Abstract
Background
We assessed the effect of automated treatment adherence support delivered via mobile-phone short message system (SMS) text-messages on blood pressure.
Methods and Results
In this pragmatic single-blind, three-arm randomized trial (StAR), undertaken in South Africa, patients treated for high blood pressure were randomly allocated in a 1:1:1 ratio to information-only or interactive SMS text-messaging, or usual care. The primary outcome was change in systolic blood pressure at 12-months from baseline measured with a validated oscillometric device. All trial staff were masked to treatment allocation. Analyses were intention to treat. Between June 26, 2012 and November 23, 2012, 1372 participants were randomized to receive information-only SMS text-messages (n=457), interactive SMS text-messages (n=458), or usual care (n=457). Primary outcome data were available for 1256 (92%) participants. At 12-months, the mean adjusted change (95% CI) in systolic blood pressure compared to usual care was −2.2 mm Hg (−4.4 to −0.04) with information-only SMS and −1.6 mm Hg (−3.7 to 0.6) with interactive SMS. Odds ratios (95% CI) for the proportion of participants with a blood pressure <140/90mm Hg were for information-only messaging 1.42 (1.03 to 1.95) and for interactive messaging 1.41 (1.02 to 1.95) compared to usual care.
Conclusions
In this randomized trial of an automated adherence support program delivered by SMS text-message in a general outpatient population of adults with high blood pressure, we found a small, reduction in systolic blood pressure control compared to usual care at 12-months. There was no evidence that an interactive intervention increased this effect.
Clinical Trial Registration Information
ClinicalTrials.gov. Identifier: South African National Clinical Trials Register number (SANCTR DOH-27-1212-386); Pan Africa Trial Register (PACTR201411000724141).
Keywords: Randomized controlled trial, adherence, health care, self-management, behavior modification
Introduction
High blood pressure is a major risk factor for global disease burden.1 Even modest reductions in blood pressure are important and would reduce the risk of associated morbidity and premature mortality.2-4 In settings where health care and medicines are freely available a substantial burden of cardiovascular disease may be attributable to sub-optimal adherence to blood pressure lowering treatments.5 Missed appointments for collection of medicine and challenges with taking lifelong treatment are some of the major reasons for sub-optimal adherence.6 Adherence support delivered via short message system (SMS) text-messages has the potential to improve treatment adherence and health outcomes.7 Some, but not all interventions delivered by SMS have been effective at improving adherence and clinical outcomes, for example in HIV infected patients in low resource settings.8 However there is no evidence that SMS-based interventions are effective improving treatment adherence and clinical outcomes for high blood pressure in low resource settings.9 We carried out an effectiveness trial (SMS-Text Adherence suppoRt, or StAR) to establish whether or not adherence support delivered via SMS text-messages through information only or interactive SMS text messaging is better than usual care in maintaining and improving treatment adherence and blood pressure control.
Methods
Study design and participants
We carried out a parallel, three-group randomized controlled trial among the general adult population attending the outpatient chronic disease services in a single large public sector clinic in Cape Town, South Africa. This primary care facility provides a range of health care services to an ethnically diverse, socioeconomically deprived population from two communities: based on 2011 census data population ethnicity of these communities are mostly black African (51%) or mixed ancestry (48%), about one third of households live in informal dwellings or shacks, and 64% of households have a monthly income of less than R3500.00 (about $270.00). The clinic is within walking distance of both communities. All primary health care services and medicines are provided free of charge. In the chronic disease services, stable patients on treatment are reviewed by a clinician (doctor or prescribing nurse) every three- to six-months although patients initiating treatment for the first time or those with a blood pressure >140/90mmHg are reviewed more frequently.
At clinical review, patients are prescribed medication on repeat until their next scheduled review. The medications available for first-line prescription are enalapril, thiazide diuretic or amlodipine. If blood pressure remains uncontrolled then beta- or alpha-blockers, and occasionally spironolactone are added. Medicines are dispensed for 28-day periods and patients collect these from the on-site pharmacy. Patients on a stable regimen, with a valid repeat prescription receive their monthly supply of medicines pre-packaged from the Chronic Dispensing Unit (CDU), a centralized service through which the supplies are delivered to the clinic pharmacy 48 hours before a patients’ scheduled collection date. Medicines not collected are returned to the CDU 72 hours after the scheduled appointment. The trial protocol, including an outline of the statistical analysis plan, has previously been published.10
We enrolled adults (age ≥21 years) who had the following characteristics: diagnosed with hypertension by a clinician using local guidelines; prescribed blood pressure lowering medication; and with a systolic blood pressure (SBP) <220 mm Hg and a diastolic blood pressure (DBP) <120 mm Hg at enrolment. Eligible patients were attending the primary care clinic, resided in one of the two study communities, and had regular access to a mobile phone (and were able to send SMS text-messages, or could do so with help of a relative). We enrolled only one member per household. The following patients were excluded: those requiring specialist care for their hypertension at a hospital (in secondary care): women who self-reported being pregnant or within three months post-partum and those with very high blood pressures (systolic BP >220 mm Hg or diastolic BP >120 mm Hg) who had symptoms suggestive of a hypertensive emergency or were otherwise acutely unwell (who were directly referred to the appropriate clinical service).
Clinic staff, who were measuring the vital signs of all patients attending the chronic disease services for a clinical review, identified potential participants. Trained research assistants assessed trial eligibility for patients who had agreed to be referred to the trial, and those eligible were enrolled. Participants received an SMS text-message at the time of recruitment to confirm their enrolment in the trial. All participants were subsequently sent non-health related messages at six-weekly intervals.
The trial was approved by the Human Research Ethics Committee of the University of Cape Town (HREC UCT 418/2011), the Oxford Tropical Research Ethics Committee (OXTREC 03–12), and the Metro District Health Services, Western Cape (RP 141/2011). Trial conduct was overseen by a trial steering committee. All participants provided written informed consent and the trial was conducted in accordance with the Helsinki Declaration of 2008.
