Abstract
Introduction:
Amyotrophic lateral sclerosis (ALS) is a fatal and most common motor neuron disease, caused by progressive loss of motor neurons. Diffusion tensor imaging (DTI) and magnetic resonance spectroscopic (MRS) studies detect pathological changes in neuronal fibers in vivo. We evaluated the role of DTI and MRS in early course of the disease, which may prove beneficial in the early diagnosis and better management.
Materials and Methods:
Twenty-one patients with ALS and 13 age-matched controls received 1.5T DTI and three-dimensional multi-voxel MRS. Fractional anisotropy (FA), apparent diffusion coefficient, N-acetyl aspartate (NAA)/Creatine (Cr), and NAA/Choline (Ch) ratios were analyzed in various regions of the brain and compared with healthy controls. ALS patients were classified as definite, possible, and probable category, and patients were also studied in limb versus bulbar onset.
Results:
Decreased FA and increase mean diffusivity values in regions of corticospinal tract (CST) and corpus callosum (CC) was consistent finding in definite and probable disease category (P < 0.05). In possible disease, CC involvement was not significant. NAA/Cr and NAA/Ch ratios were lower in CC and regions of CST. However, in possible disease, CC involvement was not significant, while regions of CST were showing significant reduction in NAA/Cr and NAA/Ch ratios (P < 0.05).
Conclusion:
DTI and MRS detect changes associated with ALS even in the early phase of the disease. Bulbar onset and limb onset ALS patients show different pattern of involvement. Extramotor involvement suggested by CC involvement is a feature seen in bulbar onset patient and can suggest poor outcome in such patients. The present findings may be helpful for designing further studies in the direction of more early diagnosis of disease and its management.
Keywords: Amyotrophic lateral sclerosis, bulbar onset, corpus callosum, diffusion tensor imaging, magnetic resonance spectroscopy, magnetic resonance imaging
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal form of the neurodegenerative disorder.[1] ALS is markedly heterogenous in its onset, clinical appearance, and progression. In past, many studies have tried to evaluate changes occurring in brain with the help of magnetic resonance imaging (MRI).[2,3,4] Conventional MR sequences have not shown significant diagnostic accuracy.[4,5] Many pathological studies have shown changes occurring in brain.[6] These changes are better studied by use of advanced MR techniques such as diffusion tensor imaging (DTI) and magnetic resonance spectroscopy (MRS).[7]
DTI quantifies diffusion characteristics and directional behavior of water in white matter hence providing information about its microstructure. Most of the studies till date have shown reduced fractional anisotropy (FA) values in various parts of corticospinal tract (CST).[8,9]
White matter tract degeneration is associated with alteration in metabolic processes and hence metabolites, which might occur silently before evidence on conventional MR sequences. 1H-MRS helps in detecting changes in these metabolites. Most of the studies have shown changes in these metabolites in ALS patients with the help of single voxel or two-dimensional multi-voxel MRS.[10,11]
The objective of this methodological study was to determine changes occurring in the brain in patients with ALS.
Materials and Methods
Subject selection
We studied 21 consecutive patients with ALS who were recruited from Department of Neurology, Indira Gandhi Medical College, Shimla, Himachal Pradesh Patients with ALS (n = 21; women = 7; men = 14; mean age = 51.28 ± 15.38 were classified according to the revised El-Escorial criteria as definite (7 patients), probable (7 patients), and possible (7 patients) ALS. Patients were also classified according to bulbar and limb onset. None of the patients had cognitive symptoms or extrapyramidal associated disorders. Subjects with progressive spinal muscular atrophy and progressive bulbar palsy were excluded. Differential diagnosis with other pathological conditions was conducted with appropriate blood tests, electrophysiological exams, and neuroimaging. Thirteen healthy volunteers formed control group (women = 5, men = 8; mean age = 42.25 ± 15.6). Control groups were chosen from patients who have come for MRI for some other indications not related to the disease being studied. These controls were chosen such that they represent each age group and can represent appropriate sex. Standard MRI prescreening procedures were used to exclude subjects with MRI contraindications, previous brain injury or surgery, or a history of neurological disease, psychiatric illness or substance abuse diabetes, hypertension or any other co-morbidity, which can add confounding factor to the study. The institutional review board of Indira Gandhi Medical College, Shimla, approved the study protocol and all subjects provided signed written consent before participation.
