Abstract
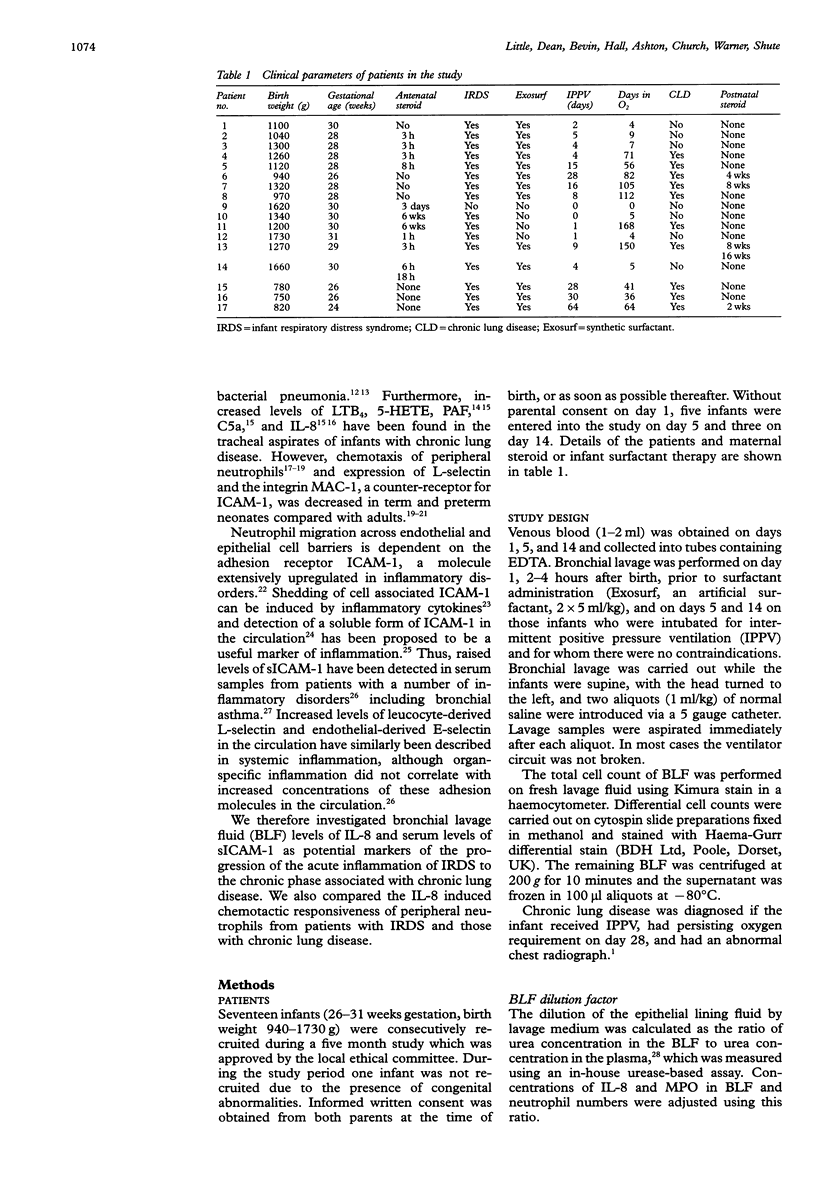
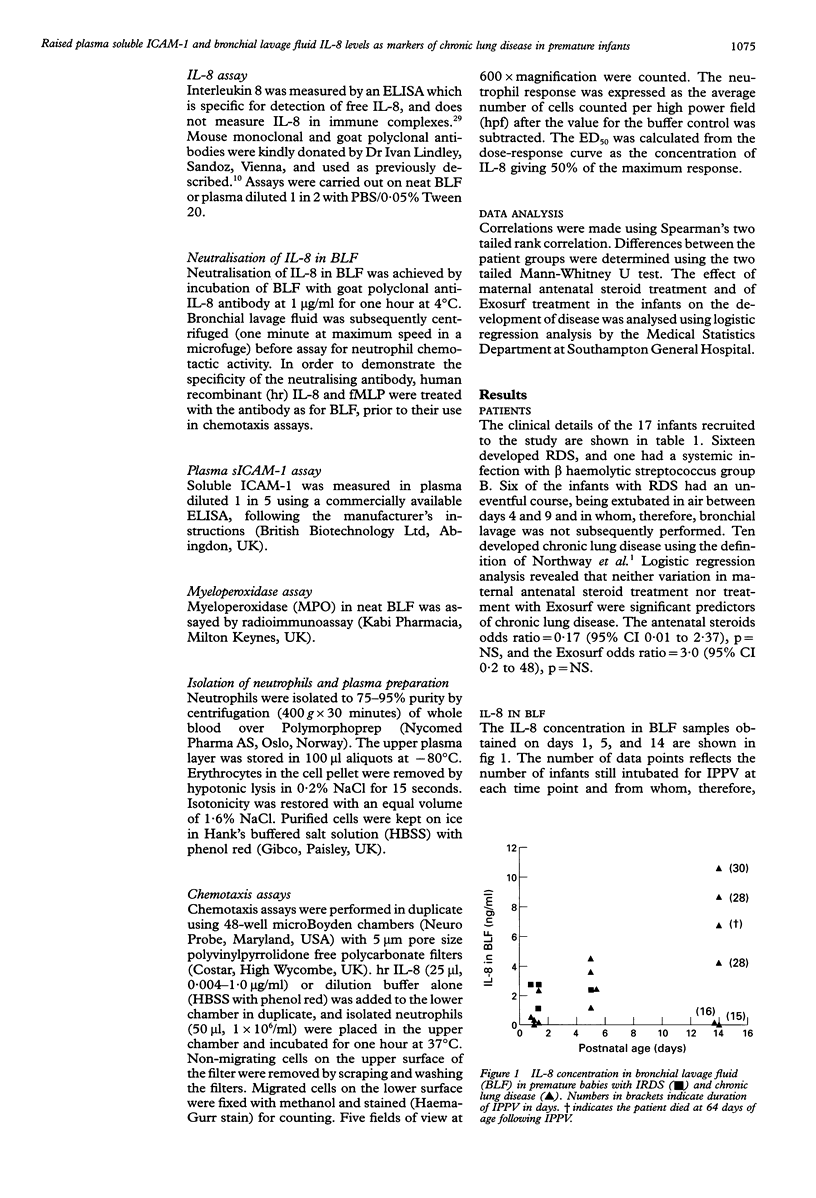
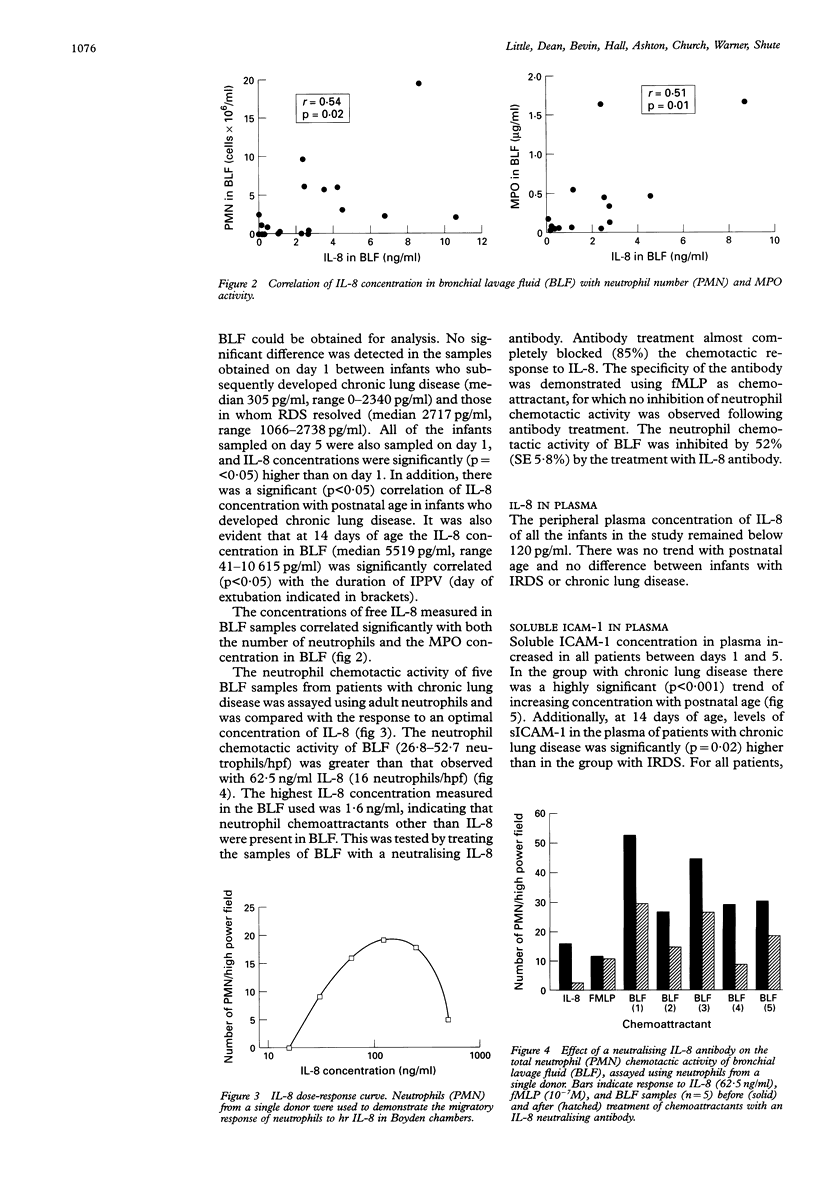
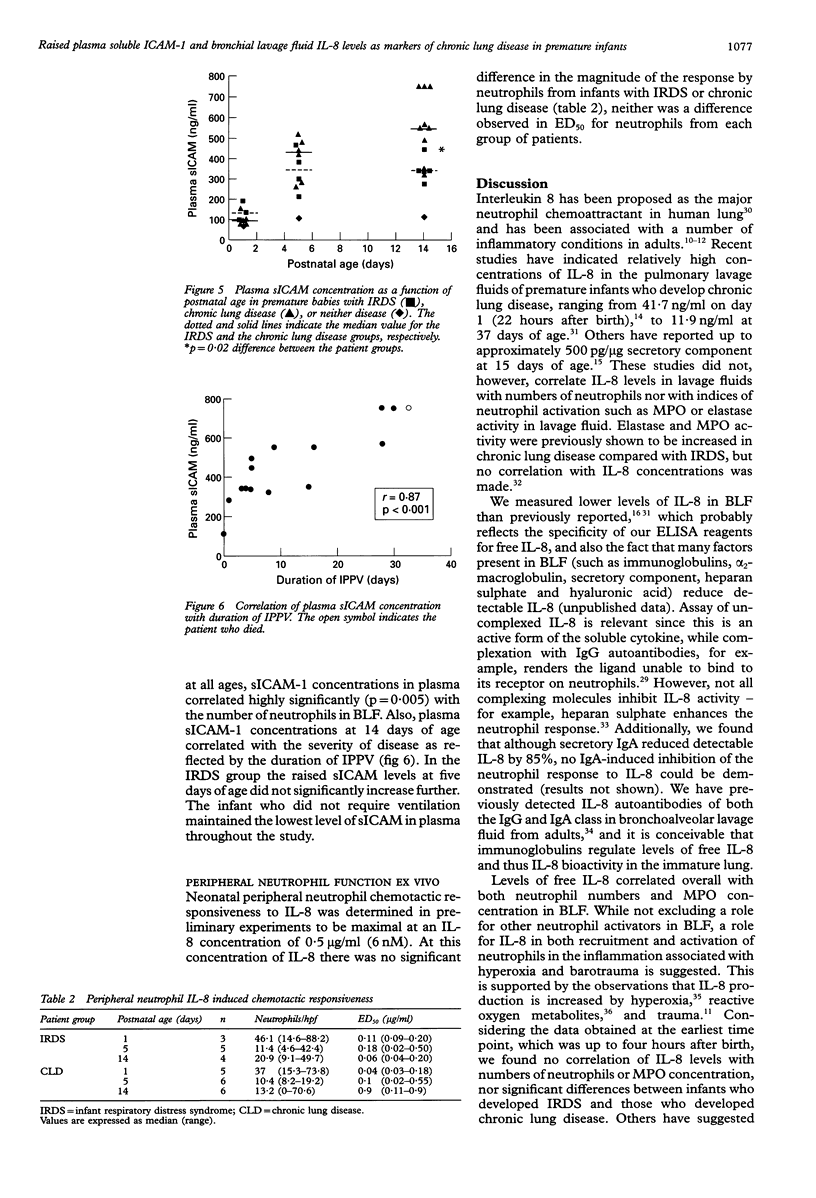
BACKGROUND--Pulmonary neutrophilia characterises both the relatively transient inflammation associated with infant respiratory distress syndrome (IRDS) and the persistent inflammation of chronic lung disease. The possibility that persistently raised markers of inflammation indicate the development of chronic lung disease in low birth weight (< 1730 g) preterm (< 31 weeks) infants was therefore investigated. METHODS--Soluble ICAM-1 (sICAM-1) levels in plasma, and interleukin (IL)-8 and myeloperoxidase (MPO) levels in bronchial lavage fluid (BLF) obtained from 17 infants on days 1, 5, and 14 following birth were measured and correlations with the number of neutrophils in BLF sought. Peripheral neutrophils were isolated on Polymorphoprep and chemotactic responsiveness to IL-8 was assessed using micro Boyden chambers. RESULTS--Sixteen infants developed IRDS and, of these, 10 infants subsequently developed chronic lung disease. Levels of IL-8 in BLF at 14 days of age correlated with the long term requirement for intermittent positive pressure ventilation (IPPV). Interleukin 8 levels in BLF correlated with neutrophil numbers and MPO concentration, suggesting both recruitment and activation in response to this cytokine. Antibody depletion studies showed that approximately 50% of total neutrophil chemotactic activity in BLF was due to IL-8. No difference in peripheral neutrophil chemotactic responsiveness at any age was observed for infants with IRDS or chronic lung disease. Plasma soluble intercellular adhesion molecule (sICAM-1) was higher at 14 days of age in infants who developed chronic lung disease than in those with resolving IRDS, and correlated with severity of disease, as indicated by duration of IPPV. CONCLUSIONS--The results indicate that high levels of plasma sICAM-1 and IL-8 in BLF at day 14 correlate with the development