Randomization and masking
Trained research staff collected baseline data immediately before enrolment and an independent administrator entered these data in a secure, web-based randomization database implemented by the Primary Care Clinical Trials Unit in Oxford. A software algorithm assigned participants independent of the research team to information-only adherence support, interactive adherence support or usual care in a 1:1:1 ratio using a non-deterministic minimization algorithm to ensure balance between groups with respect to age, sex, baseline systolic BP, years with hypertension, and recent clinic attendance.11 Trial statisticians, researchers, clinic staff and research assistants who collected outcome data were masked to allocated interventions until the trial database was locked. Researchers and clinicians were not aware of randomization assignment, were trained not to ask patients about the content of messages, and were unable to determine randomization group from casual comments by participants. Blood pressure measurements were automated and data captured directly to the trial database.
Procedures
All participants received written information about hypertension and healthy living and continued to receive care from the clinic. Personalized SMS text-messages were sent to information-only message and interactive message group participants at weekly intervals, at a time and in a language selected by the participant. We iteratively designed, developed, and tested two SMS text-messaging based interventions with clinical staff, and patients with high blood pressure working and living in low-income communities around Cape Town.12 The messages were designed to address a range of common issues with adherence to and persistence with treatment.13 We developed a library of SMS-text messages, which we mapped to a taxonomy of behavior change techniques (Supplementary Table 1).14 Most of the messages focused on the techniques of goals and planning, repetition and substitution, social support, and natural consequences. The SMS text-messages used in the interventions were developed, translated, and tested in English, isiXhosa and Afrikaans, the three languages most commonly spoken by people living in Cape Town.
The information-only adherence support group were sent messages to motivate collecting and taking medicines, and to provide education about hypertension and its treatment. Additional reminders were sent when medicines were ready for collection or about scheduled clinic appointments. All trial participants were given a phone number to contact the research team.
Participants allocated to the interactive adherence support received the same messages as the information-only group but could also respond to selected messages using free-to-user “Please-Call-Me” requests. These generated an automated series of responses from the text-message delivery system offering trial participants a number of options including cancelling or changing an appointment, and changing the timing and language of the text-messages.
All trial materials, including information sheets, consent forms and the SMS text-messages were also developed, translated, and tested in English, isiXhosa and Afrikaans.
All SMS text-messages were delivered automatically via an open-source web-based electronic medical record system (OpenMRS version 1.6.1, OpenMRS Limited, Michigan). All participant data were captured in the clinic and uploaded to OpenMRS using Sana Mobile (Sana, MIT, Massachusetts), an open-source Android platform.10,15 Timing of messages relating to clinic appointments and medicine collection was facilitated by secure linkage to computerized appointment data.
Messages were sent for one year from enrolment. Blood pressure measurements were collected from participants as they attended their routine clinic visits, but otherwise no additional measurements or interventions were carried out during the trial. Delivery of SMS text-messages was automatically tracked and if undelivered, a research assistant, blinded to group allocation, would contact the number of a friend or relative to obtain a new mobile phone number. Supplementary Table 2 provides a structured description of the intervention and its development.16
The primary clinical outcome was the change in mean SBP measured at baseline and twelve months with a validated oscillometric device,10 adapted to record six sequential readings at three-minute intervals. The mean blood pressure was calculated by discarding the initial reading and calculating the mean from the five remaining readings.17
Treatment adherence was assessed by calculating the proportion of days of medication covered (PDC), a proxy-measure of adherence, from prescribing and dispensing data routinely recorded in the clinical record, pharmacy record and CDU record.18,19 Where there were discrepancies between data about dispensing we used a computer-based algorithm, blind to randomized group to assign the status (dispensed/not dispensed) favoring any evidence that the medication had been dispensed. PDC was summarized as the proportion of patients with ≥80% of days covered with blood pressure lowering medication based on a systematic review providing evidence for the clinical importance of this threshold,5 and reported in three-monthly intervals and over twelve months.
Additional secondary outcomes measured at 12 months, specified in the protocol,10 were proportion of participants achieving a mean SBP less than 140 mm Hg and a mean DBP less than 90 mm Hg, health status measured with the EuroQol Group 5-Dimension Self-Report Questionnaire (EQ-5D),20 proportion of scheduled clinic appointments attended, retention in clinical care, satisfaction with clinic services and care, hospital admissions, self-reported adherence to medication (score range 5-10),21 and basic hypertension knowledge.22 Data were also collected on the number and type of medication changes made during the trial and numbers of clinic visits by participants.
Statistical analysis
The intended target sample size of 1215 participants, allowing for 20% loss to follow-up, (at least 405 in each group) was estimated to detect an absolute mean difference in SBP of 5mm Hg (SD 22) (a clinically important reduction in the relative risk of stroke and coronary heart disease events)23 at 12 months from baseline, with 90% power and 0.05 (two-sided) level of significance. All analyses were performed on an intention to treat (ITT) basis.
We analyzed the twelve-month primary outcome using a mixed-effect model for repeated measures including data available on all randomized patients attending follow-up visits at 6 and 12 months. The method has the advantage of implicitly accounting for the data missing at random mechanism by using maximum likelihood. In the model, participant-specific intercepts were fitted using random effects, and time and treatment were modeled using fixed effects. An interaction term between time and randomized group was also included so that possible differences of treatment effect (informational versus usual care and interactive versus usual care) could be assessed at each time point. The model was adjusted for baseline SBP and minimization factors.
