Abstract
Background
Computed tomography and serum tumor markers have limited value in detecting recurrence after curative surgery of pancreatic cancer. This study evaluated the clinical utility of 18F-fluorodeoxyglucose positron emission tomography–computed tomography (PET–CT) in diagnosing recurrence.
Methods
One hundred ten patients underwent curative resection of pancreatic cancer were enrolled. The diagnostic value of abdominal computed tomography (CT), PET–CT and serum carbohydrate antigen (CA) 19-9 concentration were compared. The prognostic value of SUVmax on PET–CT was evaluated.
Results
PET–CT showed relatively higher sensitivity (84.5% vs. 75.0%) and accuracy (84.5% vs. 74.5%) than CT, whereas PET–CT plus CT showed greater sensitivity (97.6%) and accuracy (90.0%) than either alone. In detecting distant recurrences, PET–CT showed higher sensitivity (83.1% vs. 67.7%) than CT. Nineteen patients showed recurrences only on PET–CT, with eleven having invisible or suspected benign lesions on CT, and eight had recurrences in areas not covered by CT. SUVmax over 3.3 was predictive of poor survival after recurrence.
Conclusions
PET–CT in combination with CT improves the detection of recurrence. PET–CT was especially advantageous in detecting recurrences in areas not covered by CT. If active post-operative surveillance after curative resection of pancreatic cancer is deemed beneficial, then it should include PET–CT combined with CT.
Introduction
Pancreatic cancer is a malignancy with a poor prognosis. Patients with pancreatic cancer have an estimated 5-year survival rate of 5%.1, 2 Complete surgical resection combined with adjuvant chemotherapy is the only potential curative treatment for pancreatic cancer. Unfortunately, only 15–20% of patients presenting with pancreatic cancer are eligible for such an approach2, 3 and five year survival rates of patients who undergo curative resections with adjuvant chemotherapy are only 15–25%.1, 4, 5, 6, 7, 8
Most recurrences after attempted curative resection of pancreatic cancer occur within two years.9, 10 Surveillance after surgery usually includes physical examination, measurements of serum tumor markers such as CA19-9 and radiological imaging modalities such as ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI). Guidelines of the National Comprehensive Cancer Network (NCCN) recommend regular surveillance, including history taking, physical examination, serum CA19-9 levels and CT scans, every 3–6 months for the first two years after curative resection.11 These recommendations, however, were based on low-level evidence, and only a few small studies have evaluated the optimal methods of detecting recurrence.12 In addition, it is not clear early detection translates into better quality of life or increase in survival.
Previously 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET–CT) has been reported to be helpful in detecting and staging pancreatic cancers,13, 14, 15, 16 as well as early and distant recurrences,12, 16, 17, 18 although this remains controversial. PET–CT combination with abdominal CT was reported to have limited clinical utility because additional metastases were rarely found in other study.19
The maximal standardized uptake value (SUVmax) is a quantitative marker of the uptake of 18F-fluorodeoxyglucose by the tumor and may be associated with prognosis in pancreatic cancer patients.20, 21 Less is known, however, about the prognostic value of SUVmax in recurrent pancreatic cancer.
This study was designed to compare the clinical usefulness of PET–CT with that of abdominal CT in follow-up of patients who underwent curative resection of pancreatic cancer and to explore the diagnostic potential of SUVmax as a prognostic biomarker in recurrent pancreatic cancer.
Methods
Patients
One hundred ten patients who underwent curative resection for pancreatic cancer and were followed-up at least once by PET–CT at Seoul National University Hospital between May 2004 and June 2012 were analyzed. The patients who had both abdominal CT and PET–CT performed within one month were enrolled. Clinicopathologic features, including serum tumor marker concentrations, abdominal CT and PET–CT results, pathologic results, recurrences and survival outcomes, were reviewed retrospectively.
Follow-up after resection included serum CA19-9 measurements and abdominal CT at 3, 6, and 12 months and every 6 months thereafter. Most patients also underwent PET–CT once yearly, as well as when other follow-up tests suggested tumor recurrence.
Abdominal CT images were obtained from lower lung to pelvis during the arterial and portal venous phases using a multidetector-row CT scanner (Mx 8000, Philips Healthcare; LightSpeed Ultra, GE Healthcare; Sensation 16, Siemens Healthcare) following administration of 90 mL of nonionic contrast material at a rate of 3.0–4.0 mL/s. The slice thickness was 3–5 mm.