Neurological exam
Experienced neurologist performed a neurological exam. History and risk factor evaluation were done. A complete examination of cranial nerves, sensory and motor system, superficial, and deep tendon reflexes was done. Patients were classified using El-Escorial criteria. Revised ALSFRS score was calculated for each patient. Other contributing factors if present were evaluated and necessary tests were advised and performed. Electromyographic test was also performed for each patient by treating neurologist.
Magnetic resonance imaging acquisition
MRI was obtained on a 1.5T AVANTO system (Siemens, Erlangen, Germany) by using an 8-channel head coil.
Following MRI sequences were included: T1 MPRAGE (TR = 1900 ms, TE = 3.25, bandwidth = 203 Hz, matrix size = 208 × 256, axial section thickness = 1 mm, flip angle = 8°, acquisition time = 3:40 min) and T2-weighted turbo spin-echo (TR = 3200 ms, TE = 107 ms, bandwidth = 203 Hz, number of averages = 3, FOV = 230 mm2, matrix size = 218 × 320, axial section thickness = 3 mm, flip angle = 150°, acquisition time = 2:21 min). The DTI protocol consisted of a single-shot diffusion weighted echo-planar imaging sequence. Typical acquisition parameters were as follows (TR = 5000 ms, TE = 94 ms, bandwidth = 1346 Hz, no. of directions = 30, FOV = 230 mm2, matrix size = 128 × 128, axial section thickness = 4 mm, acquisition time = 5:25 min). 1H-MRSI was obtained with a chemical shift imaging technique, using a point-resolved spectroscopy sequence (TR = 1500 ms, TE = 135 ms, bandwidth = 1000 Hz, FOV = 139 mm2, no. of directions = 30, axial section thickness = 1 mm, flip angle = 90°, acquisition time = 6:32 min).
Analysis and post processing
Diffusion tensor imaging
The DTI data were analyzed with commercial software (Siemens Syngo VE26A, Neuro 3D, Siemens, Erlangen, Germany) on an offline workstation. Eddy currents induced distortions were first corrected. The diffusion tensor was then estimated by linear regression and mean diffusivity (MD) and FA maps were generated.
One-experienced neuroradiologist and one radiologist carefully measured the FA and MD values of all patients. The regions of interest (ROIs) were manually defined as shown in Figure 1a and b. The same anatomic areas always had equal-sized ROIs over both hemispheres and over all patients. Following areas were studied. (i) White matter of primary motor cortex on both sides near knob of the central sulcus, (ii) genu and splenium of corpus callosum (CC), (iii) mid-portion of posterior limb of internal capsule, and (iv) pyramidal eminence of medulla oblongata. T1 MPRAGE images were fused with nondiffusion-weighted b0 images for better defining the structures and anatomic boundaries. The size of the ROIs was 3 mm3. For each area assessed, ROIs were manually placed on axial images of the color-coded FA maps and automatically transferred on T1 MPRAGE and apparent diffusion coefficient maps. The ROIs of the CC were drawn in the midline. The ROIs were centered in the region using color-coded directions while taking care to avoid border areas, such as areas overlapping with cerebrospinal fluid spaces and neighboring tracts.
Figure 1.
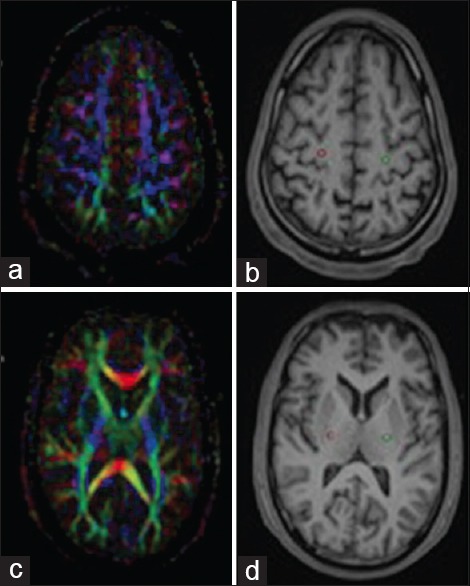
Placement of 3 mm3 sized regions of interest (ROI) in primary motor cortex (a) and mid-portion of the posterior limb of the internal capsule (c). ROIs were placed on color coded fractional anisotropy maps which were automatically transferred to fused T1 MPRAGE anatomical images (b and d)
Magnetic resonance spectroscopy
A square volume of interest (100 mm × 100 mm × 80 mm) was positioned in the centrum semiovale region to include mainly bilateral motor cortex, posterior limb of the internal capsule, and splenium of CC. Optimizing the time domain signal from the water resonance, typically at 6 Hz at a full-width half-height, localized shimming was performed on this volume. Data were acquired with water suppression to enable a first-order phase correction for the effects of eddy currents and field inhomogeneity. As shown in Figure 2a-c, MR spectra was selected from the primary motor cortex (one sub-voxel from each side of the primary motor cortex in the white matter near the knob of central sulcus), mid-portion of posterior limb of internal capsule (one sub-voxel on each side), and in splenium of CC (single sub-voxel). Using the software provided by Siemens Medical system made second-order phase correction and integral calculation of choline (Ch), creatine (Cr), and N-acetyl aspartate (NAA) peaks. As the value of Cr is stable in many disease conditions of the brain, it was selected as reference standard.
