Abstract
Background
We evaluated the effects of pre-transplant locoregional treatment on survival in living donor liver transplantation (LDLT), and the most accurate method for predicting survival after LDLT in patients who received pre-transplant locoregional treatment.
Methods
From December 2003 to December 2012, 234 patients underwent LDLT for hepatocellular carcinoma (HCC) at our transplant center. We retrospectively reviewed 86 patients newly diagnosed with HCC and who received pre-transplant locoregional treatments at our hospital.
Results
Of the 33 patients with HCC initially beyond the Milan criteria, 12 experienced successful down-staging after locoregional treatments, and the 5-year recurrence-free survival was 81.8%, which was comparable to those in patients with HCC initially within the Milan criteria. A bad responder according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [HR, 4.874 (1.059–22.442), p = 0.042], and increased AFP levels [HR 4.002 (1.540–10.397), p = 0.004] during pre-transplant locoregional treatments were independent risk factors for HCC recurrence after LDLT in multivariate analysis.
Conclusions
Liver transplantation may be considered after successful down-staging in patients with HCC initially beyond the Milan criteria. The mRECIST and serum AFP level changes are better selection criteria for LDLT in patients who have received locoregional treatments.
Introduction
Since the introduction of the Milan criteria by Mazzaferro et al.1 in 1996, recurrence-free survival (RFS) and overall survival (OS) rates after liver transplantation (LT) for patients with hepatocellular carcinoma (HCC) who meet the Milan criteria have been equivalent to those of non-HCC patients. When patients with HCC beyond the Milan criteria were treated with current treatment modalities other than LT, the outcomes were disappointing.2 This has led to studies recommending that aggressive locoregional treatments should be performed in patients with HCC beyond the Milan criteria to achieve successful down-staging of the disease.3 Moreover, locoregional treatments, including hepatic resection, transarterial chemoembolization (TACE), radiofrequency ablation (RFA), and percutaneous ethanol injection (PEI), are widely used in most transplant centers in HCC patients awaiting LT to prevent tumor progression.4, 5
It is difficult to predict the response to pre-transplant locoregional treatments, and there is controversy about the risk of tumor progression after successful down-staging. To date, there have been several reports regarding pre-transplant locoregional treatments in deceased donor liver transplantation (DDLT), but reports of living donor liver transplantation (LDLT) are rare. LDLT differs from DDLT when used as a treatment for patients with HCC. Since LDLT can be performed without waiting, based on the availability of the liver donor, the problems associated with long wait times, such as the death of those on the waiting list, the drop-out due to medical reasons, or the progression of tumors beyond the acceptable criteria, can be reduced. However, these short waiting times lead to difficulties in predicting which patients will have a poor prognosis based on their response to pre-transplant locoregional treatments.
The aim of the present study was to evaluate the effects of pre-transplant locoregional treatments on overall survival (OS) and RFS in LDLT. We also examined methods that assess and predict the responses to pre-transplant locoregional treatments.
Materials and methods
Patients
From December 2003 to December 2012, 234 patients underwent LDLT for HCC at our transplant center. We retrospectively reviewed 86 patients newly diagnosed with HCC who had received pre-transplant locoregional treatments at our hospital. We excluded 104 patients who were diagnosed and treated for HCC with locoregional treatments at another hospital and 44 patients who did not receive pre-transplant locoregional treatments. The mean age was 52.3 ± 6.5 years, and 76 (88.4%) patients were male. The most common reason for LT was hepatitis B (n = 75, 87.2%), followed by hepatitis C (n = 5, 5.8%) and other causes (n = 6, 7.0%). The mean Child-Pugh score was 6.8 ± 2.1, and the mean model for end-stage liver disease (MELD) score was 8.9 ± 6.5. Maximum tumor size and tumor number at HCC diagnosis were 3.81 ± 2.79 cm and 1.94 ± 1.69, respectively. In these 86 patients, 33 (38.4%) did not meet the Milan criteria. The median follow-up period was 37 months (range, 2–115 months). This study was approved by the Institutional Review Board of our center.
HCC diagnosis and pre-transplant locoregional treatments while awaiting LT
Diagnosis of HCC was based on the identification of typical HCC characteristics, such as hyper-vascularization in the arterial phase with washout in the portal venous or delayed phases, using a four-phase multi-detector computed tomography (CT) scan or dynamic contrast-enhanced magnetic resonance imaging (MRI). A liver biopsy was performed for radiological atypical lesions. A chest CT, bone scan, and positron emission tomography-CT (PET-CT) were performed to exclude distant metastasis and other primary malignancies. The AFP and the PIVKA-II levels were evaluated as tumor markers. Hepatitis viral markers and liver function tests were also assessed. When HCC was diagnosed, the treatment was based on the tumor stage and the liver function of the patient. Patients who were eligible for transplantation underwent DDLT or LDLT based on meeting the Milan criteria and the availability of a liver donor. For LDLT, relatively expanded selection criteria, such as distant metastasis, regional lymph node metastasis, and macroscopic main portal vein invasion, could be adopted if liver transplantation was not contraindicated. Patients who did not undergo liver transplantation received locoregional treatments for the purposes of bridging or down-staging according to the practice guidelines for HCC in Korea, which are based on the Barcelona Clinic Liver Cancer Staging System.6, 7 Pre-transplant locoregional treatments include hepatic resection, TACE, RFA, and PEI. TACE was the primary treatment modality. Among the 86 patients who received pre-transplant locoregional treatments, 79 (91.9%) received TACE, including 45 patients who received TACE only, 23 patients who received a combination of TACE and RFA or PEI, and 11 patients who received a combination of TACE and hepatic resection or radiation treatment. The mean number of TACE treatments was 3.7 (median = 3, range: 1–13). Nine patients (10.5%) underwent hepatic resection. Twenty-three patients (26.7%) received RFA, and the mean number of RFA treatments was 1.6 (median = 1, range: 1–4).
