Abstract
Introduction
Routine lymphadenectomy in the surgical treatment of intrahepatic cholangiocarcinoma (ICC) is not routinely performed. We aim to define predictive indicators of survival in patients with positive lymph nodes.
Methods
The National Cancer Data Base (NCDB) was queried for patients who underwent major hepatectomy for ICC between 1998 and 2011. Clinical and pathologic data were assessed using uni- and multi-variate analyses. A sub-analysis was performed on the 160 patients with positive lymph nodes.
Results
Of 849 patients with lymph node data, 57% had at least one lymph node examined. Median survival for lymph node negative patients was 37 months versus 15 months for lymph node positive patients. In lymph node positive patients, poorer survival was associated with not receiving chemotherapy (HR 1.83, p = 0.003), tumor size > 5 cm (p = 0.029), and older age (p < 0.0001). Lymph node positive patients age less than 45 had a median survival of 27 months.
Conclusions
Overall survival in patients with lymph node metastases from ICC is poor. Adjuvant therapy was associated with a longer survival in lymph node positive patients, although prospective data are needed. Routine lymphadenectomy should be strongly considered to provide prognostic information and guidance for adjuvant therapy.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy and constitutes 10% of liver cancers worldwide.1 The incidence of ICC is rising in many developed countries including the United States, and has doubled between the years of 1976 and 2000.2 In addition to improved diagnosis and recognition of ICC, this increase may be attributed to the prevalence of hepatitis C, alcohol use and obesity.2, 3, 4 Due to enhanced imaging modalities, resectability of ICC has also increased.5
Factors that consistently predict shorter overall survival have been established and include lymph node (LN) metastasis, large tumor size, multifocal tumors, vascular invasion, underlying cirrhosis and extremes of age.6, 7 The seventh addition of AJCC/UICC staging system includes lymph node status for staging of ICC.8 Performance of lymphadenectomy as reported in the literature, however, is highly variable and only 49–78% of patients undergoing resection of ICC have data available on lymph node status.7, 9 Of these, 35–45% are found to have LN metastasis. In addition, the 2015 NCCN guidelines on hepatobiliary malignancies state that lymphadenectomy may be considered in addition to resection and no definitive conclusion has been made regarding the role of routine lymphadenectomy.
Using the SEER database, a multivariate analysis of survival following surgery for ICC demonstrated a cumulative improvement of 34.4% between 1992 and 2002. For patients with LN metastasis, however, survival is consistently reported to be poor. A 2014 systematic review of all available evidence regarding the prognostic role of lymph node dissection (LND) reported 3 and 5-year survival among ICC patients with LNM to be 10 and 0% respectively.9 Subsequently, several recent studies have recommended consideration of routine lymphadenectomy given its prognostic implications.5, 8, 9, 10, 11 This study uses data from the NCDB to further define the predictive indicators of survival in patients with positive lymph nodes from ICC.
Methods
The National Cancer Data Base (NCDB) is a joint program of the American Cancer Society and the Commissions on Cancer of the American College of Surgeons. Established in 1989, it is a nation-wide, multicenter, comprehensive oncology outcomes database. The NCDB captures 70% of all newly diagnosed malignancies in the United States and Puerto Rico.12, 13
After obtaining an approved Participant User File from NCDB, the data base was queried for all patients in participating centers undergoing surgical resection for ICC between the years of 1998 and 2011. Patients not undergoing surgical treatment were excluded from the study. The data used in this study were derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data. All patients were included in a descriptive assessment of patient-specific variables, tumor specific variables, surgical outcomes and systemic therapeutic data.
Statistical analyses
Overall survival (OS) was defined as the time from diagnosis of cancer to date of death or censored date of last contact. Single predictor, univariate survival analyses were performed on 20 variables shown in Table 2, Table 3, including demographics, tumor characteristics, surgery outcomes and adjuvant therapy details. For categorical variables, a log rank test was used to compare Kaplan–Meier (K–M) survival curves, using a trend test when 3 or more categories were ordered. For continuous variables, Cox proportional hazards regression was used to test association with OS. Distance from facility and post surgery hospital length of stay were log transformed. Variables were considered candidates for multivariate survival analysis if p < 0.05 and at least 80% of data was non-missing. Backward stepwise modeling was used. Variables that were not statistically significant at p < 0.05 were removed from the model to obtain a final, reduced model.
Table 2.