of chronic lung disease and indicate the severity of disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Abbassi O., Kishimoto T. K., Koenig J. M., McIntire L. V., Smith C. W. Diminished lectin-, epidermal growth factor-, complement binding domain-cell adhesion molecule-1 on neonatal neutrophils underlies their impaired CD18-independent adhesion to endothelial cells in vitro. J Immunol. 1991 May 15;146(10):3372–3379. [PubMed] [Google Scholar]
- Arnon S., Grigg J., Silverman M. Association between pulmonary and gastric inflammatory cells on the first day of life in preterm infants. Pediatr Pulmonol. 1993 Jul;16(1):59–61. doi: 10.1002/ppul.1950160112. [DOI] [PubMed] [Google Scholar]
- Becker J. C., Dummer R., Hartmann A. A., Burg G., Schmidt R. E. Shedding of ICAM-1 from human melanoma cell lines induced by IFN-gamma and tumor necrosis factor-alpha. Functional consequences on cell-mediated cytotoxicity. J Immunol. 1991 Dec 15;147(12):4398–4401. [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Carr R., Pumford D., Davies J. M. Neutrophil chemotaxis and adhesion in preterm babies. Arch Dis Child. 1992 Jul;67(7 Spec No):813–817. doi: 10.1136/adc.67.7_spec_no.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet-Martin S., Montravers P., Gibert C., Elbim C., Desmonts J. M., Fagon J. Y., Gougerot-Pocidalo M. A. High levels of interleukin-8 in the blood and alveolar spaces of patients with pneumonia and adult respiratory distress syndrome. Infect Immun. 1993 Nov;61(11):4553–4559. doi: 10.1128/iai.61.11.4553-4559.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForge L. E., Fantone J. C., Kenney J. S., Remick D. G. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J Clin Invest. 1992 Nov;90(5):2123–2129. doi: 10.1172/JCI116097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean T. P., Dai Y., Shute J. K., Church M. K., Warner J. O. Interleukin-8 concentrations are elevated in bronchoalveolar lavage, sputum, and sera of children with cystic fibrosis. Pediatr Res. 1993 Aug;34(2):159–161. doi: 10.1203/00006450-199308000-00010. [DOI] [PubMed] [Google Scholar]