Assessment of the response to pre-transplant locoregional treatments
The response to pre-transplant locoregional treatments was evaluated with a 3-month interval, using the modified Response Evaluation Criteria in Solid Tumors (mRECIST)8 and evaluation of tumor marker levels, including serum AFP and PIVKA. Response based on the mRECIST was graded into four groups: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The outcomes were divided into the good response group (the CR and PR groups) and the bad response group (the SD and PD groups). Patients who received resection or RFA were classified as CR if there was no recurrence in the follow-up study. We also analyzed AFP and PIVKA-II tumor marker levels. The changes in tumor markers were defined as the difference in tumor marker levels between the time of HCC diagnosis and liver transplantation. The outcomes were classified either as an increase or decrease in tumor marker levels during pre-transplant locoregional treatments.
The patients were classified by the Milan criteria based on tumor size and the number of tumors found in the imaging studies performed at the time of HCC diagnosis and in the last imaging study before LDLT. Successful down-staging was defined as reductions in tumor size and number meeting the Milan criteria or complete tumor necrosis, indicating disappearance of the viable tumor on CT and MRI in patients with HCC initially beyond the Milan criteria.
Liver explants were sectioned into 1-cm slices to detect tumors or nodules. The histologic examination of the explanted liver included an assessment of tumor necrosis, the presence of microvascular invasion, and the tumor grade. The outcomes were classified either into ≤50% necrosis or >50% necrosis groups based on the extent of tumor necrosis.
LT and post-transplant follow-up
LDLT was performed according to a standard technique using a modified right lobe with middle hepatic vein reconstruction. For patients with ascites, aspiration and cytology were performed before the operation. When lymph node enlargement was present, or in those cases with metastatic disease suspicion, an intraoperative biopsy was performed. The operation was performed only in cases with negative biopsy results. Immunosuppression regimens consisted of a triple drug regimen that included tacrolimus or cyclosporin, mycophenolate mofetil (MMF), and prednisolone. Steroids were withdrawn one month after surgery, and MMF was withdrawn 6 months after surgery. An interleukin-2 receptor blocker was administered on both the day of surgery and on the fourth postoperative day. Patients were followed monthly for the first year, every 2 months for 5 years, and then every 3 months. Tumor markers were measured monthly during the first year, and then every 2 months thereafter. Abdomen CT, chest CT, and bone scintigraphy were routinely performed every 4 months for the first year, every 6 months for the second year, and then annually thereafter. When tumor recurrence was suspected, MRI and/or PET-CT were performed. For HBV patients, our protocol for post-transplantation HBV prophylaxis was a combination of low-dose HBIG and nucleos(t)ide analogs. All patients were taking lamivudine of adefovir (50 patients) until 2007, and then took entecavir (25 patients).
Statistical analysis
Continuous variables were reported as means ± standard deviation and were compared using the Student's t-test. Categorical variables were analyzed using the χ2 test. OS and RFS were calculated using the Kaplan–Meier method. To evaluate the risk factors for HCC recurrence, univariate analysis was performed using the Kaplan–Meier method and evaluated using the log-rank test. Candidate predictors associated with a p-value < 0.2 on univariate analysis were entered into a multivariate analysis using Cox regression analysis. The PIVKA-II level was excluded from the multivariate analysis because of the limited number of patients with an available PIVKA-II level compared with other clinicopathological variables. Statistical analysis was performed using SPSS (Chicago, IL, USA) 18.0 for Windows. A p-value < 0.05 was considered to indicate statistical significance.
Results
Outcome of pre-transplant locoregional treatments in LDLT patients
The 1-, 3-, and 5-year OS rates in all patients were 85.9, 77.8, and 75.4%, respectively. During the follow-up period, 21 patients died. The cause of death was HCC recurrence in 14 patients (66.7%), sepsis in 3 patients (14.3%), graft failure in 2 patients (9.5%), and other causes in 2 patients (9.5%). The 1-, 3-, and 5-year RFS rates were 86.3, 76.3, and 70.3%, respectively. Of the 86 patients who received pre-transplant locoregional treatments, 20 experienced HCC recurrence. Most HCC recurrences (n = 14, 70.0%) occurred within 2 years, with 11 patients (55.0%) experiencing HCC recurrence within 1 year. One patient (5.0%) experienced HCC recurrence 5 years after transplantation. The viable tumor size in the last imaging study before LDLT after the locoregional treatments was significantly different between the HCC recurrence and non-HCC recurrence groups (p = 0.019). The proportion of patients that were beyond the Milan criteria at transplantation in the HCC recurrence patient group was significantly higher than that in the non-HCC recurrence patient group (p = 0.033) (Table 1).
