Case presentation
An 8-year-old female patient was referred to a paediatrician for assessment of an episode of global muscle weakness after a 100 m sprint. There was no loss of consciousness. Proximal hip muscles and shoulder girdle weakness was noted and the possibility of a myopathy was raised. Muscle biopsy demonstrated “ultra-structurally normal muscle fibres”. There was indolent progression of proximal muscle weakness over the years, with no diagnosis made.
At the age of 18 the patient experienced three episodes of transient loss of consciousness over a two-week period. A witness described sudden “unresponsiveness and generalised muscle twitching”; a period of confusion consistent with a post-ictal period was noted. There was no urinary incontinence or tongue biting. No trigger was identified. Inpatient EEG and MRI brain were normal. A further episode of transient loss of consciousness, documented in the hospital record as ‘consistent with seizure activity’ occurred; cardiac monitoring did not reveal an arrhythmia. A clinical diagnosis of epilepsy was made based on the description of the episodes and lack of a symptom rhythm correlation. The patient was commenced on sodium valproate, which prevented further episodes.
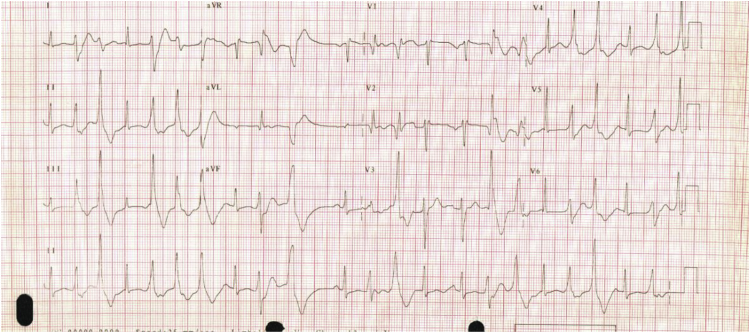
Surface electrocardiograms recorded during the admission documented asymptomatic, frequent polymorphic ectopy Fig. 1.
Fig. 1.
A 12 lead ECG recorded from the patient.
The QTc was marginally prolonged at 465ms (Bazett's formula). Echocardiogram showed no evidence of structural heart disease. An outpatient Holter monitor confirmed the background rhythm was sinus, with episodes of non-sustained bidirectional ventricular tachycardia and frequent multimorphological ventricular ectopy with a 25% ectopic burden. Ectopy was suppressed completely during treadmill exercise testing. The effect of exercise on the QT interval was not recorded. Metoprolol, verapamil, sotalol and flecainide were sequentially tried, but were either ineffective or produced intolerable side effects.
At the age of 23 years the patient moved interstate and was referred for review. She had been seizure-free for 5 years. Medical background was unremarkable. Family history was incomplete (patient had no available history from the paternal side. No abnormalities were noted on the maternal side). Physical examination revealed short stature, mandibular hypoplasia, hypertelorism and a broad nasal root. Neurological assessment demonstrated a waddling gait, with inability to rise from a sitting position without assistance. No fasciculation or myotonia was evident. Marked proximal upper and lower limb girdle weakness, Medical Research Council (MRC) 2–3/5, was found associated with hypotonia. Distal strength was largely preserved. Reflexes were symmetrically depressed. Cardiovascular examination found an irregular pulse. ECG showed frequent polymorphic ectopy as before.
A channelopathy for the cause of her frequent ventricular ectopy and syncope was postulated. Catecolaminergic polymorphic ventricular tachycardia (CPVT), was an obvious consideration, however the suppression of the ectopy during an exercise stress test, essentially excluded this diagnosis. Brugada syndrome and arrhythmogenic right ventricular cardiomyopathy were thought less likely as the ECG didn't show features typically associated with these disorders. The long QT syndrome although associated with polymorphic ventricular tachycardia, is not generally described with frequent ventricular extrasystoles at rest or bidirectional ventricular tachycardia. This patient not only had frequent multifocal ectopy, but had a documented episode of bi-directional ventricular tachycardia, a feature well described in the Andersen-Tawil syndrome. The baseline ECG didn't demonstrate the classic prominent U waves that have been documented in other ATS patients.