Blood pressure thresholds were analyzed using mixed-effect logistic regression models and treatment adherence was analyzed using multiple logistic regression. Secondary outcomes involving categorical data were analyzed using chi-squared tests and generalized linear models adjusting for minimization factors. Other continuous secondary outcomes were analyzed using analysis of covariance or non-parametric methods if the normality assumption was not satisfied. A statistical test of interaction was done to assess whether treatment effect was consistent across the pre-specified subgroups of SBP (<140, ≥140 mm Hg), age (<55, ≥55 years), sex (male, female), number of years with hypertension (<10, ≥10 years), presence of one or more co-morbid conditions and self-reported adherence at baseline (<80%, ≥80%).24
A detailed statistical analysis plan was completed before the trial database was locked (to prevent any further changes prior to analysis) and the trial allocation was disclosed.25 A statistical analysis report was prepared in line with CONSORT (Consolidated Standards of Reporting Trials) 2010 statements. No interim analysis was performed.
The content of the information only and the interactive adherence support interventions was examined in the preparatory work for the trial. (See Online Data Supplement (Expanded methods) for further details. The two interventions were considered to be distinct treatments and therefore not require adjustment for multiple comparisons.26,27,28 All statistical tests were two-sided and P values of 0.05 or less were considered to indicate statistical significance.26 Statistical analyses were performed using STATA version 13.1 (StataCorp LP, Texas, USA) and SAS version 9.3 (SAS Institute, Cary, NC, USA).
The trial was registered with the South African National Clinical Trials Register (SANCTR DOH-27-1212-386) before recruitment began, this registration was subsequently incorporated within the Pan African Trial Register (PACTR201411000724141), and we registered with ClinicalTrials.gov (NCT02019823) at the time of publishing the protocol to ensure details were widely available.
Results
Patients
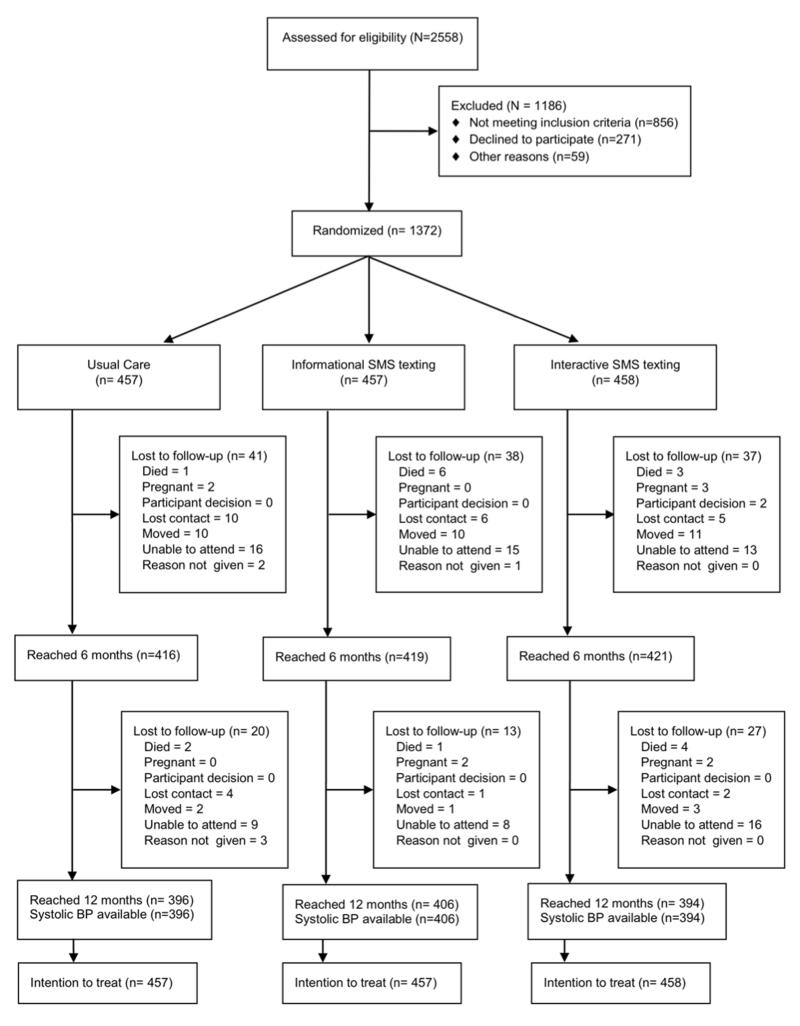
Between June 26, 2012, and November 23, 2012, we assessed 2558 patients for eligibility of whom 1372 were randomly allocated to information-only SMS text-messages (n=457), interactive SMS text-messages (n=458) and usual care (n=457). Figure 1 summarizes recruitment and follow up of participants. Attrition rates did not differ significantly between groups and those randomized were broadly similar to those screened but not randomized, although more were of black ethnicity (Supplementary Table 3).
Figure 1.
Trial profile.
Table 1 summarizes the baseline characteristics of participants. Mean (SD) age was 54.3 (11.5) years and 28% had at least ten years of hypertension. Over half of the patients had a body mass index of at least 30 kg/m2. Other baseline characteristics were similar across the three groups. Nearly all the participants owned their own phone (Supplementary Table 4). Overall, 176 (12.8%) of patients did not have a SBP measurement at 12 months. Missing SBP data at 12 months was associated with being female (P=0.03), having a BMI <30 kg/m2 (P=0.002), or having hypertension for less than ten years at enrolment (P=0.002). We sent 40,333 SMS text-messages to participants in the information-only message group, 41,450 to those in the interactive message group and 8277 to those receiving usual care. Of the messages sent, 5.5% had a “failed delivery” response. In addition, 3477 messages were not sent as planned because of technical errors. 230 (50.2%) of the participants allocated to the interactive adherence support group responded to a message at some point in the trial; in total 630 reply messages were sent by participants. There were 1231 visits by participants in the interactive group, 1109 for the information only group and 1093 for usual care. One additional blood pressure lowering medication was started by 44% of trial participants, and for this group the median (Q1, Q3) number of changes made was 2 (1,3). There were no differences between groups in the proportion of patients having medication changes (usual care - 43.6%; information only - 46.8%; and interactive - 44.0%).