PET–CT was performed using a Philips Gemini Dual (Best, The Netherlands) or Siemens Biograph TruePoint (Germany) system. After fasting for at least 8 h, patients were administered 5.18 MBq/kg (0.14 mCi/kg) of fluorodeoxyglucose (FDG). One hour later, whole body PET scan was performed from the skull base to the mid-thigh.
Recurrence
Recurrences were classified as pathologically or clinically confirmed. Clinically confirmed recurrences were defined as recurrences suspected on abdominal CT and/or PET–CT and/or elevation of serum CA19-9, which were confirmed as recurrence on subsequent follow-up. For example, a patient with suspected recurrent lesion on abdominal CT at 6 months after operation, showed more progressive lesion on abdominal CT and elevated serum CA19-9 level at 9 months after operation and thereafter. This patient was regarded as clinically confirmed recurrence at 6 months and the results of PET–CT and serum CA19-9 at 6 months post-operatively were assessed as a false negative result. On the contrary, another patient with suspected recurrent lesion on abdominal CT at 6 months post-operatively, showed similar lesion on abdominal CT and negative results on PET–CT and serum CA19-9 level at 9 months after operation and thereafter. This patient was regarded as clinically no recurrence and the result of abdominal CT at 6 months was assessed as a false positive result. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of each tests were assessed based on the recurrence definition of this study and diagnostic values of abdominal CT, PET–CT and serum CA19-9 level performed at the same period of time (within 1 month) were compared.
Survival outcome after recurrence
Survival outcome after recurrence detection was assessed to evaluated prognostic value of SUVmax. On the basis of the date when recurrence was detected first, survival duration after recurrence was calculated.
Statistical analysis
Statistical analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, USA). The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of abdominal CT, PET–CT, serum CA19-9 and their combinations were calculated and compared using McNemar's test and Fisher's exact test. Receiver operating characteristics (ROC) curve analysis was used to determine the optimal SUVmax cutoff value for 24-month survival after recurrence. Cumulative survival rates were estimated using the Kaplan–Meier method. P-values ≤0.05 were considered statistically significant.
Results
The clinicopathological findings of the 110 patients who underwent curative resection for primary pancreatic cancer are shown in Table 1.
Table 1.
Clinicopathologic characteristics of 110 patients who underwent curative resection of pancreatic cancer
| Characteristics | Number of patients (N = 110) | |
|---|---|---|
| Age | Median (range) | 62 (35–84) years |
| Gender | Male | 63 (57.3%) |
| Diabetes | 68 (61.8%) | |
| Operation | PD/PPPD | 78 (70.9%) |
| DP | 28 (25.5%) | |
| TP | 4 (3.6%) | |
| Stage | Ia | 2 (1.8%) |
| IIa | 51 (46.4%) | |
| IIb | 55 (50.0%) | |
| III | 2 (1.8%) | |
| Adjuvant treatment | 91 (82.7%) | |
| Follow-up duration | Median (range) | 26.0 (8.5–81.0) months |
| Median (range) serum CA19-9 at time of PET–CT | Recur (+) (N = 84) | 169 (1–10830) U/ml |
| Recur (−) (N = 26) | 10 (1–52) U/ml | |
The overall recurrence rate was 76.4% (84/110). Recurrences were pathologically confirmed in 31 patients (36.9%) and clinically confirmed in 53 (63.1%). Of the patients with recurrence, 19 (22.6%) had locoregional recurrence without systemic recurrence and 65 (77.4%) had systemic recurrence including liver metastasis (n = 26), peritoneal seeding (n = 13), pulmonary metastasis (n = 12), metastasis to distant LN (n = 18), bone metastasis (n = 5) and metastasis to surgical wound (n = 2). The median time to detection of recurrence was 11.0 months (range 1–54 months), and was longer in patients with locoregional than systemic recurrence with a trend toward significance (13 vs. 10 months, P = 0.091). Extra-abdominal recurrence was observed in 19 patients, with pulmonary metastasis (n = 12) being the most frequent type of extra-abdominal recurrence.
Diagnostic value
The diagnostic values of abdominal CT, PET–CT, serum CA19-9 and their combinations are shown in Table 2a. The diagnostic values of abdominal CT and PET–CT differed by site of recurrence (Table 2b).
Table 2a.
