Summary
Permanent pacing, being non physiological, often results in ventricular dysfunction over time. Narrower paced QRS duration from pacing the right ventricular outflow tract septum, might result in relatively preserved ventricular function over long term follow up.
Keywords: Right ventricular outflow tract pacing, Paced QRS duration, Equilibrium radionuclide angiography (ERNA)
1. Introduction
More than 50 years have passed since first successful human implantation of an external cardiac pacemaker [1]. However, still the optimal pacing mode and pacing site remains elusive. The normal myocardial activation is through the conduction system resulting in synchronous activation of both ventricles. Though myocardial stimulation achieved by pacing outside the conduction axis results in efficient contractions with impressive haemodynamic results, it is not physiological. Such a pacing generates a wave front which propagates slowly through the myocardium leading to ventricular dyssynchrony. This slow conduction through myocardium results in intraventricular conduction delay (IVCD), manifested on surface electrocardiogram (ECG) by a prolonged QRS duration (QRSd). Right ventricle (RV) pacing prolongs the surface ECG QRSd variably depending on the lead position.
Studies have shown that a prolonged QRSd is associated with increased congestive heart failure (CHF) risk, even in patients without any overt cardiovascular disease [2], [3]. A prolonged QRSd is also an independent risk factor for sudden cardiac death (SCD) [4], [5]. In patients with CHF, those with prolonged QRSd have lower left ventricle ejection fraction (LVEF), higher LV volumes and protracted course of heart failure, than in counterparts with shorter QRSd, indicating more advanced cardiac pathology [6], [7], [8], [9], [10], [11].
In patients receiving permanent pacemaker implants (PPI), paced QRS duration (p-QRSd) might be used as a useful indicator for impaired LV function [12], [13], [14], [15] and can predict the occurrence of congestive heart failure [14], [16], [17], [18]. Though various imaging modalities like 2D transthoracic echocardiogram (2D-TTE), 3D transthoracic echocardiogram (3D-TTE), magnetic resonance imaging (MRI) and radionuclide imaging (RNI) studies can be utilized to measure LV function, each modality has its own merits and demerits. Unlike echocardiography radionuclide imaging is objective and has a good correlation with MRI in measuring LV function and volumes [19]. Radionuclide imaging studies are safe, feasible, and readily available at many centres and scores over MRI in post pacemaker patients due to the concerns of MRI incompatibility.
The relationship between p-QRSd and left ventricular (LV) function using equilibrium radionuclide angiography (ERNA) in patients with permanent RV pacing has not been previously studied in a comprehensive manner. We intended to study the association between p-QRSd and LV function measured using ERNA in patients with permanent RV pacing who were documented to have normal baseline LV function.
2. Methods
Seventy consecutive patients who underwent permanent pacemaker implantation and had normal LV function at baseline were prospectively enrolled. Informed written consent was obtained from all participants. The study was approved by the Institute Ethics Committee. Patients with prior LV dysfunction (LV function less than 50% assessed by 2D TTE), those who required cardiac resynchronization therapy (CRT), those in atrial fibrillation, those with structural heart defects and those who were unable to provide informed consent were excluded from the study.
2.1. Pacemaker implantation protocol
We utilized fluoroscopically guided axillary vein puncture in all these patients. The details of the procedural technique have been described by our group in a previous study [20]. The type of pacemaker to be implanted whether dual or single chamber was decided by the patient and the treating physician. The position of lead implantation and site in RV was decided by the operator. Site of implantation of pacing lead at RV apex (RVA) or RV outflow tract (RVOT) was noted in all patients. The 40-degree right anterior oblique (RAO) view arbitrary divides the RV in to high RVOT, low RVOT, mid septum, low septum and RV apex. Bundle of HIS forms the inferior margin and pulmonary valve forms the superior margin of RVOT, which is further arbitrary, divided in to two equal areas, high and low RVOT. Part of RV below the bundle of HIS forms the septum, which is again equally divided into mid and low septum [21]. Cine fluoroscopy images of final lead positions were recorded in all patients.
Intra-procedural lead stimulation threshold was checked in all cases at a pulse width of 0.5 ms using a pacing system analyser. Ventricular threshold of ≤2.0 V and R-wave sensing ≥5 mV were considered acceptable pacing parameters.