Table 1.
Baseline characteristics.
| Usual Care (n=457) |
Information-only (n=457) |
Interactive (n=458) |
|
|---|---|---|---|
| *Age (years) | 54.7 (11.6) | 53.9 (11.2) | 54.2 (11.6) |
| * Sex (male) | 126 (28%) | 126 (28%) | 127 (28%) |
| Race or ethnic group | |||
| Black | 264 (58%) | 257 (56%) | 269 (59%) |
| Other † | 193 (42%) | 200 (44%) | 189 (41%) |
| *Duration of hypertension | |||
| < 10 years | 327 (72%) | 331 (72%) | 330 (72%) |
| ≥ 10 years | 130 (28%) | 126 (28%) | 128 (28%) |
| Current smoker | |||
| Non-smoker | 332 (73%) | 335 (73%) | 341 (75%) |
| Current smoker | 99 (22%) | 94 (21%) | 88 (19%) |
| Ex-smoker | 26 (5%) | 27 (6%) | 29 (6%) |
| Missing | 0 | 1 (0.2%) | 0 |
| Weight (Kg) | 84.0 (18.9) | 83.0 (18.5) | 82.8 (19.8) |
| Body mass index Kg/m2 | 33.2 (7.7) | 32.6 (7.6) | 32.5 (7.5) |
| Blood Pressure (mm Hg) | |||
| *Systolic | 135.4 (17.6) | 135.1 (16.9) | 135.6 (18.1) |
| Diastolic | 83.6 (12.4) | 83.1 (11.9) | 83.6 (12.0) |
| ‡Irregular clinic attendance | 288 (63%) | 288 (63%) | 290 (63%) |
| Highest level of education at school | |||
| No schooling | 10 (2.2) | 8 (2.2) | 4 (0.9) |
| Some primary school | 132 (28.9) | 155 (28.9) | 154 (33.6) |
| Some high school | 261 (57.1) | 229 (57.1) | 249 (54.4) |
| Completed high school | 54 (11.8) | 64 (11.8) | 51 (11.1) |
| Not reported | 0 (0) | 1 (0.2) | 0 (0) |
| Type of house | |||
| Brick/Cinder block | 378 (82.7) | 377 (82.5) | 382 (83.4) |
| Wood/Corrugated Iron | 73 (16.0) | 75 (16.4) | 73 (15.9) |
| Cardboard/Plastic | 1 (0.2) | 0 (0) | 0 (0) |
| Other | 5 (1.1) | 4 (0.9) | 3 (0.7) |
| Not reported | 0 (0) | 1 (0.2) | 0 (0) |
Data are numbers (%), mean (SD), or median (25th and 75th centiles).
Minimization variables
Race was self-reported. Other category includes (White, Colored, Indian, Other, Prefer not to say)
Appointment attendance was categorized as: (Regular: attended the last four clinic appointments, Irregular: missed at least one appointment, Unknown: no previous data on clinic appointments was found)
Primary outcome
Primary outcome data were available for 1256 (92%) participants. Table 2 summarizes change in mean SBP, which decreased from baseline to 12 months for all groups. The mean (95% CI, P value) adjusted difference in change for the information-only message group compared to usual care was −2.2 mm Hg (−4.4 to −0.04, P=0.046) and for the interactive message group compared to usual care −1.6 mm Hg (−3.7 to 0.6, P=0.16). A post-hoc sensitivity analysis was carried out excluding the 6-month measure of SBP and including BMI as a covariate. Results were consistent with the pre-specified primary ITT analysis (Supplementary Table 5).
Table 2.
Mean systolic blood pressure outcomes (including primary outcome).
| Usual Care (UC) | Information-only (IO) | Interactive (IN) | Difference in mean change (95% CI)* IO vs. UC |
P-Value* | Difference in mean change (95% CI)* IN vs. UC |
P-Value* | ||
|---|---|---|---|---|---|---|---|---|
| Baseline mean systolic blood pressure | (mm Hg) | 135.4 (17.6) | 135.1 (16.9) | 135.6 (18.1) | - | - | ||
| 6-month mean systolic blood pressure | (mm Hg) | 128.9 (17.1) | 130.1 (16.6) | 128.2 (17.5) | 0.3 (−2.5 to 3.1) | 0.82 | −0.9 (−3.7 to 1.9)* | 0.53 |
| †12-month mean systolic blood pressure | (mm Hg) | 134.3 (17.3) | 132.1 (16.6) | 132.7 (17.5) | −2.2 (−4.4 to −0.04) | 0.046 | −1.6 (−3.7 to 0.62)* | 0.16 |
Data are means of mean systolic blood pressure (SD)
Adjusted for baseline systolic blood pressure, age, gender, number of years with hypertension, appointment attendance
Numbers of participants providing data at each time-point: baseline UC n=457, IO n=457, IN n=458; six months UC n=213, IO=245, IN n=241; one year UC n=396, IO n=406, IN n=394
Primary outcome
Secondary outcomes
The adjusted odds ratios (95% CI; P-value) for participants achieving a controlled blood pressure defined as a BP <140/90 mm Hg at 12 months were, for information-only messages and interactive messages respectively compared to usual care 1.4 (1.0 to 1.9; P=0.04) and 1.4 (1.0 to 1.9; P=0.04) (Table 3).
Table 3.
