Abstract
Background
Total pancreatectomy is infrequently performed for pancreatic cancer. Perceived operative mortality and questionable survival benefit deter many surgeons. Clinical outcomes, described in single-center series, remain largely unknown.
Methods
The National Cancer Database was queried for cases of pancreatic ductal adenocarcinoma undergoing total pancreatectomy (1998–2011). Univariate survival analyses were performed for 21 variables: demographic (8), tumor characteristics (5), surgery outcomes (6), and adjuvant therapy (2). The Log-rank test of differences in Kaplan–Meier survival curves was used for categorical variables. Variables with p < 0.05 were included in a multivariate analysis. Cox proportional hazards regression was used to analyze continuous variables and multivariate models.
Results
2582 patients with staging and survival data made up the study population. 30-day mortality was 5.5%. Median overall survival was 15 months, with 1, 3, and 5-year survival rates of 60%, 22%, and 13%, respectively. Age, facility type, tumor size and grade, lymph node positivity, margin positivity, and adjuvant therapy significantly impacted survival in multivariate analysis.
Conclusion
Although total pancreatectomy is a reasonable option for selected patients with pancreatic ductal adenocarcinoma, survival of the entire group is limited. Operative mortality is improved from prior reports. Greater survival benefits were seen in younger patients with smaller, node negative tumors resected with negative margins in academic research centers.
Introduction
In the 1980's and 1990's, several reviews suggested that total pancreatectomy was inferior to partial resection in the setting of neoplastic disease.1, 2, 3 Most authors cited the low rate of peri-operative complications after Whipple resections being reported from high-volume centers, the persistently high morbidity associated with total pancreatectomy including perioperative bleeding and postoperative brittle diabetes, the benefits of preserved endocrine and exocrine pancreatic function after partial resection, and the fact that long-term cancer survival was essentially the same after either subtotal or total pancreas resection. Ingemar Ihse reported operative mortality of 27% and morbidity of 52% in a cohort of total pancreatectomy patients compared to 3% and 28% for subtotal pancreatectomy.1 Total pancreatectomy for neoplastic disease reached its nadir in the late 1990's, and was considered by most to be an overly aggressive, highly morbid procedure with little, if any, clinical benefit.
By the early 2000's, the inherent metabolic derangements associated with the apancreatic state became increasingly manageable with lifestyle modification and adherence to pharmacologic recommendations. Advances in long-acting insulin formulations and improvement in oral pancreatic enzyme supplements offered many total pancreatectomy patients a reasonable quality of life without severe glucose fluctuations and with better control of intestinal malabsorption problems. Recent studies demonstrate an acceptable quality of life after total pancreatectomy for neoplastic disease.4, 5, 6 While patients consistently score points for individual symptoms such as diarrhea, fatigue, and sleep disturbances, the surveys suggest that most patients are able to achieve a relatively high level of performance with regard to social, physical, cognitive, and emotional functioning.
Within this context, there has once again been renewed interest in total pancreatectomy for neoplastic disease over the past 10–15 years. Operative outcomes are now reported within the range of acceptability when compared to partial resection.5, 7, 8 While the debate with regards to extended resection for pancreatic ductal adenocarcinoma persists, there seems to be more compelling evidence to consider total pancreatectomy in cases of multicentric or diffuse intraductal papillary mucinous neoplasms (IPMN), multiple pancreatic neuroendocrine tumors (pNET), and multifocal pancreatic metastases from renal cell carcinoma (RCC).4, 6, 9, 10, 11, 12
Total pancreatectomy for adenocarcinoma has been addressed specifically in the literature. Nathan et al. examined mortality and long term survival after total pancreatectomy in 292 patients from the Surveillance, Epidemiology, and End Results (SEER) database, from 1998 to 2004. One month mortality ranged from 5.8 to 9.3%, depending on location of primary tumor.13, 14
To date, the vast majority of the evidence for and against total pancreatectomy has been derived from relatively small case series and meta-analyses. The impact of the procedure itself is often difficult to distinguish from that related to patient co-morbidities, surgical complications, stage of disease, and adjuvant therapies. A better understanding of the outcomes after total pancreatectomy will facilitate a more informed conversation about the risks and benefits of the operation. The aim of this study is to query a large, multi-institutional, prospective cancer database to characterize the long-term outcomes of total pancreatectomy in the treatment of neoplastic disease, and its impact on overall survival within the context of multimodality cancer care.
The National Cancer Data Base (NCDB) is a joint program of the Commission on Cancer (CoC) of the American College of Surgeons (ACoS), and the American Cancer Society (ACS). It is a multicenter oncology outcomes database for more than 1500 Commission-accredited cancer programs in the United States and Puerto Rico. These data are used to explore trends in cancer care, to create regional and state benchmarks for participating hospitals, and to serve as the basis for quality improvement. More information is available at www.facs.org/cancer/ncdb/.