All the above channelopathies, besides ATS, are not traditionally reported with muscle weakness or facial dysmorphism and given the specific documented cardiac arrhythmias a clinical diagnosis of Andersen-Tawil syndrome was made.
In light of the lack of seizures for 5 years, the anti-epileptic medication was discontinued. Six months later, the patient experienced recurrent syncope, without convulsive activity. The patient described these episodes as different from those she had suffered at the age of 18. Holter monitoring, recorded peri-event, showed non-sustained runs of bidirectional ventricular tachycardia, as well as monomorphic ventricular tachycardia Fig. 2.
Fig. 2.
Holter monitor tracing demonstrating an episode of non-sustained bidirectional ventricular tachycardia.
Due to accelerated symptomotology, positive ECG correlation and the clinical difference from previous syncope, an internal cardiac defibrillator (ICD) was advised. Immediately prior to implantation the patient became pregnant. She continued to suffer recurrent syncope and, at the beginning of the second trimester, an ICD was implanted. A pectoral muscle biopsy was sent for genetic analysis. This confirmed a mutation in c.652C > T of the KCNJ2 gene, previously reported in the Andersen-Tawil syndrome [1].
After implantation the patient experienced further syncope. The internal electrogram recorded during the episode showed no sustained arrhythmia. Witnesses described convulsive activity and symptoms consistent with a post ictal period and the possibility of a concurrent seizure disorder was raised. Sodium valproate was reinitiated and, to date, there have been no further seizures.
Eight months later the patient developed recurrent polymorphic ventricular tachycardia resulting in repeated shocks Fig. 3.
Fig. 3.
Portion of the electrogram from interrogation of the patients' ICD on admission to hospital following discharge of the device demonstrating a tachycardia in the VT/VF zone.
The majority of these occurred either at the onset of or during sleep, consistent with a hypothesis generated in a previous case report [2], that autonomic instability might precipitate haemodynamically compromising ventricular arrhythmias in patients with Andersen-Tawil syndrome. The device was reprogrammed to allow a longer delay before shock delivery resulting in long self terminating runs of polymorphic ventricular tachycardia associated with pre-syncope. Carvedilol and flecainide were commenced, and effectively suppressed ectopy but produced severe headaches requiring cessation. Mexiletine was of no benefit. Hence due to failure of medical treatment, left stellate ganglionectomy was performed. However, frequent ectopy and infrequent episodes of symptomatic polymorphic ventricular tachycardia continued unabated, though fortunately programming of the ICD has avoided recurrent shocks.
Discussion
This case report describes a 23-year old female with recurrent syncope, the Andersen-Tawil syndrome and concomitant epilepsy. ATS is a rare genetic disorder first described in 1971 by Dr. Ellen Andersen [3] in an 8-year old boy. Dr Rabi Tawil subsequently clarified the underlying genetic abnormalities as well as setting out diagnostic criteria [4]. The underlying genetic abnormality in 60% of cases relates to a missense mutation in KCNJ2 (detected on chromosome 17q23) [5], a gene that encodes for the inward potassium rectifier channel Kir 2.1 (IK1), leading to prolongation of the terminal phase of the cardiac action potential. This genetic defect can result in “terminal phase” early after depolarisations, which is thought to be demonstrated by the recurrent ventricular ectopy as well as prominent U waves on the surface ECG [4].
The inheritance of ATS is autosomal dominant, though there is incomplete penetrance [2]. Approximately 20% of those with a KCNJ2 mutation are asymptomatic. Conversely, up to 40% of patients with the clinical phenotype are negative for mutations involving KCNJ2.
The QT interval is not consistently prolonged in these patients and thus some authors have discontinued using the previous designation of long QT 7 [6].
The diagnosis of ATS is based upon three specific features: dysmorphic abnormalities such as hypognathia, clinodactyly, short stature, hypertelorism, low set ears, single palmar crease and cryptochordism [7], periodic paralysis and frequent ventricular extrasystoles [3]. A diagnosis of ATS can be made when two of the above three criteria are met. In a patient with only one of the above additional evidence, such as an affected family member is required [8].