Secondary outcomes at one year of follow up.
| Usual Care (n=385) |
Information-only (n=395) |
Interactive (n=377) |
IO vs. UC* (95% CI) |
P-Value* | IN vs. UC* (95% CI) |
P-Value* | ||
|---|---|---|---|---|---|---|---|---|
| Proportion blood pressures <140/90 mm Hg† | N (%) | 396 (58%) | 406 (65%) | 394 (65%) | 1.42 (1.03 to 1.95)‡ | 0.033 | 1.41 (1.02 to 1.95) | 0.038 |
| PDC by dispensed medicine (%)§ | median (Q1–Q3) | 79.2 (64.6–91.4) | 83.3 (69.3–91.7) | 83.3 (66.7 –91.7) | 5.2 (1.5 to 8.9) | 0.006 | 3.8 (0.03 to 7.6) | 0.048 |
| PDC ≥80%§ | N (%) | 190 (49%) | 248 (63%) | 225 (60%) | 1.86 (1.39 to 2.49) | <0.001 | 1.60 (1.20 to 2.16) | 0.002 |
| Euro-Qol 5-Dimension Index§ | median (Q1–Q3) | 0.82 (0.73–0.90) | 0.83 (0.74–0.90) | 0.81 (0.73 –0.90) | 0.01 (−0.01 to 0.02) | 0.50 | 0.003 (−0.02 to 0.02) | 0.73 |
| Retention in clinical care† | N (%) | 383 (84%) | 379 (83%) | 378 (83%) | 0.93 (0.66 to 1.32) ‡‡ | 0.69 | 0.90 (0.64 to 1.28) ‡‡ | 0.567 |
| Satisfaction with clinic services ** | median (Q1–Q3) | 28 (25–31) | 28 (25–31) | 27 (25–31) | −0.2 (−1.4 to 0.9)§§ | 0.74 | −0.1 (−1.3 to 1.0) §§ | 0.80 |
| Satisfaction with treatment ** | median (Q1–Q3) | 20 (17–20) | 20 (18–20) | 20 (17–20) | 0 (−0.3 to 0.3) §§ | >0.99 | 0 (−0.3, 0.3) §§ | >0.99 |
| Self-reported adherence ** | median (Q1–Q3) | 10 (9–10) | 10 (9 –10) | 10 (9 –10) | 0.04 (−0.1 to 0.2) §§ | 0.70 | 0.02 (−0.2 to 0.2) §§ | 0.80 |
| Hospital admissions ** | N (%) | 34 (7%) | 26 (6%) | 64 (14%) | 0.73 (0.43 to 1.24) †† | 0.24†† | 0.94 (0.57 to 1.55) †† | 0.81†† |
| Medication changes § | N (%) | 168 (44%) | 185 (47%) | 166 (44%) | 1.18 (0.88 to 1.57) ‡‡ | 0.26 | 1.04 (0.78 to 1.40) ‡‡ | 0.78 |
Data are N (%) or median (Interquartile range) unless otherwise marked. PDC - Proportion of days covered, IO – Information-only, IN – Interactive, UC – Usual Care
Adjusted comparisons for Information-only (IO) versus usual care (UC) and Interactive (IN) versus UC unless specified
For blood pressure proportion in control and retention in clinical care data, UC N=457, IO N=457, IN =458
Blood pressure comparisons are adjusted odds ratios
For dispensing data, UC N=385, IO N=395, IN =377
For satisfaction, self-reported adherence and hospital admissions UC N=395, IO N=405, IN N=390
Without any adjustment of baseline covariates as number in cells were small
Confidence intervals derived using (‡‡) multiple logistic regression or (§§) quantile regression
Adherence data were available for 1157 (86%) participants. The overall number (%) of participants who had at least 80% of PDC for blood pressure lowering medication for the 12-month period was 248 (62.8%) for the information-only message group, 225 (59.7%) for the interactive message group and 190 (49.4%) for usual care, (informative messages versus usual care P<0.001, and interactive messages versus usual care P=0.002) (Table 3). The adjusted odds ratio (95% confidence intervals) for improved availability of dispensed medicine was 1.86 (1.39 to 2.49, P<0.0001) for information-only messaging compared to usual care, and 1.60 (1.20 to 2.16, P=0.002) for interactive messaging compared to usual care.
EQ-5D scores, attendance at clinic appointments, retention in clinical care, treatment and clinic satisfaction, hypertension knowledge, self-reported adherence, hospital admissions and differences in medication changes did not differ between groups (Table 3).
Seventeen patients died (usual care=3, information-only messages=7, and interactive messages=7) during the 12-month follow-up, but we recorded no other serious events (Supplementary Table 6).
Sub-group analysis of primary data
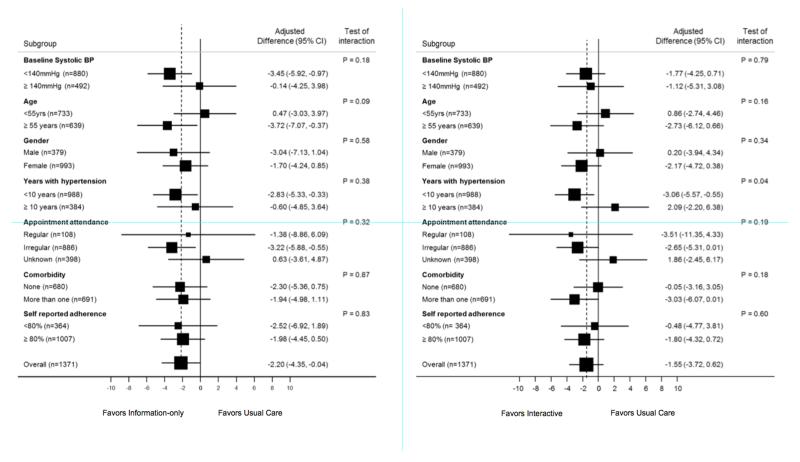
Figure 2 shows the results of pre-specified subgroup analysis of the primary outcome (SBP). There was no statistically significant heterogeneity in the treatment effects and there was an indication that active interventions were more effective among older patients (≥55 years), patients in better control at baseline (<140mmHg) and among those with a shorter duration of hypertension (<10 years).
Figure 2.