Methods
The National Cancer Data Base was queried for all patients in participating centers who underwent total pancreatectomy with or without splenectomy for pancreatic ductal adenocarcinoma between 1998 and 2011. Patients with both pylorus-preserving and classic resections were included. After obtaining an approved Participant User File from NCDB, analysis of preoperative, perioperative and postoperative data was undertaken.
All patients were included in a descriptive assessment of patient-specific variables. Demographic characteristics analyzed (some of which are shown in Table 1) were age, gender, race, medical comorbidities, insurance type, surgical facility type, regional location, urban/rural household, distance from household to surgical facility, and year of diagnosis. Tumor-specific characteristics (Table 1) were tumor size, tumor grade, tumor location within the pancreas, number of nodes positive for invasive cancer, lymph node ratio (nodes positive/nodes examined), pathologic and clinical TNM stage, and analytic stage group. Surgical outcomes (Table 1) included number of nodes examined, margin status, length of hospital stay, hospital readmission, and 30-day mortality. Margin assessment was made by NCDB standards, which did not change during the study reporting period, defined as microscopic involvement that “cannot be seen by the naked eye” and macroscopic being “visible to the naked eye”. These correspond to “R1” and “R2” resections, and are standard definitions utilized in reporting in North America. Systemic therapeutic data analyzed (Table 1) included administration of chemotherapy and/or radiation therapy and the sequencing of additional therapies as neoadjuvant, adjuvant, or both.
Table 1.
Descriptive statistics
| IA. Patient characteristic | Patients |
|---|---|
| N | 5726 |
| Age, Mean ± SD (years) | 65.5 ± 10.8 |
| Gender | |
| Male | 52% (2989) |
| Female | 48% (2737) |
| Facility type | |
| Academic research center | 53% (3024) |
| Comprehensive community cancer center | 41% (2329) |
| Community cancer center | 5% (296) |
| Other cancer program | 1% (77) |
| Comorbidities (N = 4724) | |
| None | 68% (3191) |
| One or more | 32% (1533) |
| IB. Tumor characteristic | Patients |
|---|---|
| Tumor grade (N = 5178) | |
| 1 | 9% (485) |
| 2 | 52% (2708) |
| 3 | 37% (1925) |
| 4 | 1% (60) |
| Surgical margins (N = 5506) | |
| Negative | 76% (4197) |
| Microscopic positive | 14% (758) |
| Involved, not specified | 9% (481) |
| Macroscopic positive | 1% (70) |
| Lymph nodes (N = 5415) | |
| Negative | 36% (1935) |
| Positive | 64% (3480) |
| Analytic stage (N = 5521) | |
| 0 | 1% (45) |
| 1 | 15% (824) |
| 2 | 66% (3646) |
| 3 | 12% (635) |
| 4 | 7% (371) |
| IC. Operative outcome | Patients |
|---|---|
| Length of stay (Days) (N = 4197) | |
| Median (min, max) | 10 (0, 180) |
| Readmission 30 days (N = 4543) | |
| None | 90% (4067) |
| Unplanned | 8% (364) |
| Planned | 2% (112) |
| Mortality (N = 5711) | |
| 30-day mortality | 5.5% (316) |
| ID. Adjuvant treatment | Patients |
|---|---|
| N | 5158 |
| Chemotherapy | 23% (1211) |
| Radiation | 2% (104) |
| Chemotherapy and radiation | 39% (2007) |
| None | 36% (1836) |
Survival analysis
Survival data was available for patients diagnosed between 1998 and 2006. Univariate and multivariate analyses were performed in these patients to determine the degree to which the various patient-specific, tumor-specific, procedure-specific, and treatment-specific variables impacted overall survival. Patients without documented cancer staging and those with stage 0 non-invasive neoplasms were excluded from the survival analyses. Overall survival was measured from time of pancreatic cancer diagnosis to time of death or censored at last date of follow-up.
Effect of each variable on survival in univariate predictor models was tested with Cox proportional hazards regression for continuous variables and a Log-rank test comparing Kaplan–Meier curves for categorical variables. Then Cox proportional hazards regression was used for survival analysis of multivariate full and reduced models, with reduced models determined by forward step-wise modeling. Complete case analysis was used multivariate modeling and subjects were included if they had non-missing data for all variables.
Variables included in the full models and as candidates for the final model were all variables tested in univariate survival models that were statistically significant at p < 0.05 and that had values for at least 60% of patients. Only one of a pair of highly correlated variables (positive nodes but not nodes ratio) or those with overlapping definitions (nodes and tumor size but not analytic stage) was included. Variables excluded due to having missing values for >40% of patients were: comorbidity, 30-day readmission, length of stay after surgery, and sequence of surgery and systemic therapy.
Global p-values for categorical variables are reported and then paired comparisons used to describe the significant findings, reporting the unadjusted contrast p-values. Since type one error rate increases as the number of tests increases, the Dunn-Sidak method was used to determine levels of significance of p < 0.0170 for 3 comparisons, and p < 0.0127 for 4 comparisons. SAS 9.3 was used for statistical analysis (SAS Institute Inc., Cary, NC).