Diagnostic values of abdominal CT, PET–CT, CA19-9 and their combinations overall
| CT | PET | CA19-9 | CT + PET–CT | CT + CA19-9 | PET–CT + CA19-9 | P-Value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CT vs. PET–CT | CT vs. CA19-9 | PET–CT vs. CA19-9 | CT vs. CT + PET–CT | PET vs. CT + PET–CT | |||||||
| Sensitivity | 75.0% (63/84) | 84.5% (71/84) | 67.9% (57/84) | 97.6% (82/84) | 97.6% (82/84) | 92.9% (78/84) | 0.180 | 0.397 | 0.019 | <0.001 | 0.007 |
| Specificity | 73.1% (19/26) | 84.6% (22/26) | 88.5% (23/26) | 65.4% (17/26) | 65.4% (17/26) | 76.9% (20/26) | 0.499 | 0.290 | 0.995 | 0.763 | 0.201 |
| PPV | 90.0% (63/70) | 94.7% (71/75) | 95.0% (57/60) | 90.1% (82/91) | 90.1% (82/91) | 92.9% (78/84) | 0.450 | 0.462 | 0.754 | 0.807 | 0.419 |
| NPV | 47.5% (19/40) | 62.8% (22/35) | 46.0% (23/50) | 89.5% (17/19) | 89.5% (17/19) | 76.9% (20/26) | 0.273 | 0.944 | 0.191 | 0.005 | 0.076 |
| Accuracy | 74.5% (82/110) | 84.5% (93/110) | 72.7% (80/110) | 90.0% (99/110) | 90.0% (99/110) | 89.1% (98/110) | 0.095 | 0.881 | 0.049 | 0.005 | 0.307 |
Table 2b.
Diagnostic values of abdominal CT, PET–CT, CA19-9 and their combinations according to the recurrence site
| CT | PET–CT | CT + PET–CT | P-Value |
||||
|---|---|---|---|---|---|---|---|
| CT vs. PET–CT | CT vs. CT + PET–CT | PET–CT vs. CT + PET–CT | |||||
| Local recurrence | Sensitivity | 82.5% (33/40) | 80.0% (32/40) | 97.5% (39/40) | 1.000 | 0.062 | 0.033 |
| Specificity | 95.7% (67/70) | 92.9% (65/70) | 88.6% (62/70) | 0.726 | 0.212 | 0.558 | |
| PPV | 91.7% (33/36) | 86.5% (32/37) | 83.0% (39/47) | 0.736 | 0.405 | 0.891 | |
| NPV | 90.5% (67/74) | 89.0% (65/73) | 98.4% (62/63) | 0.978 | 0.110 | 0.064 | |
| Accuracy | 90.9% (100/110) | 88.2% (97/110) | 91.8% (101/110) | 0.664 | 0.998 | 0.506 | |
| Distant recurrence | Sensitivity | 67.7% (44/65) | 83.1% (54/65) | 95.4% (62/65) | 0.067 | <0.001 | 0.048 |
| Specificity | 88.9% (40/45) | 86.7% (39/45) | 80.0% (36/45) | 1.000 | 0.382 | 0.569 | |
| PPV | 89.8% (44/49) | 90.0% (54/60) | 87.3% (62/71) | 0.776 | 0.897 | 0.835 | |
| NPV | 65.6% (40/61) | 78.0% (39/50) | 92.3% (36/39) | 0.221 | 0.005 | 0.122 | |
| Accuracy | 76.4% (84/110) | 84.5% (93/11) | 89.1% (98/110) | 0.179 | 0.021 | 0.419 | |
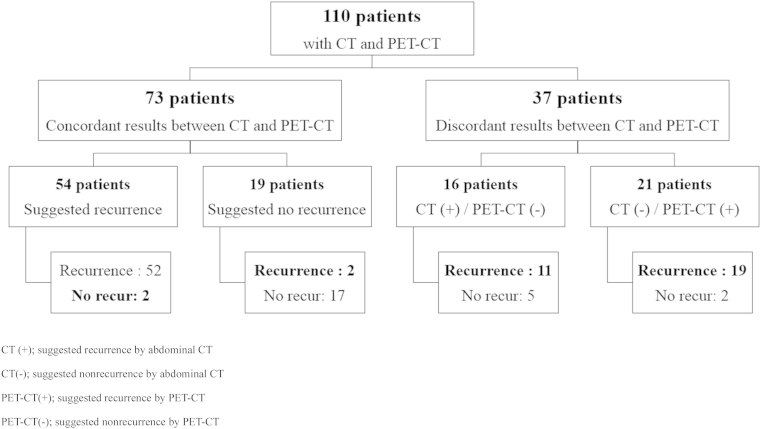
The results of abdominal CT and PET–CT are compared in Fig. 1.
