Abstract
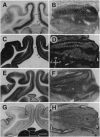
There is considerable physiological evidence for the compartmentalization of mammalian visual cortex into functional columnar modules, representing features of visual information processing such as eye and orientation specificity. However, anatomical markers of visual cortical compartmentalization have been described only for primate visual cortex. In this report, we describe an interdigitated mosaic of four neuroactive molecules which demarcate two distinct columnar systems in the kitten visual cortex. Serotonin 1C receptors and synaptic zinc were found to demarcate columns within layer IV of kitten visual cortex, which were interdigitated with a second, patchy system characterized by increased levels of cytochrome oxidase and acetylcholinesterase. In primate visual cortex, as well as in the kitten, synaptic zinc was periodically distributed in a manner precisely complementary to cytochrome oxidase. These findings provide an anatomical framework on which unifying hypotheses of the functional organization of columnar systems in mammalian visual cortex can be built.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaulieu C., Dyck R., Cynader M. Enrichment of glutamate in zinc-containing terminals of the cat visual cortex. Neuroreport. 1992 Oct;3(10):861–864. doi: 10.1097/00001756-199210000-00010. [DOI] [PubMed] [Google Scholar]
- Blasdel G. G. Differential imaging of ocular dominance and orientation selectivity in monkey striate cortex. J Neurosci. 1992 Aug;12(8):3115–3138. doi: 10.1523/JNEUROSCI.12-08-03115.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G. G., Salama G. Voltage-sensitive dyes reveal a modular organization in monkey striate cortex. Nature. 1986 Jun 5;321(6070):579–585. doi: 10.1038/321579a0. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T., Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991 Oct 3;353(6343):429–431. doi: 10.1038/353429a0. [DOI] [PubMed] [Google Scholar]
- Condo G. J., Casagrande V. A. Organization of cytochrome oxidase staining in the visual cortex of nocturnal primates (Galago crassicaudatus and Galago senegalensis): I. Adult patterns. J Comp Neurol. 1990 Mar 22;293(4):632–645. doi: 10.1002/cne.902930408. [DOI] [PubMed] [Google Scholar]
- Dyck R. H., Cynader M. S. Autoradiographic localization of serotonin receptor subtypes in cat visual cortex: transient regional, laminar, and columnar distributions during postnatal development. J Neurosci. 1993 Oct;13(10):4316–4338. doi: 10.1523/JNEUROSCI.13-10-04316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck R., Beaulieu C., Cynader M. Histochemical localization of synaptic zinc in the developing cat visual cortex. J Comp Neurol. 1993 Mar 1;329(1):53–67. doi: 10.1002/cne.903290105. [DOI] [PubMed] [Google Scholar]
- Horton J. C. Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex. Philos Trans R Soc Lond B Biol Sci. 1984 Jan 17;304(1119):199–253. doi: 10.1098/rstb.1984.0021. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hedley-Whyte E. T. Mapping of cytochrome oxidase patches and ocular dominance columns in human visual cortex. Philos Trans R Soc Lond B Biol Sci. 1984 Jan 17;304(1119):255–272. doi: 10.1098/rstb.1984.0022. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hubel D. H. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981 Aug 20;292(5825):762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Pazos A., Probst A., Palacios J. M. Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res. 1986 Jun 18;376(1):97–107. doi: 10.1016/0006-8993(86)90903-0. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- LeVay S., Stryker M. P., Shatz C. J. Ocular dominance columns and their development in layer IV of the cat's visual cortex: a quantitative study. J Comp Neurol. 1978 May 1;179(1):223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- Livingstone M., Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988 May 6;240(4853):740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Löwel S., Singer W. Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science. 1992 Jan 10;255(5041):209–212. doi: 10.1126/science.1372754. [DOI] [PubMed] [Google Scholar]
- Martinez-Guijarro F. J., Soriano E., Del Rio J. A., Lopez-Garcia C. Zinc-positive boutons in the cerebral cortex of lizards show glutamate immunoreactivity. J Neurocytol. 1991 Oct;20(10):834–843. doi: 10.1007/BF01191734. [DOI] [PubMed] [Google Scholar]
- Pazos A., Palacios J. M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985 Nov 4;346(2):205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Swindale N. V., Matsubara J. A., Cynader M. S. Surface organization of orientation and direction selectivity in cat area 18. J Neurosci. 1987 May;7(5):1414–1427. doi: 10.1523/JNEUROSCI.07-05-01414.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H., Lam D. M. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974 Oct 18;79(2):273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N. Postnatal development of the visual cortex and the influence of environment. Nature. 1982 Oct 14;299(5884):583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]