We present the case of an 18-year old male with a history of isolated noncompaction of the ventricular myocardium (INVM). The patient was originally diagnosed at age 14, following a collapse at school when intermittent complete heart block was seen on ECG. A cardiac MRI at this time showed mild noncompaction of the entire left ventricular (LV) free wall and the distal third of the septum, mild dilatation of both ventricles and a mildly impaired LV ejection fraction (45–55%). On this basis a dual chamber defibrillator (Boston Scientific, Incepta®) was implanted.
Four years later, he began to experience frequent episodes of non-sustained ventricular tachycardia (NSVT) and ventricular tachycardia (VT), which terminated with anti-tachycardia pacing. The patient was started on Sotalol 80 mg bid at this time but went on to have an episode of ventricular storm with frequent monomorphic and polymorphic VT, including one episode that degenerated into ventricular fibrillation and required 9 shocks to terminate. At this time the patient was loaded with intravenous amiodarone and was eventually discharged home on 200 mg of amiodarone daily and 25 mg of metoprolol bid.
Following discharge, he continued to have highly symptomatic runs of NSVT with a 12 lead Holter monitor revealing 3 different VT morphologies (RBBB with positive concordance and inferior axis, RBBB with mid precordial transition and superior axis and RBBB with near positive concordance and superior axis). A VT ablation was planned.
The study was performed under general anaesthesia with use of the CARTO® Navigation System (BiosenseWebster, Diamond Bar, CA, USA). A VT induction was performed from the RV apex initially and rapid polymorphic VT only was induced with a pace train at 400 ms and two extra-stimuli. This required a 200 J biphasic shock to terminate.
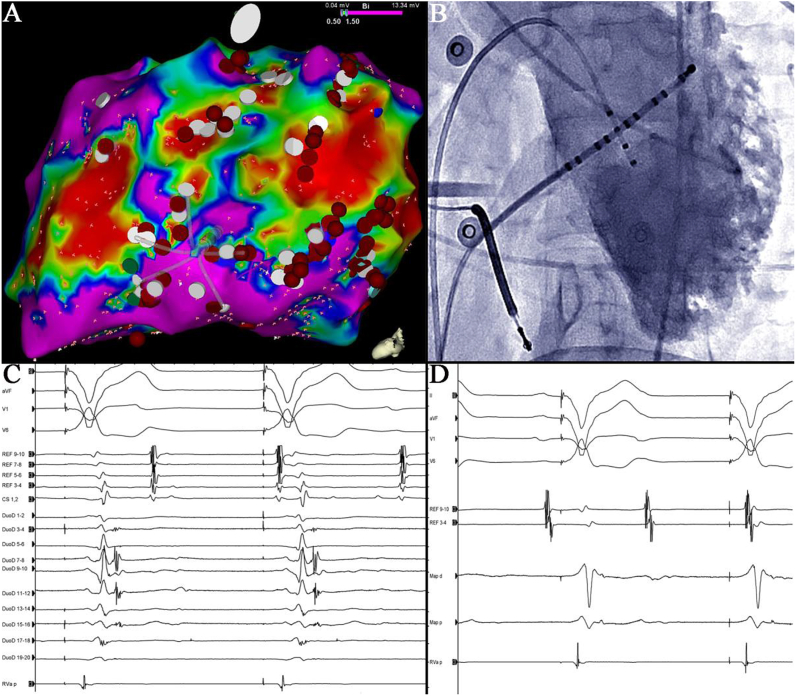
Following this, a substrate-based approach was taken. Electroanatomic mapping of the left ventricle (LV) showed diffuse, patchy scar over its lateral border from the base to the apex (Fig. 1, Panel A). This corresponded to the region of non-compacted myocardium seen on MRI and left ventriculography (Panel B). Extensive heterogeneity of conduction was seen in these regions, as demonstrated by multiple fractionated electrograms and late potentials during right ventricular pacing (Panel C). Identification of fractionated potentials was facilitated by use of the narrow calibre PentaRay® Nav catheter (BiosenseWebster, Diamond Bar, CA, USA) as shown in Panel C.
Fig. 1.
