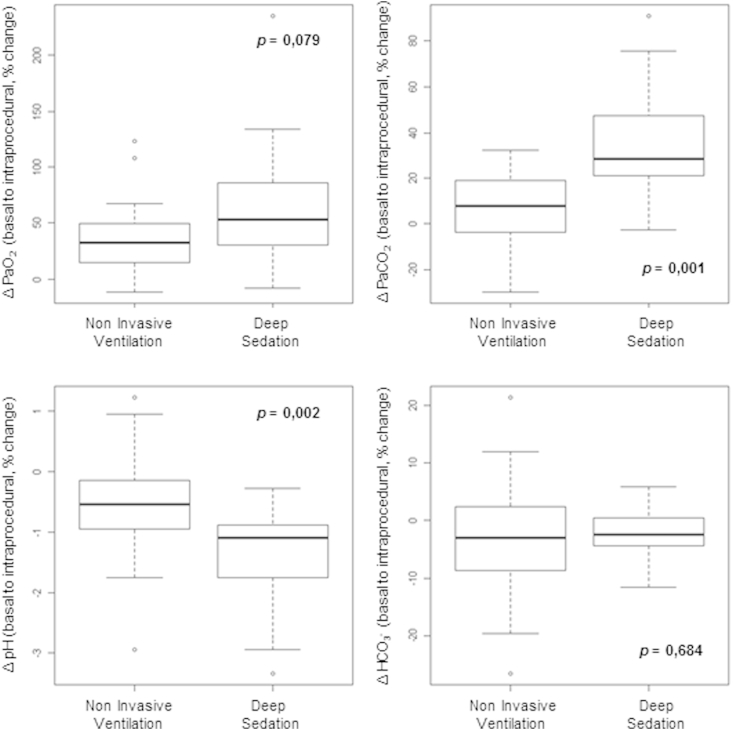
Analysis of the percentage change between basal to intraprocedural blood gas parameters (Fig. 3) shows a statistical variation in the changing of pH and PaCO2 (p = 0.002 and 0.001, respectively).
Fig. 3.
Intra-procedural changes in arterial blood gas variables (percentage variation compared to basal values).
During NIV procedures there were no problems due to mask-related difficulties, gastric distention, NIV discomfort or significant hemodynamic alterations related to positive pressure ventilation. In the deep sedation procedure there were two episodes of respiratory complications with severe hypercapnia (achieved a PaCO2 greater than 60 mmHg) treated with application of NIV. Endotracheal intubation was not necessary in any case.
Adverse events, focused on periprocedural complications, were reported in 26 patients (36%). Among the complications, vascular access complications occurred in nine cases (iatrogenic femoral pseudoaneurysms that required percutaneous treatment [17] in 5 cases and femoral hematoma with decrease in hemoglobin levels greater than 2 g/dl in four cases); none of these cases required transfusion support. Pleuro-pericardial effusion was documented in 8 cases; exacerbation of bronchial asthma in three cases; bradyarrhythmia requesting definitive pacemaker implantation in two cases; pulmonary embolism in one case; procedural myocardial infarction in one case; post-procedural stroke in one case and permanent paralysis of the phrenic nerve in one case.
The recurrence of AF within 48 h of the electrophysiological procedure was recorded in 21 patients (29%) and was treated by electrical or pharmacological cardioversion.
Discussion
Deep sedation for catheter ablation of AF is feasible and safe [4] while maintaining spontaneous ventilation. In our sedation procedures in catheter ablation of AF the NIV was safe as well, and maintained (although with respiratory parameters in the physiological range) a better homeostasis in these parameters compared to deep sedation in breathing spontaneously.
Data shown in Fig. 2, Fig. 3 confirmed a lower impact of NIV on arterial blood gas tensions and hematic pH balance [18] compared to deep sedation.
While statistically significant differences were found, the absolute value differences had a clinically limited benefit; using NIV made it possible to reduce the flow of oxygen administered to the patient during the procedure and maintain better respiratory dynamics. These conditions allowed us to maintain effective CO2 elimination with minimum impact on the pH compared to the deep sedation group.
Furthermore, in the 2 cases of patients who developed severe hypercapnia during deep sedation, the application of NIV was effective in solving the respiratory problem without the need for endotracheal intubation. This data shows an important and clinically relevant difference between the groups, although the small sample size does not allow to compare this end point between the two arms of the study.