Figure 1.
Comparisons of abdominal CT and PET–CT results
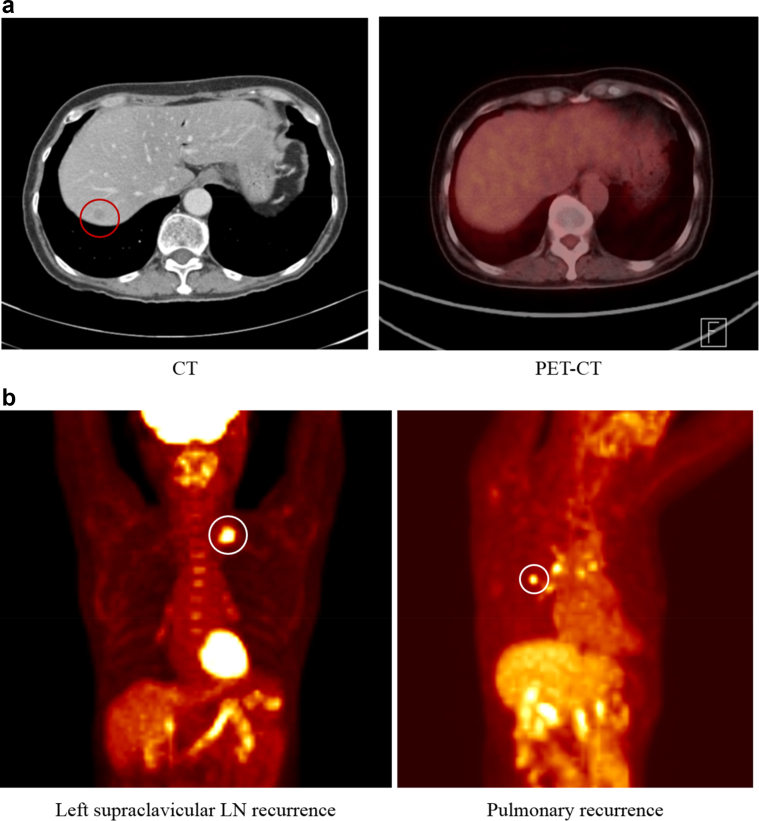
Fig. 2 shows false negative PET–CT findings (Fig. 2a) and recurrences undetected by abdominal CT since they occurred in areas not covered by abdominal CT (Fig. 2b).
Figure 2.
a) False negative finding on a PET–CT image. Eleven patients with false negative PET-CT findings showed suspected lesions on abdominal CT but no or only mildly hypermetabolic lesions on PET–CT. b) PET–CT detection of recurrences in areas not covered by abdominal CT. Of 19 patients with false negative CT findings, 11 showed no recurrent lesion or only a benign reactive change, while eight patients had recurrences in areas not covered by abdominal CT such as the supraclavicular LN and lungs
Prognostic value of SUVmax
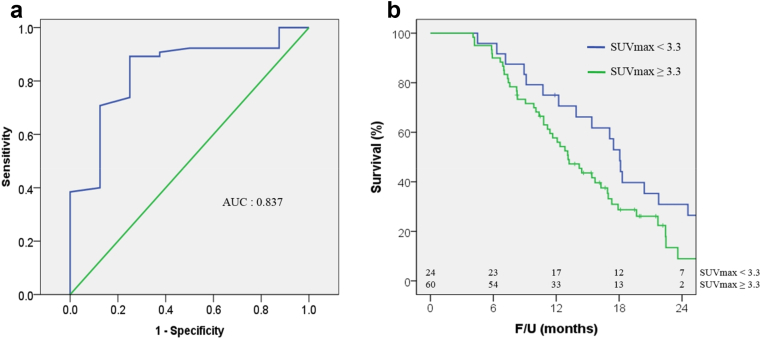
The median survival time after detection of recurrence was 14 months (range 4–44 months). Of the 84 patients with tumor recurrence, 69 received palliative chemotherapy. Construction of an ROC curve for 24-month survival after diagnosis of recurrence (Fig. 3a) yielded an area under the curve (AUC) of 0.837. The cutoff value of SUVmax was 3.3 with a sensitivity of 89.2% and a specificity of 75.0%. Median overall survival after recurrence was longer in the 24 patients with SUVmax <3.3 than in the 60 patients with SUVmax ≥3.3 (Fig. 3b). Median time to recurrence of SUVmax <3.3 group and SUVmax ≥3.3 group was not significantly different (10.6 vs. 11.0 months, P = 0.350). Subgroup analysis of the 65 patients who experienced systemic recurrence showed that median overall survival after systemic recurrence was significantly longer in the 19 patients with SUVmax <3.3 than in the 46 with SUVmax ≥3.3 (18 vs. 13 months, P = 0.029). Among the 19 patients with local recurrence alone, median survival after recurrence was similar in those with SUVmax <3.3 and ≥3.3 (19 vs. 16 months, P = 0.858).