Univariate survival statistics – all patients
| II-A. Univariate survival – Patient characteristics | Median (months) | 3 Yr (%) | 5 Yr (%) | p |
|---|---|---|---|---|
| Overall survival | ||||
| n = 881 | 26.6 | 41.5 | 27.1 | |
| Univariate demographics | ||||
| Age (10 years), HR (95% CI) | 1.02 (0.96, 1.09) | 0.5100 | ||
| Gender | <0.0001 | |||
| Male | 31.3 | 37.4 | 20.0 | |
| Female | 21.3 | 44.7 | 33.0 | |
| Comorbidities | 0.0468 | |||
| None | 30.8 | 44.50 | 31.43 | |
| One or more | 21.1 | 36.61 | 27.07 | |
| II-B. Univariate tumor characteristics | Median (months) | 3 Yr (%) | 5 Yr (%) | p |
| Grade | <0.0001 | |||
| 1–2 | 32.3 | 46.9 | 31.8 | |
| 3–4 | 21.4 | 33.7 | 19.4 | |
| Surgical margin | <0.0001 | |||
| Negative | 31.0 | 45.4 | 30.4 | |
| Micro or macro positive | 19.6 | 29.4 | 14.1 | |
| Involved – not specified | 15.3 | 27.3 | 14.8 | |
| Regional nodes examined | 0.0108 | |||
| 0 | 29.0 | 45.4 | 30.2 | |
| 1–3 | 29.1 | 43.0 | 28.1 | |
| ≥4 | 21.4 | 30.7 | 17.7 | |
| Lymph nodes positive | <0.0001 | |||
| 0 | 36.5 | 50.5 | 34.7 | |
| 1–3 | 15.0 | 18.1 | 5.8 | |
| 4–11 | 15.4 | 10.5 | 0.0 | |
| Tumor size | 0.0004 | |||
| <5 cm | 40.2 | 54.1 | 36.3 | |
| ≥5 cm | 23.8 | 37.9 | 24.3 | |
| II-C. Univariate – operative outcome | Median (months) | 3 Yr (%) | 5 Yr (%) | p |
| Length of stay (10 fold increase), HR(95% CI) | 2.13 (1.35, 3.36) | 0.0012 | ||
| Readmission 30 days | 0.3494 | |||
| None | 29.0 | 43.5 | 31.7 | |
| Readmission | 26.6 | 37.7 | 22.3 | |
| II-D. Univariate adjuvant therapy | Median (months) | 3 Yr (%) | 5 Yr (%) | p |
| Systemic treatment | 0.2177 | |||
| Chemotherapy only | 22.6 | 36.8 | 18.8 | |
| Chemotherapy and radiation | 27.8 | 44.3 | 22.0 | |
| None | 27.8 | 42.5 | 30.5 | |
Missing data reported in Table 1. Kaplan–Meier curves of categorical variables compared with log rank test of homogeneity or trend test for 3 or more ordered levels. Continuous variables tested with Cox proportion hazards regression. HR, hazard ratio.
Table 3.
Univariate survival statistics – positive node patients
| III-A. Univariate survival – patient characteristics | Median (months) | 3 Yr (%) | 5 Yr (%) | p |
|---|---|---|---|---|
| Overall survival | ||||
| n = 160 | 15.4 | 16.6 | 4.6 | |
| Univariate demographics | ||||
| AGE (10 years), HR (95% CI) | 1.34 (1.19, 1.57) | <0.0001 | ||
| Gender | 0.1641 | |||
| Male | 17.5 | 16.2 | 3.0 | |
| Female | 11.7 | 16.9 | 6.1 | |
| Comorbidities | 0.5284 | |||
| None | 18.0 (13.5, 21.5) | 14.5 | 3.9 | |
| One or more | 9.3 (3.3, 21.4) | 10.0 | 10.0 | |
| III-B. Univariate tumor characteristics | Median (months) | 3 Yr (%) | 5 Yr (%) | p |
| Grade | 0.9956 | |||
| 1–2 | 14.2 | 16.7 | 6.8 | |
| 3–4 | 16.6 | 15.7 | 1.9 | |
| Surgical margin | 0.3986 | |||
| Negative | 18.3 | 18.8 | 4.7 | |
| Micro or macro positive | 10.4 | 13.3 | 4.4 | |
