Abstract
Background
Fluid collections (FC) at the resection margin of the pancreatic stump after distal pancreatectomy (DP) are common radiological findings in follow-up scans. No recommendations exist regarding the management of such findings. The aim was to characterise incidence, risk factors, clinical impact and therapy of FC.
Method
Data of 209 patients who underwent DP between 07/2009 and 06/2011 were prospectively collected and analysed, regarding follow-up CT or MRI scan findings of FC at the resection margin. FC was defined as a cyst-like lesion >1 cm in diameter.
Results
A follow-up with at least two cross-sectional images was available in 159/209 patients. In the first postoperative control, 68 patients showed an FC (43%). FC size was classified as <5 cm (n = 38 pat.), 5–10 cm (n = 24 pat.) and >10 cm (n = 6 pat.). 20 patients (30%) showed clinical symptoms. Six patients (9%) required specific treatment, all other FC showed spontaneous regression. No correlation with stump closure techniques or preceding postoperative pancreatic fistula was found (4/68 patients, 6%). Multivariate analysis revealed standard resections as the only significant factor for FC.
Conclusions
FCs at the resection margin after DP are frequent and harmless findings. Therapeutic interventions are required in only 9% of all FC patients.
Introduction
Distal pancreatectomy (DP) with or without splenectomy is a standard procedure for the treatment of benign and malignant lesions of the pancreatic corpus and tail. The closure of the pancreatic remnant still remains a surgical challenge with regard to the risk of postoperative pancreatic fistula (POPF) development, which represents the most frequent procedure-related complication. Large series of DP have described POPF rates ranging from 12% to 31%.1, 2 Different surgical techniques of transection and stump closure have been examined and discussed3, 4, 5, 6 without showing the superiority of any of these approaches in terms of POPF prevention. Therefore, although most DP-associated POPFs are non-complicated type A fistulas,7 they remain a relevant clinical problem with regard to morbidity, the risk for other complications and increasing healthcare costs for prolonged hospitalisation or readmission.8 In our centre, follow-up examinations after pancreatic resections are routinely carried out to evaluate the patients' status with regard to potential late complications as well as surveillance for recurrence of tumours or cystic lesions on an outpatient care basis and include cross-sectional imaging with MRI or CT scans in defined intervals. Depending on the histopathological diagnosis, intervals of 3–6 months are commonly chosen for follow-up imaging, although only a few recommendations exist regarding this topic. In contrast to the management of POPF after DP, which has been well-defined, the phenomenon of delayed fluid collections (FC) located at the resection margin in follow-up imaging has not yet been described. Therefore, the pathophysiology of FC – especially with regard to a possible POPF association – remains unclear, as does the clinical impact of these findings.
The aim of the study was to evaluate the incidence of FCs and risk factors for their development, as well as clinical symptoms, therapeutic measures and the outcome of DP-associated FC in the postoperative follow-up.
Methods
Data of all patients who underwent DP between July 2009 and June 2011 at the Department of Surgery, University of Heidelberg, Germany, were prospectively collected and retrospectively analysed with regard to postoperative follow up in the outpatient department until October 2014. Patients with at least two consecutively available postoperative cross-sectional follow-up imaging examinations (CT or MRI scans) were included in the analysis. The study was approved by the local ethics committee (S 011/15).
Baseline data acquisition included patients' gender and age, histopathological diagnosis, surgical parameters of DP (basic operative procedure and closure type, operation time, blood loss, extended resection), postoperative complications (bleeding, re-operation, POPF, lymphatic fistula, delayed gastric emptying (DGE), other complications, mortality) and duration of hospital stay.
Extended resections were defined according to the recent consensus conference of ISGPS as the additional resection of one or more organs, other than the pancreatic tail, spleen and gall bladder.9 POPF and DGE were defined according to the former ISGPS definitions.7, 10 Lymphatic fistula was defined as milky drain fluid, concurrent with the beginning of postoperative oral food intake, showing a triglyceride concentration >1.2 mmol/l.11, 12
Outpatient follow-up examinations included anamnesis, clinical examination, quality of life assessment (EORTC Q30 questionnaire), serum analysis for routine parameters and tumour markers (CEA, CA 19-9), as well as CT or MRI scan. Scans were independently examined with regard to FC at the resection margin by two radiologists (F.F., L.G.). FC was defined as lesions of ≥1 cm in diameter with a typical cyst-like appearance located at the pancreatic resection margin (Fig. 3). FC diameters were documented and FC-related symptoms were defined as abdominal pain, pressure sensation or elevated C-reactive protein levels. FC-associated therapeutic measures were documented.