Sub-group analysis of pre-specified subgroups of the primary outcome (systolic blood pressure) for information-only versus usual care (2a) and interactive versus usual care (2b).
Discussion
Summary of trial findings
This trial provides evidence that support delivered via SMS text-messages could improve collection of medicines and may have a small impact on blood pressure as compared to usual care in a general outpatient population of adults with high blood pressure. There was no evidence of differences in intervention effectiveness between men and women; younger and older patients, and patients with and without co-morbid conditions.
Strengths and limitations
This randomized trial of a behavioral intervention delivered via SMS text-message was carried out in a low-resource setting and designed to test for the impact of the intervention on pre-specified measurable intermediate variables that are linked to important clinical outcomes.29,30,31
We prepared a detailed statistical analysis plan and specified, in advance, adjustment by covariates to ensure transparent analysis procedures.25 We have provided detailed descriptions of the intervention and its delivery, in-line with recent reporting guidelines16 that will enable comparison with other SMS text-messaging based systems and support development of new interventions.
The system for delivering the text-messages was innovative; combining low-cost technology, open-source software and interoperability with external data sources, including the Department of Health of the Western Cape appointment data.
The trial had a number of limitations inherent in its design. The provision of free medication and recommendations for regular follow up at the clinic may have reduced some supply-side barriers to non-adherence, although up to half the participants were not collecting regular treatment. The trial was not powered to identify the observed difference in systolic blood pressure observed between groups although our sample size calculation was based on the effect sizes found in published trials of other behavioral interventions to support adherence to treatments for high blood pressure.32 In addition, the measure of adherence used only reflects dispensing in the clinic, and not the act of taking medication.
Targeting a group of people diagnosed with hypertension rather than those with a diagnosis of poorly controlled pressure may have also limited the extent to which improvements in blood pressure were possible. However, we could not reliably predict those with poor control due to poor adherence, nor identify when non-adherence might take place in a previously adherent patient. Our decision not to target non-adherent patients was informed by formative work in which we identified the possibility that non-adherence could start at any time, and also patient feedback that SMS messages targeted at people who were non-adherent might reduce their acceptability.
Comparisons with other literature
This is the first study to report a small impact on objectively measured blood pressure using support via text-messaging without additional blood pressure monitoring,33 and without health care providers contacting non-adherent patients.30 We found no evidence that an interactive intervention delivered with the same frequency as an information-only intervention had a greater effect on adherence or blood pressure, in contrast to findings from other mobile phone-based interventions.34,35 This might be explained by the older average age of participants in this trial compared with other mobile phone based studies and could indicate either an age-based or experience based difference in how people make use of such technology.
Both trial interventions in StAR were delivered with similar fidelity to the trial protocol. Slightly more SMS texts-messages were sent in the interactive intervention group compared to the information-only group because of a small number of additional follow-up messages. Fifty percent of participants allocated to the interactive intervention responded, while in comparison 38.5% of participants in a smoking cessation trial sent un-prompted SMS-texts requesting support.36 In a trial of weekly interactive SMS-text messages to support treatment adherence among HIV-infected people initiating anti-retroviral treatment, 68% of participants responded, with non-responders receiving a phone call from a health care worker.8
The effect size observed on systolic blood pressure was smaller than anticipated, particularly since the impact on treatment adherence was similar to others reported.9,35 However, those taking additional medication and achieving a lower blood pressure through better adherence may have only been a small proportion of participants. A smaller standard deviation for systolic blood pressure, higher follow up of participants than anticipated and use of a mixed model for analysis may have all contributed to detecting a difference smaller than originally anticipated.
Results from a recent systematic review of interventions, delivered via SMS text-message, to support adherence to anti-retroviral therapy suggest that the dose-response relationship for SMS text-message frequency, if it exists, may not be linear, although counter-intuitively, daily SMS text-messages were less effective than weekly messages.35 More research is needed to identify the optimal frequency for SMS text-messages to support treatment adherence.
The decrease in blood pressure observed in all participants at six months, may be due to seasonal variation in blood pressure.37 However, this finding should be interpreted with caution as six-month data were intended to supplement the multi-level modeling of the primary outcome where available, and was only collected on a small proportion of participants.
Clinical interpretation and research implications
High blood pressure is the leading risk factor for global disease burden and even modest reductions in blood pressure are important and would reduce the risk of associated morbidity and premature mortality.1,2 Although availability of medicines and access to care remain important structural barriers to adherence in settings where health care and medicines are freely available (including many low and middle-income countries like South Africa) a substantial burden of cardiovascular disease may be attributable to sub-optimal adherence to blood pressure lowering treatments.
The difference in blood pressure observed in this trial was similar to that of intensive face-to-face behavioral counseling in which a 1 mm Hg difference between intervention and control was observed,38 and the difference in adherence was similar to other adherence support interventions delivered via SMS-text.39,40 Pooled results from a systematic review of the literature suggest that the relative risk (95% CI) of development of cardiovascular disease for those with good versus poor (<80%) adherence to blood pressure lowering medication is 0.81 (0.76 to 0.86) and for all-cause mortality is 0.71 (0.64 to 0.78) with an estimated absolute risk difference for any cardiovascular disease associated with poor medication adherence of 13 cases per 100,000 individuals per year.5 Though not reported here we think that the costs of delivering messages via an automated SMS text-messages at scale are likely to be low in comparison to community based face-to-face interventions or training programs for clinicians.8,41,42
Conclusion
This trial has demonstrated that a behavioral intervention to support adherence to blood pressure treatment delivered via SMS text-message can improve adherence and may modestly decrease blood pressure at 12 months. The delivery of pre-defined messages can be achieved by an automated system consistently and without the need for additional training programs for clinical staff. The optimal frequency of the different categories of text-messages; the incremental costs of modifying messages so that they remain effective; and the wider implementation of these messages in different communities, for different long-term conditions, and for patients with multiple conditions needs further study.