Figure 3.
a) ROC curve of SUVmax for 24-month survival after tumor recurrence. b) Survival after recurrence according to SUVmax. ROC curve, receiver operating characteristics curve; SUVmax, maximum standard uptake value. b) Median overall survival time after tumor recurrence tended to be higher in the 24 patients with SUVmax <3.3 (n = 24) than in the 60 patients with SUVmax ≥3.3 (19 vs. 14 months, P = 0.051)
Discussion
Recurrence rates after curative resection of pancreatic cancer are high. Although no definitive treatment option has shown clear survival benefits in patients with recurrent pancreatic cancer, repeated radical resection or local ablation therapy of locally recurrent tumors has been found to improve survival outcomes in selected patients.22, 23, 24, 25 Moreover, the development of novel systemic chemotherapy regimens has improved treatment responses and survival outcomes of patients with recurrent pancreatic cancer.26, 27, 28, 29 Early detection and treatment of recurrence before systemic dissemination occurs and patient's general condition deteriorates may lead to improved survival. Therefore, it can be argued that efforts should be made to detect tumor recurrence following pancreatic cancer resection.
Generally, serum tumor markers and abdominal CT are the most widely utilized follow-up modalities after pancreatic cancer resection. Serum CA19-9 has shown clinical significance, with a sensitivity of 81% and a specificity of 77% in detecting recurrences.12 However, measurements of serum CA19-9 cannot provide any information about specific recurrence sites, which could be useful to establish treatment plan such as repeated resection or local ablation therapy when single local recurrence occurred. CT scanning has shown a sensitivity of 55% and a specificity of 75% in detecting recurrences,12 but has shown difficulties in differentiating between local recurrences and benign post-operative changes. Moreover, abdominal CT scan, which is routinely used for follow-up, may miss unsuspected metastatic recurrences in other areas.
PET–CT provides a combination of functional (PET) and anatomic (CT) images and may provide information about glucose metabolism in anatomical structures. Moreover, as a whole body scan, PET–CT has an advantage than abdominal CT. PET–CT can assess areas not covered by abdominal CT scan alone. PET–CT has also shown clinical significances in preoperative staging, especially in detecting metastases and monitoring responses to chemotherapy.13, 30, 31, 32 A meta-analysis showed that preoperative PET–CT had good diagnostic accuracy, with a sensitivity of approximately 90% and a specificity of approximately 80% for pancreatic cancer.33
Previous studies have reported that PET–CT had a sensitivity of 83.3–98%, a specificity of 90–90.5% and an accuracy of 86.7–96% for detecting recurrent pancreatic cancer.12, 18 The results of this study showed that PET–CT had greater sensitivity and accuracy than abdominal CT or serum CA19-9 concentration alone. The combination of PET–CT and abdominal CT showed significantly greater sensitivity, but lower specificity, than either alone. The diagnostic value of PET–CT plus abdominal CT was similar to that of abdominal CT plus serum CA19-9. PET–CT was reported to have much higher sensitivity than CT (96% vs. 39%) in detecting local recurrences, whereas CT/MRI was more sensitive than PET in detecting liver metastases (92% vs. 42%).17 This study found that abdominal CT and PET–CT had comparable diagnostic accuracy, and their combination showed improved sensitivity and tolerable specificity, in detecting locoregional recurrences. In detecting systemic recurrences, PET–CT showed higher sensitivity with a trend toward significance and similar specificity compared with abdominal CT, whereas the combination of PET–CT and abdominal CT had greater sensitivity and accuracy than abdominal CT alone. In this study, eight patients showed recurrent lesions in areas not covered by abdominal CT scan. The findings of this study therefore indicate that the combination of PET–CT and abdominal CT after curative resection of pancreatic cancer improves the detection of recurrences, especially of distant recurrences in areas not covered by abdominal CT. Prospective randomized controlled studies are needed to evaluate the clinical usefulness of PET–CT as routine follow-up of patients who undergo curative resection of pancreatic cancer.