No specific features of the syndrome predict the severity of arrhythmic burden or risk of sudden cardiac death (SCD). In one study consisting of 96 patients, the incidence of Torsades de pointes was approximately 3% [9].
Typically, periodic paralysis presents before other components of the disorder manifest themselves. The average age of onset of muscle weakness is 5 years of age compared to 13 years of age for cardiac arrhythmias. This may explain the difficulty in formal diagnosis. There is no documented relation to other potassium sensitive forms of periodic paralysis. As in this patient, weakness following prolonged exertion is common and muscle biopsies, generally, have no specific abnormalities [10]. The underlying defect in skeletal muscle is also due to a mutation in the IK1 channel.
The cardiac features are now thought to be due to a channelopathy that results in varying degrees of ventricular arrhythmias [10] ranging from frequent multimorphological ectopy to bidirectional ventricular tachycardia to polymorphic ventricular tachycardia. Despite the impressive arrhythmic burden (up to 65% of patients in one cohort had evidence of non-sustained ventricular tachycardia) [9]; the majority of patients with ATS are asymptomatic [7]. Changes on the surface ECG include: prominent U waves in the anterior leads (not associated with bradycardia or hypokalemia), increased T-U junction and prolongation of the terminal, down sloping portion of the T wave [9]. Sudden death is rare and much less than that associated with other channelopathies such as the Long QT or Brugada syndromes [2].
Differentiating cardiac syncope from other forms of transient loss of consciousness can be difficult. Features more suggestive of cardiac origin include preceding feelings of light-headedness/diaphoresis, palpitations or chest pain. They may also be associated with activities such as prolonged standing/sitting or micturition etc. Transient loss of consciousness associated with a seizure may demonstrate a preceding aura (visual/auditory/olfactory), involve tongue biting, urinary incontinence, post ictal period and even focal neurological deficits.
Convulsive syncope can be particularly difficult to discern a cause as global hypoperfusion of the brain leads to syncope, but also associated convulsive activity.
Although it was initially felt that this patients' recurrent syncope was cardiac in origin, the absence of a recorded arrhythmia, convulsive activity and post-ictal periods during some of the episodes and eyewitness accounts, strongly suggested epilepsy as an additional diagnosis. Since recommencing sodium valproate, no further seizures have occurred. Although, an increased incidence of epilepsy is not reported in patients with ATS and occurrence in this patient may reflect the background prevalence of epilepsy in the general population. It could also represent the expression of her ion channelopathy in neural tissues. Recent publications have intimated a deficit in abstract reasoning and executive function suggesting subtle cerebral abnormalities in patients with ATS [11]. Although it is possible that the Kir 2.1 mutation, which leads to the missense mutation in potassium channels in cardiac myocytes, may also affect similar channels in the brain. To the author's knowledge, it has not been proven whether this defect results in seizures.
This case is unusual because of the significant resistance to anti-arrhythmic medication. Only flecainide suppressed the frequency of ectopy and bidirectional ventricular tachycardia, but could not be tolerated by the patient (The efficacy of flecainide in reducing ectopy has been noted in other individual cases of ATS) [12], [13]. Beta-blockers also could not be tolerated. Stellate ganglionectomy was considered even though the episodes occurred at rest, because she was symptomatic with frequent episodes of polymorphic ventricular tachycardia and had failed available medical treatment.
Currently there are no specific guidelines for the implantation of an ICD in patients with Andersen-Tawil syndrome. Current consensus is that patients who are survivors of a cardiac arrest, have recurrent syncope or sustained, symptomatic ventricular tachyarrhythmias should be considered for ICD implantation [4].
The fetus was tested and demonstrated the same mutation as the proband, but to date has not experienced cardiac arrhythmias, although she does have the proximal muscle weakness and the waddling gait (currently 5 years-old).