Supplementary Material
Clinical Perspectives.
There is some evidence that clinical outcomes for treatment of long-term conditions can be improved through interventions targeting adherence behaviour. This has been achieved in a small number of clinical trials for people with high blood pressure, but successful strategies can be costly, for example through case management and pharmacy-based education. These approaches may not be practical in a low-resource setting. Some studies report that mobile phone messaging interventions may offer benefit through supporting self-management and also have potential to support lifestyle change, for example in supporting smoking cessation. However randomised trials of the effectiveness of mobile phone messaging in the management of people with high blood pressure are few, often focussed on high risk groups such as stroke survivors and renal transplant recipients, with only one small trial in the general population. This randomised clinical trial is the first to show there may be a modest benefit from improved adherence to medication regimens and reduction in blood pressure compared to usual care from a targeted mobile phone messaging intervention used to support people treated for high blood pressure. In addition, this intervention was delivered in a setting where providing health care is challenging. The extent of blood pressure lowering is similar to that observed in other community studies aimed at improving blood pressure management. Delivering an automated behavioural intervention to people treated for high blood pressure via mobile phones is acceptable to recipients can be carried out at a wide scale.
Acknowledgements
We are grateful to the patients; health-care workers; pharmacist; clinic administrative staff and field staff under Sister Carmen Delport for taking part in this trial. We are grateful to the Department of Health of the Western Cape for their support of the trial and access to routinely collected administrative data.
Funding Sources: This trial is supported by the Oxford Centre of Excellence in Medical Engineering funded by the Wellcome Trust and the Engineering and Physical Sciences Research Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
There are no competing interests held by any of the authors.
Disclosures: None.
References
- 1.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 2.Lawes CMM, Vander Hoorn S, Rodgers A, for the International Society of Hypertension Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, for the Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S. Unresolved Issues in the Management of Hypertension. BMJ. 2010;55:832–834. doi: 10.1161/HYPERTENSIONAHA.109.142349. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, Stricker B, Mendis S, Hofman A, Mant J, Franco OH. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–8. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 6.Gwadry-Sridhar FH, Manias E, Lal L, Salas M, Hughes DA, Ratzki-Leewing A, Grubisic M. Impact of Interventions on Medication Adherence and Blood Pressure Control in Patients with Essential Hypertension. Value in Health. 2013;16:863–871. doi: 10.1016/j.jval.2013.03.1631. [DOI] [PubMed] [Google Scholar]
- 7.Beratarrechea A, Lee AG, Willner JM, Jahangir E, Ciapponi A, Rubinstein A. The Impact of Mobile Health Interventions on Chronic Disease Outcomes in Developing Countries: A Systematic Review. Telemed J E Health. 2014;20:75–82. doi: 10.1089/tmj.2012.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, Jack W, Habyarimana J, Sadatsafavi M, Najafzadeh MN, Marra CA, Estambale B, Ngugi E, Blake Ball T, Thabane L, Gelmon LJ, Kimani J, Ackers M, Plummer FA. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 9.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Patel V, Haines A. The Effectiveness of Mobile-Health Technology-Based Health Behaviour Change or Disease Management Interventions for Health Care Consumers: A Systematic Review. PLoS Med. 2013;10:e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bobrow K, Brennan T, Springer D, Levitt NS, Rayner B, Namane M, Yu L-M, Tarassenko L, Farmer A. Efficacy of a text messaging (SMS) based intervention for adults with hypertension: protocol for the StAR (SMS Text-message Adherence suppoRt trial) randomised controlled trial. BMC Public Health. 2014;14:28. doi: 10.1186/1471-2458-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altman DG, Bland JM. Treatment allocation by minimisation. BMJ. 2005;330:843. doi: 10.1136/bmj.330.7495.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig P, Dieppe P, MacIntyre S, S M, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655–a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vries H, Mudde A, Leijs I, Charlton A, Vartiainen E, Buijs G, Clemente MP, Storm H, González Navarro A, Nebot M, Prins T, Kremers S. The European Smoking Prevention Framework Approach (EFSA): an example of integral prevention. Health Educ Res. 2003;18:611–626. doi: 10.1093/her/cyg031. [DOI] [PubMed] [Google Scholar]
- 14.Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008;27:379–387. doi: 10.1037/0278-6133.27.3.379. [DOI] [PubMed] [Google Scholar]
- 15.Springer DB, Bobrow KL, Levitt N, Farmer A, Tarassenko L. The SMS-text adherence support (StAR) study: hardware and software infrastructure. International Conference on Information and Communication Technologies and Development. 2013;6:147–150. [Google Scholar]
- 16.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan A-W, Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 17.Schulze MB, Kroke A, Boeing H. Differences of blood pressure estimates between consecutive measurements on one occasion: implications for inter-study comparability of epidemiologic studies. Eur J Epidemiol. 2000;16:891–898. doi: 10.1023/a:1011020823807. [DOI] [PubMed] [Google Scholar]