Figure 2.
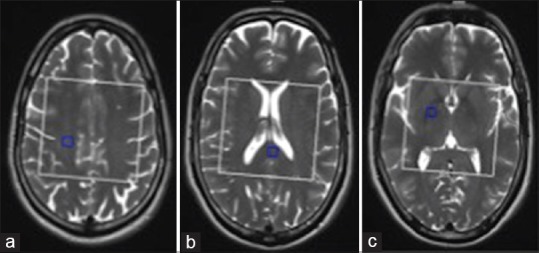
(a-c) Placement of regions of interest (ROI) in right primary motor cortex, splenium of corpus callosum and posterior limb of right internal capsule in three-dimensional multi-voxel spectroscopic images. White box represents voxel of interest and blue box represents activated ROI
Statistical analysis
Obtained data were analyzed using standard statistical tests. Mean, standard error of mean of the FA and MD values, NAA/Cr, NAA/Cho ratios were calculated for control and cases. P < 0.05 was considered as statistically significant. Statistical data were analyzed on QI Macros version, KnowWare International inc., USA. 2014.09 extension tool for Microsoft Excel for Mac 2011.
Results
Clinical characterization
A total of 21 patients were enrolled in the study, and 13 healthy volunteers formed control group. Based on clinical profile and El-Escorial score patients were classified in definite (n = 7), probable (n = 7), possible (n = 7). Eleven patients showed limb onset and 10 patients showed bulbar onset.
Diffusion tensor imaging
ALS patients showed significantly decreased FA and increased MD values in primary motor cortex and in the posterior limb of the internal capsule on both sides (P < 0.05). In genu and splenium of CC, FA values were decreased and MD values were increased (P < 0.05). Compared with healthy controls, patients showed decreased FA values in left side of pyramidal eminence of medulla oblongata (P = 0.007), however FA value on right side and MD values on either side did not show statistical significance.
FA and MD values from patients who were classified in definite disease group showed significant difference between cases and control in each region of the brain as shown in Table 1. Patients having probable disease showed significant difference in primary motor cortex, posterior limb of the internal capsule, and in CC. Pyramid shows significant difference in FA values on left side in probable group and on right side in definite group. Patients having possible disease showed significant decrease in FA and increase in MD values in primary motor cortex and in the posterior limb of the internal capsule (P ≤ 0.05). In patients with possible disease, CC and pyramid did not show significant difference in either FA or MD values.
Table 1.
P values in various regions of brains and their respected groups
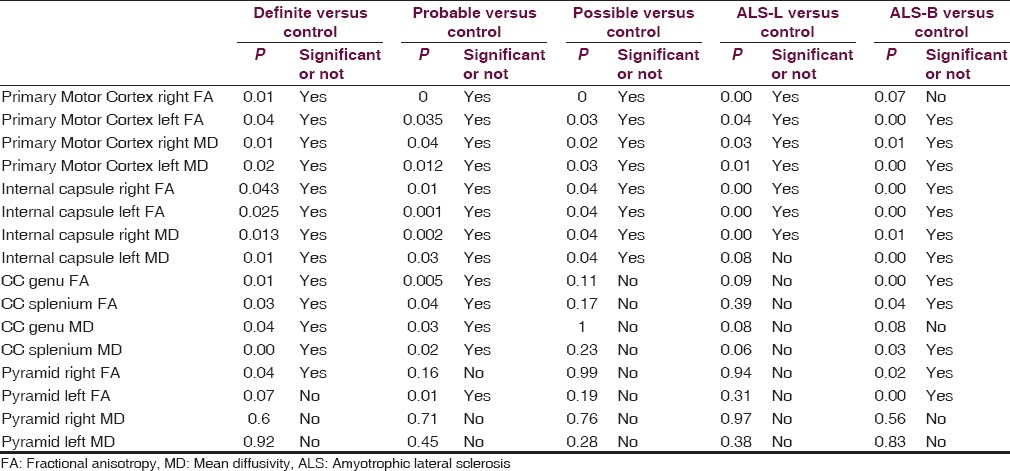
Patients with bulbar onset ALS showed significant reduction in FA values and increase in MD values, except FA values in the region of right side of the primary cortex. ALS-L patients also show significant decrease in FA values and increase in MD values, except increase in MD values. CC show significant decrease in FA values and increase in MD values in ALS-B patients.