2.2. Equilibrium radionuclide angiography protocol
All patients underwent Technetium-99m labelled Red Blood Cells (Tc-99m RBC) ERNA studies both at rest (with pacing parameters as at the time of pacemaker implantation) and under forced cardiac pacing at 100 beats per minute (bpm). Forced pacing at 100 bpm was conducted to ensure uniformity so that p-QRSd does not vary owing of different pacing rates. The RBC labelling was performed by 0.5–0.9 mg (15 μg/Kg of body weight) of stannous ions as stannous chloride via intravenous route. The stannous ions prime the RBCs, which were tagged with 15–20 mCi (550–740 MBq) of Tc99m pertechnate injected intravenously. Rest ERNA acquisition was performed 10–15 min later with a dual head gamma camera (Infinia Hawkeye 7; General Electric Company, Milwaukee, Wisconsin, USA) fitted with a Low energy general purpose (LEGP) collimator. Images were acquired in left anterior oblique view (for best septal view, approx. LAO 40–50°) using energy discrimination centered on 140 ± 10% kilo electron volts (keV). The projection was gated with the ECG to get 32 frames spanning the cardiac cycle. Images were acquired in 64*64 matrix, with a zoom factor of 1.6; the view was acquired for 300–400 s (approx. 500–600 Kilo counts). Elimination of ventricular premature beats was obtained with a window threshold of 15% around the mean RR interval during acquisition of projections. Extrasystolic and post extrasystolic cycles were excluded.
Each patient had baseline rest (S0) & baseline forced paced (pS0) study within 5 days of pacemaker implantation with follow-up studies at 6 month (rest S1 and forced paced pS1) and 12 month (rest S2 and forced paced pS2). The rest study was conducted with default pacing parameters, while the forced paced study was conducted at a forced pacing rate of 100 bpm. LV ejection fraction (LVEF) was analysed as a parameter of LV function.
Paced QRS duration on surface ECG was measured in all patients at a forced pacing rate of 100 bpm, after recording ECG strips at a paper speed of 50 mm per sec to minimize error. Patients were divided into two groups based on p-QRSd using a cutoff value of ≤130 ms (ms). Group 1 included patients with PQRSD ≤130 ms while group 2 had PQRSD >130 ms. We studied various parameters between these two groups including LV function, the percentage of ventricular pacing, development of atrial fibrillation and any other pacemaker related events documented during routine pacemaker interrogation.
2.3. Statistical analysis
Data are presented as mean with standard deviations or median with ranges where appropriate. Chi Square test was used for comparison of categorical (qualitative) data between groups. For normally distributed continuous variables, the Student's t-test was applied for comparison between two groups. If variables did not follow normal distribution, the Mann–Whitney test was applied. Statistical significance was analysed using the Paired T test for comparisons of paired quantitative data if the data was normally distributed. If the data was not normally distributed, Wilcoxon log rank test was used for analysis. Comparison of repeated measurements was done using general linear model (One-way analysis of variance was used to compare the repeated measures of continuous variables between groups, followed by a post hoc bonferroni's test, as appropriate.). A p value of <0.05 was considered as significant. All the data analyses were performed using the statistical software packages SPSS 11.5 (SPSS Inc., Chicago, Illinois, USA).
3. Results
Out of seventy patients who were enrolled in the study, forty-eight who completed 12 months of follow up were included in the final analysis. The baseline characteristics of the study population are shown in Table 1. Mean age of the patients was 56.21 ± 12.04 years with 28 male patients (58.33%). Thirteen patients were lost to follow-up or did not adhere to the schedule of study protocol and nine patients whose studies failed quality control due to high percentage of rejected beats; were excluded from the final analysis.
Table 1.
Baseline characteristics of study population.
| Characteristic | Total (N = 48) | N (%) | |
|---|---|---|---|
| Age (years) | 56.21 ± 12.04 | ||
| Sex | Male | 28 | 58.33 |
| pQRSd (ms) | 141.46 ± 22.54 | ||
| Indication | CHB | 44 | 91.77 |
| SSS | 4 | 8.33 | |
| Pacing | Dual chamber | 33 | 68.75 |
| Single chamber | 15 | 31.25 | |
| Site | RVOT | 27 | 56.25 |
| RVA | 21 | 43.75 | |
| Baseline LVEF | 56.80 ± 4.53 | ||
pQRSd: paced QRS duration, CHB: complete heart block, SSS: sick sinus syndrome, RVOT: right ventricle outflow tract, RVA: right ventricle apex, LVEF: left ventricle ejection fraction.