- Donnelly S. C., Strieter R. M., Kunkel S. L., Walz A., Robertson C. R., Carter D. C., Grant I. S., Pollok A. J., Haslett C. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet. 1993 Mar 13;341(8846):643–647. doi: 10.1016/0140-6736(93)90416-e. [DOI] [PubMed] [Google Scholar]
- Dos Santos C., Davidson D. Neutrophil chemotaxis to leukotriene B4 in vitro is decreased for the human neonate. Pediatr Res. 1993 Mar;33(3):242–246. doi: 10.1203/00006450-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993 Oct;14(10):506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- Groneck P., Götze-Speer B., Oppermann M., Eiffert H., Speer C. P. Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics. 1994 May;93(5):712–718. [PubMed] [Google Scholar]
- Groneck P., Reuss D., Götze-Speer B., Speer C. P. Effects of dexamethasone on chemotactic activity and inflammatory mediators in tracheobronchial aspirates of preterm infants at risk for chronic lung disease. J Pediatr. 1993 Jun;122(6):938–944. doi: 10.1016/s0022-3476(09)90024-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Imai K., Kobayashi T., Amemiya E., Takahashi Y., Tomita Y., Iwata T., Suguro H., Yamaguchi M., Yachi A. Elevated levels of soluble ICAM-1 in sera from patients with bronchial asthma. Allergy. 1993 Jul;48(5):370–372. doi: 10.1111/j.1398-9995.1993.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Hopkins H., Stull T., Von Essen S. G., Robbins R. A., Rennard S. I. Neutrophil chemotactic factors in bacterial pneumonia. Chest. 1989 May;95(5):1021–1027. doi: 10.1378/chest.95.5.1021. [DOI] [PubMed] [Google Scholar]
- Kamran S., Usmani S. S., Wapnir R. A., Mehta R., Harper R. G. In vitro effect of indomethacin on polymorphonuclear leukocyte function in preterm infants. Pediatr Res. 1993 Jan;33(1):32–35. doi: 10.1203/00006450-199301000-00007. [DOI] [PubMed] [Google Scholar]