Results
Descriptive statistics
The study population includes 5726 patients with pancreatic ductal adenocarcinoma who underwent total pancreatectomy between 1998 and 2011 at 934 separate surgical facilities. 53% of patients had surgery at an academic research program, 41% at a comprehensive community cancer program, and 5% of patients had their surgery done in a community cancer program. The average age was 65.5 years, ranging from 28 to 90 years. 88% (4914/5581) of the patients were Caucasian. Significant comorbidities were reported in 32% of patients. 51% of patients had Medicare insurance while 39% were privately insured, and 10% of patients had some other source of insurance or were uninsured. There was a gradual increase in the number of cases recorded in the database as time progressed, rising from 210 cases in 1998, to 464 in 2005, to 664 in 2011.
Final analytic staging data was available in 5521/5726 patients. 45 (0.8%) were Stage 0, 824 (15%) were Stage 1, 3646 (66%) were Stage 2, 635 (12%) were Stage 3, and 371 (7%) were Stage 4. The primary site of disease was the pancreatic head in 72% of patients, the neck/body/tail in 14%, and overlapping or other site within the pancreas in 14% of patients. Tumors were defined as grade 1 in 9% patients, grade 2 in 52%, grade 3 in 37%, and grade 4 in 1% of patients. Tumor size was <2 cm in 611 (11%) of the patients, 2–5 cm in 3823 patients (72%), and >5 cm in 898 patients (17%). The median number of lymph nodes examined was 12 nodes, ranging from 0 to 77 nodes. 3480 patients had nodal metastasis with 1–3 positive nodes in 42% of patients and >3 positive nodes in 22%. 36% of patients had node negative disease.
Microscopically negative margins (R0) were reported in 76% of the patients, while 14% had microscopically positive (R1) margins and 1% patients had grossly positive (R2) margins. Median length of hospital stay was 10 days. 476 patients (10%) were readmitted to the hospital within 30 days of surgery. 316 patients died within 30 days of surgery, resulting in an overall operative mortality rate of 5.5%.
With regard to adjuvant therapies, 39% of patients received radiation and chemotherapy in addition to surgery. Chemotherapy alone was given to 23% of patients. 2% of patients received radiation alone. 36% of the patients received no systemic treatment. Systemic therapy was administered on a neoadjuvant basis in 8% (264/3410) of patients, both before and after surgery in 3% (93) of patients, and on an adjuvant basis in 51% (1739) of patients. Unfortunately, the sequence of surgery and systemic therapy was not recorded in a large number of patients (40% of 5726).