All patients had successful, fluoroscopically guided axillary vein puncture without any procedural complication. Complete heart block was the most common indication for pacing (44/48 patients; 91.66%). Thirty three patients (68.75%) received dual chamber pacemaker; while the rest had isolated RV pacing. 28 (58.33%) patients received RVOT pacing. Mean pQRSd of the study population was 141.46 ± 22.54 ms.
Nineteen patients had a pQRSd ≤130 ms (Group 1) and 29 patients had pQRSd >130 ms (Group 2). Baseline characteristics were similar between the two groups with respect to age, sex, percentage of ventricular pacing, presence of predisposing cardiac disease like hypertension, coronary artery disease, valvular heart disease and diabetes mellitus (Table 2). There was no significant difference in the number of patients with respect to dual v/s single chamber pacemaker between the two groups. Left ventricular ejection fraction (LVEF) at baseline did not differ significantly between both groups (57.2 ± 4.1% vs 56.5 ± 4.8%; P = 0.615). It was observed that the majority of patients (14/19, 73.68%) in group 1 had pacing leads implanted in the RVOT, as compared to 14/29 (48.27%) in group 2. pQRSd (mean ± S.D.) was significantly greater in group 2 than in group 1 (155.5 ± 17.31 ms vs. 120 ± 7.45 ms; p < 0.0001) (Fig. 1). At the end of one year follow-up, a statistically significant decline in LVEF was observed in group 2 (54.7 ± 5.6% to 52.9 ± 6.3%; P = 0.031); but LVEF was found to be preserved in group 1 (56.6 ± 5.5% to 54.9 ± 7.5%; P = 0.151) (Fig. 2). No significant difference in LVEF between the two groups was observed after one year of follow-up (54.8 ± 7.3 v/s 52.3 ± 6.9%, p = 0.946). However when paired differences between both groups were analysed, the change in LVEF was not found to be significant. A small study group could be the possible reason for this discrepancy. None of the patient in either group developed new onset atrial fibrillation, congestive heart failure or had acute coronary syndrome during this period.
Table 2.
Characteristics between group 1 (pQRSD ≤130 ms) and group 2 (pQRSd >130 ms).
| Parameters | Group 1 (N = 19) Mean ± S.D |
Group 2 (n = 29) Mean ± S.D |
P value | |||
|---|---|---|---|---|---|---|
| Age | 54.79 ± 14.62 | 57.13 ± 10.18 | p = 0.515 | |||
| Sex (male/female) | 8/11 | 20/9 | p = 0.065 | |||
| pQRSd (msec) | 120 ± 7.4 | 155.5 ± 17.3 | P < 0.0001 | |||
| Mode | Dual chamber | 16 | 17 | P = 0.061 | ||
| Single chamber | 3 | 12 | ||||
| Site | RVOT | 14 | 14 | P = 0.080 | ||