- Kang B. H., Crapo J. D., Wegner C. D., Letts L. G., Chang L. Y. Intercellular adhesion molecule-1 expression on the alveolar epithelium and its modification by hyperoxia. Am J Respir Cell Mol Biol. 1993 Oct;9(4):350–355. doi: 10.1165/ajrcmb/9.4.350. [DOI] [PubMed] [Google Scholar]
- Kawano T., Mori S., Cybulsky M., Burger R., Ballin A., Cutz E., Bryan A. C. Effect of granulocyte depletion in a ventilated surfactant-depleted lung. J Appl Physiol (1985) 1987 Jan;62(1):27–33. doi: 10.1152/jappl.1987.62.1.27. [DOI] [PubMed] [Google Scholar]
- Kojima T., Sasai M., Kobayashi Y. Increased soluble ICAM-1 in tracheal aspirates of infants with bronchopulmonary dysplasia. Lancet. 1993 Oct 23;342(8878):1023–1024. doi: 10.1016/0140-6736(93)92880-3. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Standiford T., Kasahara K., Strieter R. M. Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Exp Lung Res. 1991 Jan-Feb;17(1):17–23. doi: 10.3109/01902149109063278. [DOI] [PubMed] [Google Scholar]
- Kwon O. J., Au B. T., Collins P. D., Baraniuk J. N., Adcock I. M., Chung K. F., Barnes P. J. Inhibition of interleukin-8 expression by dexamethasone in human cultured airway epithelial cells. Immunology. 1994 Mar;81(3):389–394. [PMC free article] [PubMed] [Google Scholar]
- Mallard E. C., Williams C. E., Gunn A. J., Gunning M. I., Gluckman P. D. Frequent episodes of brief ischemia sensitize the fetal sheep brain to neuronal loss and induce striatal injury. Pediatr Res. 1993 Jan;33(1):61–65. doi: 10.1203/00006450-199301000-00013. [DOI] [PubMed] [Google Scholar]
- Marcy T. W., Merrill W. W., Rankin J. A., Reynolds H. Y. Limitations of using urea to quantify epithelial lining fluid recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1987 Jun;135(6):1276–1280. doi: 10.1164/arrd.1987.135.6.1276. [DOI] [PubMed] [Google Scholar]
- McColm J. R., McIntosh N. Interleukin-8 in bronchoalveolar lavage samples as predictor of chronic lung disease in premature infants. Lancet. 1994 Mar 19;343(8899):729–729. doi: 10.1016/s0140-6736(94)91606-3. [DOI] [PubMed] [Google Scholar]