| Involved – not specified | 11.8 | 17.2 | 8.6 | |
| Regional nodes examined | 0.7122 | |||
| 0 | – | – | – | |
| 1–3 | 11.7 | 16.6 | 7.2 | |
| ≥4 | 63.4 | 1.7 | 0.0 | |
| Lymph nodes positive | 0.3613 | |||
| 0 | – | – | – | |
| 1–3 | 15.0 | 18.1 | 5.8 | |
| 4–11 | 15.4 | 10.5 | 0.0 | |
| Tumor size | 0.0488 | |||
| <5 cm | 18.23 | 25.3 | 12.6 | |
| ≥5 cm | 15.0 | 13.4 | 2.2 | |
| III-C. Univariate – operative outcome | Median (months) | 3 Yr (%) | 5 Yr (%) | p |
| Length of stay (10 fold increase), HR (95% CI) | 1.29 (0.5, 3.1) | 0.5613 | ||
| Readmission 30 days | 0.5376 | |||
| None | 17.7 | 15.2 | 5.3 | |
| Readmission | 15.5 | 0.0 | 0.0 | |
| III-D. Univariate adjuvant therapy | Median (months) | 3 Yr (%) | 5 Yr (%) | p |
| Systemic treatment | 0.0033 | |||
| Chemotherapy only | 19.1 | 23.2 | 2.9 | |
| Chemotherapy and radiation | 20.1 | 31.0 | 12.1 | |
| None | 9.3 | 10.2 | 3.4 | |
Missing data reported in Table 1. Kaplan–Meier curves of categorical variables compared with log rank test of homogeneity or trend test for 3 or more ordered levels. Continuous variables tested with Cox proportion hazards regression. HR, hazard ratio.
Tumor stage variables were not used in multivariate analysis for clearer interpretation of tumor size and number of lymph nodes positive. For the 27 patients for which number of LN examined was missing, number examined was set to the number of positive LN or 1 if there were zero positive nodes. This was deemed appropriate given that prior to imputation, a small number of lymph nodes were examined for the majority of patients (median = 1, 75th percentile = 2).
Both single predictor univariate and multivariate Cox proportional hazards survival analyses were initially performed for the entire cohort. The same analyses were then performed on the subgroup of patients found to have positive lymph nodes. SAS 9.3 (SAS Institute Inc., Cary, NC) was used for statistical analysis.
Results
Descriptive statistics: all patients
The study population includes 881 total patients surgically treated for ICC with curative intent. Median follow up of survival was 87.4 mo and ranged from 0.0 to 168.8 mo. The clinicopathologic features are summarized in Table 1. 66.4% of patients were treated at academic research centers. The primary site of tumor was liver in 53% of patients and 47% was intra-hepatic bile duct. Patients with hilar cholangiocarcinoma were not included in this study. In addition, data on macroscopic subtypes of ICC was not available. No data was available for lymphovascular invasion. The tumor characteristics and their frequencies are summarized in Table 1. 491/881 (55.7%) of the cohort had data available on lymph node status, and this number did not significantly change over the time span of the study. Of these 491 patients, 32.6% had positive lymph nodes (LN+) (26.3% with 1–3 LN+, 6.3% with 4–11).
Table 1.