Figure 3.
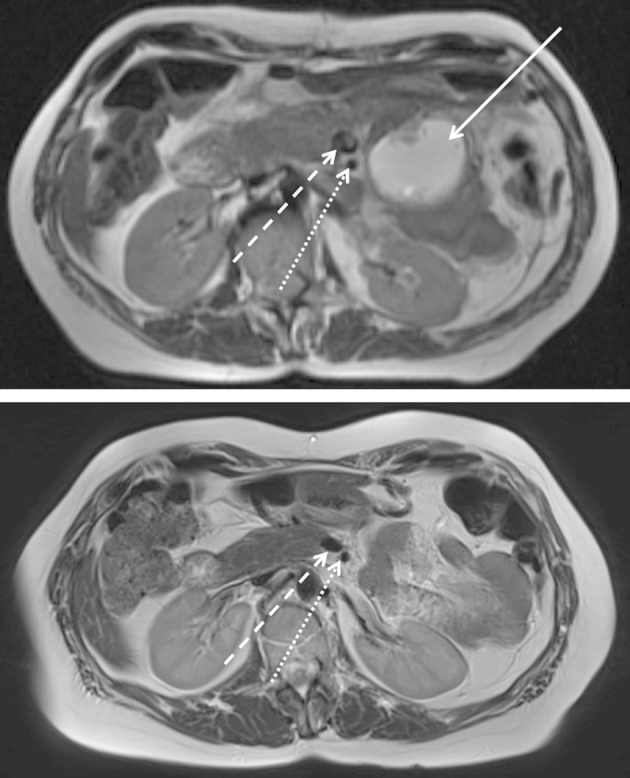
Follow-up MRI scans (T2-weighted) showing FC (54 mm, white arrow) at the resection margin three months after DP (above). Complete resolution without FC-directed therapy after six months (below). Resection margin at the level of superior mesenteric vein (broke white arrow) and superior mesenteric artery (dotted white arrow)
Data management and statistical analysis were carried out by SAS® software release 9.1 (SAS Institute, Cary, North Carolina, USA). The distributions of the quantitative parameters are presented with the median and the interquartile range (iqr), unless otherwise stated. Regarding the quantitative parameters, the Kruskal–Wallis test was used to compare the groups POPF, LC, and non-leakage, and the Mann–Whitney U test was used for pairwise comparison between the groups. To analyse categorical parameters, Fisher's exact test was used. All tests were performed two-sided. Significance was accepted at a level of 95%.
Results
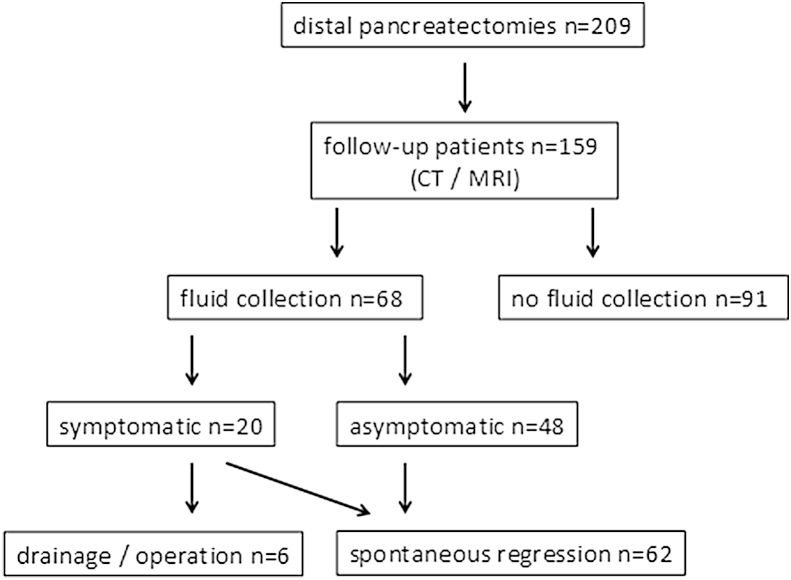
During the observation period, a total of 209 patients underwent DP. Four patients (1.9%) died during the postoperative course (heart attack n = 1, pulmonary embolism n = 1, post-pancreatectomy haemorrhage n = 2) and 46 patients were lost to follow-up Overall, 159 of 209 patients (76%) were available for follow-up as described above and were included in the study (Fig. 1). The median age was 58.3 years with a range of 17.1–82.3 years and 75 patients (47%) were male. Patients' baseline demographic data, regarding age distribution and preoperative ASA classification, are given in detail in Table 1. Indications for DP included pancreatic cancer (35%), neuroendocrine tumours (14%), chronic pancreatitis (13%), IPMN (11%) as well as other pancreatic tumours (14%) and extrapancreatic malignancies (13%), as outlined in Table 1.