Table 1.
Clinicopathological factors associated with hepatocellular carcinoma recurrence in patients with locoregional treatments
| Total (n = 86) | No recurrence (n = 66) | Recurrence (n = 20) | p | |
|---|---|---|---|---|
| Mean age (yr)a | 52.3 ± 6.5 | 52.4 ± 6.4 | 51.9 ± 6.8 | 0.755 |
| Male, n (%) | 76 (88.4%) | 58 (87.9%) | 18 (90.0%) | 0.792 |
| Etiology, hepatitis B, n (%) | 75 (87.2%) | 59 (89.4%) | 16 (80.0%) | 0.331 |
| GRWRa | 1.16 ± 0.33 | 1.15 ± 0.34 | 1.20 ± 0.29 | 0.592 |
| MELD scorea | 8.9 ± 6.5 | 8.8 ± 6.3 | 9.5 ± 7.4 | 0.680 |
| At HCC diagnosis | ||||
| Maximal tumor size (cm)a | 3.81 ± 2.79 | 3.52 ± 2.48 | 4.74 ± 3.55 | 0.089 |
| Tumor numbera | 1.94 ± 1.69 | 1.97 ± 1.78 | 1.85 ± 1.38 | 0.784 |
| AFP (ng/ml)a | 666.2 ± 4829.4 | 797.4 ± 5507.9 | 233.3 ± 547.3 | 0.650 |
| PIVKA (mAU/ml)a | 743.0 ± 1993.9 | 846.8 ± 2159.0 | 189.4 ± 183.5 | 0.369 |
| Milan criteria (beyond), n (%) | 33 (38.4%) | 24 (36.4%) | 9 (45.0%) | 0.487 |
| At transplantation | ||||
| Maximal tumor size (cm)a | 2.37 ± 1.93 | 2.83 ± 0.02 | 4.42 ± 0.03 | 0.088 |
| Maximal tumor size, enhanced (cm)a | 2.07 ± 2.65 | 1.51 ± 1.69 | 3.90 ± 4.10 | 0.019 |
| Tumor numbera | 2.37 ± 1.93 | 2.21 ± 1.85 | 2.90 ± 2.12 | 0.164 |
| AFP (ng/ml)a | 100.4 ± 385.4 | 32.6 ± 89.6 | 335.8 ± 767.1 | 0.103 |
| PIVKA (mAU/ml)a | 245.6 ± 1478.5 | 32.1 ± 47.1 | 1462.2 ± 3752.9 | 0.259 |
| Milan criteria (Beyond), n (%) | 34 (39.5%) | 22 (33.3%) | 12 (60.0%) | 0.033 |
| Microvascular inv. (+), n (%) | 18 (21.7%) | 11 (17.5%) | 7 (35.0%) | 0.097 |
| E–S grade (III–IV), n (%) | 25 (36.8%) | 17 (33.3%) | 8 (47.1%) | 0.309 |
| Transplant waiting time (days)a | 495.5 ± 468.9 | 514.2 ± 502.0 | 434.1 ± 341.3 | 0.507 |
| Post-operative complication (Clavien-Dindo classification ≥ III) | 22 (25.6%) | 16 (24.2%) | 6 (30.0%) | 0.575 |
AFP, Alpha-fetoprotein; E–S grade, Edmondson–Steiner grade; GRWR, graft-to-recipient body weight ratio; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; PIVKA-II, proteins induced by vitamin K antagonism or absence-II.
Values are shown as means ± standard deviation except where stated otherwise.
