Abstract
Background
Curative liver resection is the treatment of choice for both primary and secondary liver malignancies. However, an inadequate future liver remnant (FLR) frequently precludes successful surgery. Portal vein embolization is the gold-standard modality for inducing hypertrophy of the FLR. In recent times, unilobar Yttrium-90 selective internal radiation therapy (SIRT) has been reported to induce hypertrophy of the contralateral, untreated liver lobe. The aim of this study is to review the current literature reporting on contralateral liver hypertrophy induced by unilobar SIRT.
Methods
A systematic review of the English-language literature between 2000 and 2014 was performed using the search terms “Yttrium 90” OR “selective internal radiation therapy” OR “radioembolization” AND “hypertrophy”.
Results
Seven studies, reporting on 312 patients, were included. Two hundred and eighty four patients (91.0%) received treatment to the right lobe. Two hundred and fifteen patients had hepatocellular carcinoma (HCC), 12 had intrahepatic cholangiocarcinoma, and 85 had liver metastases from mixed primaries. Y90 SIRT resulted in contralateral liver hypertrophy which ranged from 26 to 47% at 44 days–9 months. All studies were retrospective in nature, and heterogeneous, with substantial variations relative to pathology treated, underlying liver disease, dosage and delivery of Y90, number of treatment sessions and time to measurement of hypertrophy.
Conclusion
Unilobar Y90 SIRT results in significant hypertrophy of the contralateral liver lobe. The rate of hypertrophy seems to be slower than that achieved by other methods.
Introduction
Liver resection (LR) with negative margins is the only potentially curative treatment in the majority of patients with both primary and secondary malignant disease.1 However, an adequate future liver remnant (FLR) is imperative to avoid postoperative liver failure. In patients with a preserved liver function, a FLR of at least 25–30% is deemed sufficient by most clinicians to prevent liver failure.2 However, in patients with impaired liver function (e.g. cirrhosis), a FLR of up to 40% should be preserved.3, 4 Inadequate FLR is one of the most common reasons for precluding otherwise suitable patients from potentially curative LR.
At present, the two techniques most commonly used to induce FLR hypertrophy in patients with inadequate FLR are portal vein embolization (PVE) and portal vein ligation (PVL). In head-to-head comparisons, the two techniques have been shown provide equivalent degrees of hypertrophy,5, 6 estimated to be between 10 and 46% at 2–8 weeks.7 PVE is thus preferentially utilised as it is minimally invasive in nature and avoids a laparotomy.
Presently, selective internal radiation therapy (SIRT) with Yttrium-90 (also known as radioembolization) has become an increasingly utilized treatment modality for locally advanced liver tumours, with radiological tumour response rates of between 42 and 70% reported.8, 9, 10 In addition to documented efficacy for local tumour control, recent reports have described that the delivery of unilobar SIRT may result in a significant hypertrophy of the contralateral liver lobe.11, 12, 13, 14, 15, 16, 17, 18, 19 This finding is relatively recent and has the potential of increasing resectability rates as it allows both tumour down-staging and induces FLR hypertrophy.
To date, there have been multiple reports—largely heterogeneous case series—describing this phenomenon. The aim of this study was to perform a systematic review of the English language literature to summarize the current evidence on liver hypertrophy following unilobar SIRT.
Methods
Systematic literature search
A systematic literature search was performed from the PubMed and Scopus databases from January 1 2000 to August 20 2014. SIRT with Y90 is a relatively new technology, and searches extending earlier than this would not yield additional results. The search terms “Yttrium 90” OR “selective internal radiation therapy” OR “radioembolization” AND “hypertrophy” were used. From the titles identified, all abstracts were screened by two authors (Teo JY and Goh BKP) to identify studies reporting on the degree of liver hypertrophy after Y90 SIRT. Subsequently, full-text articles of potentially eligible articles were screened. All references of the included studies were screened for potential relevant studies not identified by the initial literature search. The final decision on eligibility was reached by consensus between the two screening authors. There were no cases of disagreement and hence no requirement for adjudication by an independent third reviewer. When more than one study was published from the same centre, and the cohorts were overlapping, only the most recent study was included in the analysis.