Figure 1.
Study flow chart
Table 1.
Patient characteristics
| All (n = 159) | FC (n = 64) | No leakage (n = 57) | POPF (n = 38) | p-value | |
|---|---|---|---|---|---|
|
Sex |
0.0760 | ||||
| M | 75 (47.2%) | 28 (43.8%) | 23 (40.4%) | 24 (63.2%) | |
| F | 84 (52.8%) | 36 (64.2%) | 34 (59.6%) | 14 (36.8%) | |
| Age | 0.4744 | ||||
| ≤40 | 18 (11.3%) | 6 (9.4%) | 5 (8.8%) | 7 (18.4%) | |
| >40 < 70 | 106 (66.7%) | 45 (70.3%) | 40 (70.2%) | 21 (55.3%) | |
| ≥70 | 35 (22.0%) | 13 (20.3%) | 12 (21.0%) | 10 (26.3%) | |
| Median body mass index (iqr) | 24.7 (21.6–27.3) | 24.6 (22.6–27.4) | 24.3 (20.6–26.4) | 26.1 (22.6–27.5) | 0.1890 |
| ASA | 0.6686 | ||||
| I | 7 (2.8%) | 4 (6.3%) | 2 (3.7%) | 1 (2.8%) | |
| II | 90 (58.3%) | 40 (63.5%) | 29 (53.7%) | 21 (58.3%) | |
| III | 56 (38.9%) | 19 (30.2%) | 23 (42.6%) | 14 (38.9%) | |
| Diagnosis | 0.2240 | ||||
| PDAC | 55 (34.6%) | 19 (29.7%) | 26 (45.6%) | 10 (26.3%) | |
| Chronic pancreatitis | 21 (13.2%) | 9 (14.1%) | 4 (7.0%) | 8 (21.0%) | |
| IPMN | 18 (11.3%) | 9 (14.1%) | 6 (10.5%) | 3 (7.9%) | |
| NET | 22 (13.8%) | 7 (10.9%) | 7 (12.3%) | 8 (21.1%) | |
| Other pancreatic tumours | 23 (14.5%) | 13 (20.3%) | 5 (8.8%) | 5 (13.2%) | |
| Extrapancreatic malignancies | 20 (12.6%) | 7 (10.9%) | 9 (15.8%) | 4 (10.5%) | |
| Operative procedures | 0.0114 | ||||
| DP with splenectomy | 124 (78.0%) | 57 (89.1%) | 42 (73.7%) | 25 (65.8%) | |
| extended DP | 24 (15.1%) | 3 (4.7%) | 10 (17.5%) | 11 (28.9%) | 0.0024 |
| Spleen-preserving DP | 11 (6.9%) | 4 (6.2%) | 5 (8.8%) | 2 (5.3%) | |
| Closure methods | 0.0688 | ||||
| Stapler/suture closure | 42 (26.4%) | 14 (21.9%) | 15 (26.3%) | 13 (34.2%) | |
| Lig. teres patch | 80 (50.3%) | 34 (53.1%) | 27 (47.4%) | 19 (50.0%) | |
| Omentum patch | 23 (14.5%) | 12 (18.7%) | 6 (10.5%) | 5 (13.2%) | |
| Serosa patch | 8 (5.0%) | 4 (6.3%) | 3 (5.3%) | 1 (2.6%) | |
| Pancreatico-jejunostomy | 6 (3.8%) | 0 0.0 | 6 (10.5%) | 0 0.0 | |
| Median blood loss, ml (iqr) | 350 (200–600) | 350 (200–500) | 375 (250–600) | 275 (200–900) | 0.81545 |
| Median operative time, min (iqr) | 180 (140–246) | 180 (140–228) | 171 (140–218) | 222.5 (165–290) | 0.0115 |
| Median hospital stay, days (iqr) | 10.5 (8–15) | 9 (8–13) | 9.5 (8–12.5) | 21 (14–31) | <0.0001 |
| Morbidity (except POPF) | 49 (30.8%) | 18 (28.1%) | 17 (29.8%) | 14 (36.8%) | 0.6394 |
| DGE | 16 (10.1%) | 5 (7.8%) | 6 (10.5%) | 5 (13.2%) | 0.6680 |
| Lymphatic fistula | 10 (6.3%) | 3 (4.7%) | 6 (10.5%) | 1 (2.6%) | 0.2814 |
| Prolonged wound healing | 10 (6.3%) | 5 (7.8%) | 0 0.0 | 5 (13.2%) | 0.0136 |
| Other complications | 13 (8.1%) | 5 (7.8%) | 5 (8.8%) | 3 (7.8%) | 1.0 |
p-values in bold were considered statistically significant at p < 0.05.