Survival statistics
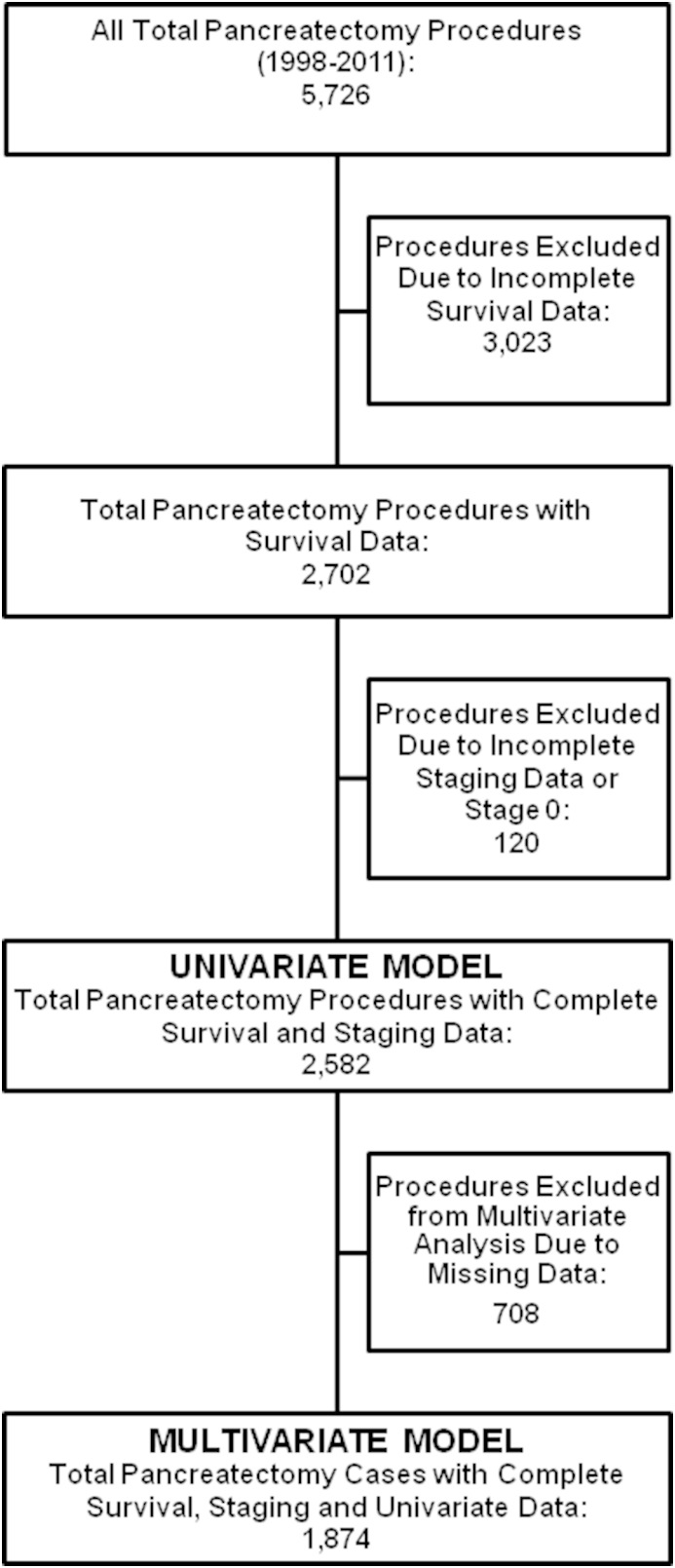
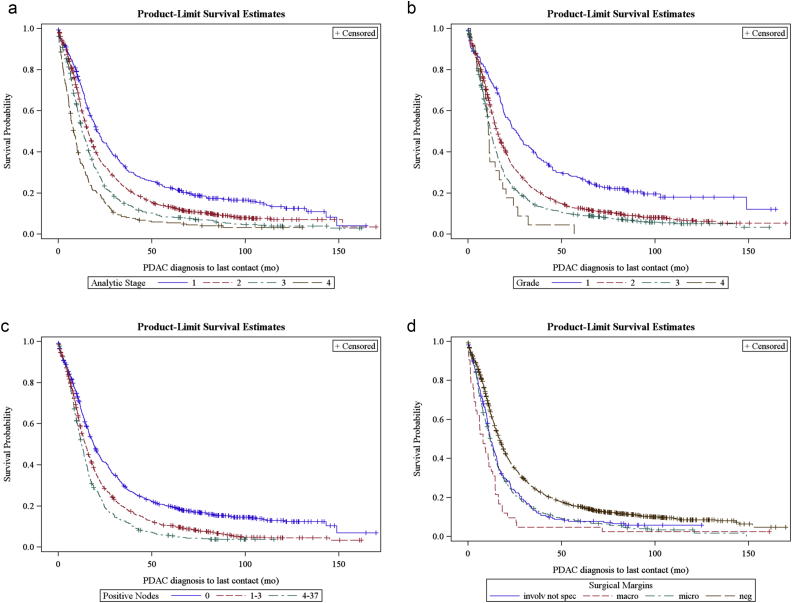
Of the 5726 patients selected for the study, 2582 patients (45%) were included in the univariate survival analysis. All patients that had survival data and were stage 1–4 (Fig. 1). The median overall survival was 15 months. One, three, and five-year survival was 59%, 21%, and 12% respectively. 12 factors were found to have a significant impact on survival (Table 2). Improved survival was associated with patient-specific characteristics younger age, more recent year of diagnosis, academic facility, and private insurance. Tumor-specific characteristics associated with improved survival were smaller tumor size, lower tumor grade, earlier analytic stage, and less cancer spread to lymph nodes. Treatment-specific characteristics associated with improved survival were negative surgical margins, lymph node ratio, and administration of systemic therapy (Fig. 2).
Figure 1.
Inclusion criteria
Table 2.
Univariate survival statistics
| Univariate survival | Median (months) | 1 yr (%) | 3 yr (%) | 5 yr (%) | p |
|---|---|---|---|---|---|
| Overall survival | |||||
| N = 2582 | 15.15 | 59.49 | 21.24 | 12.95 | |
| Univariate demographics | |||||
| Facility type | <0.0001 | ||||
| Academic | 16.33 | 62.19 | 23.04 | 14.29 | |
| Comprehensive | 14.00 | 57.09 | 19.58 | 11.78 | |
| Community | 12.85 | 53.39 | 20.48 | 11.89 | |
| Other | 10.91 | 40.10 | 12.13 | 6.07 | |
| Insurance group | <0.0001 | ||||
| Medicare | 13.77 | 55.18 | 17.82 | 10.71 | |
| Private | 17.48 | 65.47 | 25.95 | 16.16 | |
| Uninsured/other | 14.03 | 57.02 | 19.47 | 11.28 | |
| Univariate tumor characteristics | Median (months) | 1 yr (%) | 3 yr (%) | 5 yr (%) | p |
|---|---|---|---|---|---|
| Analytic stage | <0.0001 | ||||
| 1 | 20.67 | 72.79 | 32.37 | 22.44 | |
| 2 | 16.00 | 62.20 | 22.64 | 13.16 | |
| 3 | 12.71 | 53.57 | 14.70 | 8.31 | |
| 4 | 8.15 | 34.61 | 8.47 | 5.42 | |
| Grade (N = 2386) | <0.0001 | ||||
| 1 | 25.76 | 74.80 | 39.87 | 27.12 | |
| 2 | 15.61 | 61.72 | 21.51 | 12.56 | |
| 3 | 12.06 | 50.22 | 14.05 | 9.20 | |
| 4 | 10.91 | 35.19 | 4.40 | 0.00 | |
| Tumor size (N = 2332) | <0.0001 | ||||
| <2 cm | 23.13 | 70.87 | 35.00 | 25.78 | |
| 2–5 cm | 15.67 | 61.49 | 21.11 | 12.01 | |
| >5 cm | 11.50 | 47.33 | 14.18 | 8.83 |
| Univariate tumor characteristics | Median (months) | 1 yr (%) | 3 yr (%) | 5 yr (%) | p |
|---|---|---|---|---|---|
| Positive nodes (N = 2411) | <0.0001 | ||||