Magnetic resonance spectroscopy
Metabolite ratios are shown in Table 2 within each disease categories and limb vs bulbar onset disease. Patients having definite disease showed reduced NAA/Cr and NAA/Ch ratio in primary motor cortex, posterior limb of internal capsule, and splenium of CC (P < 0.04) Patients having probable disease also showed reduced NAA/Cr ratio (P < 0.04). However, NAA/Ch ratio was not having statistical significance. Patients having possible disease or relatively early phase of the disease showed no reduction in either ratio.
Table 2.
Various regions of brain and comparison of cases and controls in various groups and related P values
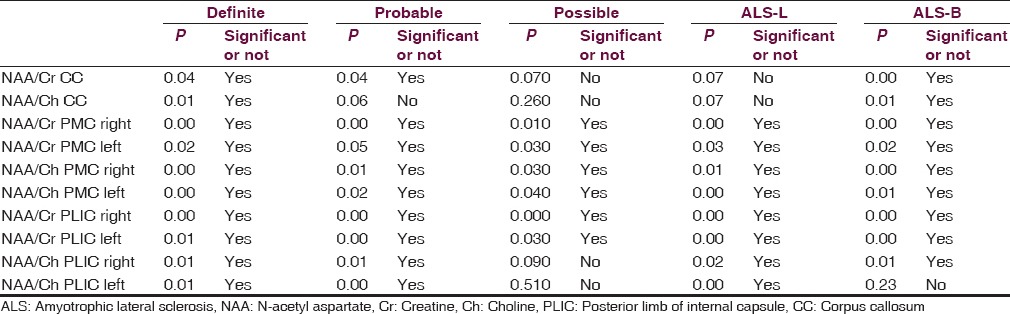
In all three phase of disease, NAA/Cr and NAA/Ch ratio showed significant (P ≤ 0.05) reduction on either side in white matter of the primary motor cortex.
In definite and probable disease group posterior limb of the internal capsule showed reduced NAA/Cr and NAA/Ch ratios on either side. In patients with possible disease, decrease in NAA/Cr ratio was found. However in this group decrease in NAA/Ch ratio was not statistically significant.
Patients having limb onset ALS showed reduced NAA/Cr and NAA/Ch ratios in primary motor cortex, posterior limb of the internal capsule but not in splenium of CC.
Patients having bulbar onset ALS showed reduced NAA/Cr and NAA/Ch in splenium of CC, primary motor cortex, and posterior limb of the internal capsule (P < 0.02) except for NAA/Ch ratio on left side of internal capsule.
Discussion
Motor neuron disease is one of the most common adult onset neurodegenerative diseases.[1] Pathological changes in ALS show atrophy of anterior horn cells in the spinal cord, atrophy of motor cortex, atrophy of cranial nerve nuclei in brain stem, etc. These changes microscopically show degeneration of nerve fibers with gliosis.[12] Many studies have tried to detect such changes with the help of modern MR sequences such as DTI and MRS.[7] These changes differ in different disease category, that is, definite, probable, and possible, and also between bulbar versus limb onset of disease.