Operative parameters
In this study, 151 of the operations were open DPs (95%); a laparoscopic approach was chosen in 8 patients. The majority of resections were carried out with an additional splenectomy (93%), and extended resections were performed in 24 patients (15.1%). These included distal or total gastrectomy, and resections of the small bowel, colon, adrenal gland, left kidney, celiac trunk or portal vein.
Pancreatic remnant closure
In 80 patients, the pancreatic remnant was covered by a lig. teres hepatis patch13 in 8 patients, jejunal or gastric serosa was used to cover the resection margin, and in 23 patients, omentum was used. In 42 patients, the transection was performed by stapling or suturing alone without any further covering, while 6 patients received an anastomosis with a pancreatico-jejunostomy.
A soft silicon drain was routinely placed at the pancreatic transection margin and another drain in the left subphrenic space. Drains were routinely removed on the third postoperative day if secretion was <200 ml/day and provided that there were no signs of POPF.
Operation time and blood loss
Median operation time was 180 min (iqr 140–246 min) and median blood loss was 350 ml (iqr 200–600 ml). Detailed data are given in Table 1.
Postoperative morbidity
The most frequent postoperative complication was POPF development, which occurred in 38 patients (24%). POPF grade A and B were seen in 21 (55%) and 11 (29%) patients, respectively; POPF grade C was reported in 6 patients (16%). Analysis of risk factors for POPF development revealed that male patients, extended resections and longer operation times were associated with a significantly higher POPF rate (Table 1). Complications other than POPF were observed in 49 patients (31%), including DGE (16 pat., 10%), wound infections (10 pat., 6%) and lymphatic fistula (10 pat., 6%). Overall, the re-operation rate was 2.5% (4/159 pat.); indications were bleeding (n = 3) or burst abdomen (n = 1).
Hospital stay
Median hospital stay was 10.5 days (iqr 8–15 days); patients suffering from POPF had a significantly longer stay than patients without this complication (p < 0.0001, Table 1).
Follow up
Mean interval between operation and first follow-up imaging was 5.5 months; the majority of patients presented 3 months postoperatively. On average, 2.7 follow-up scans (range 2–9) were available per patient. Mean follow-up time was 16.1 months (range 3–53 months).
Fluid collections – size, symptoms, therapy and course
FCs at the resection margin were observed in 68 of 159 patients (43%). Four of these patients had shown a preceding POPF. All FCs in POPF patients were <5 cm and did not cause any symptoms. After 12 months, three of them resolved completely. The fourth POPF-related FC was still visible in the patients' latest follow-up 19 months postoperatively, but had decreased from an initial transverse diameter of 49 mm–16 mm.
In contrast, 64 FCs were observed in the first follow-up scan without any history of POPF. Transverse FC diameter ranged from 1.4 to 11.3 cm. For further analysis, FC were classified into 3 groups: <5 cm (n = 34 pat.), 5–10 cm (n = 24 pat.) and >10 cm (n = 6 pat.). The size was significantly associated with the occurrence of symptoms, as shown in Table 2 (p < 0.0001). In the group of small FCs (<5 cm), 9% of the patients showed mild symptoms. In contrast, 54% (5–10 cm) and 67% (>10 cm) of patients with larger FCs suffered from mild to severe symptoms, including abdominal discomfort and pain, as well as elevated serum CRP levels (Table 2).
Table 2.