| Apical | 5 | 15 | ||||
| Hypertension | 7 | 16 | P = 0.250 | |||
| Diabetes | 1 | 5 | P = 0.381 | |||
| Coronary Artery Disease | 1 | 6 | P = 0.219 | |||
| Valvular heart disease | 0 | 2 | P = 0.512 | |||
| Ventricular Pacing (%) | 53.8 ± 43.0 | 63.1 ± 44.7 | P = 0.541 | |||
| LVEF (Baseline %) | 57.2 ± 4.1 | 56.5 ± 4.8 | P = 0.615 | |||
| LVEF (at 12 months %) | 54.8 ± 7.3 | 52.3 ± 6.9 | P = 0.946 | |||
| LVEF (at paced rate 100 bpm) baseline % | 56.6 ± 5.5 | P = 0.151 | 54.7 ± 5.6 | P = 0.031 | P = 0.272 | |
| LVEF (at paced rate 100 bpm) 1 Year % | 54.9 ± 7.5 | 52.9 ± 6.3 | P = 0.239 | |||
| Paired difference in LVEF at 1 year % | −1.67 ± 4.70 | −1.75 ± 4.07 | P = 0.95 | |||
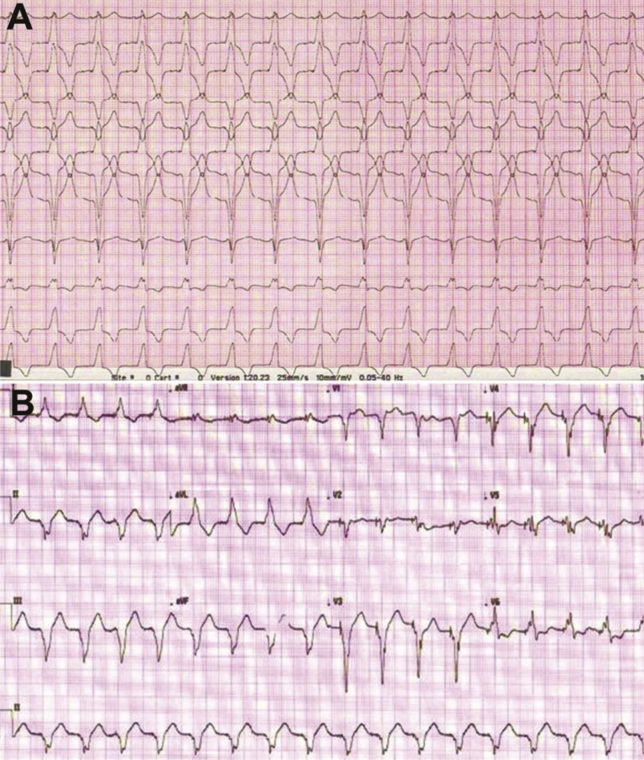
Fig. 1.
A. Electrocardiogram of a patient in group 1 with RVOT pacing having narrow paced QRS duration (pQRSd) of <130 ms. B. Electrocardiogram of patient in group 2 with RV apical pacing having pQRSd of >130 ms.
Fig. 2.
There is significant fall in left ventricle ejection fraction (LVEF) in group 2 with paced QRD duration (pQRSd) >130 ms (54.7 ± 5.6 to 52.9 ± 6.3, p = 0.031). Whereas there is insignificant fall in LVEF in group 1 with pQRSd ≤130 ms (56.6 ± 5.5 to 54.9 ± 7.5, p = 0.151).
4. Discussion
4.1. Paced QRS duration and left ventricular function
Studies have consistently shown that narrow paced QRS duration is associated with preservation of LV function and reduced incidence of heart failure events. Sumiyoshi et al. [12] did a retrospective analysis of 114 patients implanted with permanent pacemaker for atrio-ventricular block. Patients with pQRSd of more than 180 ms had significantly high incidence of underlying heart disease (83% vs 32%, p < 0.01), reduced LVEF (49 ± 17% vs 68 ± 10%, p < 0.01) and elevated LV end diastolic dimension (57.1 ± 7.9 mm vs 48.5 ± 5.6 mm, p < 0.01), as compared to those with pQRSd of less than 180 ms. The authors concluded that prolonged pQRSd is associated with more advanced underlying heart disease and can be valuable in predicting impaired LV systolic function. A case control study in patients receiving permanent RV apical pacing for at least 1 year [13] showed pQRSd to have significant negative correlation with LVEF (β = −109.25, P < 0.001) and significant positive correlations with LVDd (LV end-diastolic) and LVDs (LV end-systolic) dimensions (β = 1.59 and 1.54, respectively; P < 0.001). A pQRSd value of 200 ms was 71.72% sensitive and 86.71% specific to detect LV systolic dysfunction. Similar results were reiterated by Yangang et al. [22].