| 0 | 18.92 | 67.77 | 29.19 | 19.77 | |
| 1-3 | 14.46 | 57.68 | 18.76 | 10.42 | |
| >3 | 12.52 | 51.69 | 12.60 | 5.42 | |
| Nodes ratio (N = 2370) | <0.0001 | ||||
| 0 | 19.29 | 68.19 | 29.37 | 19.91 | |
| 0.01–0.14 | 15.44 | 61.60 | 20.40 | 13.08 | |
| 0.15–0.29 | 14.46 | 58.22 | 15.51 | 6.93 | |
| 0.30–1 | 11.83 | 49.49 | 14.92 | 7.26 | |
| Surgical margin (N = 2457) | <0.0001 | ||||
| Negative | 16.79 | 63.71 | 24.36 | 15.30 | |
| Micro positive | 11.96 | 49.16 | 13.05 | 7.80 | |
| Not specified | 11.89 | 49.31 | 14.39 | 7.38 | |
| Macro positive | 8.08 | 35.71 | 4.76 | 4.76 |
| Univariate adjuvant therapy | Median (months) | 1 yr (%) | 3 yr (%) | 5 yr (%) | p |
|---|---|---|---|---|---|
| Adjuvant Rx (N = 2386) | <0.0001 | ||||
| Chemo + Rads | 19.09 | 71.43 | 27.79 | 16.83 | |
| Chemo | 15.97 | 63.07 | 22.12 | 14.20 | |
| Rads | 12.85 | 51.88 | 8.65 | 5.40 | |
| No adjuvant Rx | 11.73 | 48.79 | 17.43 | 10.38 |
| Univariate continuous | Unit | Hazard ratio | 95% Confidence interval | p |
|---|---|---|---|---|
| Age | ||||
| 10 years | 1.125 | 1.083–1.170 | <0.0001 | |
| Distance to hosp (N = 2495) | ||||
| 10 fold increase in distance | 0.931 | 0.867–0.999 | 0.0474 | |
| Yr of diagnosis | ||||
| 1 year | 0.981 | 0.965–0.997 | 0.0222 |
*Paired comparisons are statistically significant after controlling for multiple comparisons when p < 0.0170 for 3 comparisons and p < 0.0127 for 4 comparisons. Thus, note that facility type “Other vs. Academic” is not statistically significant.
Figure 2.
Kaplan–Meier survival by (a) Analytic stage, (b) Tumor grade, (c) Positive node status, and (d) Surgical margins.
A multivariate survival analysis was performed to independently evaluate the 12 factors determined to predict survival in the univariate analysis. 1874 patients (33% of the full cohort and 73% of patients included in univariate survival analyses) had sufficient data in all fields to be included in the multivariate analysis (Fig. 1). We elected to exclude analytic stage and lymph node ratio from this analysis, because these data are directly related to other variables including tumor size and lymph node positivity. Therefore we determined that their inclusion would potentially confound the results of the analysis. With the remaining ten factors included, seven factors were found to have a statistically significant impact on improved patient survival (Table 3). These factors include younger age, academic facility, smaller tumor size, lower tumor grade, less lymph node positivity, negative surgical margin status, and administration of systemic therapy.
Table 3.