Descriptive statistics
| All patients | Subgroup with positive nodes | |
|---|---|---|
| I-A. Patient characteristics | % (Frequency) | % (Frequency) |
| N | 881 | 160 |
| Age, mean ± SD (years) | 62.2 ± 12.4 | 57.3 ± 13.1 |
| Gender | ||
| Male | 44.5% (392) | 49.4% (79) |
| Female | 55.5% (489) | 50.6% (81) |
| Comorbidities | ||
| None | 77.3% (364) | 88.7% (79) |
| One or more | 22.7% (107) | 11.3%10 |
| I-B. Tumor characteristic | % (Frequency) | % (Frequency) |
| Grade | ||
| 1 | 12.0% (85) | 5.8%8 |
| 2 | 50.9% (361) | 46.4% (64) |
| 3 | 35.3% (250) | 44.9% (62) |
| 4 | 1.8%13 | 2.9%4 |
| Resection type | ||
| Hepatectomy NOS | 34.4% (303) | 31.2% (50) |
| Right hepatectomy | 15.9% (140) | 15.7% (25) |
| Left hepatectomy | 15.9% (140) | 18.1% (29) |
| Extended hepatectomy NOS | 14.3% (126) | 12.5%20 |
| Extended right hepatectomy | 5.8% (51) | 8.8%14 |
| Extended left hepatectomy | 4.4% (39) | 6.2%10 |
| Hepatectomy + additional procedure | 9.3% (82) | 7.5%12 |
| Surgical margins | ||
| Negative | 76.1% (594) | 66.7% (98) |
| Microscopic positive | 13.8% (108) | 18.4% (27) |
| Involved, not specified | 8.7% (68) | 12.9%19 |
| Macroscopic positive | 1.4%11 | 2.0%3 |
| Regional nodes examined | ||
| 0 | 42.0% (357) | – |
| 1–3 | 41.3% (351) | 53.8% (86) |
| ≥4 | 16.6% (141) | 46.2% (74) |
| Lymph nodes positive | ||
| 0 | 67.4% (331) | – |
| 1–3 | 26.3% (129) | 80.6% (129) |
| 4–11 | 6.3% (31) | 19.4% (31) |
| Tumor size | ||
| <2 cm | 5.6% (44) | 3.5%5 |
| 2–5 cm | 28.3% (225) | 24.5% (35) |
| >5 cm | 66.1% (525) | 72.0% (103) |
| I-C. Operative outcome | % (Frequency) | % (Frequency) |
| Length of stay (days) | ||
| Median (min, max) | 8 (0, 124) | 8 (0, 45) |
| Readmission 30 days | ||
| None | 90.8% (411) | 92.9% (79) |
| Readmission | 9.2% (42) | 7.1%6 |
| Mortality | ||
| 30-Day mortality | 6.0% (52) | 10.0%16 |
Missing data were: systemic treatment before/after surgery, 88% for all patients and 84% for patients with positive lymph nodes; length of stay, 55% for all patients and 52% for patients with positive nodes; 30 day readmission, 49% and 47%; comorbidities, 46% and 44%; lymph nodes positive and positive nodes ratio, 44% for all patients; grade, 20% and 14%; pathological T-stage, 12% and 6%; surgical margins, 11% and 8%; tumor size, 10% and 11%; chemotherapy, 6% and 7%; distance to medical facility, 6% and 8%; 30 day mortality, 5.3% and 0.6%; nodes examined, 4% for all patients.
In our cohort of LN + patients, 70.4% did not receive adjuvant therapy. 14.9% received chemotherapy alone, while 2.9% received radiation alone and 11.9% received both chemotherapy and radiation therapy.
Variables associated with OS: univariate analysis
Median overall survival (OS) for the entire patient population examined was 26.6 months. 3 and 5-year survival rates are presented in Table 2. Longer survival times were associated with female gender, metro (compared to urban + rural), no comorbidities, more recent year of diagnosis (though there is less follow up for more recent diagnoses), lower analytic stage, lower pathology T stage, tumor size < 5 cm, liver primary site (but differences in rates compared to IH bile duct site may not be clinically relevant), lower grade, negative lymph nodes, positive nodes ratio of zero, negative surgical margins, fewer nodes examined, and shorter time to surgical discharge (length of stay). Univariable results for the entire patient population examined are listed in Table 2.
Six variables were then tested in the multivariate model: sex, urban/rural, primary site, tumor size, surgical margins, and regional nodes examined. Candidates for multivariate analysis were variables that were significant on univariate analysis and had complete data.
Multivariate survival analysis
Multivariable survival analysis was performed on N = 400/881 patients, those with complete data for all variables in the model. Patients that had missing data on lymph node status (N = 481) were excluded from the multivariable analysis. Primary site, lymph nodes examined, and urban/rural location were significant in univariate survival, but not in the multivariable model. In the reduced multivariate model, after controlling for effects of each other, poorest survival was associated with ≥4 lymph nodes positive (p < 0.0001), positive surgical margins whether micro or macro (p = 0.0021), male gender (p = 0.013), and tumor size ≥ 5 cm (p = 0.04). These results are reported in Table 4a.
Table 4a.
Multivariate analysis – all patients
| Parameter | Hazard ratio (Confidence Interval) | p Value |
|---|---|---|
| Lymph node positive | ||
| 4–11 versus 0 | 2.61 (1.57–4.35) | <0.0001 |
| 1–3 versus 0 | 2.29 (1.75–3.10) | <0.0001 |
| Surgical margins | ||
| Involved versus negative | 1.64 (1.15–2.34) | 0.0021 |
| Male versus female | 1.34 (1.06–1.69) | 0.0132 |
| >5 cm tumor versus < 5 cm | 1.30 (1.01–1.65) | 0.0452 |
*Other variables tested and not significant: primary site, regional lymph nodes examined, urban/rural location.