Inclusion and exclusion criteria
Inclusion criteria were (i) case series reporting on ≥ 2 patients; (ii) undergoing unilobar SIRT with Y90 microspheres; (iii) and reporting on hypertrophy of the contralateral lobe at any time point. When volume changes at more than one independent time point were reported, the maximal volume increase was extracted and analysed.
Exclusion criteria were (i) case reports; (ii) studies which did not report volumetric changes; (iii) review articles which did not present unique data. (iv) Articles not published in English.
Data extraction
From the included studies, the following data were extracted: number of patients, pathology of disease being treated, modality and site of Y90 delivery, number of treatment sessions, method of volumetric measurement, time to determination of liver hypertrophy and degree of hypertrophy achieved.
Results
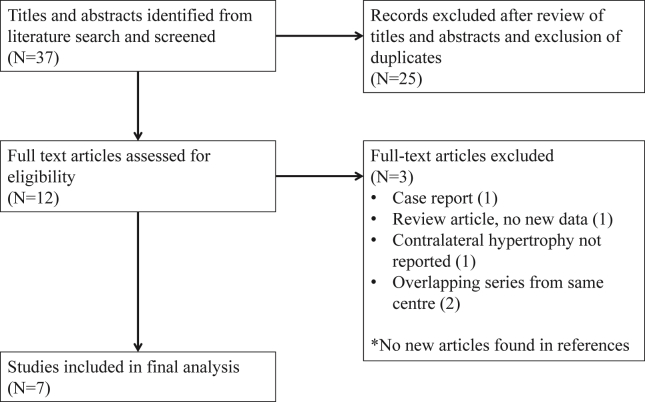
Fig. 1 shows the PRISMA flow chart for the study.20 Nine studies, published between 2008 and 2014 were identified.11, 12, 13, 14, 15, 16, 17, 18, 19 Three studies11, 12, 15 were reported from the same centre with overlapping patient cohorts. Two studies11, 12 were excluded; and only the most recent (and largest) report15 was included. Finally, 7 studies reporting on a total of 312 patients were included in the final analysis. Table 1 shows a summary of the data variables collected. All identified studies were retrospective in nature. As there was clearly a great degree of clinical heterogeneity among studies—most notably in terms of time to volumetric measurement—a meta-analysis was not performed as any result obtained would be of questionable value and difficult to interpret.
Figure 1.
Flow diagram of study identification and selection
Table 1.
Summary of studies reporting on post-SIRT hypertrophy after unilobar SIRT
| Paper | Number of patients | Age | Pathology treated | SIRT modality | Site of Y90 delivery | Number of treatment sessions | Method of volume measurement | Time to measurement | Percentage hypertrophy (mean/median (range)) |
|---|---|---|---|---|---|---|---|---|---|
| Ahmadzadehfar et al. 2013 Germany13 |
24 | Median 53 (range 44–78) | Metastatic disease (mixed) 17 – bi-lobar |
Resin microspheres | Right lobe | Single | FDG PET/CT | Mean 44 days, median 36 days | Mean 47%, median 34% Only right lobe disease – mean 57%, median 70% |
| Edeline et al. 2013 France14 |
34 | Not stated | Primary – HCC | 30 Glass, 4 resin microspheres | 23 right, 11 left | Single | CT | 3 months Not stated |
Mean 29% Mean 42% (maximal) |
| Vouche et al. 2013 USA15 |
83 | Median 68 (range 36–89) | 67 HCC, 8 IHC, 8 CRC mets | Glass microspheres | Right lobe | Single | MRI/CT | 1- >9 months | Median overall 26% (−14–86) Median 45% at 9 months (5–186) |
| Theysohn et al. 2013 Germany16 |
45 | Mean 71.9 | HCC | Glass microspheres | Right lobe | Single | CT | 6 Months | Mean 30.8% |
| Fernandez-Ros et al. 2013 Spain17 |
83 | Median 66 | 52 HCC, 4 IHC, 13 CRC mets, 14 others | Resin microspheres | 66 right, 17 left | Single | CT/MRI | 26 weeks | Mean 45% |
| Garlipp et al. 2013 Germany, France18 |
26 | Mean 59.2 | Metastatic disease (mixed) | Resin microspheres | Right lobe | Single | MRI | Median 46 days (27–79 days) | Mean 29%, median 25.3% |
| Teo et al. 2014 Singapore19 |
17 | Median 72 (range 42–78) | HCC | Resin microspheres | Right lobe | Single | CT | Median 5 months | Mean 34.2% |
HCC, hepatocellular carcinoma; IHC, intrahepatic cholangiocarcinoma; CRC mets, colorectal cancer metastases; FDG PET, fluorodeoxyglucose positron-emission-tomography; CT, computed tomography; MRI, magnetic resonance imaging.