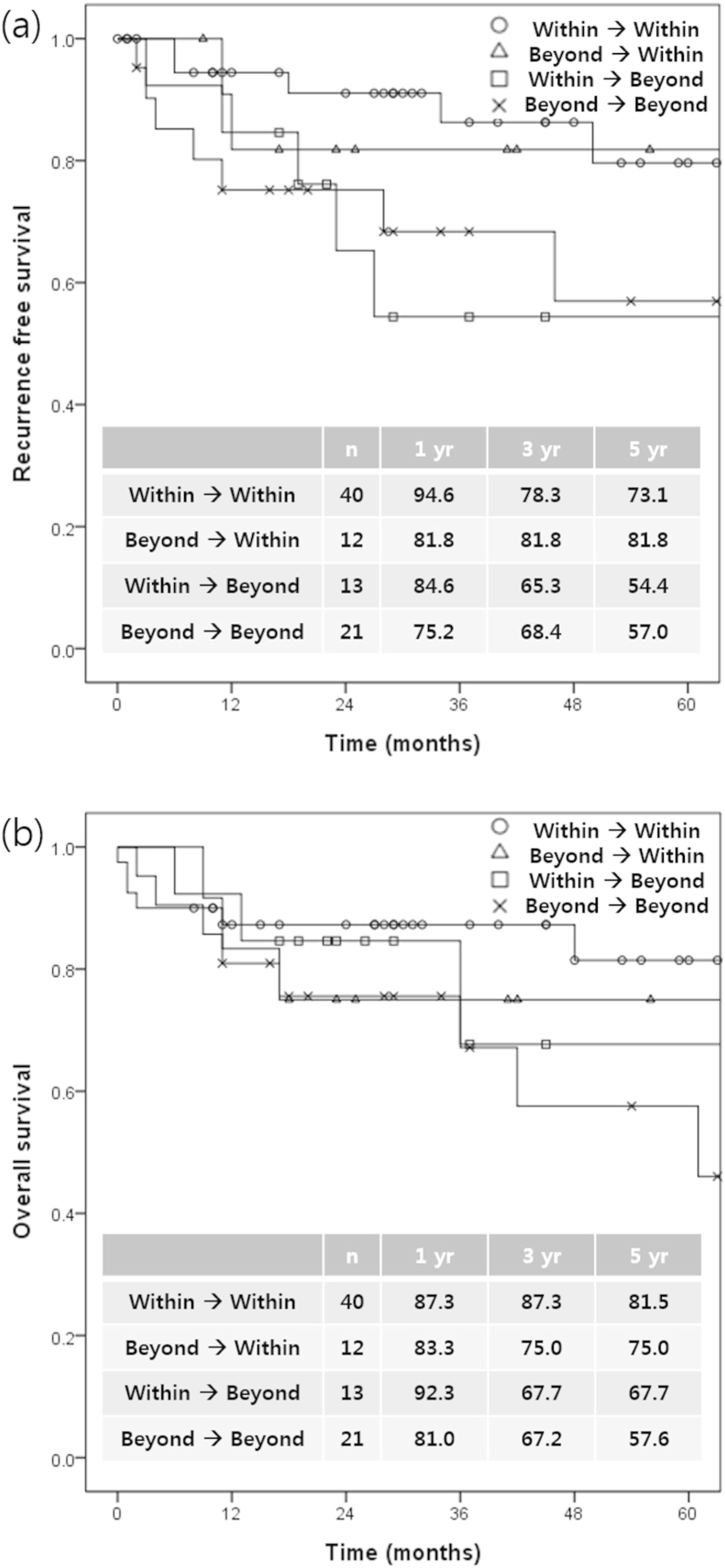
The rates of tumor progression and down-staging after locoregional treatments and their survival rates after LDLT were evaluated retrospectively. Of the 33 patients with HCC beyond the Milan criteria, 12 (36.4%) experienced successful down-staging. The 1-, 3-, and 5-year RFS rates in the patients with successful down-staging were each 81.8% and were comparable to those in patients with HCC within Milan criteria at both HCC diagnosis and transplantation (94.6, 86.4, and 79.8%, respectively). Of the 53 patients initially within the Milan criteria, 13 (24.5%) experienced HCC progression during locoregional treatment with tumors beyond the Milan criteria. The 1-, 3-, and 5-year DFS rates in patients with HCC progression were 84.6, 65.3 and 54.4%, respectively, and were comparable to those of the patients with HCC beyond Milan criteria at both HCC diagnosis and transplantation (75.2, 68.4, and 57.0%, respectively) (Fig. 1).
Figure 1.
Recurrence-free survival (a) and overall survival (b) following Milan criteria changes after locoregional treatments
Evaluation of several methods to assess tumor response after locoregional treatment
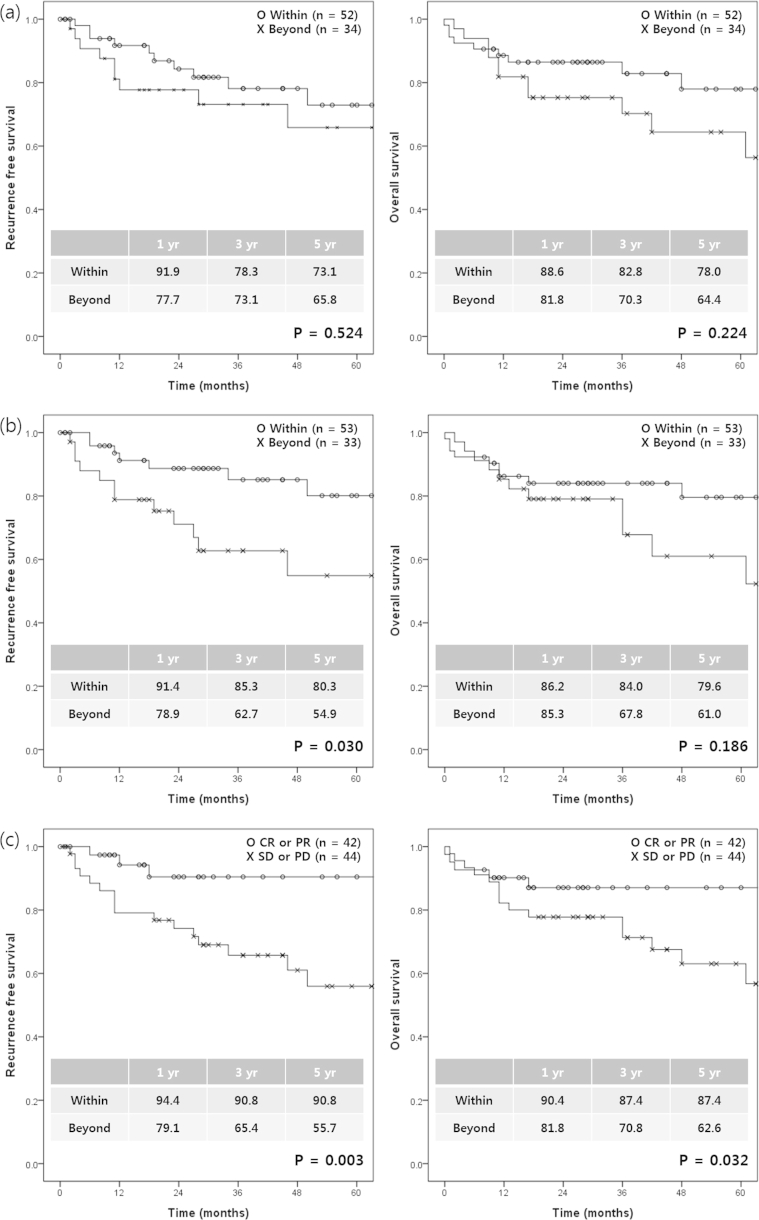
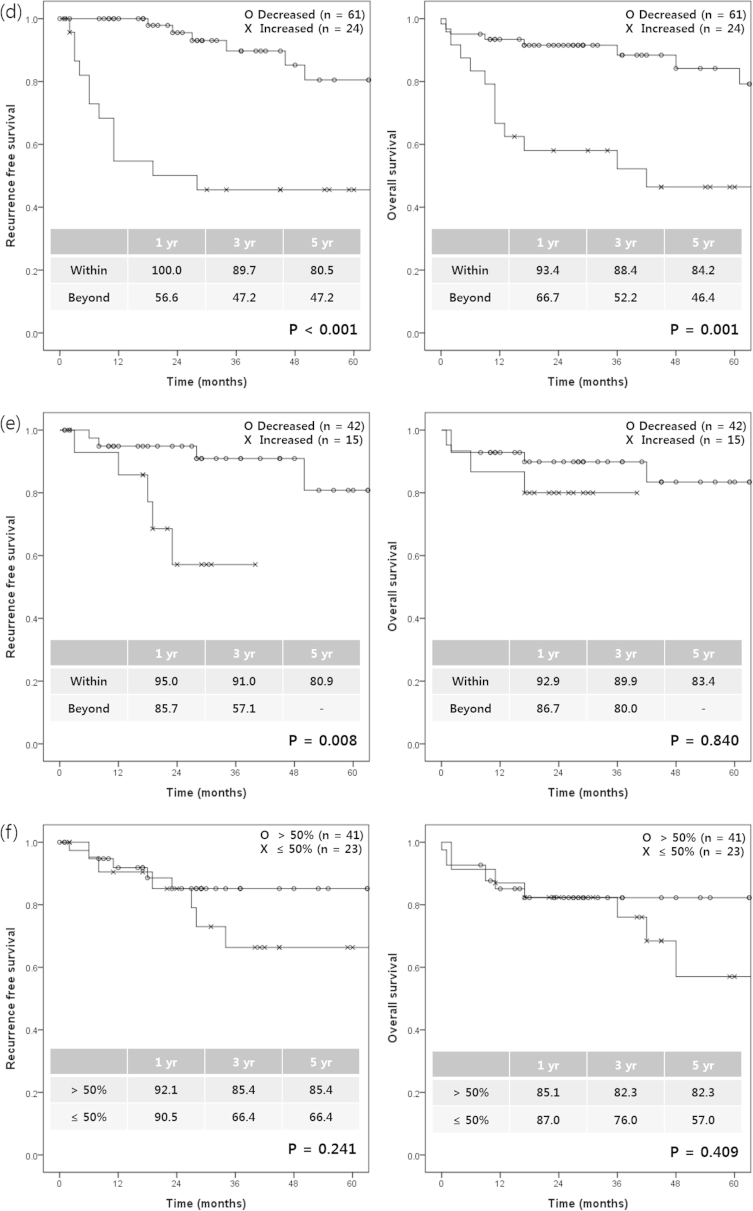
We investigated the most accurate method for predicting survival after LDLT among the several used for evaluating tumor response to pre-transplant locoregional treatments. According to the Milan criteria, the RFS and OS rates were not significantly different between the groups within and beyond the Milan criteria