Diffusion tensor imaging
Involvement of white matter in ALS patients is increasingly being recognized.[13] Our study tries to quantify the difference of water diffusion characteristics along CST. For these two quantifiable variables, FA and MD were used. Spatial changes in the CST have been suggested by many studies.[8,13] We have studied four regions of the brain, that is, primary motor cortex, posterior limb of internal capsule, CC, and pyramid. In our study, controls show decreasing FA and increasing MD values from motor cortex up to pyramid. However, pyramid shows lower FA values and higher MD values with a broader range, which can be suggested by the presence of other tracts and nuclei at brain stem level.[14] These findings of our study are in well concordance from studies done by Virta et al., Stieltjes et al., Pierpaoli et al., and Toosy et al.[8,14,15,16,17,18]
In ALS patients, we found significant reduction in FA values on either side of primary motor cortex compared to healthy control (P < 0.04). This finding has been suggested by many previous studies.[19,20,21,22] We also found that the reduction in FA values is correlating with stage of disease. More reduction in FA value and increase in MD values were found in patients having definite disease than probable or possible category, which corresponds to the extent of axonal degeneration.[22,23] Similar changes were also noted in the posterior limb of the internal capsule. FA and MD values show significant changes in all disease stages (P < 0.015). Even in early disease, that is, in possible disease primary motor cortex and posterior limb of internal capsule show significant changes.[24,25,26]
Changes in white matter have also been documented to extend well farther from classic motor network, that is, CC.[7,27] Our study found that CC show decreased FA and increased MD values along genu and splenium. These changes are consistent with progression of the disease. We found significant difference in definite and probable disease. In patients with possible, that is, relative early course of disease CC did not show significant difference. Due to tightly packed bundle interhemispheric association appears to involve late in disease. It might be due to secondary disease progression through callosal fibers or independent feature of bicortical progression.[27] Genu of CC showed more decrease in FA value compared to splenium in all three groups. This subtle finding can suggest that the involvement of anterior CC might be one of the causative factors in the development of frontotemporal dementia, which is recognized now more frequently in patients of motor neurone disease (MND).[27,28,29]
ALS-L patients showed significant reduction in FA and increase in MD values in primary motor cortex and posterior limb of the internal capsule. These changes were consistent with previous studies done.[30,31] CC and pyramidal eminence of medulla oblongata did not showed consistent and significant changes associated with the disease.
ALS-B patients showed reduced FA and increased MD values in the posterior limb of the internal capsule and primary motor cortex except for reduction in FA values in right side of the primary motor cortex. CC showed reduced FA and increase MD values along its splenium and genu except for an increase in MD values in the genu. Changes in CC, that is, in extramotor area in bulbar onset patients may be suggestive of more advanced involvement of nervous system and could be related to grave outcome.[32]
Magnetic resonance spectroscopy
H-MRS has been shown to be useful in patients with MND for the early detection of neuronal loss/dysfunction in the motor cortex, CST, and extramotor cortex. It can also be used for the assessment of longitudinal progression of the disease and the effect of drug therapy. In this study, comprehensive evaluation of MRS observed alterations in cerebral metabolites in primary motor cortex, splenium of CC, and in the posterior limb of the internal capsule.[33]
Our study showed reduced mean absolute value of NAA in ALS patients with 4.38 ± 0.90 ppm on right side and 4.32 ± 0.80 ppm on left side in the primary motor cortex. We also found a modest increase in Ch and Cr values as compared to healthy controls. Although increased Ch is not substantiated by other investigators in ALS and it could be reflective of cerebral astrogliosis or cellular membrane degeneration.[34,35,36,37] Absolute values of metabolites did not show significant difference in the internal capsule.
NAA/Ch ratio among patients was 1.9 ± 0.69 on right side and 2.03 ± 0.75 on left side in the primary motor cortex. On comparing with healthy control, these ratios were reduced significantly on either side (P < 0.05). This findings are consistent with various previous studies done.[10,38,39] Interestingly, our study also found that these changes are seen in all three groups of patients; signifying that even in early stages, that is, in possible disease NAA/Cr and NAA/Ch ratios are altered.
NAA/Cr and NAA/Ch ratios were significantly reduced in definite and probable group of patients. NAA/Cr ratio was reduced in patients with possible disease, while NAA/Ch ratio was not significantly reduced. These changes can be interpreted as slightly delayed involvement of lower portion of CST in corona radiata in the course of disease progression. Reason for increased Cr and Ch concentrations in the motor cortex is probably due to increased gliosis and membrane phospholipid breakdown. Suhy et al. however, showed increased Ch and Cr values in the early stage of disease followed by a decrease in these values on follow-up MRI.[40] These changes can provide insight into heterogenous nature of involvement of CST by the disease. As pathological studies and diffusion tensor MRI have shown that CST involvement is not a contiguous process, reduction in above mentioned ratios along CST cannot be predicted by mere craniocaudal model of CST.[8,25] This can suggest that there can be multiple site of disease onset within CST.
