Abstract
Background
Air pollution in Beijing, especially PM2.5, has received increasing attention in the past years. Although exposure to PM2.5 has been linked to many health issues, few studies have quantified the impact of PM2.5 on the risk of influenza-like illness (ILI). The aim of our study is to investigate the association between daily PM2.5 and ILI risk in Beijing, by means of a generalized additive model.
Methods
Daily PM2.5, meteorological factors, and influenza-like illness (ILI) counts during January 1, 2008 to December 31, 2014 were retrieved. An inverse Gaussian generalized additive model with log link function was used to flexibly model the nonlinear relationship between the PM2.5 (single- and multiday lagged exposure) and ILI risk, adjusted for the weather conditions, seasonal and year trends. We also assessed if the effect of PM2.5 differs during flu season versus non-flu season by including the interaction term between PM2.5 and flu season in the model. Furthermore, a stratified analysis by age groups was conducted to investigate how the effect of PM2.5 differs across age groups.
Results
Our findings suggested a strong positive relationships between PM2.5 and ILI risk at the flu season (October-April) (p-value < 0.001), after adjusting for the effects of ambient daily temperature and humidity, month and year; whereas no significant association was identified at the non-flu season (May-September) (p-value = 0.174). A short term delayed effect of PM2.5 was also identified with 2-day moving average (current day to the previous day) of PM2.5 yielding the best predictive power. Furthermore, PM2.5 was strongly associated with ILI risk across all age groups (p-value < 0.001) at the flu season, but the effect was the most pronounced among adults (age 25–59), followed by young adults (age 15–24), school children (age 5–14) and the elderly (age 60+) and the effect of PM2.5 was the least pronounced for children under 5 years of age (age < 5).
Conclusions
Ambient PM2.5 concentrations were significantly associated with ILI risk in Beijing at the flu season and the effect of PM2.5 differed across age groups, in Beijing, China.
Keywords: PM2.5, Influenza, Meteorological factor, Spline, Generalized additive model
Background
Air pollution has been well documented as a major public health issue for many areas of the world, as a growing body of epidemiological and clinical evidence has shown that pollutants increase the risks of numerous diseases [1–7]. Airborne particulate matter is a mixture of liquid and solid material of varying size and chemical characteristics, which includes dust, dirt, soot, smoke, and liquid droplets emitted into the air. The sizes of the inhalable particles are limited to be those within aerodynamic diameters of 10 μm or less (PM10) in aerodynamic diameter. PM10 consists of two size fractions, fine and coarse, which have both different physiologic and different source characteristics. The particles mechanically generated from agriculture, mining, road traffic, and related sources are generally larger than 2.5 μm, which are usually referred to as coarse mass particles (PM2.5-10). In contrast, particles resulting from combustion processes are generally less than 2.5 μm, which are defined as fine particles (PM2.5).
Toxicological and epidemiological studies suggest that PM2.5 are especially harmful [2, 4–6, 8, 9], since smaller particles are more likely to penetrate deeper into the lungs and blood streams unfiltered [10]. Studies have shown exposure to PM2.5 is associated with a number of adverse health outcomes ranging from respiratory disease [9, 11, 12] to cardiovascular disease [1, 4, 13]. Elevated fine-particulate concentrations are also the cause of mortality [2, 3, 14, 15]. This is also one of the important reasons for WHO designating all countries to have standards for PM2.5. In 2013, a longitudinal study involving 312,944 people in nine European countries revealed that that the lung cancer rate rose 22 % for every increase of 10 μg/m3 in PM10. The smaller PM2.5 were particularly deadly, with a 36 % increase in lung cancer per 10 μg/m3and the effect of PM2.5 was not affected by adjustment for PM2.5-10 [9]. Other studies also revealed the similar findings showing that PM10 and PM2.5 are significantly associated with all cause and cause-specific mortality [2]; whereas no such associations were observed for PM2.5-10 [2, 6, 8, 16]. Those studies suggested that the proportion of PM2.5 in the PM10 composition is more important and might be more strongly related to adverse health effects. Thus, PM2.5 pollution has gained increasing attention, especially for those living in metropolitan areas [17].
Beijing, the capital city in China, has been suffered with severe air pollution in the last decade due to rapid industrial expansion and the increased number of automobiles on the road. The number of heavy or more severe pollution days (PM2.5 > 75 μg/m3) has been hovering over hundred days annually in Beijing [18, 19]. In China, much attention on air pollution has been focused on PM10 [20, 21]. Few studies have devoted to study the PM2.5 exposure on health impact, partly because of lack of such information, until recently China has released PM2.5 concentrations in major cities to the public [22].
Researchers believe that airborne pollution particles provide “condensation nuclei” to which virus droplets attach; however, the quantitative research on the association between air pollution and influenza is still rare, considering that extreme ambient pollution is a biologically plausible risk factor, and that more intense pollution are imminent this century. Despite a recent study showing that PM2.5 was associated with monthly influenza cases [22], there are few studies using daily pollution and influenza data, and no study has been conducted to date investigating the effects of PM2.5 on influenza risk by age group. Such research is needed, as influenza epidemics constitute a serious public health problem associated with increased morbidity and mortality, especially in high risk populations, with children, the elderly, and patients with chronic diseases being particularly vulnerable to air pollution [23]. As such, it is important to determine if the effect of PM2.5 varies over different age groups. Meteorological factors, in particular temperature and humidity, have also been shown contributing to the risk of influenza infections, such that both low temperature and humidity increase the spread of influenza viruses [24, 25]. However, to our knowledge, no studies have precisely examined the association between PM2.5 and influenza by age groups, after controlling the confounding effects of meteorological factors.