Miyoshi et al. [14] prospectively analysed p-QRSd during RV pacing in 92 permanently paced patients over a mean period of 53 ± 16 months. Prolonged p-QRSd using a cut-off valve of ≥190 ms significantly predicted the development of CHF(46.6% v/s 11.6%, p < 0.05) and was associated with significant worsening of LV function parameters, with lower LVEF (53.0 ± 14.6% vs 62.8 ± 12.3%, p < 0.05), higher LVDd (60.0 ± 10.4 mm v/s 48.9 ± 7.4 mm, p < 0.05) and LVDs (43.2 ± 11.5 vs 32.1 ± 8.1 mm, p < 0.05) dimensions, as compared to those with pQRSd of less than 190 ms A recent observational cohort study PREDICT-HF [16] enrolled 194 patients receiving RV apical pacing and stratified them to groups based on pQRSd. Over a mean follow-up period of 3 years significantly higher number of patients (56.8%) with pQRSd >190 ms developed overt heart failure as compared to 9.4% with pQRSd <160 ms, p < 0.001. Linear regression analyses showed falling LVEF to correlate with prolonged pQRSd (RR = 0.423, p < 0.05).
The prolonged pQRSd results in haemodynamic deterioration not only in long term but also results in acute haemodynamic worsening. Hong et al. [23] studied the acute haemodynamic consequences of RV pacing and its relationship with pQRSd. They found that systolic blood pressure (SBP) decrease during pacing was larger in the group exhibiting pQRSd of greater than 140 msec. The SBP decrease during pacing showed significant correlation with pQRSd (r = 0.500, p = 0.001). They concluded that ventricular pacing should be performed from a site with narrowest pQRSd possible, preferably <140 msec.
Consistent with prior studies, our study also shows that in patients with permanent RV pacing and normal baseline LV function, presence of narrow paced QRS duration (pQRSd <130 ms) is associated with preservation of LV function at 12 month follow-up. However the change of LVEF in group 2 with pQRSd >130 ms in our study group was less than that reported by previous authors. We also did not found any event of congestive heart failure at 12 month follow-up. These observations could be explained by the various factors. Firstly, RV pacing induced adverse left ventricular remodelling in patients with normal baseline LV function may manifest after a long time [24], [25]. A shorter follow-up period of 12 months as compared to other clinical studies explains this nil incidence of new onset heart failure and relative preservation of LVEF in our study group. Moreover, the mean pQRSd of our study group (141.46 ± 22.54 ms) was far less than that in other studies, suggesting far less advanced underlying cardiac disease in our study population. Prior studies have included a large number of patients with underlying heart disease as compared to relatively healthy population in our study. Our study population was relatively young as compared to the western studies, which could have affected these outcomes. Finally there were no events of acute coronary syndrome or development of new onset atrial fibrillation in the follow-up period which could have predisposed to LV dysfunction.
4.2. Role of paced site and QRS duration
Site of RV Pacing determines the myocardial activation sequence, and thus pacing at different RV sites result in varied paced QRS duration. As prolong pQRSd is associated with worsening of LV function and higher risk of heart failure hospitalisations, right ventricular outflow tract (RVOT) pacing has been proposed as an alternative RV pacing site to preserve LV function [26], [27].
Nakmura et al. [21] studied the effect on pQRSd on pacing RV from five different sites in 50 patients. They divided RV pacing sites into high RVOT, low RVOT, mid septum, low septum and RV apex. The pQRSd was significantly shorter during low RVOT (143 ± 17 ms) and mid-septal (151 ± 20 ms) pacing compared to apical (167 ± 18 ms) pacing site (p < 0.001). QRS duration was shortest when the septum was paced in the right ventricle. However in 32% patients, pQRSd was no different with low-RVOT, mid-septal, or apical pacing. The authors concluded that low RVOT or mid septal pacing is likely to lead to less dyssynchrony than RV apical pacing in almost 70% of the patients requiring RV pacing. A recent study [28] reported significantly longer pQRSd in patients receiving the right ventricular apical pacing (160 ± 15 ms) as compared to RVOT (140 ± 15 ms, P = 0.02) and RV inflow tract pacing (133 ± 17 ms, P < 0.001).
In our study, presence of majority of leads at RVOT (14/19) in patients with narrow pQRSd <130 ms, suggests that RVOT pacing might well be associated with less prolongation of pQRSd and lesser deterioration in LV function as compared to RVA pacing. Although alternate site RV pacing leads to narrow pQRSd as compared to RV apical pacing, long term clinical benefit of non-apical pacing are still not completely clear. Though there are studies which show clinical benefit of alternate site RV pacing at para-Hisian [29], septal [24] and RVOT [30] sites as compared to RV apical position, there are others which refute this notion [31], [32]. Most of these are small non randomized studies with relatively short follow-up period and variable clinical endpoints, making interpretation difficult. Moreover most studies have used echocardiography as imaging modality to follow-up changes in LV function, which is highly subjective and is bound to have inter as well as intra observer variability. Three on-going large randomized control trials (PROTECT PACE, RASP, and OPTIMISE RV) randomizing patients to apical versus septal pacing, may shed light on issue of significance of alternate RV pacing site in patients requiring long term RV pacing [33].