Symptoms and FC-directed therapy
| FC size < 5 cm | FC size 5–10 cm | FC size >10 cm | |
|---|---|---|---|
| Total (n = 64) | 34 | 24 | 6 |
| FC-associated symptomatic pat (n = 20) | 3 (8.8%) | 13 (54.2%) | 4 (67%) |
| Pain | 1 | 8 | 1 |
| Pressure | 2 | 5 | 3 |
| Elevated CRP | 0 | 4 | 1 |
| Therapy | n = 0 | CT-guided puncture (n = 2) CT-guided drainage (n = 1) Reoperation (n = 1) |
CT-guided drainage (n = 2) |
An FC-specific therapy was required in six symptomatic patients. Two patients underwent a percutaneous puncture three and eight months after the operation, respectively, leading to a relief of symptoms and complete FC resolution two months later. The third patient with a 56 mm FC got an CT-guided drainage for 12 days without further follow up Two patients received percutaneous CT-guided drainage of >10 cm FC due to pain or elevated serum CRP 3 months postoperatively. For one patient, complete resolution was observed after drain removal within 4 months. The other showed a slow decrease of initially 10 cm to a 2.9 cm residuum after 10 months. In the sixth patient, surgical re-exploration and cysto-jejunostomy was required 8 months postoperatively due to persisting abdominal discomfort and increasing FC diameter from 7.3 cm to 9.1 cm. In four of six patients who underwent a therapeutic intervention, FC fluid was examined and contained pancreatic juice (lipase 832–30,040 U/ml; amylase 807–10,519 U/ml).
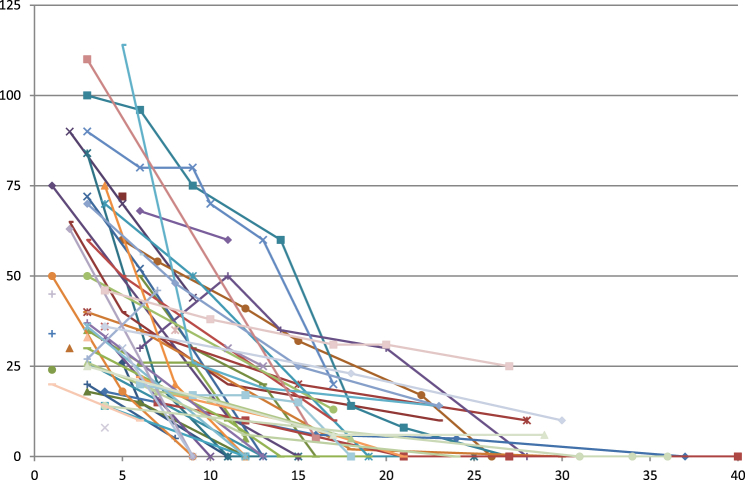
In all other patients, FC diameter decreased in the further follow-up without any therapy and was <1.0 cm within 12 months of operation in 49% (n = 31) of the patients (Fig. 2).
Figure 2.
Course of 58 FCs without therapy. Size in mm (left axis) at the different time points of follow-up (2–5 follow-up examinations)
Risk factors for POPF and FC
Multivariate risk factor analysis for FC development between patients with and without POPF showed no differences with regard to age, BMI and ASA classification. Male patients showed a higher POPF rate; however, this was not reflected in FC incidence. Histopathological diagnoses were not associated with significant differences in either POPF or FC incidence. Analysis of the operation methods revealed a significantly higher POPF rate after extended resections, whereas FC occurrence was observed significantly more often after standard resections without any difference in spleen-preserving procedures or DP with splenectomy. No correlation with stump closure techniques was found for POPF or FC incidence (Table 1).
Discussion
DP as a surgical option for benign and malignant lesions of the pancreatic body and tail is a standard procedure performed as an open or laparoscopic procedure.14, 15, 16 The most frequent complication of POPF is observed in app. 30% of all patients and has been extensively investigated in recent years with regard to different remnant closure methods.3, 4, 5, 6 In contrast, very little is known about the common finding of FCs in the further follow-up.17
This is the first series evaluating the incidence and clinical impact of FC following DP. Nearly half of the analysed patients developed FCs at the resection margin within the first weeks postoperatively. Furthermore, the vast majority of FCs shows little or mild symptoms and resolve without further therapy, even in larger lesions. A specific therapy is required only in exceptional cases.