The published series were heterogeneous in terms of pathology treated, dosage and delivery of Y90, and time to measurement of hypertrophy. However, it was clear that unilobar Y90 SIRT resulted in significant hypertrophy of the contralateral lobe—the reported average hypertrophy achieved ranged from 26 to 47% over time periods of 44 days to 9 months. Of the 312 patients 284 (91.0%) received SIRT to the right lobe. In terms of underlying pathology, 215 (68.9%) patients were treated for hepatocellular carcinoma (HCC), 12 (3.8%) for intrahepatic cholangiocarcinoma and 85 (27.2%) for liver metastases from various primaries.
Comparison between SIRT and PVE
Only one study18 attempted a direct head-to-head comparison between SIRT and PVE. Garlipp et al. performed a matched-pair analysis of patients with secondary liver malignancy confined to the right hemiliver. Patients were well matched for (i) baseline FLR; (ii) history of platinum-based chemotherapy; (iii) platelet count and (iv) extent of embolization. Although subject to the usual biases inherent in such a study, PVE was reported to result in significantly greater hypertrophy (PVE: 61.5%; SIRT: 29.0%) within a shorter median time frame (PVE: 33 days (range 24–56 days); SIRT: 46 days (range 27–79 days). In this study, tumour growth in both arms was not reported.
Rate of hypertrophy with SIRT
Two studies attempted to describe the time-dependant changes in liver volume.15, 17 The studies by both Vouche et al.15 and Fernandez-Ros et al.17 suggested that the kinetics of post-Y90 hypertrophy are slow, with gradual increases in volume, and no demonstrated plateau. However, due to differences in patient populations and treatment specifics, the Vouche et al.15 study reported 45% hypertrophy at 9 months, whereas the Fernandez-Ros et al.17 study reported 45% hypertrophy in 26 weeks, thus clearly demonstrating the importance of underlying patient and disease characteristics in influencing the eventual degree of growth achieved. Importantly, in the study by Vouche et al.,15 which reported in detail the percentage hypertrophy at various time points, FLR growth above baseline was only 7% at 1 month and 24% at 3 months.
Other findings
The study by Teo et al.16 was the first to report that HCC patients with hepatitis B experience a significantly greater degree of hypertrophy after SIRT (44.5%) compared to those with hepatitis C or alcoholic cirrhosis (7.7%). Although acknowledging the limitation of their small sample size, the authors postulated that the difference in hypertrophy was due to differences in underlying pathogenesis, with cirrhosis being a more important factor in patients with hepatitis C or alcoholic liver disease.
All identified studies reported predominantly on the phenomenon of post-SIRT hypertrophy as the primary outcome, and therefore outcomes on the treated tumours were not explicitly reported. However, a consistent finding was that hypertrophy of the untreated lobe is accompanied by a corresponding decrease in size of the tumour-bearing hemiliver, resulting in no net change in liver volume. This suggested that Y90 radioembolization resulted in good local tumour control which is consistent with previous studies reporting on oncological outcomes of Y90 radioembolization.8, 9
Discussion
An adequate FLR is essential for a safe and successful major hepatectomy. The safety and efficacy of PVE for reliably producing significant hypertrophy in the FLR prior to the planned LR has been well-established.7 This method should be regarded as the “gold standard” against which novel techniques are judged. However, a major drawback of PVE is that tumour growth continues unabated while awaiting hypertrophy, which may eventually preclude resection especially in tumours which are in close proximity to major bilio-vascular structures. This is far from being a merely theoretical concern as increased tumour growth rates after PVE have been reported in animal models21, 22 and humans.23
Given these concerns, a sequential approach combining transarterial chemoembolization (TACE) and PVE has been advocated, with proponents claiming both a significant rate of FLR hypertrophy as well as increased local tumour control. This approach was first shown to result in good FLR hypertrophy, with no increased risk of liver failure, as might be expected after occlusion of the liver's dual blood supply.24 These findings were replicated in subsequent larger studies, which also showed an improvement in both overall and disease-free survival in patients undergoing sequential treatment as opposed to PVE alone.25, 26 However, in these studies, the mean increase in percentage of FLR achieved in the PVE + TACE arms was only 7.3–22%, which was significantly less than that usually reported with PVE in the literature.7
The current systematic review demonstrated that unilobar Y90 SIRT resulted in significant hypertrophy of the contralateral liver lobe. However, all studies to date have been retrospective and observational in nature. The true degree and kinetics of hypertrophy, as well as the impact on these by tumour type, underlying liver disease, previous hepatotoxic chemotherapy, dose and delivery of radiation and other factors are as yet relatively unstudied and unknown.