Smith in 1960 showed the involvement of CC in its mid anterior and to a lesser extent posterior part.[6] The structural changes occurring in CC is beyond the conventional MRI.[41] A study done by Chapman et al. showed no significant difference in CC size among healthy controls and ALS patients. However, as DTI data has shown disease related changes in ALS patients with altered FA and MD values as described previously.[27,29] Possible explanation suggested by Chapman et al. were that changes detected by DTI are too subtle, or do not manifest in a way to be observed with the resolution of structural MRI. Alternate explanation can be that any fiber atrophy occurring in the CC due to neuronal degeneration is offset by a consequent reactive gliosis, a characteristic proliferative response of local astrocytes to various brain insults.[41] These subtle metabolite changes can be studied in vivo by application of MRS to CC. Our study also showed reduced absolute NAA values in the splenium of CC in cases. However, Cr and Ch level were not reduced. Mean NAA/Cr ratio was 1.59 ± 0.28 and mean NAA/Ch ratio was 1.7 ± 0.29. These were significantly reduced as compared to healthy controls. Patients having definite disease showed significant reduction in NAA/Cr (P = 0.05) and NAA/Ch (P < 0.01). While patients in probable category showed significant reduction in NAA/Cr (P = 0.04) ratio, and no significant changes were seen in NAA/Ch. However, patients with possible category showed no significant reduction in either ratio. These findings show that CC involvement is not seen in the early disease. As the disease progresses and more widespread involvement of neurons occur, fibers entering CC are involved.
Amyotrophic lateral sclerosis limb versus bulbar onset
Our study showed that patients with limb onset of disease were having consistent involvement of primary motor cortex and posterior limb of internal capsule with significant reduction in NAA/Cr and NAA/Ch ratios (P < 0.03). However, CC shows no significant changes in limb onset.
Patient having the bulbar onset of disease showed consistent changes with statistical significance in primary motor cortex and posterior limb of the internal capsule (P < 0.02). Splenium of CC also showed significant reduction in FA and MD values as opposed to ALS-L patients. Hence, both of these techniques throw insight on differing pattern of brain involvement.
Our study has few limitations, that is, we have studied small sample size as few patients having definite disease and involvement of respiratory muscles were excluded. Furthermore, the incidence of disease is smaller in our region than west. In a technical aspect of DTI, we have used manual ROI method which has little limitation,[42] on the aspect of reproducibility but our strenuous efforts and consistent approach has helped to limit this bias.
Conclusion
DTI and MRS detect changes associated with ALS even in early phase of the disease. Bulbar onset and limb onset ALS patients show different pattern of involvement. Extramotor involvement suggested by CC involvement is a feature seen in bulbar onset patient and can suggest poor outcome in such patients. The present findings may be helpful for designing further studies in the direction of more early diagnosis of disease and its management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Talbot K. Motor neurone disease. Postgrad Med J. 2002;78:513–9. doi: 10.1136/pmj.78.923.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 3.Hecht MJ, Fellner C, Schmid A, Neundörfer B, Fellner FA. Cortical T2 signal shortening in amyotrophic lateral sclerosis is not due to iron deposits. Neuroradiology. 2005;47:805–8. doi: 10.1007/s00234-005-1421-5. [DOI] [PubMed] [Google Scholar]
- 4.Comi G, Rovaris M, Leocani L. Review neuroimaging in amyotrophic lateral sclerosis. Eur J Neurol. 1999;6:629–37. doi: 10.1046/j.1468-1331.1999.660629.x. [DOI] [PubMed] [Google Scholar]
- 5.Charil A, Corbo M, Filippi M, Kesavadas C, Agosta F, Munerati E, et al. Structural and metabolic changes in the brain of patients with upper motor neuron disorders: A multiparametric MRI study. Amyotroph Lateral Scler. 2009;10:269–79. doi: 10.3109/17482960902777339. [DOI] [PubMed] [Google Scholar]