With regard to the diagnoses leading to DP, the patient collective of this study shows a smaller proportion of pancreatic cancer than patients undergoing pancreatic head resection, as pancreatic cancer is mainly located in the head of the gland. This is consistent with other large studies.15, 18, 19 Neither a correlation of histopathological diagnosis nor other patient-related factors and FC development could be demonstrated.
The closure method of the pancreatic remnant – although examined in large clinical studies – remains the subject of ongoing debate, as it probably represents the most important factor for POPF development and possibly FC occurrence in the longer follow-up. Despite various technical options including suture and stapler application, the POPF incidence remains approximately 30%.3, 5, 18, 20, 21 In the present study, the POPF rate (24%) was comparable with previous publications and showed no dependency on closure methods, which included suture, stapler and additional patch (lig. teres hepatis, omentum, intestinal or gastric serosa) closure. Furthermore, closure methods had no influence on FC occurrence, which showed a higher overall incidence than POPF. This underlines that FCs – which is observed in nearly half of the patients – occur independently from clinically relevant POPF and that POPF disappears during the healing process without a residual FC in the long-term observation. The fact that high pancreatic enzyme levels were found in FCs once they had to be drained, could indicate a pathomechanism of subclinical leakage with much slower and prolonged secretion in FC than in clinically evident POPF. On the other hand, it could be speculated that two different types of FCs can occur: an uncomplicated type consisting mainly of haemato-seroma and resolving asymptomatically, and a complicated type, representing the above-mentioned pathomechanism of low-flow but prolonged pancreatic juice leakage, finally leading to FCs requiring an intervention, as observed in six patients in this study. As uncomplicated FCs were not investigated with regard to their fluid enzyme concentrations, a final conclusion on this question is not possible.
In contrast to the closure methods, only one risk factor for FC development was identified, namely standard DP, compared to extended resections, what has to be further elucidated. While the relation of POPF and FC in standard resections was 20%:46% in the study collective, it turned out to be 46%:13% in the extended resection group, suggesting that patients suffer from either one or the other complication, but not from both simultaneously. This observation could additionally underline the “subclinical leakage” theory for FC development: POPF as the more severe form of leakage is less frequent after standard DP but shows a higher incidence after extended surgery, while the less severe leakage leading to FC shows an inverse distribution, reflecting the extent of surgical trauma quite well.
With regard to treatment recommendations, a specific FC therapy is rarely necessary, even for large (>10 cm) findings. Nearly all FCs resolve within one year postoperatively. Only 9% of all patients in the present study received an FC-directed intervention due to symptoms, persisting high CRP levels or increasing FC size. Successful interventional puncture or drainage was the treatment of choice in five patients and operative revision was chosen in one patient. All approaches can be performed consecutively, which suggests that an individual decision needs to be taken in these situations.
In conclusion, FC after DP is observed more frequently than POPF, but – although a common radiological finding - its clinical relevance is limited. FCs are not associated with clinically relevant POPF or different closure methods of the pancreatic stump. However, due to high levels of pancreatic enzymes in some FCs, their development may be attributed to a subclinical and self-limiting leakage of pancreatic juice. FCs are mostly asymptomatic and harmless, without further complications, and show a strong tendency to resolve. Even in large FCs, a specific therapy is rarely required.
Funding
All authors declare no financial support.
Conflict of interest
None declared.