Multivariate survival statistics
| Multivariate N = 1874 |
Comparison | Hazard ratio | 95% Confidence interval | p* |
|---|---|---|---|---|
| Age | <0.0001 | |||
| Unit = 10 years | 1.134 | 1.083–1.189 | ||
| Facility type | 0.0042 | |||
| Comprehensive vs. | Academic | 1.159 | 1.046–1.285 | 0.0048 |
| Community vs. | Academic | 1.192 | 0.963–1.476 | 0.1074 |
| Other vs. | Academic | 1.568 | 1.092–2.252 | 0.0149 |
| Community vs. | Comprehensive | 1.028 | 0.828–1.276 | 0.8014 |
| Grade | <0.0001 | |||
| 2 vs. | 1 | 1.448 | 1.210–1.732 | <0.0001 |
| 3 vs. | 1 | 1.795 | 1.490–2.164 | <0.0001 |
| 4 vs. | 1 | 2.862 | 1.738–4.711 | <0.0001 |
| Tumor size | <0.0001 | |||
| 2–5 cm vs. | <2 cm | 1.422 | 1.206–1.676 | <0.0001 |
| >5 cm vs. | <2 cm | 1.725 | 1.424–2.089 | <0.0001 |
| >5 cm vs. | 2-5 cm | 1.213 | 1.070–1.376 | 0.0026 |
| Node status | <0.0001 | |||
| 1–3 | Negative | 1.300 | 1.164–1.453 | <0.0001 |
| >3 | Negative | 1.632 | 1.415–1.883 | <0.0001 |
| >3 | 1–3 | 1.256 | 1.098–1.435 | 0.0009 |
| Margin status | <0.0001 | |||
| R1 vs. | R0 | 1.470 | 1.274–1.695 | <0.0001 |
| R2 vs. | R0 | 1.858 | 1.241–2.74 | 0.0027 |
| Not specified vs. | R0 | 1.318 | 1.100–1.581 | 0.0028 |
| R2 vs. | R1 | 1.264 | 0.831–1.93 | 0.2730 |
| Adjuvant Rx | <0.0001 | |||
| Chemo vs. | Chemo + Rads | 1.131 | 0.971–1.317 | 0.1133 |
| Rads vs. | Chemo + Rads | 1.566 | 1.170–2.096 | 0.0026 |
| No adjuvant Rx vs. | Chemo + Rads | 1.767 | 1.582–1.973 | <0.0001 |
*Paired comparisons are statistically significant after controlling for multiple comparisons when p < 0.0170 for 3 comparisons and p < 0.0127 for 4 comparisons. Thus, note that facility type “Other vs. Academic” is not statistically significant.
Discussion
The decision to perform a total pancreatectomy in the setting of invasive cancer continues to be a difficult one for many surgeons. Once considered to be superior to partial pancreatectomy, total pancreatectomy fell out of favor in the 1990's and early 2000's because of persistently high mortality rates and a vastly decreased quality of life without native endocrine and exocrine pancreatic function. Recently, however, the appropriateness of the procedure has been reconsidered in the context of improved perioperative care, recognition that high-volume centers are capable of limiting peri-operative mortality, and the widespread availability and effectiveness of long-acting insulin and exogenous pancreatic enzyme formulations. Patient selection for this procedure is challenging, as survival for the entire group is suboptimal, and selection criteria are currently being proposed in the literature. Although, Nathan et al. have published a similar powered series in the literature, specific comparisons of prognostic factors and relative patient survival rates are lacking.8 The current series addresses these issues and is the largest to date in the literature.
Our multivariate analysis shows that seven factors impact survival in patients undergoing total pancreatectomy for pancreatic ductal adenocarcinoma. Advancing age is a non-modifiable factor that likely reflects patients' overall health and physiologic reserve. The type of surgical facility was important, and this remains an important consideration in the management of patients with pancreatic neoplasms. Academic research centers demonstrated the best survival results, followed by comprehensive community cancer centers. Community cancer centers were under-represented in the analysis and this is a reflection of national referral patterns. These data support consideration of referral of patients with pancreatic cancer to a high-volume cancer center, a position supported by numerous previous studies.15, 16
Tumor size and grade are tumor-specific factors that were significant predictors of overall survival in the multivariate analysis. Tumor size is included in the current AJCC T-staging guidelines for pancreatic cancer, but the current criteria are only for tumors greater or smaller than 2 cm. The current study shows a survival difference between patients with tumors <2 cm, tumors 2–5 cm, and tumors >5 cm suggesting that there may be some prognostic advantage to including these size parameters in the future staging algorithms. Similarly, tumor grade was significant in this analysis, and should be similarly considered.
Patients with lymphatic metastases also have worse survival outcomes compared with those without lymphatic spread, which is not surprising. However, the data demonstrate a survival difference between patients with 1–3 nodal metastases and those with >3 positive lymph nodes. Again, this finding suggests that the number of positive nodes impacts prognosis, and should be considered in the nodal staging algorithm for pancreatic adenocarcinoma. Positive surgical margins are an important factor in estimating survival in patients undergoing resection for pancreatic adenocarcinoma. This has been questioned in previous studies.7, 17, 18 The current NCDB data shows that there is a significant difference in survival between patients with negative and positive margins. Those with microscopic positive (R1) margins showed a survival advantage over those with macroscopic margins, but this did not achieve statistical significance in the multivariate analysis. Finally, patients survived longer if they received combined systemic chemotherapy and radiation compared to alternative adjuvant treatments, but this is arguably influenced by selection bias. The frequency of patients in this series that did not receive systemic treatment seems high, but is typical for reports for postoperative adjuvant strategy at major institutions.19, 20 One series from the Mayo Clinic examined prognostic factors in their patients resected between 1995 and 2005, finding that 46% (69 of 151) patients did not receive systemic therapy.21 One potential advantage of a neoadjuvant approach for chemotherapy in this group of patients is that more patients actually receive their systemic therapy; usually about 80–90% of patients with resectable tumors that receive neoadjuvant therapy with intent of getting to resection are able to undergo R0 or R1 resection.19
This NCDB review suggests that total pancreatectomy should be a consideration for selected patients with pancreatic ductal adenocarcinoma. Survival for the entire cohort is limited, at a mean of 15 months. Yet median survival for some subsets of the group is acceptable, which we would define as 18 months or more, a number similar to most large prospective studies that report overall survival for pancreaticoduodenectomy for pancreatic adenocarcinoma.22, 23 Although not assessed specifically in this review, patients that have adenocarcinoma in the setting of IPMN can achieve longer survival, as published elsewhere.24 Indications to perform a total pancreatic resection include large tumors that cannot be extirpated with a more limited operation, centrally located tumors that leave marginal options for pancreatic reconstruction, and for patients in whom partial pancreatectomy is not possible for anatomic or technical reasons. Younger patients are more likely to survive longer. Specifically, each decade of advancing age yielded an increase of death of 13%. Therefore patients with age >70 experience a significant decline in potential benefit of the procedure, especially if other tumor factors are not favorable. These data suggest that survival is improved if these procedures are performed in high-volume centers of excellence. The fact that survival is improved in patients with smaller, node negative tumors is more a reflection of the stage of disease rather than the operation that was performed. However, the impact of achieving a negative surgical margin is highlighted in this review, and is probably one of the few significant factors impacting survival that can be controlled by the surgeon. The benefits of systemic therapy in combination with surgery have been illustrated in other studies, and this review is consistent with those conclusions.