at the time of HCC diagnosis (p = 0.524 for RFS and p = 0.224 for OS). However, at the time of transplantation, the 1-, 3-, and 5-year RFS rates in patients within the Milan criteria at transplantation were significantly higher than in those beyond the Milan criteria (p = 0.030). According to the mRECIST criteria, the RFS and OS rates were significantly higher in the good responder group than in the bad responder group (p = 0.003 for RFS and p = 0.032 for OS). Increased AFP was significantly associated with both decreased RFS (p < 0.001) and OS (p = 0.001). Increased PIVKA was also significantly associated with decreased RFS (p = 0.008). The degree of tumor necrosis was not associated with RFS (p = 0.241) or OS (p = 0.409) (Fig. 2). On multivariate analysis, bad responders according to the mRECIST criteria (hazard ratio [HR], 4.874 [1.059–22.442], p = 0.042) and increased AFP during pre-transplant locoregional treatments (HR, 4.002 [1.540–10.397], p = 0.004) were independent risk factors for HCC recurrence after LDLT. In addition, increased AFP during pre-transplant locoregional treatments (HR, 3.509 [1.416–8.698], p = 0.007) was an independent risk factor for only overall survival after LDLT (Table 2). When the combined criteria for the bad responders according to the mRECIST criteria and increased AFP during pre-transplant locoregional treatments were met, the sensitivity and specificity, positive predictive value, and negative predictive value of these predictors on the likelihood of HCC recurrence rate were 57.9%, 87.9%, 57.9%, and 87.9%, respectively.