Lymphadenectomy: incidence of LN metastasis and impact of nodal status
To define predictive indicators of survival in patients with positive lymph metastases from ICC, we identified 160 patients with positive lymph nodes and complete survival data and performed a sub-analysis. Descriptive statistics as well as clinicopathologic features are described in Table 1. Most patients had either grade 2 or 3 tumors (45 and 45% respectively). Data on lymph vascular invasion is missing in the data set. Tumor size >5 cm was identified in 72% of patients while 24.5% had tumor size between 2 and 5 cm, and 3.5% had tumors that were less than 2 cm. In this subgroup, 80.6% of patients had 1–3 LN positive while 19.4% had 4–11 positive LN. 10% of patients died within the first 30 days postoperative. 66.7% had negative surgical margins. Number of LN examined was 1–3 in 53.8% of patients while LN examined was ≥4 in 46.2%. In the LN positive subgroup of patients, 58.4% of patients did not receive adjuvant chemotherapy.
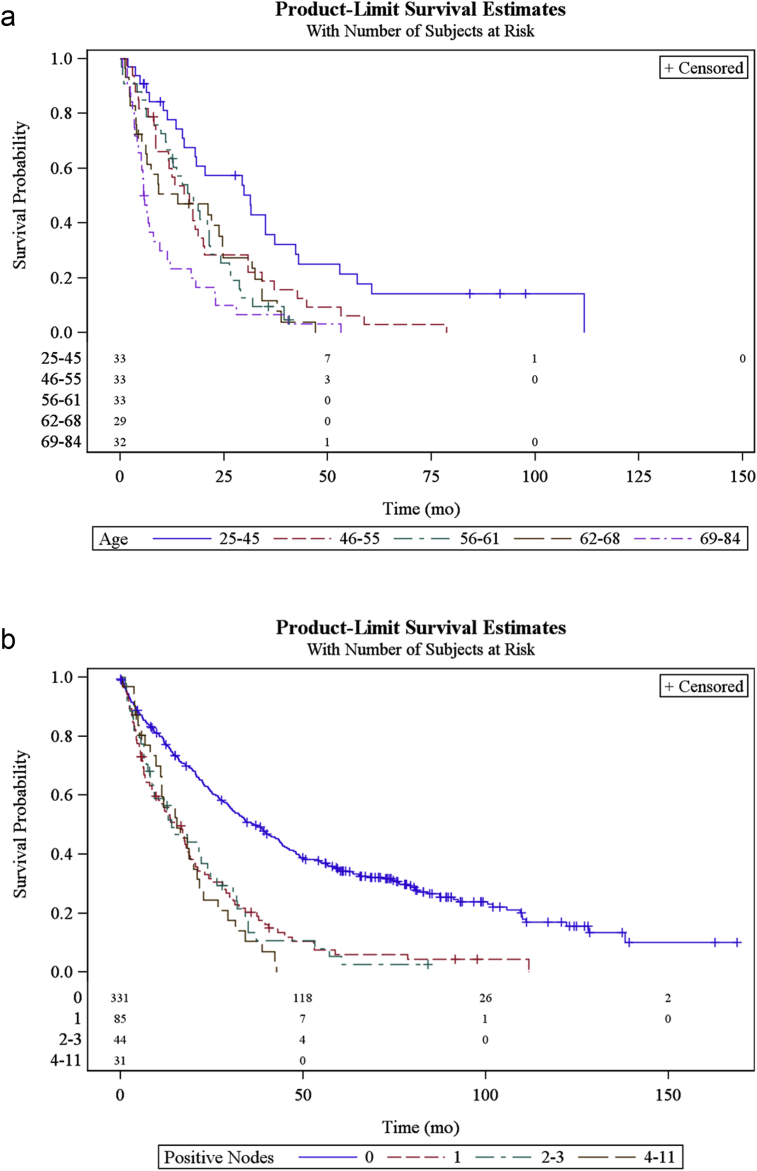
Univariate analysis of OS performed on the subgroup of patients with positive LN (n = 160) showed that longer survival times were associated with facility location, younger age (Fig. 1a), tumor size < 5 cm, and receiving adjuvant therapy. When stratifying by N1 disease, grade was not significant in univariate analysis; grade 1–2 median survival was 14.2 months compared to 16.6 months for grade 3–4. The number of lymph nodes positive was also not significant. Median OS for 1 LN positive, 2–3 LN positive and 4–11 LN positive was similar, 15 months for each group (Fig. 1b). In addition, margin status was not statistically different on univariate analysis, though median OS was 18 months (95% CI) in patients with negative margins and 11 months for patients with positive margins. Furthermore, the number of LN examined was not statistically significant on univariate survival analysis in the LN positive group. Receiving adjuvant therapy was statistically significant with OS being 20 months for patients receiving adjuvant chemotherapy compared to 9.2 months for patients not receiving chemotherapy. Patients that received radiation therapy were low in number (<4% of total patients) and were thus excluded from the analysis. The univariable analysis results for patients with positive lymph nodes are shown in Table 3.