5. Limitations
This was a single centre non randomized observational study involving small study group with a relatively short follow-up duration. Though Equilibrium radionuclide angiography (ERNA) derived LV function was assessed by independent observers having no access to other patient data, still the potential of observer bias remains. Equilibrium radionuclide angiography (ERNA) findings were not validated against routinely used modality for assessment of LV function like echocardiography. However, since same imaging modality was used in follow-up, each patient served as his/her own control and therefore, this was not mandatory. The study exclusively included patients with normal baseline LV function, so whether the findings of this study are applicable to patients with LV dysfunction remains an issue for further investigation. Though the change in LVEF at 1 year when both groups were compared was significant, there was no significant change in LVEF when paired difference in LVEF was analysed. This discrepancy is a major limitation of this study which could be explained by a small sample size. May be a larger prospective study with longer follow-up address this anomaly.
6. Conclusion
Equilibrium radionuclide angiography (ERNA) can be used for quantitative assessment of left ventricular ejection fraction in patients with permanent pacemaker and provides reliable objective assessment of LV function in follow-up studies. Paced QRS (pQRSd) duration of ≥130 ms may be associated with significant decline in left ventricular ejection fraction over a period of 1 year as compared to those with pQRSd of <130 ms. RVOT pacing might be associated with less prolongation of pQRSd and lesser deterioration in LV function as compared to RVA pacing, and should be more fully assessed in a large randomized control trial setting.
Conflict of interests
None.
Disclosures
Dr Sanders is supported by the National Heart Foundation of Australia and by a Practitioner Fellowship from the National Health and Medical Research Council of Australia. Dr Sanders reports having served on the advisory board of Biosense-Webster, Medtronic, St Jude Medical, Sanofi-Aventis and Merck, Sharpe and Dohme. Dr Sanders reports having received lecture and/or consulting fees from Biosense-Webster, Medtronic, St Jude Medical, Boston Scientific, Merck, Sharpe and Dohme, Biotronik and Sanofi-Aventis. Dr Sanders reports having received research funding from Medtronic, St Jude Medical, Boston Scientific, Biotronik and Sorin.
No any other author has anything to declare.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Luderitz B. We have come a long way with device therapy: historical perspectives on antiarrhythmic electrotherapy. J Cardiovasc Electrophysiol. 2002 Jan;13(1 Suppl.):S2–S8. doi: 10.1111/j.1540-8167.2002.tb01945.x. [DOI] [PubMed] [Google Scholar]
- 2.Dhingra R., Pencina M.J., Wang T.J. Electrocardiographic QRS duration and the risk of congestive heart failure: the Framingham Heart Study. Hypertension. 2006;47:861–867. doi: 10.1161/01.HYP.0000217141.20163.23. [DOI] [PubMed] [Google Scholar]
- 3.Ilkhanoff L., Liu K., Ning H. Association of QRS duration with left ventricular structure and function and risk of heart failure in middle-aged and older adults: the Multi-Ethnic Study of Atherosclerosis (MESA) Eur J Heart Fail. 2012 Nov;14(11):1285–1292. doi: 10.1093/eurjhf/hfs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurl S., Mäkikallio T.H., Rautaharju P., Kiviniemi V., Laukkanen J.A. Duration of QRS complex in resting electrocardiogram is a predictor of sudden cardiac death in men. Circulation. 2012;125:2588–2594. doi: 10.1161/CIRCULATIONAHA.111.025577. [DOI] [PubMed] [Google Scholar]