Deep sedation requires frequent control and titration by anesthesiologist due to minor problems such as requiring high oxygen dose, great difference between basal and intraprocedural gas properties and occasional hypercapnia. The NIV is useful to mitigate these problems [6], [7], [8], [9], [10], [11], [12]. Furthermore, NIV allows to reduce the risk of hemodynamic, respiratory or neurological problems secondary to improper ventilation. We didn't have problems related to mask-related difficulties, gastric distention, NIV discomfort or significant hemodynamic alterations related to positive pressure ventilation.
Also, consideration must be given to the demographic characteristics of own patients: our population had a high number of overweight patients (overweight 39%, obese 25%) and with a history of smoking (44%). In relation to these conditions, even when outside the guidelines [13], [14], our patients' baseline respiratory function characterization was performed. This characterization allowed us to exclude patients with respiratory insufficiency from the study.
A limit of our study is the relatively small number of patients evaluated, which did not arrive at the number of patients evaluated in other studies that assessed the safety of deep sedation [4] or high-frequency jet ventilation [5].
As previously described, AF catheter ablation procedure has substantial procedural risks [2], [3], [19]. Most of the complications (12/26 cases) that we reported were due to the difficult balance between bleeding (due to systemic anticoagulation) and thrombotic (ablation procedure and recurrence of AF) processes. In our cases we did not report massive effusion or cardiac tamponade. Pleuro-pericardial effusion is due to the irritation of the serosa secondary to the ablation procedure as well as paralysis of the phrenic nerve and the damage occurring to the conduction system.
Incidence of major procedural complications (5/26 cases) was comparable to the previous reports [20], [21], do not show differences in the two groups and are imputable to electrophysiological procedure rather than anesthetic management. Of the minor complications, the only one related to anesthetic management was exacerbation of bronchial asthma. We did not find significant differences between the two anesthetic regimens tested (two cases in NIV group vs one case in the deep sedation group). These patients responded rapidly to treatment with inhaled β2-agonists and showed no alterations in chest X-ray compatible with bronchopneumonia.
Own data on the early recurrence of AF are in line with the literature, and this event was not related to the long-term success but was due to edema and inflammation of the myocardium subjected to ablation [1]. In relation to the thromboembolic risk of early relapse of AF, international guidelines [1] recommend oral anticoagulation therapy in the first few months post-ablation.
In conclusion, our study suggests that in catheter AF ablation, NIV proved to be a safe practice in anesthesiological management, as it can treat respiratory depression induced by sedation and maintain better respiratory homeostasis and better arterial blood gas balance when added to deep sedation. Furthermore, during the procedure patient safety was also ensured by a ventilator, which allowed continuous control of Tidal Volume, amount of ventilation lost to leak and effective minute ventilation.
Funding sources
No financial support was received.
Disclosures
No conflict of interest for any authors.
Acknowledgments
The Authors of this manuscript gratefully acknowledge Ms. Alison Frank for her kind and professional support in editing the English of the manuscript.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Calkins H., Kuck K.H., Cappato R., Brugada J., Camm A.J., Chen S.A. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson W.G., Albert C.M. Catheter ablation for paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1648–1649. doi: 10.1056/NEJMe1210548. [DOI] [PubMed] [Google Scholar]
- 3.Lee G., Sparks P.B., Morton J.B., Kistler P.M., Vohra J.K., Medi C. Low risk of major complications associated with pulmonary vein antral isolation for atrial fibrillation: results of 500 consecutive ablation procedures in patients with low prevalence of structural heart disease from a single center. J Cardiovasc Electrophysiol. 2011;22:163–168. doi: 10.1111/j.1540-8167.2010.01870.x. [DOI] [PubMed] [Google Scholar]