- Merritt T. A., Cochrane C. G., Holcomb K., Bohl B., Hallman M., Strayer D., Edwards D. K., 3rd, Gluck L. Elastase and alpha 1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. Role of inflammation in the pathogenesis of bronchopulmonary dysplasia. J Clin Invest. 1983 Aug;72(2):656–666. doi: 10.1172/JCI111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metinko A. P., Kunkel S. L., Standiford T. J., Strieter R. M. Anoxia-hyperoxia induces monocyte-derived interleukin-8. J Clin Invest. 1992 Sep;90(3):791–798. doi: 10.1172/JCI115953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefort S., Feather I. H., Wilson S. J., Haskard D. O., Lee T. H., Holgate S. T., Howarth P. H. The expression of leukocyte-endothelial adhesion molecules is increased in perennial allergic rhinitis. Am J Respir Cell Mol Biol. 1992 Oct;7(4):393–398. doi: 10.1165/ajrcmb/7.4.393. [DOI] [PubMed] [Google Scholar]
- Montefort S., Holgate S. T. Adhesion molecules and their role in inflammation. Respir Med. 1991 Mar;85(2):91–99. doi: 10.1016/s0954-6111(06)80284-2. [DOI] [PubMed] [Google Scholar]
- Montefort S., Roche W. R., Howarth P. H., Djukanovic R., Gratziou C., Carroll M., Smith L., Britten K. M., Haskard D., Lee T. H. Intercellular adhesion molecule-1 (ICAM-1) and endothelial leucocyte adhesion molecule-1 (ELAM-1) expression in the bronchial mucosa of normal and asthmatic subjects. Eur Respir J. 1992 Jul;5(7):815–823. [PubMed] [Google Scholar]
- Murch S. H., MacDonald T. T., Wood C. B., Costeloe K. L. Tumour necrosis factor in the bronchoalveolar secretions of infants with the respiratory distress syndrome and the effect of dexamethasone treatment. Thorax. 1992 Jan;47(1):44–47. doi: 10.1136/thx.47.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Yoshimura K., McElvaney N. G., Crystal R. G. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J Clin Invest. 1992 May;89(5):1478–1484. doi: 10.1172/JCI115738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northway W. H., Jr, Rosan R. C., Porter D. Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967 Feb 16;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- Ogden B. E., Murphy S. A., Saunders G. C., Pathak D., Johnson J. D. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis. 1984 Nov;130(5):817–821. doi: 10.1164/arrd.1984.130.5.817. [DOI] [PubMed] [Google Scholar]