Figure 2.
Recurrence-free survival and overall survival according to the various methods used to assess tumor response after locoregional treatments. Milan criteria at HCC diagnosis (a), Milan criteria at transplantation (b), the mRECIST criteria (c), the change of AFP (d), the change of PIVKA-II (e), and tumor necrosis (f). AFP, Alpha-fetoprotein; mRECIST, modified Response Evaluation Criteria in Solid Tumors; PIVKA-II, proteins induced by vitamin K antagonism or absence-II
Table 2.
Multivariate analysis of recurrence-free survival and overall survival according to the various methods used to assess tumor response after locoregional treatments
| Factors | Recurrence-free survival |
Overall survival |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| Milan criteria at transplantation (beyond) | 1.614 (0.611–4.267) | 0.334 | 1.081 (0.425–2.747) | 0.870 |
| mRECIST criteria (bad responder; SD or PD) | 4.874 (1.059–22.442) | 0.042 | 1.968 (0.661–5.858) | 0.224 |
| Serum AFP(increased AFP) | 4.002 (1.540–10.397) | 0.004 | 3.509 (1.416–8.698) | 0.007 |
AFP, Alpha-fetoprotein; CR, complete response; HCC, hepatocellular carcinoma; mRECIST, modified Response Evaluation Criteria in Solid Tumors; PD, progressive disease; PIVKA-II, proteins induced by vitamin K antagonism or absence-II; PR, partial response; SD, stable disease.
We evaluated the factors associated with treatment response according to the mRECIST criteria. The maximal viable tumor size (p < 0.001), tumor number (p < 0.001), and AFP level (p = 0.043) at transplantation were significantly different between the good response group and the bad response group. The proportion of patients with decreased AFP level in the good response group was significantly higher in the good response group (p = 0.002) and microvascular invasion was significantly higher in the bad response group (p = 0.017). However, the patients' age, gender, etiology, liver function measured by the MELD score and total bilirubin, and the tumor stage at HCC diagnosis, indicated by maximal tumor size, number, and tumor markers, were not significantly different between the two groups of patients (Table 3).
Table 3.
Factors associated with the treatment response (mRECIST) to pre-transplant locoregional treatments
| Good responder (CR or PR, n = 42) | Bad responder (SD or PD, n = 44) | p | |
|---|---|---|---|
| Mean age (yr)a | 51.8 ± 6.4 | 52.7 ± 6.6 | 0.518 |
| Male, n (%) | 36 (85.7%) | 40 (90.9%) | 0.516 |
| Etiology, hepatitis B, n (%) | 38 (90.5%) | 37 (84.1%) | 0.413 |
| MELD scorea | 10.1 ± 7.4 | 7.8 ± 5.4 | 0.112 |
| Total Bilirubin (mg/dl)a | 2.97 ± 6.33 | 2.58 ± 6.09 | 0.771 |
| Transplant waiting time (mo)a | 385.1 ± 435.9 | 601.0 ± 479.8 | 0.032 |
| At HCC diagnosis | |||
| Maximal tumor size (cm)a | 3.82 ± 2.64 | 3.81 ± 2.95 | 0.984 |
| Tumor numbera | 2.10 ± 2.10 | 1.80 ± 1.19 | 0.416 |
| AFP (ng/ml)a | 1216.0 ± 6983.4 | 169.0 ± 431.7 | 0.316 |
| PIVKA (mAU/ml)a | 1071.9 ± 2565.2 | 322.1 ± 699.5 | 0.121 |
| Milan criteria (beyond), n (%) | 17 (40.5%) | 16 (36.4%) | 0.695 |
| At transplantation | |||
| Maximal tumor size, enhanced (cm)a | 0.73 ± 1.33 | 3.34 ± 2.95 | <0.001 |
| Tumor numbera | 1.38 ± 1.18 | 3.32 ± 2.03 | <0.001 |
| AFP (ng/ml)a | 14.8 ± 24.0 | 180.1 ± 525.4 | 0.043 |
| PIVKA (mAU/ml)a | 34.4 ± 59.1 | 490.7 ± 2165.5 | 0.250 |
| Milan criteria (beyond), n (%) | 8 (19.0%) | 26 (59.1%) | <0.001 |
| Pathology | |||
| Microvascular invasion (+), n (%) | 4 (10.3%) | 14 (31.8%) | 0.017 |
| E–S Grade (III–IV), n (%) | 7 (25.9%) | 18 (43.9%) | 0.133 |
| Tumor necrosis, n (%) | 0.093 | ||
| >50% | 25 (73.5%) | 16 (53.3%) | |
| ≤50% | 9 (26.5%) | 14 (46.7%) | |
| Decreased AFP (n = 85), n (%) | 36 (87.8%) | 25 (56.8%) | 0.002 |
| Decreased PIVKA (n = 57), n (%) | 26 (81.3%) | 16 (64.0%) | 0.142 |
AFP, Alpha-fetoprotein; CR, complete response; E–S grade, Edmondson–Steiner grade; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; mRECIST, modified Response Evaluation Criteria in Solid Tumors; PIVKA-II, proteins induced by vitamin K antagonism or absence-II; PD, progressive disease; PR, partial response; SD, stable disease.