- 5.Teodorescu C., Reinier K., Uy-Evanado A. Prolonged QRS duration on the resting ECG is associated with sudden death risk in coronary disease, independent of prolonged ventricular repolarization. Heart Rhythm. 2011;8(10):1562–1567. doi: 10.1016/j.hrthm.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenyo A., Zaręba W. Prognostic significance of QRS duration and morphology. Cardiol J. 2011;18(1):8–17. [PubMed] [Google Scholar]
- 7.Kalra P.R., Sharma R., Shamim W. Clinical characteristics and survival of patients with chronic heart failure and prolonged QRS duration. Int J Cardiol. 2002;86:225–231. doi: 10.1016/s0167-5273(02)00270-x. [DOI] [PubMed] [Google Scholar]
- 8.Sandhu R., Bahler R.C. Prevalence of QRS prolongation in a community hospital cohort of patients with heart failure and its relation to left ventricular systolic dysfunction. Am J Cardiol. 2004 Jan 15;93(2):244–246. doi: 10.1016/j.amjcard.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 9.Shenkman H.J., Pampati V., Khandelwal A.K. Congestive heart failure and QRS duration: establishing prognosis study. Chest. 2002;122:528–534. doi: 10.1378/chest.122.2.528. [DOI] [PubMed] [Google Scholar]
- 10.Iuliano S., Fisher S.G., Karasik P.E., Fletcher R.D., Singh S.N. QRS duration and mortality in patients with congestive heart failure. Am Heart J. 2002;143:1085–1091. doi: 10.1067/mhj.2002.122516. [DOI] [PubMed] [Google Scholar]
- 11.Murkofsky R.L., Dangas G., Diamond J.A., Mehta D., Schaffer A., Ambrose J.A. A prolonged QRS duration on surface electrocardiogram is a specific indicator of left ventricular dysfunction. J Am Coll Cardiol. 1998;32:476–482. doi: 10.1016/s0735-1097(98)00242-3. [DOI] [PubMed] [Google Scholar]
- 12.Sumiyoshi M., Nakata Y., Tokano T. Clinical significance of QRS duration during ventricular pacing. Pacing Clin Electrophysiol. 1992 Jul;15(7):1053–1064. doi: 10.1111/j.1540-8159.1992.tb03099.x. [DOI] [PubMed] [Google Scholar]
- 13.Pan W., Su Y., Gong X., Sun A., Shu X., Ge J. Value of the paced QRS duration. J Card Fail. 2009;15:347–352. doi: 10.1016/j.cardfail.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi F., Kobayashi Y., Itou H. Prolonged paced QRS duration as a predictor for congestive heart failure in patients with right ventricular apical pacing. Pacing Clin Electrophysiol. 2005;28:1182–1188. doi: 10.1111/j.1540-8159.2005.50181.x. [DOI] [PubMed] [Google Scholar]
- 15.Sweeney M.O., Hellkamp A.S. Heart failure during cardiac pacing. Circulation. 2006;113:2082–2088. doi: 10.1161/CIRCULATIONAHA.105.608356. [DOI] [PubMed] [Google Scholar]
- 16.Chen S., Yin Y., Lan X. PREDICT-Heart Failure study international group. Paced QRS duration as a predictor for clinical heart failure events during right ventricular apical pacing in patients with idiopathic complete atrioventricular block: results from an observational cohort study (PREDICT-HF) Eur J Heart Fail. 2013;15(3):352–359. doi: 10.1093/eurjhf/hfs199. [DOI] [PubMed] [Google Scholar]
- 17.Shukla H.H., Hellkamp A.S., James E.A., Mode Selection Trial (MOST) Investigators Heart failure hospitalization is more common in pacemaker patients with sinus node dysfunction and a prolonged paced QRS duration. Heart Rhythm. 2005;2:245–251. doi: 10.1016/j.hrthm.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X.H., Chen H., Siu C.W. New onset heart failure after permanent right ventricular apical pacing in patients with acquired high-grade atrioventricular block and normal left ventricular function. J Cardiovasc Electrophysiol. 2008;19:136–141. doi: 10.1111/j.1540-8167.2007.01014.x. [DOI] [PubMed] [Google Scholar]
- 19.Clements I.P., Akerem Khan S.K., Chen H.H., Mullan B.P. Measurement of left and right ventricular volumes with tomographic equilibrium radionuclide angiocardiography and cardiac MRI. Nucl Med Commun. 2012 May;33(5):481–485. doi: 10.1097/MNM.0b013e32835167ae. [DOI] [PubMed] [Google Scholar]