Panel A is a tilted left lateral view of the LV electroanatomic voltage map, showing dense scar (<0.5 mV) and the PentaRay® mapping catheter in good contact with the lateral endocardium. Fractionated and late potentials were tagged with white dots and points of ablation are tagged in red. Panel B is a left ventriculogram in LAO showing deep trabeculation along the lateral LV margin from the base to the apex. Panel C shows apical right ventricular pacing without retrograde conduction to the atrium (REF channels represent coronary sinus electrograms). Splines on the PentaRay® mapping catheter are shown as DuoD 1–20 with frequent fractionated potentials seen across multiple poles. Panel D shows the ablation catheter positioned at DuoD poles 7,8 from Panel C and the same degree of fractionation can not be appreciated. Mapping and ablation catheters were all gained to 2500. Signal filtering on all panels was set at 8 Hz and 500 Hz for low pass and high pass respectively.
Three sites with good pace maps to the clinical VTs (the baso-lateral, apico-lateral and infero-basal LV) were determined. Radiofrequency (RF) ablation was performed at these sites (30–40 Watts, 55 °C) using a 4 mm irrigated tip ablation catheter (Ez Steer® Thermocool®, BiosenseWebster, Diamond Bar, CA, USA). Electrograms from the ablation catheter positioned over DuoD 7,8 in Panel C are shown in Panel D for comparison. All sites with fractionated and late potentials were also targeted with a total of 46 RF lesions delivered (60 s each). During more than 12 months of follow up, the patient has not had any symptomatic VT and a 4 beat run of NSVT only has been detected by his ICD.
Discussion
Isolated noncompaction of the ventricular myocardium is an uncommon cause of cardiomyopathy. Previous case reports of catheter ablation in patients with INVM have not described confluent scar and extensive endocardial substrate. We present a case of catheter ablation in a patient with INVM where confluent scar and frequent late potentials on the endocardium, enabled a successful substrate based approach to ablation of multiple VT morphologies.
At autopsy, patchy regions of fibrosis and elastic tissue have been identified on the endocardium and in inter-trabecular recesses in INVM [1]. Trabecular hyper-enhancement that is indicative of fibrosis and scar has also been shown in MRI studies of INVM [2]. This is consistent with the diffuse endocardial substrate found in our case. The presence of inter-trabecular fibrosis may explain the greater efficacy of the fine fronds of the PentaRay® mapping catheter (3 French calibre) in identifying scar and late potentials when compared to the ablation catheter [3] (Panel C compared with Panel D).
This patient had 3 different VT morphologies on Holter monitor from distinctly different regions of the myocardium in the setting of a cardiomyopathy that can produce a diffuse and progressive substrate. Given his recurrent, symptomatic VT despite amiodarone, however, catheter ablation at an experienced centre was a reasonable course of action. At electrophysiology study, polymorphic VT was easily induced and a diffuse endocardial VT substrate with patchy scar and frequent late potentials was seen, increasing his risk for recurrent VT and VF during follow up [4]. This case demonstrates that catheter ablation in a patient with INVM and diffuse endocardial substrate can be successful in treating recurrent VT using a substrate-guided approach. The novel use of the PentaRay® mapping catheter was effective in identifying scar and late potentials in inter-trabecular regions in this patient with INVM.
Conflict of interest disclosures
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Chin T.K., Perloff J.K., Williams R.G., Jue K., Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507–513. doi: 10.1161/01.cir.82.2.507. [DOI] [PubMed] [Google Scholar]
- 2.Dodd J.D., Holmvang G., Hoffmann U., Ferencik M., Abbara S., Brady T.J. Quantification of left ventricular noncompaction and trabecular delayed hyperenhancement with cardiac MRI: correlation with clinical severity. AJR Am J Roentgenol. 2007;189:974–980. doi: 10.2214/AJR.07.2364. [DOI] [PubMed] [Google Scholar]
- 3.Stinnett-Donnelly J.M., Thompson N., Habel N., Petrov-Kondratov V., Correa de Sa D.D., Bates J.H. Effects of electrode size and spacing on the resolution of intracardiac electrograms. Coron artery Dis. 2012;23:126–132. doi: 10.1097/MCA.0b013e3283507a9b. [DOI] [PubMed] [Google Scholar]
- 4.Steffel J., Kobza R., Namdar M., Wolber T., Brunckhorst C., Luscher T.F. Electrophysiological findings in patients with isolated left ventricular non-compaction. Europace. 2009;11:1193–1200. doi: 10.1093/europace/eup187. [DOI] [PubMed] [Google Scholar]