Figure 1.
(a) Overall survival curve of patients underling major hepatectomy for ICC stratified by age. (b) Overall survival curve of patients undergoing major hepatectomy for ICC stratified by number of positive lymph nodes. Patients with lymph node metastases experience shorter overall survival, regardless of number of positive lymph nodes.
Multivariable survival analysis was performed on N = 135/160 patients with positive nodes that had complete data records. After controlling for effects of each other, poorest survival was associated with not receiving adjuvant chemotherapy (p = 0.006). Additionally, patients with tumor size >5 cm compared to those with tumors <5 cm (p = 0.029), and older age (p < 0.0001) had increased risk of death. For every ten-year increase in age, there was on average a 43% increase in the hazard of death (HR = 1.43 CI = (1.21, 1.69)). Results are shown in Table 4b.
Discussion
The understanding of prognostic factors for surgically treated ICC is improving, though remains incomplete. Prognostic factors that consistently demonstrate importance are tumor number, vascular invasion and lymph node metastasis.1 Lymph node metastasis has been established as an independent prognostic factor for shorter overall survival by multiple retrospective studies with relatively large cohorts.8, 14, 15 In addition, lymph node metastasis is a reliable predictor of recurrence after surgical resection of ICC, as documented by several authors.4, 16, 17, 18, 19, 20, 21 Aspects of surgical treatment remain poorly defined, specifically with regard to the role of routine lymphadenectomy.
Assessment of LN metastasis
Lymph node status has been defined as a strong prognosticator by multiple studies including ours. We found that the number of patients with at least one lymph node examined was 57%, and is similar to rates reported from US and European studies. This number is lower than most studies reported in the Japanese literature, where approximately 85% of patients undergo LN sampling.9 Several Japanese centers now perform routine lymphadenectomy.5 The potential for stage migration due to inconsistent LN sampling may underestimate the survival of patients without LN metastasis and may lead to an inaccurate estimate of survival for LN positive patients.
Evaluation of lymph node status with pre-operative imaging has been examined. In a study of 120 patients with liver tumors, Ercolani et al. concluded that the sensitivity, specificity, and diagnostic accuracy of cross-sectional CT imaging to reveal LN metastasis was 35.2%, 91.8% and 46.1% respectively.11 Choi et al. showed that the accuracy of diagnosis was 66% in the hepatoduodenal ligament basin and 82% in the common hepatic artery LN, with sensitivities of 45.5 and 55% in each nodal group respectively.10 Both authors concluded that the addition of lymphadenectomy is safe and can be performed without an increased risk of complications and with an acceptable increase in operative time.10, 11 We agree that LN metastases in ICC cannot be predicted with enough accuracy on pre-operative imaging and thus a thorough lymphadenectomy is necessary.
Do LN positive patients benefit from surgery
The present study is to our knowledge the largest in the literature of ICC patients undergoing resection demonstrating that patients with positive LN have much poorer OS compared to patients without lymph node metastasis, 15 months and 37 months respectively. Published literature regarding the benefit of surgical resection for patients with positive lymph node disease is conflicting. Some authors propose that patients do not benefit from resection given that their overall survival approaches that of unresectable patients.20
Other authors advocate that a selected group of patients with positive lymph node status benefit from surgery and have identified 5-year survivors. Nakagawa et al. reported that curative resection with lymph node dissection improved survival in patients with no more than two positive lymph nodes.5 The authors found 3-year survival rates in patients with zero positive LN, one or two positive LN removed, and three or more positive LN removed to be 62, 50 and 0%. Additional authors have reported 5-year survival rates as high as 26% in patients with solitary tumors and lymph node metastasis.22, 23
While we found that the OS in the lymph node positive subgroup was poor (15 months), NCDB data has significant potential for selection bias. This is data from centers in which lymphadenectomy is not performed routinely, thus it could be that the patients that underwent lymph node sampling had bulky lymphadenopathy or other high risk and concerning features at the time of their operation. The difference in survival between our results and the authors discussed above could be explained by stage migration. Our overall survival may represent patients with grossly positive lymph nodes rather than all patients with positive lymph nodes. Therefore, we are unable to determine whether patients with positive lymph nodes benefit from resection. The available data do show that performing lymphadenectomy is at the very least a strong prognostic indicator and allows for accurate staging.