- 20.Sharma G., Senguttuvan N.B., Thachil A. A comparison of lead placement through the subclavian vein technique with fluoroscopy-guided axillary vein technique for permanent pacemaker insertion. Can J Cardiol. 2012 Sep-Oct;28(5):542–546. doi: 10.1016/j.cjca.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura H., Mine T., Kanemori T., Ohyanagi M., Masuyama T. Effect of right ventricular pacing site on QRS Width. Asian Cardiovasc Thorac Ann. 2011;19:339–345. doi: 10.1177/0218492311422485. [DOI] [PubMed] [Google Scholar]
- 22.Su Y., Pan W., Gong X., Cui J., Shu X., Ge J. Relationships between paced QRS duration and left cardiac structures and function. Acta Cardiol. 2009;64(2):231–238. doi: 10.2143/AC.64.2.2036143. [DOI] [PubMed] [Google Scholar]
- 23.Hong Y.J., Yang B.R., Sim D.S. The effects of QRS duration and pacing sites on the acute hemodynamic changes during right ventricular pacing. Korean J Intern Med. 2005 Mar;20(1):15–20. doi: 10.3904/kjim.2005.20.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tse H.F., Yu C., Wong K.K. Functional abnormalities in patients with permanent right ventricular pacing: the effect of sites of electrical stimulation. J Am Coll Cardiol. 2002;40:1451–1458. doi: 10.1016/s0735-1097(02)02169-1. [DOI] [PubMed] [Google Scholar]
- 25.Tse H.F., Lau C.P. Selection of permanent ventricular pacing site: how far should we go? J Am Coll Cardiol. 2006;48:1649–1651. doi: 10.1016/j.jacc.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 26.De Cock C.C., Meyer A., Kamp O., Visser C.A. Hemodynamic benefits of right ventricular outflow tract pacing: comparison with right ventricular apex pacing. Pacing Clin Electrophysiol. 1998;21:536–541. doi: 10.1111/j.1540-8159.1998.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 27.Buckingham T.A., Candinas R., Schlapfer J. Acute hemodynamic effects of atrioventricular pacing at different sites in the right ventricle individually and simultaneously. Pacing Clin Electrophysiol. 1997;20:909–915. doi: 10.1111/j.1540-8159.1997.tb05493.x. [DOI] [PubMed] [Google Scholar]
- 28.Kawakami T., Tanaka N., Ohno H. The relationship between right ventricular lead position and paced QRS duration. Pacing Clin Electrophysiol. 2013 Feb;36(2):187–193. doi: 10.1111/pace.12047. [DOI] [PubMed] [Google Scholar]
- 29.Orchetta E., Bortnik M., Magnani A. Prevention of ventricular desynchronization by permanent para-Hisian pacing after atrioventricular node ablation in chronic atrial fibrillation: a crossover, blinded, randomized study versus apical right ventricular pacing. J Am Coll Cardiol. 2006;47:1938–1945. doi: 10.1016/j.jacc.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 30.De Cock C.C., Giudici M.C., Twisk J.W. Comparison of the haemodynamic effects of right ventricular outflow-tract pacing with right ventricular apex pacing: a quantitative review. Europace. 2003;5:275–278. doi: 10.1016/s1099-5129(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 31.Ten Cate T.J., Scheffer M.G., Sutherland G.R., Verzijlbergen J.F., van Hemel N.M. Right ventricular outflow and apical pacing comparably worsen the echocardiographic normal left ventricle. Eur J Echocardiogr. 2008;9:672–677. doi: 10.1093/ejechocard/jen108. [DOI] [PubMed] [Google Scholar]
- 32.Riahi S., Nielsen J.C., Hjortshøj S., DANPACE Investigators Heart failure in patients with sick sinus syndrome treated with single lead atrial or dual-chamber pacing: no association with pacing mode or right ventricular pacing site. Europace. 2012 Oct;14(10):1475–1482. doi: 10.1093/europace/eus069. [DOI] [PubMed] [Google Scholar]
- 33.Kaye G., Stambler B.S., Yee R. Search for the optimal right ventricular pacing site: design and implementation of three randomized multicenter clinical trials. Pacing Clin Electrophysiol. 2009;32:426–433. doi: 10.1111/j.1540-8159.2009.02301.x. [DOI] [PubMed] [Google Scholar]