Several studies have reported the prognostic value of preoperative PET–CT in pancreatic cancer patients with SUVmax cutoff values of 3.5–7.0 after resection20, 34, 35 or for locally advanced pancreatic cancer.36 Other PET–CT parameters, including metabolic tumor volume (MTV) and total lesion glycolysis (TLG), which reflect both metabolic activity and tumor volume, have been shown to be independent preoperative risk factors for overall and recurrence free survival.37, 38 However, no previous study assessed the predictive value of PET–CT in patients with recurrent pancreatic cancer. In evaluating the prognostic value of SUVmax in these patients, this study found that a cutoff of 3.3 was significantly associated with 24-month survival rates after tumor recurrence, with a SUVmax over 3.3 being a predictor of poor survival after recurrence.
This study had several limitations. Because it was based on retrospective data, it was susceptible to selection and informational biases, resulting in possible overestimations of the sensitivity and specificity of PET–CT. In addition, most recurrences were confirmed clinically, not pathologically. Although there may have been errors in defining clinically confirmed recurrent pancreatic cancer, the authors precisely reviewed all follow-up records to clarify clinical recurrences, minimizing the possibility of errors. Indeed, median overall post-recurrence survival time was similar in patients with pathologically and clinically confirmed tumors (33.3 vs. 26.2 months; P = 0.151) and this could support reliability of defining clinically confirmed recurrent pancreatic cancer in this study.
In conclusion, follow-up PET–CT after curative resection of pancreatic cancer is clinically useful, when combined with abdominal CT, in detecting tumor recurrences, especially distant recurrences involving areas not covered by abdominal CT. In addition, SUVmax of PET–CT was predictive of survival following recurrence. These findings suggest that PET–CT in combination with abdominal CT should be the preferred method of follow-up of patients who undergo curative resection of pancreatic cancer if it is thought that early detection likely to change subsequent management. There will be a need of following studies about proper interval of PET–CT and its cost effectiveness.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C2640).
Conflicts of interest
None declared.
References
- 1.Vincent A., Herman J., Schulick R., Hruban R.H., Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wray C.J., Ahmad S.A., Matthews J.B., Lowy A.M. Surgery for pancreatic cancer: recent controversies and current practice. Gastroenterology. 2005;128:1626–1641. doi: 10.1053/j.gastro.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Li D., Xie K., Wolff R., Abbruzzese J.L. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 4.Yeo C.J., Cameron J.L., Lillemoe K.D., Sitzmann J.V., Hruban R.H., Goodman S.N. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–731. doi: 10.1097/00000658-199506000-00011. discussion 731–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winter J.M., Cameron J.L., Campbell K.A., Arnold M.A., Chang D.C., Coleman J. 1423 Pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210–1191. [DOI] [PubMed] [Google Scholar]
- 6.Nitecki S.S., Sarr M.G., Colby T.V., van Heerden J.A. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang M.J., Jang J.Y., Lee S.E., Lim C.S., Lee K.U., Kim S.W. Comparison of the long-term outcomes of uncinate process cancer and non-uncinate process pancreas head cancer: poor prognosis accompanied by early locoregional recurrence. Langenbecks Arch Surg. 2010;395:697–706. doi: 10.1007/s00423-010-0593-6. [DOI] [PubMed] [Google Scholar]
- 8.Jang J.Y., Kang M.J., Heo J.S., Choi S.H., Choi D.W., Park S.J. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg. 2014;259:656–664. doi: 10.1097/SLA.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 9.Sperti C., Pasquali C., Piccoli A., Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 10.Shimada K., Sakamoto Y., Sano T., Kosuge T. The role of paraaortic lymph node involvement on early recurrence and survival after macroscopic curative resection with extended lymphadenectomy for pancreatic carcinoma. J Am Coll Surg. 2006;203:345–352. doi: 10.1016/j.jamcollsurg.2006.05.289. [DOI] [PubMed] [Google Scholar]