Furthermore, we found that the overall survival in patients with N1 disease was 9 months in the group not receiving adjuvant chemotherapy, compared to 20 months in the patients receiving adjuvant chemotherapy, a statistically significant finding on both univariate and multivariate analysis. These results may also have been influenced by selection bias, such as the physical performance scores and age of the patients selected to receive adjuvant therapy. In addition, insurance type variability and its influence on receiving adjuvant therapy may have also played a role in this selection bias.
We advocate the performance of routine lymphadenectomy as it would facilitate collection of LN data into tumor registries, eliminate under staging of these patients and help improve our understanding of lymph node positive patients. Lymph node assessment is well described in most other gastrointestinal malignancies, as is the number of LN needed to stage these patients accurately.
Study limitations
There are several limitations to our study beyond the potential for selection bias. These include the potential for coding errors, missing data and absence of several variables commonly used as prognostic factors for ICC. In addition, data on recurrence and disease-specific survival is not available. The 5th, 6th and 7th edition of AJCC staging are used in the current dataset and there is no consistency to staging. Thus, information regarding tumor number and vascular invasion (both important prognostic factors for ICC) are difficult to examine because they are not coded as separate variables.
Conclusion
Lymph node sampling in patients undergoing hepatectomy for ICC may provide important prognostic information. We know that non-routine or selective lymphadenectomy can lead to selection bias and overall survival rates that may not accurately reflect the survival for all lymph node positive patients. We believe that there is a strong argument for performing routine lymphadenectomy in order to appropriately stage patients and potentially identify a subset of patients in which adjuvant chemotherapy may be of benefit. Further prospective studies are needed to define the association between receiving adjuvant therapy on survival.
Disclosures
The authors of this manuscript do not have anything to disclose.
Funding
The authors would like to acknowledge support from the Foundation for Surgical Fellowships (FSF) and Providence Cancer Center.
Conflict of interest
None declared.
Table 4b.
Multivariate analysis – lymph node positive subgroup
| Parameter | Hazard ratio (Confidence Interval) | p Value |
|---|---|---|
| Age (Unit = 10 years) | 1.57 (1.24–2.0) | 0.0002 |
| No adjuvant therapy | 1.73 (1.07–3.25) | 0.006 |
| >5 cm tumor size versus < 5 cm | 1.68 (1.08–2.62) | 0.018 |
• Other variables tested and not significant: insurance group.
Footnotes
This study was presented at the Annual Meeting of the AHPBA, 11-15 March 2015, Miami, Florida.