- 6.Smith MC. Nerve fibre degeneration in the brain in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1960;23:269–82. doi: 10.1136/jnnp.23.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foerster BR, Welsh RC, Feldman EL. 25 years of neuroimaging in amyotrophic lateral sclerosis. Nat Rev Neurol. 2013;9:513–24. doi: 10.1038/nrneurol.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toosy AT, Werring DJ, Orrell RW, Howard RS, King MD, Barker GJ, et al. Diffusion tensor imaging detects corticospinal tract involvement at multiple levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2003;74:1250–7. doi: 10.1136/jnnp.74.9.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agosta F, Pagani E, Petrolini M, Caputo D, Perini M, Prelle A, et al. Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: A diffusion tensor MR imaging tractography study. AJNR Am J Neuroradiol. 2010;31:1457–61. doi: 10.3174/ajnr.A2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Block W, Karitzky J, Träber F, Pohl C, Keller E, Mundegar RR, et al. Proton magnetic resonance spectroscopy of the primary motor cortex in patients with motor neuron disease: Subgroup analysis and follow-up measurements. Arch Neurol. 1998;55:931–6. doi: 10.1001/archneur.55.7.931. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Poptani H, Woo JH, Desiderio LM, Elman LB, McCluskey LF, et al. Amyotrophic lateral sclerosis: Diffusion-tensor and chemical shift MR imaging at 3.0 T. Radiology. 2006;239:831–8. doi: 10.1148/radiol.2393050573. [DOI] [PubMed] [Google Scholar]
- 12.Piao YS, Wakabayashi K, Kakita A, Yamada M, Hayashi S, Morita T, et al. Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol. 2003;13:10–22. doi: 10.1111/j.1750-3639.2003.tb00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keil C, Prell T, Peschel T, Hartung V, Dengler R, Grosskreutz J. Longitudinal diffusion tensor imaging in amyotrophic lateral sclerosis. BMC Neurosci. 2012;13:141. doi: 10.1186/1471-2202-13-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virta A, Barnett A, Pierpaoli C. Visualizing and characterizing white matter fiber structure and architecture in the human pyramidal tract using diffusion tensor MRI. Magn Reson Imaging. 1999;17:1121–33. doi: 10.1016/s0730-725x(99)00048-x. [DOI] [PubMed] [Google Scholar]
- 15.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–48. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 16.Peled S, Gudbjartsson H, Westin CF, Kikinis R, Jolesz FA. Magnetic resonance imaging shows orientation and asymmetry of white matter fiber tracts. Brain Res. 1998;780:27–33. doi: 10.1016/s0006-8993(97)00635-5. [DOI] [PubMed] [Google Scholar]
- 17.Stieltjes B, Kaufmann WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M, et al. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001;14:723–35. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- 18.Wong JC, Concha L, Beaulieu C, Johnston W, Allen PS, Kalra S. Spatial profiling of the corticospinal tract in amyotrophic lateral sclerosis using diffusion tensor imaging. J Neuroimaging. 2007;17:234–40. doi: 10.1111/j.1552-6569.2007.00100.x. [DOI] [PubMed] [Google Scholar]
- 19.Ellis CM, Suckling J, Amaro E, Jr, Bullmore ET, Simmons A, Williams SC, et al. Volumetric analysis reveals corticospinal tract degeneration and extramotor involvement in ALS. Neurology. 2001;57:1571–8. doi: 10.1212/wnl.57.9.1571. [DOI] [PubMed] [Google Scholar]
- 20.Graham JM, Papadakis N, Evans J, Widjaja E, Romanowski CA, Paley MN, et al. Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology. 2004;63:2111–9. doi: 10.1212/01.wnl.0000145766.03057.e7. [DOI] [PubMed] [Google Scholar]
- 21.Cosottini M, Giannelli M, Siciliano G, Lazzarotti G, Michelassi MC, Del Corona A, et al. Diffusion-tensor MR imaging of corticospinal tract in amyotrophic lateral sclerosis and progressive muscular atrophy. Radiology. 2005;237:258–64. doi: 10.1148/radiol.2371041506. [DOI] [PubMed] [Google Scholar]
- 22.Thivard L, Pradat PF, Lehéricy S, Lacomblez L, Dormont D, Chiras J, et al. Diffusion tensor imaging and voxel based morphometry study in amyotrophic lateral sclerosis: Relationships with motor disability. J Neurol Neurosurg Psychiatry. 2007;78:889–92. doi: 10.1136/jnnp.2006.101758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlsborg M, Rosenbaum S, Wiegell M, Simonsen H, Larsson H, Werdelin L, et al. Corticospinal tract degeneration and possible pathogenesis in ALS evaluated by MR diffusion tensor imaging. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:136–40. doi: 10.1080/14660820410018982. [DOI] [PubMed] [Google Scholar]
- 24.Schimrigk SK, Bellenberg B, Schlüter M, Stieltjes B, Drescher R, Rexilius J, et al. Diffusion tensor imaging-based fractional anisotropy quantification in the corticospinal tract of patients with amyotrophic lateral sclerosis using a probabilistic mixture model. AJNR Am J Neuroradiol. 2007;28:724–30. [PMC free article] [PubMed] [Google Scholar]