PVE has been reported to give rise to FLR hypertrophy of 10–46% after 2–8 weeks.5 The current systematic review showed hypertrophy of 26–47% at time intervals of from 44 days to 9 months after unilobar SIRT with Y90. Thus while the degree of growth achieved is comparable to that achieved with PVE alone, and superior to that achieved after PVE + TACE, the kinetics of hypertrophy after SIRT are likely to be different from PVE. This is further borne out in the study by Vouche et al.,15 which showed only limited hypertrophy in the early post-treatment period.
The recent development of another novel technique for inducing liver hypertrophy, i.e. associating liver partition with portal vein ligation for staged hepatectomy (ALPPS), should also be mentioned. This technique allows for extremely rapid hypertrophy of the FLR, but at the risk of increased morbidity and a significant mortality rate. Two recent review papers27, 28 concluded that mean FLR hypertrophy in excess of 80% at 7–10 days was achievable, but at the risk of a 35–44% rate of significant morbidity, a 30-day mortality rate of 6% and 90-day mortality of 11%. In view of the significant morbidity and mortality, ALPPS is therefore at present best considered to be an experimental technique. It should only be used in highly selected patients in a clinical trial setting.29
The technique of Y90 SIRT is relatively safe and, in contrast to PVE, has the theoretical benefit of providing concomitant tumour control, with tumour response ranging from 42 to 70%,8, 9 by the RECIST criteria. In situations where a large, bulky tumour abuts major vascular and/or biliary structures that must be saved, or when the ability to achieve adequate oncological margins are a concern, then Y90 SIRT is theoretically advantageous in providing both tumour control/downsizing while increasing the FLR.29 Unfortunately, in the only study to date which has attempted a direct head-to-head comparison between these modalitiees,18 tumour growth in both arms was not reported.
In addressing the mechanism of hypertrophy, several11, 17, 19 studies have described changes consistent with portal hypertension following Y90 SIRT. These include increases in portal vein and spleen diameter with corresponding decreases in platelet count. Whether these changes reflect the underlying mechanism of hypertrophy, or if they are an indirect consequence of radiation-induced atrophy of the treated lobe, is unknown.
This review has several limitations. Most importantly, the studies identified are all retrospective in nature and are likely to be subject to selection and reporting bias. The patient cohorts are also vastly heterogeneous, with great variations in pathology treated, underlying liver disease, dosage and delivery of Y90, number of treatment sessions and time to measurement of hypertrophy—many of which may well influence the magnitude of treatment effect. Lastly, owing to study heterogeneity, a meta-analysis was not attempted as it would be difficult to interpret the data in any meaningful way.
Conclusion
Administration of unilobar SIRT results in significant hypertrophy of the contralateral liver lobe. The rate of hypertrophy seems to be slower than that achieved by other methods. Nevertheless, as SIRT is most often utilised in a palliative setting, on patients with presumably inferior functional reserve and liver function, the published literature may well underestimate the true potential of this modality. In conclusion, the phenomenon of post-Y90 hypertrophy provides a novel and exciting option in the multidisciplinary management of patients with liver tumours. Prospective studies are required to determine the kinetics of hypertrophy, as well as pre-procedural factors predictive of hypertrophy. In addition, the functional capacity of the hypertrophied liver is yet to be ascertained, and the oncological outcomes of patients undergoing LR after Y90 SIRT must be established.