References
- 1.Goh B.K., Tan Y.M., Chung Y.F., Cheow P.C., Ong H.S., Chan W.H. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg. 2008;143:956–965. doi: 10.1001/archsurg.143.10.956. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J., Diener M.K., Z'graggen K., Hinz U., Wagner M., Bachmann J. Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg. 2007;245:573–582. doi: 10.1097/01.sla.0000251438.43135.fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hackert T., Büchler M.W. Remnant closure after distal pancreatectomy: current state and future perspectives. Surgeon. 2012;10:95–101. doi: 10.1016/j.surge.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Diener M.K., Seiler C.M., Rossion I., Kleeff J., Glanemann M., Butturini G. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet. 2011;377:1514–1522. doi: 10.1016/S0140-6736(11)60237-7. [DOI] [PubMed] [Google Scholar]
- 5.Klein F., Glanemann M., Faber W., Gül S., Neuhaus P., Bahra M. Pancreatoenteral anastomosis or direct closure of the pancreatic remnant after a distal pancreatectomy: a single-centre experience. HPB. 2012;14:798–804. doi: 10.1111/j.1477-2574.2012.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walters D.M., Stokes J.B., Adams R.B., Bauer T.W. Use of a falciform ligament pedicle flap to decrease pancreatic fistula after distal pancreatectomy. Pancreas. 2011;40:595–599. doi: 10.1097/MPA.0b013e3182153a4e. [DOI] [PubMed] [Google Scholar]
- 7.Bassi C., Dervenis C., Butturini G., Fingerhut A., Yeo C., Izbicki J. International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Hackert T., Werner J., Büchler M.W. Postoperative pancreatic fistula. Surgeon. 2011;9:211–217. doi: 10.1016/j.surge.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Hartwig W., Vollmer C.M., Fingerhut A., Yeo C.J., Neoptolemos J.P., Adham M. Forth International Study Group on Pancreatic Surgery: extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS) Surgery. 2014;156:1–14. doi: 10.1016/j.surg.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Wente M.N., Bassi C., Dervenis C., Fingerhut A., Gouma D.J., Izbicki J.R. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Staats B.A., Ellefson R.D., Budahn L.L., Dines D.E., Prakash U.B., Offord K. The lipoprotein profile of chylous and non-chylous pleural effusions. Mayo Clin Proc. 1980;55:700–704. [PubMed] [Google Scholar]
- 12.van der Gaag N.A., Verhaar A.C., Haverkort E.B., Busch O.R., van Gulik T.M., Gouma D.J. Chylous ascites after pancreaticoduodenectomy: introduction of a grading system. J Am Coll Surg. 2008;207:751–757. doi: 10.1016/j.jamcollsurg.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Hassenpflug M., Hartwig W., Strobel O., Hinz U., Hackert T., Fritz S. Decrease in clinically relevant pancreatic fistula by coverage of the pancreatic remnant after distal pancreatectomy. Surgery. 2012:164–171. doi: 10.1016/j.surg.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Lillemoe K.D., Kaushal S., Cameron J.L., Sohn T.A., Pitt H.A., Yeo C.J. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229:693–698. doi: 10.1097/00000658-199905000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song K.B., Kim S.C., Park J.B., Kim Y.H., Jung Y.S., Kim M.H. Single-centre experience of laparoscopic left pancreatic resection in 359 consecutive patients: changing the surgical paradigm of left pancreatic resection. Surg Endosc. 2011;25:3364–3372. doi: 10.1007/s00464-011-1727-9. [DOI] [PubMed] [Google Scholar]
- 16.Vijan S.S., Ahmed K.A., Harmsen W.S., Que F.G., Reid-Lombardo K.M., Nagorney D.M. Laparoscopic vs. open distal pancreatectomy: a single-institution comparative study. Arch Surg. 2010;145:616–621. doi: 10.1001/archsurg.2010.120. [DOI] [PubMed] [Google Scholar]
- 17.Sierzega M., Kulig P., Kolodziejczyk P., Kulig J. Natural history of intra-abdominal fluid collections following pancreatic surgery. J Gastrointest Surg. 2013;17(8):1406–1413. doi: 10.1007/s11605-013-2234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter T.I., Fong Z.V., Hyslop T., Lavu H., Tan W.P., Hardacre J. A dual-institution randomized controlled trial of remnant closure after distal pancreatectomy: does the addition of a falciform patch and fibrin glue improve outcomes? J Gastrointest Surg. 2013;17:102–109. doi: 10.1007/s11605-012-1963-x. [DOI] [PubMed] [Google Scholar]
- 19.Stauffer J.A., Rosales-Velderrain A., Goldberg R.F., Bowers S.P., Asbun H.J. Comparison of open with laparoscopic distal pancreatectomy: a single institution's transition over a 7-year period. HPB. 2013;15:149–155. doi: 10.1111/j.1477-2574.2012.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrone C.R., Warshaw A.L., Rattner D.W., Berger D., Zheng H., Rodriguez R. Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg. 2008;12:1691–1697. doi: 10.1007/s11605-008-0636-2. discussion 1697–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J.H., Li G., Baek N.H., Hwang J.C., Hong J., Yoo B.M. Surgical outcomes of distal pancreatectomy. Hepatogastroenterology. 2013;60:1263–1267. doi: 10.5754/hge13260. [DOI] [PubMed] [Google Scholar]