- Philip A. G. Oxygen plus pressure plus time: the etiology of bronchopulmonary dysplasia. Pediatrics. 1975 Jan;55(1):44–50. [PubMed] [Google Scholar]
- Rolfe M. W., Kunkel S. L., Standiford T. J., Chensue S. W., Allen R. M., Evanoff H. L., Phan S. H., Strieter R. M. Pulmonary fibroblast expression of interleukin-8: a model for alveolar macrophage-derived cytokine networking. Am J Respir Cell Mol Biol. 1991 Nov;5(5):493–501. doi: 10.1165/ajrcmb/5.5.493. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Mainolfi E. A., Czajkowski M., Marlin S. D. A form of circulating ICAM-1 in human serum. J Immunol. 1991 Dec 1;147(11):3788–3793. [PubMed] [Google Scholar]
- Seth R., Raymond F. D., Makgoba M. W. Circulating ICAM-1 isoforms: diagnostic prospects for inflammatory and immune disorders. Lancet. 1991 Jul 13;338(8759):83–84. doi: 10.1016/0140-6736(91)90077-3. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Rolfe M. W., Evanoff H. L., Allen R. M., Strieter R. M. Regulation of human alveolar macrophage- and blood monocyte-derived interleukin-8 by prostaglandin E2 and dexamethasone. Am J Respir Cell Mol Biol. 1992 Jan;6(1):75–81. doi: 10.1165/ajrcmb/6.1.75. [DOI] [PubMed] [Google Scholar]
- Stenmark K. R., Eyzaguirre M., Westcott J. Y., Henson P. M., Murphy R. C. Potential role of eicosanoids and PAF in the pathophysiology of bronchopulmonary dysplasia. Am Rev Respir Dis. 1987 Sep;136(3):770–772. doi: 10.1164/ajrccm/136.3.770. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Lukacs N. W., Standiford T. J., Kunkel S. L. Cytokines. 2. Cytokines and lung inflammation: mechanisms of neutrophil recruitment to the lung. Thorax. 1993 Jul;48(7):765–769. doi: 10.1136/thx.48.7.