- 18.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: Methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 19.Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, Lewek P, Matyjaszczyk M, Mshelia C, Clyne W, Aronson JK, Urquhart J, The ABC Project Team A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmac. 2012;73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EuroQol Group EuroQol: a new facility for the measurement of health related quality of life. Health Pol. 2001;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 21.Morisky DE, Green LW, Levine DM. Concurrent and Predictive Validity of a Self-reported Measure of Medication Adherence. Med Care. 1986;24:67. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Scheepers E, Christofides N. Soul City 4 Impact Evaluation Hypertension. Johannesburg; Soul City: 2001. [Google Scholar]
- 23.Collins R, Peto R, MacMahon S, Hebert P, Fiebach N, Eberlein K, Godwin J, Qizilbash N, Taylor J, Hennekens C. Blood pressure, stroke, and coronary heart disease. Part 2. The Lancet. 1990;335:827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 24.Matthews JN, Altman DG. Interaction 3: How to examine heterogeneity. BMJ. 1996;313:862. doi: 10.1136/bmj.313.7061.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ICH harmonised tripartite guideline: statistical principles for clinical trials. Stat Med. 1999;18:1905–1942. [PubMed] [Google Scholar]
- 26.Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. The Lancet. 2005;365:1591–1595. doi: 10.1016/S0140-6736(05)66461-6. [DOI] [PubMed] [Google Scholar]
- 27.Cook RJ, Farewell VT. Multiplicity considerations in the design and analysis of clinical trials. J R Statist Soc. 1996;159:93–110. [Google Scholar]
- 28.Wason JMS, Stecher L, Mander AP. Correcting for multiple-testing in multi-arm trials: is it necessary and is it done? Trials. 2014;15:364–7. doi: 10.1186/1745-6215-15-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Contreras EEM, la Figuera von Wichmann de MM, Guillén VVG, Ylla-Catalá AA, Figueras MM, Balaña MM, Naval JJ. Effectiveness of an intervention to provide information to patients with hypertension as short text messages and reminders sent to their mobile phone (HTA-Alert) Aten Primaria. 2004;34:399–405. doi: 10.1016/S0212-6567(04)78922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wald DS, Bestwick JP, Raiman L, Brendell R, Wald NJ. Randomised Trial of Text Messaging on Adherence to Cardiovascular Preventive Treatment (INTERACT Trial) PLoS ONE. 2014;9:e114268. doi: 10.1371/journal.pone.0114268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logan AG, Irvine MJ, McIsaac WJ, Tisler A, Rossos PG, Easty A, Feig DS, Cafazzo JA. Effect of home blood pressure telemonitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension. 2012;60:51–57. doi: 10.1161/HYPERTENSIONAHA.111.188409. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder K, Fahey T, Ebrahim S. How Can We Improve Adherence to Blood Pressure– Lowering Medication in Ambulatory Care?: Systematic Review of Randomized Controlled Trials. Arch Intern Med. 2004;164:722–732. doi: 10.1001/archinte.164.7.722. [DOI] [PubMed] [Google Scholar]
- 33.Carrasco MP, Salvador CH, Sagredo PG, Marquez-Montes J, de Mingo MAG, Fragua JA, Rodriguez MC, Garcia-Olmos LM, Garcia-Lopez F, Carrero AM, Monteagudo JL. Impact of Patient-General Practitioner Short-Messages-Based Interaction on the Control of Hypertension in a Follow-up Service for Low-to-Medium Risk Hypertensive Patients: A Randomized Controlled Trial. IEEE Trans Inform Technol Biomed. 2008;12:780–791. doi: 10.1109/TITB.2008.926429. [DOI] [PubMed] [Google Scholar]
- 34.Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, Rodgers A, Cairns J, Kenward MG, Roberts I. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet. 2011;378:49–55. doi: 10.1016/S0140-6736(11)60701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finitsis DJ, Pellowski JA, Johnson BT. Text Message Intervention Designs to Promote Adherence to Antiretroviral Therapy (ART): A Meta-Analysis of Randomized Controlled Trials. PLoS ONE. 2014;9:e88166–10. doi: 10.1371/journal.pone.0088166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devries KM, Kenward MG, Free CJ. Preventing Smoking Relapse Using Text Messages: Analysis of Data From the txt2stop Trial. Nicotine Tob Res. 2012;15:77–82. doi: 10.1093/ntr/nts086. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Li LM, Lewington S, Guo Y, Sherliker P, Bian Z, Collins R, Peto R, Liu Y, Yang R, Zhang Y, Li G, Liu S, Chen Z, China Kadoorie Biobank Study Collaboration Outdoor temperature, blood pressure and cardiovascular disease mortality among 23,000 individuals with diagnosed cardiovascular diseases from China. Eur Heart J. 2015;36:1178–85. doi: 10.1093/eurheartj/ehv023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pladevall M, Brotons C, Gabriel R, Arnau A, Suarez C, la Figuera de M, Marquez E, Coca A, Sobrino J, Divine G, Heisler M, Williams LK, Writing Committee on behalf of the COM99 Study Group Multicenter cluster-randomized trial of a multifactorial intervention to improve antihypertensive medication adherence and blood pressure control among patients at high cardiovascular risk (the COM99 study) Circulation. 2010;122:1183–1191. doi: 10.1161/CIRCULATIONAHA.109.892778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedberg JP, Rodriguez MA, Watsula ME, Lin I. Effectiveness of a Tailored Behavioral Intervention to Improve Hypertension Control Primary Outcomes of a Randomized Controlled Trial. Hypertension. 2015;65:440–6. doi: 10.1161/HYPERTENSIONAHA.114.03483. [DOI] [PubMed] [Google Scholar]
- 40.Baena CP, Olandoski M, Younge JO, Buitrago-Lopez A, Darweesh SKL, Campos N, Sedaghat S, Sajjad A, van Herpt TTW, Freak-Poli R, van den Hooven E, Felix JF, Faria-Neto JR, Chowdhury R, Franco OH. Effects of lifestyle-related interventions on blood pressure in low and middle-income countries: systematic review and meta-analysis. J Hypertens. 2014;32:961–973. doi: 10.1097/HJH.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 41.Zurovac D, Larson BA, Sudoi RK, Snow RW. Costs and Cost-Effectiveness of a Mobile Phone Text-Message Reminder Programmes to Improve Health Workers' Adherence to Malaria Guidelines in Kenya. PLoS ONE. 2012;7:e52045. doi: 10.1371/journal.pone.0052045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerriero C, Cairns J, Roberts I, Rodgers A, Whittaker R, Free C. The cost-effectiveness of smoking cessation support delivered by mobile phone text messaging: Txt2stop. Eur J Health Econ. 2013;14:789–797. doi: 10.1007/s10198-012-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.