The overall 30-day mortality rate for cancer patients undergoing total pancreatectomy is 5.5%. This is consistent with recently reported mortality data collected from the ACS NSQIP that shows total pancreatectomy to have a nearly two-fold increase in mortality risk compared to proximal pancreatectomy.25, 26 These mortality rates are lower than those reported in previous decades, but high enough to discourage elective total pancreatectomy if a partial resection would be oncologically and technically feasible. Hospital lengths of stay and readmission rates are comparable to other major pancreatic resections. Quality of life was not included in this analysis, but the results of other recent studies demonstrate overall improvement in patient satisfaction compared to historical reviews. Considering the consistently poor survival outcomes of patients who undergo margin-positive resections, an effort to obtain clear margins via progression to total pancreatectomy should be considered in patients with favorable characteristics. There are several limitations to this study, which include the retrospective nature of the review, the lack of morbidity data, and missing data, although expected in a database of this size and nature. No clinically relevant analysis included variables with missing data, as discussed in the methods section. In addition, there is a need for more specific oncologic outcome data such as local recurrence rates, and lack of tolerance data specific to total pancreatectomy, such as readmissions for endocrine deficiencies, details of hypoglycemic control, and quality of life.
Conclusion
The question of whether to proceed with a total pancreatic resection becomes one based on the benefits of removing the entire gland with the intention of clearing all gross and microscopic disease, and minimizing the likelihood for local recurrence. Large comparative studies looking at partial versus total pancreatectomy are not currently available, but an NCDB review comparing total pancreatic resection with partial resection would be informative. This review serves to define the survival characteristics of patients with invasive pancreatic tumors who underwent a total pancreatectomy in the last 14 years in the United States, and may provide a baseline for future comparative analyses on this topic.
Disclosures
The authors of this manuscript do not have anything to disclose.
Support
The authors would like to acknowledge support from the Foundation for Surgical Fellowships (FSF) and Providence Cancer Center.
Conflicts of interest
None to declare.
Footnotes
Presented at the Scientific Papers Sessions at the American College of Surgeons 2014 Clinical Congress.
References
- 1.Sarr M.G., Behrns K.E., van Heerden J.A. Total pancreatectomy. An objective analysis of its use in pancreatic cancer. Hepatogastroenterology. 1993;40:418–421. [PubMed] [Google Scholar]
- 2.Trede M. The surgical treatment of pancreatic carcinoma. Surgery. 1985;97:28–35. [PubMed] [Google Scholar]
- 3.Ihse I., Anderson H., Andren S. Total pancreatectomy for cancer of the pancreas: is it appropriate? World J Surg. 1996;20:288–293. doi: 10.1007/s002689900046. discussion 94. [DOI] [PubMed] [Google Scholar]
- 4.Casadei R., Monari F., Buscemi S., Laterza M., Ricci C., Rega D. Total pancreatectomy: indications, operative technique, and results: a single centre experience and review of literature. Updates Surg. 2010;62:41–46. doi: 10.1007/s13304-010-0005-z. [DOI] [PubMed] [Google Scholar]
- 5.Muller M.W., Friess H., Kleeff J., Dahmen R., Wagner M., Hinz U. Is there still a role for total pancreatectomy? Ann Surg. 2007;246:966–974. doi: 10.1097/SLA.0b013e31815c2ca3. discussion 74–75. [DOI] [PubMed] [Google Scholar]
- 6.Barbier L., Jamal W., Dokmak S., Aussilhou B., Corcos O., Ruszniewski P. Impact of total pancreatectomy: short- and long-term assessment. HPB. 2013;15:882–892. doi: 10.1111/hpb.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy S., Wolfgang C.L., Cameron J.L., Eckhauser F., Choti M.A., Schulick R.D. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg. 2009;250:282–287. doi: 10.1097/SLA.0b013e3181ae9f93. [DOI] [PubMed] [Google Scholar]