- 25.Senda J, Ito M, Watanabe H, Atsuta N, Kawai Y, Katsuno M, et al. Correlation between pyramidal tract degeneration and widespread white matter involvement in amyotrophic lateral sclerosis: A study with tractography and diffusion-tensor imaging. Amyotroph Lateral Scler. 2009;10:288–94. doi: 10.3109/17482960802651717. [DOI] [PubMed] [Google Scholar]
- 26.Iwata NK, Kwan JY, Danielian LE, Butman JA, Tovar-Moll F, Bayat E, et al. White matter alterations differ in primary lateral sclerosis and amyotrophic lateral sclerosis. Brain. 2011;134:2642–55. doi: 10.1093/brain/awr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filippini N, Douaud G, Mackay CE, Knight S, Talbot K, Turner MR. Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology. 2010;75:1645–52. doi: 10.1212/WNL.0b013e3181fb84d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao YP, Cho KH, Yeh CH, Chou KH, Chen JH, Lin CP. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp. 2009;30:3172–87. doi: 10.1002/hbm.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: An [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15:601–9. doi: 10.1016/j.nbd.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Prell T, Peschel T, Hartung V, Kaufmann J, Klauschies R, Bodammer N, et al. Diffusion tensor imaging patterns differ in bulbar and limb onset amyotrophic lateral sclerosis. Clin Neurol Neurosurg. 2013;115:1281–7. doi: 10.1016/j.clineuro.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 31.Cardenas-Blanco A, Machts J, Acosta-Cabronero J, Kaufmann J, Abdulla S, Kollewe K, et al. Central white matter degeneration in bulbar- and limb-onset amyotrophic lateral sclerosis. J Neurol. 2014;261:1961–7. doi: 10.1007/s00415-014-7434-4. [DOI] [PubMed] [Google Scholar]
- 32.Chapman MC, Jelsone-Swain L, Johnson TD, Gruis KL, Welsh RC. Diffusion tensor MRI of the corpus callosum in amyotrophic lateral sclerosis. J Magn Reson Imaging. 2014;39:641–7. doi: 10.1002/jmri.24218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma KR, Saigal G, Maudsley AA, Govind V. 1H MRS of basal ganglia and thalamus in amyotrophic lateral sclerosis. NMR Biomed. 2011;24:1270–6. doi: 10.1002/nbm.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gredal O, Rosenbaum S, Topp S, Karlsborg M, Strange P, Werdelin L. Quantification of brain metabolites in amyotrophic lateral sclerosis by localized proton magnetic resonance spectroscopy. Neurology. 1997;48:878–81. doi: 10.1212/wnl.48.4.878. [DOI] [PubMed] [Google Scholar]
- 35.Sarchielli P, Pelliccioli GP, Tarducci R, Chiarini P, Presciutti O, Gobbi G, et al. Magnetic resonance imaging and 1H-magnetic resonance spectroscopy in amyotrophic lateral sclerosis. Neuroradiology. 2001;43:189–97. doi: 10.1007/s002340000472. [DOI] [PubMed] [Google Scholar]
- 36.Kalra S, Vitale A, Cashman NR, Genge A, Arnold DL. Cerebral degeneration predicts survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2006;77:1253–5. doi: 10.1136/jnnp.2006.090696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller BL, Chang L, Booth R, Ernst T, Cornford M, Nikas D, et al. In vivo 1H MRS choline: Correlation with in vitro chemistry/histology. Life Sci. 1996;58:1929–35. doi: 10.1016/0024-3205(96)00182-8. [DOI] [PubMed] [Google Scholar]
- 38.Rooney WD, Miller RG, Gelinas D, Schuff N, Maudsley AA, Weiner MW. Decreased N-acetylaspartate in motor cortex and corticospinal tract in ALS. Neurology. 1998;50:1800–5. doi: 10.1212/wnl.50.6.1800. [DOI] [PubMed] [Google Scholar]
- 39.Pohl C, Block W, Karitzky J, Träber F, Schmidt S, Grothe C, et al. Proton magnetic resonance spectroscopy of the motor cortex in 70 patients with amyotrophic lateral sclerosis. Arch Neurol. 2001;58:729–35. doi: 10.1001/archneur.58.5.729. [DOI] [PubMed] [Google Scholar]
- 40.Suhy J, Miller RG, Rule R, Schuff N, Licht J, Dronsky V, et al. Early detection and longitudinal changes in amyotrophic lateral sclerosis by (1) H MRSI. Neurology. 2002;58:773–9. doi: 10.1212/wnl.58.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapman MC, Jelsone-Swain L, Fling BW, Johnson TD, Gruis K, Welsh RC. Corpus callosum area in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13:589–91. doi: 10.3109/17482968.2012.708935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakulinen U, Brander A, Ryymin P, Öhman J, Soimakallio S, Helminen M, et al. Repeatability and variation of region-of-interest methods using quantitative diffusion tensor MR imaging of the brain. BMC Med Imaging. 2012;12:30. doi: 10.1186/1471-2342-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]