Conflicts of interest
Brian Goh has received travel grants from Sirtex Medical.
David Ng has received travel grants from Sirtex Medical.
Pierce Chow has received travel and research grants from Sirtex Medical.
References
- 1.Goh B.K., Chow P.K., Teo J.Y., Wong J.S., Chan C.Y., Cheow P.C. Number of nodules, Child-Pugh status, margin positivity, and microvascular invasion, but not tumor size, are prognostic factors of survival after liver resection for multifocal hepatocellular carcinoma. J Gastrointest Surg. 2014 Aug;18:1477–1485. doi: 10.1007/s11605-014-2542-0. [DOI] [PubMed] [Google Scholar]
- 2.Goh B.K. Measured versus estimated total liver volume to preoperatively assess the adequacy of future liver remnant: which method should we use? Ann Surg. 2015;262:e72. doi: 10.1097/SLA.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka K., Shimada H., Matsuo K., Ueda M., Endo I., Togo S. Remnant liver regeneration after two-stage hepatectomy for multiple bilobar colorectal metastases. Eur J Surg Oncol. 2007 Apr;33:329–335. doi: 10.1016/j.ejso.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 4.Hemming A.W., Reed A.I., Howard R.J., Fujita S., Hochwald S.N., Caridi J.G. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003 May;237:686–691. doi: 10.1097/01.SLA.0000065265.16728.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capussotti L., Muratore A., Baracchi F., Lelong B., Ferrero A., Regge D. Portal vein ligation as an efficient method of increasing the future liver remnant volume in the surgical treatment of colorectal metastases. Arch Surg. 2008 Oct;143:978–982. doi: 10.1001/archsurg.143.10.978. [DOI] [PubMed] [Google Scholar]
- 6.Aussilhou B., Lesurtel M., Sauvanet A., Farges O., Dokmak S., Goasguen N. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg. 2008 Feb;12:297–303. doi: 10.1007/s11605-007-0410-x. [DOI] [PubMed] [Google Scholar]
- 7.Liu H., Zhu S. Present status and future perspectives of preoperative portal vein embolization. Am J Surg. 2009 May;197:686–690. doi: 10.1016/j.amjsurg.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Kulik L.M., Carr B.I., Mulcahy M.F., Lewandowski R.J., Atassi B., Ryu R.K. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008 Jan;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 9.Salem R., Lewandowski R.J., Mulcahy M.F., Riaz A., Ryu R.K., Ibrahim S. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010 Jan;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Khor A.Y., Toh Y., Allen J.C., Ng D.C., Kao Y.H., Zhu G. Survival and pattern of tumor progression with yttrium-90 microsphere radioembolization in predominantly hepatitis B Asian patients with hepatocellular carcinoma. Hepatol Int. 2014 Aug:395–404. doi: 10.1007/s12072-014-9533-9. [DOI] [PubMed] [Google Scholar]
- 11.Jakobs T.F., Saleem S., Atassi B., Reda E., Lewandowski R.J., Yaghmai V. Fibrosis, portal hypertension, and hepatic volume changes induced by intra-arterial radiotherapy with 90yttrium microspheres. Dig Dis Sci. 2008 Sep;53:2556–2563. doi: 10.1007/s10620-007-0148-z. Epub 2008 Jan 31. [DOI] [PubMed] [Google Scholar]
- 12.Gaba R.C., Lewandowski R.J., Kulik L.M., Riaz A., Ibrahim S.M., Mulcahy M.F. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009 Jun;16:1587–1596. doi: 10.1245/s10434-009-0454-0. Epub 2009 Apr 9. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadzadehfar H., Meyer C., Ezziddin S., Sabet A., Hoff-Meyer A., Muckle M. Hepatic volume changes induced by radioembolization with 90Y resin microspheres. A single-centre study. Eur J Nucl Med Mol Imaging. 2013 Jan;40:80–90. doi: 10.1007/s00259-012-2253-2. Epub 2012 Oct 13. [DOI] [PubMed] [Google Scholar]