- 8.Nathan H., Wolfgang C.L., Edil B.H., Choti M.A., Herman J.M., Schulick R.D. Peri-operative mortality and long-term survival after total pancreatectomy for pancreatic adenocarcinoma: a population-based perspective. J Surg Oncol. 2009;99:87–92. doi: 10.1002/jso.21189. [DOI] [PubMed] [Google Scholar]
- 9.Karpoff H.M., Klimstra D.S., Brennan M.F., Conlon K.C. Results of total pancreatectomy for adenocarcinoma of the pancreas. Arch Surg. 2001;136:44–47. doi: 10.1001/archsurg.136.1.44. discussion 8. [DOI] [PubMed] [Google Scholar]
- 10.Crippa S., Tamburrino D., Partelli S., Salvia R., Germenia S., Bassi C. Total pancreatectomy: indications, different timing, and perioperative and long-term outcomes. Surgery. 2011;149:79–86. doi: 10.1016/j.surg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Raut C.P., Cleary K.R., Staerkel G.A., Abbruzzese J.L., Wolff R.A., Lee J.H. Intraductal papillary mucinous neoplasms of the pancreas: effect of invasion and pancreatic margin status on recurrence and survival. Ann Surg Oncol. 2006;13:582–594. doi: 10.1245/ASO.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 12.D'Angelica M., Brennan M.F., Suriawinata A.A., Klimstra D., Conlon K.C. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–408. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan H., Cameron J.L., Choti M.A., Schulick R.D., Pawlik T.M. The volume-outcomes effect in hepato-pancreato-biliary surgery: hospital versus surgeon contributions and specificity of the relationship. J Am Coll Surg. 2009;208:528–538. doi: 10.1016/j.jamcollsurg.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Li V.K., Yum J.L., Yeung Y.P. Optimal timing of elective laparoscopic cholecystectomy after acute cholangitis and subsequent clearance of choledocholithiasis. Am J Surg. 2010;200:483–488. doi: 10.1016/j.amjsurg.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Birkmeyer J.D., Finlayson S.R., Tosteson A.N., Sharp S.M., Warshaw A.L., Fisher E.S. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125:250–256. [PubMed] [Google Scholar]
- 16.Finks J.F., Osborne N.H., Birkmeyer J.D. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raut C.P., Tseng J.F., Sun C.C., Wang H., Wolff R.A., Crane C.H. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt C.M., Glant J., Winter J.M., Kennard J., Dixon J., Zhao Q. Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin positive pancreatic adenocarcinoma. Surgery. 2007;142:572–578. doi: 10.1016/j.surg.2007.07.016. discussion 8–80. [DOI] [PubMed] [Google Scholar]
- 19.Heinrich S., Schafer M., Weber A., Hany T., Bhure U., Pestalozzi B. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: results of a prospective phase II trial. Ann Surg. 2008;248:1014–1022. doi: 10.1097/SLA.0b013e318190a6da. [DOI] [PubMed] [Google Scholar]
- 20.Klinkenbijl J.H., Jeekel J., Sahmoud T., van Pel R., Couvreur M.L., Veenhof C.H. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782. doi: 10.1097/00000658-199912000-00006. discussion 82–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallemeier C., Botros M., Corsini M., Haddock M., Gunderson L., Miller R. Preoperative CA 19-9 level is an important prognostic factor in patients with pancreatic adenocarcinoma treated with surgical resection and adjuvant concurrent chemoradiotherapy. Am J Clin Oncol. 2011;34:567–572. doi: 10.1097/COC.0b013e3181f946fc. [DOI] [PubMed] [Google Scholar]
- 22.Regine W.F., Winter K.A., Abrams R.A., Safran H., Hoffman J.P., Konski A. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 23.Oettle H., Post S., Neuhaus P., Gellert K., Langrehr J., Ridwelski K. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 24.Koh Y.X., Zheng H.L., Chok A.Y., Tan C.S., Wyone W., Lim T.K. Systematic review and meta-analysis of the spectrum and outcomes of different histologic subtypes of noninvasive and invasive intraductal papillary mucinous neoplasms. Surgery. 2015;157:496–509. doi: 10.1016/j.surg.2014.08.098. [DOI] [PubMed] [Google Scholar]
- 25.Parikh P., Shiloach M., Cohen M.E., Bilimoria K.Y., Ko C.Y., Hall B.L. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB. 2010;12:488–497. doi: 10.1111/j.1477-2574.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kneuertz P.J., Pitt H.A., Bilimoria K.Y., Smiley J.P., Cohen M.E., Ko C.Y. Risk of morbidity and mortality following hepato-pancreato-biliary surgery. J Gastrointest Surg. 2012;16:1727–1735. doi: 10.1007/s11605-012-1938-y. [DOI] [PubMed] [Google Scholar]