- 11.Tempero M.A., Arnoletti J.P., Behrman S., Ben-Josef E., Benson A.B., 3rd, Berlin J.D. Pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2010;8:972–1017. doi: 10.6004/jnccn.2010.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperti C., Pasquali C., Bissoli S., Chierichetti F., Liessi G., Pedrazzoli S. Tumor relapse after pancreatic cancer resection is detected earlier by 18-FDG PET than by CT. J Gastrointest Surg. 2010;14:131–140. doi: 10.1007/s11605-009-1010-8. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich S., Goerres G.W., Schafer M., Sagmeister M., Bauerfeind P., Pestalozzi B.C. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg. 2005;242:235–243. doi: 10.1097/01.sla.0000172095.97787.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kauhanen S.P., Komar G., Seppanen M.P., Dean K.I., Minn H.R., Kajander S.A. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957–963. doi: 10.1097/SLA.0b013e3181b2fafa. [DOI] [PubMed] [Google Scholar]
- 15.Wakabayashi H., Nishiyama Y., Otani T., Sano T., Yachida S., Okano K. Role of 18F-fluorodeoxyglucose positron emission tomography imaging in surgery for pancreatic cancer. World J Gastroenterol. 2008;14:64–69. doi: 10.3748/wjg.14.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asagi A., Ohta K., Nasu J., Tanada M., Nadano S., Nishimura R. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas. 2013;42:11–19. doi: 10.1097/MPA.0b013e3182550d77. [DOI] [PubMed] [Google Scholar]
- 17.Ruf J., Lopez Hanninen E., Oettle H., Plotkin M., Pelzer U., Stroszczynski C. Detection of recurrent pancreatic cancer: comparison of FDG-PET with CT/MRI. Pancreatology. 2005;5:266–272. doi: 10.1159/000085281. [DOI] [PubMed] [Google Scholar]
- 18.Kitajima K., Murakami K., Yamasaki E., Kaji Y., Shimoda M., Kubota K. Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent pancreatic cancer: comparison with integrated FDG-PET/non-contrast-enhanced CT and enhanced CT. Mol Imaging Biol. 2010;12:452–459. doi: 10.1007/s11307-009-0271-7. [DOI] [PubMed] [Google Scholar]
- 19.Pappas S.G., Christians K.K., Tolat P.P., Mautz A.P., Lal A., McElroy L. Staging chest computed tomography and positron emission tomography in patients with pancreatic adenocarcinoma: utility or futility? HPB. 2014;16:70–74. doi: 10.1111/hpb.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto K., Koyama I., Miyazawa M., Toshimitsu Y., Aikawa M., Okada K. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts early recurrence after pancreatic cancer resection. Int J Clin Oncol. 2011;16:39–44. doi: 10.1007/s10147-010-0124-z. [DOI] [PubMed] [Google Scholar]
- 21.Moon S.Y., Joo K.R., So Y.R., Lim J.U., Cha J.M., Shin H.P. Predictive value of maximum standardized uptake value (SUVmax) on 18F-FDG PET/CT in patients with locally advanced or metastatic pancreatic cancer. Clin Nucl Med. 2013;38:778–783. doi: 10.1097/RLU.0b013e31829f8c90. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki M., Yoshitomi H., Shimizu H., Ohtsuka M., Yoshidome H., Furukawa K. Repeat pancreatectomy for pancreatic ductal cancer recurrence in the remnant pancreas after initial pancreatectomy: is it worthwhile? Surgery. 2014;155:58–66. doi: 10.1016/j.surg.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 23.Habermehl D., Brecht I.C., Bergmann F., Welzel T., Rieken S., Werner J. Chemoradiation in patients with isolated recurrent pancreatic cancer – therapeutical efficacy and probability of re-resection. Radiat Oncol. 2013;8:27. doi: 10.1186/1748-717X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strobel O., Hartwig W., Hackert T., Hinz U., Berens V., Grenacher L. Re-resection for isolated local recurrence of pancreatic cancer is feasible, safe, and associated with encouraging survival. Ann Surg Oncol. 2013;20:964–972. doi: 10.1245/s10434-012-2762-z. [DOI] [PubMed] [Google Scholar]