- 14.Edeline J., Lenoir L., Boudjema K., Rolland Y., Boulic A., Le Du F. Volumetric changes after (90)y radioembolization for hepatocellular carcinoma in cirrhosis: an option to portal vein embolization in a preoperative setting? Ann Surg Oncol. 2013 Aug;20:2518–2525. doi: 10.1245/s10434-013-2906-9. Epub 2013 Mar 15. [DOI] [PubMed] [Google Scholar]
- 15.Vouche M., Lewandowski R.J., Atassi R., Memon K., Gates V.L., Ryu R.K. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013 Nov;59:1029–1036. doi: 10.1016/j.jhep.2013.06.015. Epub 2013 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theysohn J.M., Ertle J., Müller S., Schlaak J.F., Nensa F., Sipilae S. Hepatic volume changes after lobar selective internal radiation therapy (SIRT) of hepatocellular carcinoma. Clin Radiol. 2014 Feb;69:172–178. doi: 10.1016/j.crad.2013.09.009. Epub 2013 Oct 25. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Ros N., Silva N., Bilbao J.I., Iñarrairaegui M., Benito A., D'Avola D. Partial liver volume radioembolization induces hypertrophy in the spared hemiliver and no major signs of portal hypertension. HPB. 2014 Mar;16:243–249. doi: 10.1111/hpb.12095. Epub 2013 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garlipp B., de Baere T., Damm R., Irmscher R., van Buskirk M., Stübs P. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology. 2014 May;59:1864–1873. doi: 10.1002/hep.26947. Epub 2014 Apr 1. [DOI] [PubMed] [Google Scholar]
- 19.Teo J.Y., Goh B.K., Cheah F.K., Allen J.C., Lo R.H., Ng D.C. Underlying liver disease influences volumetric changes in the spared hemiliver after selective internal radiation therapy with (90) Y in patients with hepatocellular carcinoma. J Dig Dis. 2014 Aug;15:444–450. doi: 10.1111/1751-2980.12162. [DOI] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda Y., Matsumata T., Takenaka K., Sasaki O., Soejima K., Sugimachi K. Preliminary report of tumor metastasis during liver regeneration after hepatic resection in rats. Eur J Surg Oncol. 1995 Apr;21:188–190. doi: 10.1016/s0748-7983(95)90468-9. [DOI] [PubMed] [Google Scholar]
- 22.Mizutani J., Hiraoka T., Yamashita R., Miyauchi Y. Promotion of hepatic metastases by liver resection in the rat. Br J Cancer. 1992 Jun;65:794–797. doi: 10.1038/bjc.1992.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pamecha V., Levene A., Grillo F., Woodward N., Dhillon A., Davidson B.R. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br J Cancer. 2009 Feb 24;100:617–622. doi: 10.1038/sj.bjc.6604872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoki T., Imamura H., Hasegawa K., Matsukura A., Sano K., Sugawara Y. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004 Jul;139:766–774. doi: 10.1001/archsurg.139.7.766. [DOI] [PubMed] [Google Scholar]
- 25.Ogata S., Belghiti J., Farges O., Varma D., Sibert A., Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006 Sep;93:1091–1098. doi: 10.1002/bjs.5341. [DOI] [PubMed] [Google Scholar]
- 26.Yoo H., Kim J.H., Ko G.Y., Kim K.W., Gwon D.I., Lee S.G. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol. 2011 May;18:1251–1257. doi: 10.1245/s10434-010-1423-3. [DOI] [PubMed] [Google Scholar]
- 27.Ielpo B., Caruso R., Ferri V., Quijano Y., Duran H., Diaz E. ALPPS procedure: our experience and state of the art. Hepatogastroenterology. 2013 Nov–Dec;60:2069–2075. [PubMed] [Google Scholar]
- 28.Schadde E., Schnitzbauer A.A., Tschuor C., Raptis D.A., Bechstein W.O., Clavien P.A. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol. 2015;22:3109. doi: 10.1245/s10434-014-4213-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Teo J.Y., Goh B.K. Contra-lateral liver lobe hypertrophy after unilobar Y90 radioembolization: an alternative to portal vein embolization? World J Gastroenterol. 2015;21:3170. doi: 10.3748/wjg.v21.i11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]