References
- 1.Tsai S., Nathan H., Pawlik T.M. Primary liver cancer: intrahepatic cholangiocarcinoma emerges from the shadows. Updat Surg. 2010;62:5–9. doi: 10.1007/s13304-010-0011-1. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn K.A., Tarone R.E., El-Serag H.B. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2006;15:1198–1203. doi: 10.1158/1055-9965.EPI-05-0811. [DOI] [PubMed] [Google Scholar]
- 3.Nathan H., Pawlik T.M., Wolfgang C.L., Choti M.A., Cameron J.L., Schulick R.D. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg. 2007;11:1488–1496. doi: 10.1007/s11605-007-0282-0. discussion 96–7. [DOI] [PubMed] [Google Scholar]
- 4.Endo I., Gonen M., Yopp A.C., Dalal K.M., Zhou Q., Klimstra D. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa T., Kamiyama T., Kurauchi N., Matsushita M., Nakanishi K., Kamachi H. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005;29:728–733. doi: 10.1007/s00268-005-7761-9. [DOI] [PubMed] [Google Scholar]
- 6.Mavros M.N., Economopoulos K.P., Alexiou V.G., Pawlik T.M. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149:565–574. doi: 10.1001/jamasurg.2013.5137. [DOI] [PubMed] [Google Scholar]
- 7.Hyder O., Marques H., Pulitano C., Marsh J.W., Alexandrescu S., Bauer T.W. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg. 2014;149:432–438. doi: 10.1001/jamasurg.2013.5168. [DOI] [PubMed] [Google Scholar]
- 8.de Jong M.C., Nathan H., Sotiropoulos G.C., Paul A., Alexandrescu S., Marques H. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 9.Amini N., Ejaz A., Spolverato G., Maithel S.K., Kim Y., Pawlik T.M. Management of lymph nodes during resection of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: a systematic review. J Gastrointest Surg. 2014;18:2136–2148. doi: 10.1007/s11605-014-2667-1. [DOI] [PubMed] [Google Scholar]
- 10.Choi S.B., Kim K.S., Choi J.Y., Park S.W., Choi J.S., Lee W.J. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16:3048–3056. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]
- 11.Ercolani G., Grazi G.L., Ravaioli M., Grigioni W.F., Cescon M., Gardini A. The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg. 2004;239:202–209. doi: 10.1097/01.sla.0000109154.00020.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilimoria K.Y., Stewart A.K., Winchester D.P., Ko C.Y. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winchester D.P., Stewart A.K., Bura C., Jones R.S. The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol. 2004;85:1–3. doi: 10.1002/jso.10320. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Li J., Xia Y., Gong R., Wang K., Yan Z. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 15.Ribero D., Pinna A.D., Guglielmi A., Ponti A., Nuzzo G., Giulini S.M. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg. 2012;147:1107–1113. doi: 10.1001/archsurg.2012.1962. [DOI] [PubMed] [Google Scholar]
- 16.Li T., Qin L.X., Zhou J., Sun H.C., Qiu S.J., Ye Q.H. Staging, prognostic factors and adjuvant therapy of intrahepatic cholangiocarcinoma after curative resection. Liver Int Off J Int Assoc Study Liver. 2014;34:953–960. doi: 10.1111/liv.12364. [DOI] [PubMed] [Google Scholar]
- 17.Chen L.P., Li C., Wang C., Wen T.F., Yan L.N., Li B. Predictive factors of recurrence for patients with intrahepatic cholangiocarcinoma after hepatectomy. Hepatogastroenterology. 2012;59:1765–1768. doi: 10.5754/hge11820. [DOI] [PubMed] [Google Scholar]
- 18.Morine Y., Shimada M., Utsunomiya T., Imura S., Ikemoto T., Mori H. Clinical impact of lymph node dissection in surgery for peripheral-type intrahepatic cholangiocarcinoma. Surg Today. 2012;42:147–151. doi: 10.1007/s00595-011-0057-9. [DOI] [PubMed] [Google Scholar]
- 19.Shirabe K., Mano Y., Taketomi A., Soejima Y., Uchiyama H., Aishima S. Clinicopathological prognostic factors after hepatectomy for patients with mass-forming type intrahepatic cholangiocarcinoma: relevance of the lymphatic invasion index. Ann Surg Oncol. 2010;17:1816–1822. doi: 10.1245/s10434-010-0929-z. [DOI] [PubMed] [Google Scholar]
- 20.Shimada M., Yamashita Y., Aishima S., Shirabe K., Takenaka K., Sugimachi K. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:1463–1466. doi: 10.1046/j.0007-1323.2001.01879.x. [DOI] [PubMed] [Google Scholar]
- 21.Okabayashi T., Yamamoto J., Kosuge T., Shimada K., Yamasaki S., Takayama T. A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer. 2001;92:2374–2383. doi: 10.1002/1097-0142(20011101)92:9<2374::aid-cncr1585>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Miwa S., Miyagawa S., Kobayashi A., Akahane Y., Nakata T., Mihara M. Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J Gastroenterol. 2006;41(9):893–900. doi: 10.1007/s00535-006-1877-z. [DOI] [PubMed] [Google Scholar]
- 23.Uenishi T., Kubo S., Yamazaki O., Yamada T., Sasaki Y., Nagano H. Indications for surgical treatment of intrahepatic cholangiocarcinoma with lymph node metastases. J Hepatobiliary Pancreat Surg. 2008;15:417–422. doi: 10.1007/s00534-007-1315-5. [DOI] [PubMed] [Google Scholar]