In summary this case highlights the complexities of managing a patient with Andersen-Tawil syndrome manifesting as refractory arrhythmia and profound proximal weakness. The patient had concurrent epilepsy, which resulted in clinically different episodes from those caused by the arrhythmia. The case illustrates the importance of awareness of the clinical features of this syndrome, and in managing concomitant medical issues. We encourage other groups to report their experience of seizures, in patients with the ATS, to promote further research into the link between KCNJ2 mutations and neurological disorders.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society
Contributor Information
Michael David Fryer, Email: mfryer006@hotmail.com.
Gerald Kaye, Email: Gerald.kaye@health.qld.gov.au.
Susan Tomlinson, Email: susan.tomlinson@sydney.edu.au.
References
- 1.Van der Werf C., Hofman N., Tan H.L. Diagnostic yield in sudden unexplained death and aborted cardiac arrest in the young: the experience of a tertiary referral center in The Netherlands. Heart Rhythm. 2010;7:1383–1389. doi: 10.1016/j.hrthm.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 2.Airey K.J., Etheridge S.P., Tawil R., Tristani-Firouzi M. Resuscitated sudden cardiac death in Andersen-Tawil syndrome. Heart Rhythm. 2009;6:1814–1817. doi: 10.1016/j.hrthm.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen E.D., Krasilnikoff P.A., Overvad H. Intermittent muscular weakness, extrasystoles, and multiple developmental anomalies. A new syndrome? Acta Paediatr Scand. 1971;60:559–564. doi: 10.1111/j.1651-2227.1971.tb06990.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith A.H., Fish F.A., Kannankeril P.J. Andersen-tawil syndrome. Indian Pacing Electrophysiol J. 2006;6:32–43. [PMC free article] [PubMed] [Google Scholar]
- 5.Venance S.L., Cannon S.C., Fialho D. The primary periodic paralyses: diagnosis, pathogenesis and treatment. Brain. 2006;129:8–17. doi: 10.1093/brain/awh639. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson M.R., Yoon G., Fu Y.H., Ptacek L.J. Andersen-Tawil syndrome: a model of clinical variability, pleiotropy, and genetic heterogeneity. Ann Med. 2004;36:92–97. doi: 10.1080/17431380410032490. [DOI] [PubMed] [Google Scholar]
- 7.Tristani-Firouzi M., Jensen J.L., Donaldson M.R. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome) J Clin Invest. 2002;110:381–388. doi: 10.1172/JCI15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Statland J.M., Tawil R., Venance S.L. University of Washington; Seattle: 2004. Andersen-tawil syndrome. GeneReviews. [PubMed] [Google Scholar]
- 9.Zhang L., Benson D.W., Tristani-Firouzi M. Electrocardiographic features in Andersen-Tawil syndrome patients with KCNJ2 mutations: characteristic T-U-wave patterns predict the KCNJ2 genotype. Circulation. 2005;111:2720–2726. doi: 10.1161/CIRCULATIONAHA.104.472498. [DOI] [PubMed] [Google Scholar]
- 10.Tawil R., Ptácek L.J., Pavlakis S.G. Andersen's syndrome: potassium- sensitive periodic paralysis, ventricular ectopy, and dysmorphic features. Ann Neurol. 1994;35:326–330. doi: 10.1002/ana.410350313. [DOI] [PubMed] [Google Scholar]
- 11.Yoon G., Quitania L., Kramer J.H., Fu Y.H., Miller B.L., Ptacek L.J. AndersenTawil syndrome: definition of a neurocognitive phenotype. Neurology. 2006;66:1703–1710. doi: 10.1212/01.wnl.0000218214.64942.64. [DOI] [PubMed] [Google Scholar]
- 12.Fox D., Klein G., Hahn A. Reduction of complex ventricular ectopy and improvement in exercise capacity with flecainide therapy in Andersen-Tawil syndrome. Europace. 2008;10:1006–1008. doi: 10.1093/europace/eun180. [DOI] [PubMed] [Google Scholar]
- 13.Bokenkamp R., Wilde A., Schalij M., Blom N. Flecainide for recurrent malignant ventricular arrhythmias in two siblings with the Andersen Tawil syndrome. Heart Rhythm. 2007;4:508–511. doi: 10.1016/j.hrthm.2006.12.031. [DOI] [PubMed] [Google Scholar]