Values are shown as means ± standard deviation except where stated otherwise.
Discussion
The purpose of this study was to assess the effect of pre-transplant locoregional treatments on survival after LDLT and to determine the most accurate method for predicting survival after LDLT among the several methods used for the evaluation of the tumor response to pre-transplant locoregional treatments. In contrast to Western countries, where DDLT is performed more frequently, LDLT is performed more often in Eastern countries because of a lack of deceased organ donors. Unlike DDLT, wait times can be controlled based on donor availability in LDLT, since LT can be performed without a waiting period when a living donor is available. This reduces the problems associated with long waiting times. However, it is difficult to predict tumor biology or aggressiveness prior to LT without pre-transplant locoregional treatments. Furthermore, reports of pre-transplant locoregional treatments in LDLT are scarce, while the results of pre-transplant locoregional treatments in DDLT have been reported widely. In addition, the recurrence rate was reported to be higher for LDLT compared with DDLT in HCC patients.9 Therefore, this study evaluated the effect of pre-transplant locoregional treatments on LDLT according to the records at an active transplant center that primarily performs LDLT.
In this study, among the 86 patients who received pre-transplant locoregional treatments, 20 (23.3%) experienced recurrence of HCC, and the recurrence rate in this study was higher than that in previous studies conducted in Western countries. The major differences in LT between Eastern and Western countries were the etiology of LT and the proportion of LDLT performed. In the present study, the most common cause of LT was hepatitis B (n = 75, 87.2%), followed by hepatitis C (n = 5, 5.8%) and other causes (n = 6, 7.0%). However, hepatitis B was not related to HCC recurrence (p = 0.331). We determined that broader selection criteria could be adopted for LDLT. Although the recurrence rate of the entire population in this study was high, the recurrence rate of patients within the Milan criteria was 15.4% (8/52), which is similar to rates presented in previous studies. The reason for the high recurrence rate may have been the high proportion of patients beyond the Milan criteria.
The Milan criteria are strict guidelines. Therefore, many centers are making efforts to expand the selection criteria.10, 11 There are also reports that survival after DDLT in patients with HCC beyond the Milan criteria successfully treated by down-staging was similar to the survival of patients who initially met the criteria for transplantation.3, 12 In this study we found that, if liver transplantation was not contraindicated, relatively expanded selection criteria could be adopted because we performed primarily LDLT. Thirty-three (38.4%) patients did not meet the Milan criteria at HCC diagnosis, and the 5-year overall survival rate was 64.4%. In addition, among the patients who received pre-transplant locoregional treatments, 12 (36.4%) of the 33 patients with HCC initially beyond the Milan criteria experienced successful down-staging. The 5-year RFS and OS rates of the 12 patients in whom down-staging was successful were comparable to those of patients with HCC initially within the Milan criteria. Moreover, the Milan criteria after pre-transplant locoregional treatments were more predictable of post-transplant recurrence (p = 0.030) than those at the time of HCC diagnosis (p = 0.524). Therefore, transplantation may be considered after successful down-staging.