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester I., Yoshimura T., Sticherling M., Schröder J. M., Ceska M., Peichl P., Leonard E. J. Neutrophil attractant protein-1-immunoglobulin G immune complexes and free anti-NAP-1 antibody in normal human serum. J Clin Invest. 1992 Aug;90(2):471–481. doi: 10.1172/JCI115883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler A., Meier R., Seitz M., Dewald B., Baggiolini M., Fey M. F. Glucocorticoids downregulate gene expression of GM-CSF, NAP-1/IL-8, and IL-6, but not of M-CSF in human fibroblasts. Blood. 1992 Jan 1;79(1):45–51. [PubMed] [Google Scholar]
- Török C., Lundahl J., Hed J., Lagercrantz H. Diversity in regulation of adhesion molecules (Mac-1 and L-selectin) in monocytes and neutrophils from neonates and adults. Arch Dis Child. 1993 May;68(5 Spec No):561–565. doi: 10.1136/adc.68.5_spec_no.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A., Burgener R., Car B., Baggiolini M., Kunkel S. L., Strieter R. M. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp Med. 1991 Dec 1;174(6):1355–1362. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb L. M., Ehrengruber M. U., Clark-Lewis I., Baggiolini M., Rot A. Binding to heparan sulfate or heparin enhances neutrophil responses to interleukin 8. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7158–7162. doi: 10.1073/pnas.90.15.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welty S. E., Rivera J. L., Elliston J. F., Smith C. V., Zeb T., Ballantyne C. M., Montgomery C. A., Hansen T. N. Increases in lung tissue expression of intercellular adhesion molecule-1 are associated with hyperoxic lung injury and inflammation in mice. Am J Respir Cell Mol Biol. 1993 Oct;9(4):393–400. doi: 10.1165/ajrcmb/9.4.393. [DOI] [PubMed] [Google Scholar]
- Zakrzewski J. T., Barnes N. C., Piper P. J., Costello J. F. Detection of sputum eicosanoids in cystic fibrosis and in normal saliva by bioassay and radioimmunoassay. Br J Clin Pharmacol. 1987 Jan;23(1):19–27. doi: 10.1111/j.1365-2125.1987.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