- 25.Roeder F., Timke C., Uhl M., Habl G., Hensley F.W., Buechler M.W. Aggressive local treatment containing intraoperative radiation therapy (IORT) for patients with isolated local recurrences of pancreatic cancer: a retrospective analysis. BMC Cancer. 2012;12:295. doi: 10.1186/1471-2407-12-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniyama T.K., Morizane C., Nakachi K., Nara S., Ueno H., Kondo S. Treatment outcome for systemic chemotherapy for recurrent pancreatic cancer after postoperative adjuvant chemotherapy. Pancreatology. 2012;12:428–433. doi: 10.1016/j.pan.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi N., Shimamura T., Tokuhisa M., Goto A., Mori R., Matsuyama R. A case of post-operative recurrence of pancreatic cancer in the residual pancreas treated by resection of the residual pancreas following radiological complete response achieved with second-line FOLFIRINOX. Gan To Kagaku Ryoho. 2014;41:901–904. [PubMed] [Google Scholar]
- 28.Saji O., Ishii M., Kobayashi H., Sumiyoshi K., Asano T., Iwata K. A case of recurrent pancreatic cancer effectively responding to S-1 combined irinotecan third-line chemotherapy with PSK. Gan To Kagaku Ryoho. 2013;40:1409–1412. [PubMed] [Google Scholar]
- 29.Oh D.Y., Lee K.W., Lee K.H., Sohn C.H., Park Y.S., Zang D.Y. A phase II trial of erlotinib in combination with gemcitabine and capecitabine in previously untreated metastatic/recurrent pancreatic cancer: combined analysis with translational research. Invest New Drugs. 2012;30:1164–1174. doi: 10.1007/s10637-011-9651-3. [DOI] [PubMed] [Google Scholar]
- 30.Farma J.M., Santillan A.A., Melis M., Walters J., Belinc D., Chen D.T. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15:2465–2471. doi: 10.1245/s10434-008-9992-0. [DOI] [PubMed] [Google Scholar]
- 31.Kim M.J., Lee K.H., Lee K.T., Lee J.K., Ku B.H., Oh C.R. The value of positron emission tomography/computed tomography for evaluating metastatic disease in patients with pancreatic cancer. Pancreas. 2012;41:897–903. doi: 10.1097/MPA.0b013e318252f4f5. [DOI] [PubMed] [Google Scholar]
- 32.Kittaka H., Takahashi H., Ohigashi H., Gotoh K., Yamada T., Tomita Y. Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in predicting the pathologic response to preoperative chemoradiation therapy in patients with resectable T3 pancreatic cancer. World J Surg. 2013;37:169–178. doi: 10.1007/s00268-012-1775-x. [DOI] [PubMed] [Google Scholar]
- 33.Tang S., Huang G., Liu J., Liu T., Treven L., Song S. Usefulness of 18F-FDG PET, combined FDG-PET/CT and EUS in diagnosing primary pancreatic carcinoma: a meta-analysis. Eur J Radiol. 2011;78:142–150. doi: 10.1016/j.ejrad.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Maemura K., Takao S., Shinchi H., Noma H., Mataki Y., Kurahara H. Role of positron emission tomography in decisions on treatment strategies for pancreatic cancer. J Hepatobiliary Pancreat Surg. 2006;13:435–441. doi: 10.1007/s00534-006-1102-8. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto T., Sugiura T., Mizuno T., Okamura Y., Aramaki T., Endo M. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2014;22:677–684. doi: 10.1245/s10434-014-4046-2. [DOI] [PubMed] [Google Scholar]
- 36.Chang J.S., Choi S.H., Lee Y., Kim K.H., Park J.Y., Song S.Y. Clinical usefulness of (1)(8)F-fluorodeoxyglucose-positron emission tomography in patients with locally advanced pancreatic cancer planned to undergo concurrent chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2014;90:126–133. doi: 10.1016/j.ijrobp.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 37.Xu H.X., Chen T., Wang W.Q., Wu C.T., Liu C., Long J. Metabolic tumour burden assessed by (1)(8)F-FDG PET/CT associated with serum CA19-9 predicts pancreatic cancer outcome after resection. Eur J Nucl Med Mol Imaging. 2014;41:1093–1102. doi: 10.1007/s00259-014-2688-8. [DOI] [PubMed] [Google Scholar]
- 38.Lee J.W., Kang C.M., Choi H.J., Lee W.J., Song S.Y., Lee J.H. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med. 2014;55:898–904. doi: 10.2967/jnumed.113.131847. [DOI] [PubMed] [Google Scholar]