To succeed in such a treatment strategy, it is necessary that the treatment response method accurately evaluates the results of locoregional treatments. The method currently used is the Milan criteria; however, according to the literature, many patients who met these criteria experienced recurrence after transplantation. This is because not only tumor size and number but also tumor biology can affect tumor recurrence.13 Since it is difficult to determine tumor biology such as microvascular invasion and tumor cell differentiation before transplantation, other criteria are needed. In patients receiving locoregional treatment before LT, tumor biology can be indirectly predicted through the evaluation of treatment response.14 This study showed that the mRECIST criteria [HR, 4.874 (1.059–22.442), p = 0.042] and the change in serum AFP level [HR 4.002 (1.540–10.397), p = 0.004] correspond well with HCC recurrence after LT in patients who have received pre-transplant locoregional treatment. The mRECIST criteria have become the standard method of evaluating treatment response after locoregional treatment in HCC. However, studies analyzing the survival rate after LT using the mRECIST criteria and studies comparing the mRECIST criteria with other methods, such as the Milan criteria, are rare. The importance of this study is that the treatment response after pre-transplant locoregional treatments and the survival rates after LDLT were evaluated using the mRECIST criteria and compared with those of other evaluation methods. Additionally, about half of HCC patients show an abnormal AFP level, which is used to monitor tumor change. An elevated AFP level is known to be a poor prognostic factor, but the exact cut-off level has yet to be determined.15 In patients receiving pre-transplant locoregional treatments, the trend in serum AFP during the transplant wait time correlated more with survival than did the absolute value of serum AFP at a single time point.16 In this study, elevated levels of serum AFP during the pre-transplant locoregional treatment correlated with HCC recurrence and poor overall survival after transplantation.
This study had several limitations. First, we retrospectively analyzed only patients who received LDLT, rather than all patients diagnosed with HCC. Second, the heterogeneity of the study population in terms of modalities of locoregional treatments represents a possible bias. For the treatment of HCC, many patients received a combination of several locoregional treatments because of the technical limitations of the various locoregional treatments. Because of the current lack of donor organs, it is important to predict HCC recurrence and survival after transplantation in advance. This study showed that the mRECIST criteria and serum AFP changes are important factors in predicting HCC recurrence after LDLT by comparing methods for evaluating treatment responses of locoregional treatment. These factors can complement the Milan criteria, which relies on tumor size and number.
In conclusion, LT may be considered after successful down-staging in patients with HCC initially beyond the Milan criteria. mRECIST and serum AFP level changes are better selection criteria for LDLT in patients who have received locoregional treatments relative to the initial assessment of tumor size or number.
Conflicts of interest
The authors have declared no conflicts of interest.
References
- 1.Mazzaferro V., Regalia E., Doci R., Andreola S., Pulvirenti A., Bozzetti F. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 2.Marrero J.A. Multidisciplinary management of hepatocellular carcinoma: where are we today? Semin Liver Dis. 2013;33(Suppl. 1):S3–S10. doi: 10.1055/s-0033-1333631. [DOI] [PubMed] [Google Scholar]
- 3.Yao F.Y., Kerlan R.K., Jr., Hirose R., Davern T.J., 3rd, Bass N.M., Feng S. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzaferro V., Battiston C., Perrone S., Pulvirenti A., Regalia E., Romito R. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900–909. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millonig G., Graziadei I.W., Freund M.C., Jaschke W., Stadlmann S., Ladurner R. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13:272–279. doi: 10.1002/lt.21033. [DOI] [PubMed] [Google Scholar]
- 6.Choi J.Y. Treatment algorithm for intermediate and advanced stage hepatocellular carcinoma: Korea. Oncology. 2011;81(Suppl. 1):141–147. doi: 10.1159/000333277. [DOI] [PubMed] [Google Scholar]
- 7.An H.J., Jang J.W., Bae S.H., Choi J.Y., Yoon S.K., You U.K. Serum C-reactive protein is a useful biomarker for predicting outcomes after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2012;18:1406–1414. doi: 10.1002/lt.23512. [DOI] [PubMed] [Google Scholar]
- 8.Lencioni R., Llovet J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant R.C., Sandhu L., Dixon P.R., Greig P.D., Grant D.R., McGilvray I.D. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transpl. 2013;27:140–147. doi: 10.1111/ctr.12031. [DOI] [PubMed] [Google Scholar]
- 10.Kaido T., Ogawa K., Mori A., Fujimoto Y., Ito T., Tomiyama K. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. 2013;154:1053–1060. doi: 10.1016/j.surg.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 11.Yao F.Y., Ferrell L., Bass N.M., Watson J.J., Bacchetti P., Venook A. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 12.Ravaioli M., Grazi G.L., Piscaglia F., Trevisani F., Cescon M., Ercolani G. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transpl. 2008;8:2547–2557. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]
- 13.Sumie S., Kuromatsu R., Okuda K., Ando E., Takata A., Fukushima N. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375–1382. doi: 10.1245/s10434-008-9846-9. [DOI] [PubMed] [Google Scholar]
- 14.Otto G., Herber S., Heise M., Lohse A.W., Monch C., Bittinger F. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260–1267. doi: 10.1002/lt.20837. [DOI] [PubMed] [Google Scholar]
- 15.Farinati F., Marino D., De Giorgio M., Baldan A., Cantarini M., Cursaro C. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 16.Vibert E., Azoulay D., Hoti E., Iacopinelli S., Samuel D., Salloum C. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transpl. 2010;10:129–137. doi: 10.1111/j.1600-6143.2009.02750.x. [DOI] [PubMed] [Google Scholar]