- 4.Kottkamp H., Hindricks G., Eitel C., Müller K., Siedziako A., Koch J. Deep sedation for catheter ablation of atrial fibrillation: a prospective study in 650 consecutive patients. J Cardiovasc Electrophysiol. 2011;22:1339–1343. doi: 10.1111/j.1540-8167.2011.02120.x. [DOI] [PubMed] [Google Scholar]
- 5.Elkassabany N., Garcia F., Tschabrunn C., Raiten J., Gao W., Chaichana K. Anesthetic management of patients undergoing pulmonary vein isolation for treatment of atrial fibrillation using high-frequency jet ventilation. J Cardiothorac Vasc Anesth. 2012;26:433–438. doi: 10.1053/j.jvca.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Hill N.S., Brennan J., Garpestad E., Nava S. Noninvasive ventilation in acute respiratory failure. Crit Care Med. 2007;35:2402–2407. doi: 10.1097/01.CCM.0000284587.36541.7F. [DOI] [PubMed] [Google Scholar]
- 7.Huang Z., Chen Y.S., Yang Z.L., Liu J.Y. Dexmedetomidine versus midazolam for the sedation of patients with non-invasive ventilation failure. Intern Med. 2012;51:2299–2305. doi: 10.2169/internalmedicine.51.7810. [DOI] [PubMed] [Google Scholar]
- 8.Cabrini L., Antonelli M., Savoia G., Landriscina M. Non-invasive ventilation outside of the intensive care unit: an Italian survey. Minerva Anestesiol. 2011;77:313–322. [PubMed] [Google Scholar]
- 9.Guarracino F., Ambrosino N. Non invasive ventilation in cardio-surgical patients. Minerva Anestesiol. 2011;77:734–741. [PubMed] [Google Scholar]
- 10.Guarracino F., Covello R.D., Landoni G., Baldassarri R., Stefani M., Cariello C. Anesthetic management of transcatheter aortic valve implantation with transaxillary approach. J Cardiothorac Vasc Anesth. 2011;25:437–443. doi: 10.1053/j.jvca.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Guarracino F., Cabrini L., Baldassarri R., Cariello C., Covello R.D., Landoni G. Non-invasive ventilation-aided transoesophageal echocardiography in high-risk patients: a pilot study. Eur J Echocardiogr. 2010;11:554–556. doi: 10.1093/ejechocard/jeq019. [DOI] [PubMed] [Google Scholar]
- 12.Landoni G., Zangrillo A., Cabrini L. Noninvasive ventilation after cardiac and thoracic surgery in adult patients: a review. J Cardiothorac Vasc Anesth. 2012;26:917–922. doi: 10.1053/j.jvca.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Calderini E., Accorsi A., Adrario E., Bettelli G., Carrani L., Cornara G. Guidelines for completing the perioperative anesthesia record. Minerva Anestesiol. 2002;68:879–892. 892-904. [PubMed] [Google Scholar]
- 14.SIAARTI Study Group for Safety in Anesthesia and Intensive Care Recommendations for anesthesia and sedation in nonoperating room locations. Minerva Anestesiol. 2005;71:11–20. [PubMed] [Google Scholar]
- 15.Ramsay M.A., Savege T.M., Simpson B.R., Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sbrana F., Ripoli A., Formichi B. Safety and utility of noninvasive ventilation during deep sedation for catheter ablation of atrial fibrillation. J Cardiothorac Vasc Anesth. 2014;28 doi: 10.1053/j.jvca.2013.09.003. e6-8. [DOI] [PubMed] [Google Scholar]
- 17.Del Corso A., Vergaro G. Percutaneous treatment of iatrogenic pseudoaneurysms by cyanoacrylate-based wall-gluing. Cardiovasc Interv Radiol. 2013;36:669–675. doi: 10.1007/s00270-012-0502-1. [DOI] [PubMed] [Google Scholar]
- 18.Flenley D.C. Arterial blood gas tensions and pH. Br J Clin Pharmacol. 1980;9:129–135. doi: 10.1111/j.1365-2125.1980.tb05822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cosedis Nielsen J., Johannessen A., Raatikainen P., Hindricks G., Walfridsson H., Kongstad O. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 20.Spragg D.D., Dalal D., Cheema A., Scherr D., Chilukuri K., Cheng A. Complications of catheter ablation for atrial fibrillation: incidence and predictors. J Cardiovasc Electrophysiol. 2008;19:627–631. doi: 10.1111/j.1540-8167.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- 21.Dagres N., Hindricks G., Kottkamp H., Sommer P., Gaspar T., Bode K. Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern? J Cardiovasc Electrophysiol. 2009;20:1014–1019. doi: 10.1111/j.1540-8167.2009.01493.x. [DOI] [PubMed] [Google Scholar]