In the present study, we provide direct evidence to support the role of ambient fine particulate matter exposure, after adjusting for the effects of weather conditions in the dynamics of influenza and thereby address an emerging question fundamental to the understanding of influenza epidemiology. A generalized additive model was utilized to flexibly model the nonlinear relationship between the daily PM2.5 and daily influenza risk in Beijing from year 2008 to 2014, while adjusting for the effects of ambient daily temperature and humidity, status of being week day or weekend/holiday, month and year. We also assessed if the effect of PM2.5 differs across various age groups. To explore the delayed impact of PM2.5, lag effect of PM2.5 was also considered.
Methods
Data sources and description
Influenza data consisted of reports of daily number of patients seeking medical attention with influenza-like illness (ILI), defined as the one with body temperature more than 38° Celsius and cough or sore throat, from January 1, 2008 to December 31, 2014 in the capital city of China, Beijing. The data was retrieved from the surveillance system at the Beijing Centre of Disease Control [26]. The influenza surveillance system has been reported elsewhere [27]. In brief, the surveillance is conducted in 150 level two and level three hospitals in Beijing, which consists of hospitals from national, city and district level. The data has a reasonable representativeness given that the sentinel hospitals cover all the 16 districts in Beijing, and the data were from the all outpatients related to respiratory disease treatment. Health care system in each sentinel hospital reports the data to the Beijing Centre of Disease Control every day from online system, and staffs in the district Centre of Disease Controls are responsible for data validation.
Average daily measurements of PM2.5 from January 1, 2008 to December 31, 2014 were retrieved from an air quality monitoring site at the US Embassy in Beijing, which is located at the Chaoyang district. The Embassy’s air pollution data was used because it recorded detailed measurements of PM2.5 over a long period of time, despite originating from only one location. The data was validated and used by other paper [22]. Temperature and relative humidity were considered as the potential confounders of the association between PM2.5 and ILI risk. Daily temperatures and relative humidity, spanning the study period, were obtained through the China Weather Network’s outdoor weather reports. Daily counts of ILI, air pollution levels and weather data were linked by date and analyzed. This study was approved by the Institutional Review Board at Beijing Centre of Disease Control [28].
Statistical analysis
In epidemiological research, one most frequently used model for modeling counts data is the Poisson regression. A severe limitation of the Poisson model is that the mean and variance of the dependent variable are assumed to be equal, conditional on any covariates [29]. In practice, a very common complication when modeling discrete responses is the presence of overdispersion, when the variance of the response is greater than the mean [30]. It is generally caused by positive correlation between responses or by an excess variation between response counts. If overdispersion is present in a dataset, the standard errors of the estimates could be underestimated (i.e. a variable may appear to be significant predictor when it is in fact not significant) [29]. Negative binomial (NB) regression has been suggested as an alternative to the Poisson, which accounts for overdispersion by adding an additional dispersion (variance) parameter to the Poisson model [31]. However, the negative binomial distribution also imposes some constraints on the mean and variance relationship, whose validation also needs to be seriously assessed. Over the past decades, the family of inverse Gaussian distributions [32, 33] has attracted the attention of many researchers in studying the number of event occurrences for a wide range of field. The inverse Gaussian distribution is particularly useful for dealing with data of considerable skewness [34]. In such cases, the choice is made upon the basis of goodness of fit and upon the ease of working with the distribution. As such, we carefully examined the Poisson, the negative binomial and the inverse Gaussian regression models to identify which model fits the data well and fits the data the best. In fact, all three types of distributions belong to the exponential family in a generalized linear modeling framework [35]; therefore, all the interpretation of the regression coefficients are the same, if the same link function is applied. Here, we used the most commonly used log link function for ease of interpretation [36].
To allow for comparability, all models were adjusted for the same meteorological variables (temperature and humidity) and time variables (year and day of the week). We screened all variables for multi-collinearity. All the three types of models can be written in the following form:
where μt represents the expected mean number of individuals reporting ILI on day t and α0 denote the intercept. We let nt denote the population size on day t, which is estimated by fitting a sigmoid function to the annual population size of Beijing spanning the study period [37–39], since only annual population of Beijing can be retrieved for this study. We define the ILI incidence rate as the ratio of μt relative to nt. Following the standard practice in generalized additive models, fj(x), j = 1,…,5, are the penalized smoothing spline functions for PM2.5 at flu season (October-April), non- flu season (May-September), temperature, humidity and month, respectively. That is, fj(x) = ∑qi = 1bi(x)δi, where bi(x), i = 1, … q are a set of basis functions and δi are the corresponding regression coefficients. These basis functions are sections of polynomials that join at a number of knot locations. Common type of basis functions include cubic B-splines or thin-plate splines [36]. The smoothness of the spline functions were automatically estimated using unbiased risk estimation [36]. To explore the delayed impact of PM2.5 on ILI risk, we lagged PM2.5 by p days, denoted by PM2.5,t − p representing the measurement of PM2.5 taken at day p prior to ILI case report date t, p = 0, 1, … 5. For example, a lag of 0 days (lag 0) corresponds to the current day PM2.5, and a lag of 1 day (lag 1) refers to the previous-day PM2.5. We also investigated the effect of accumulated exposures of PM2.5 on ILI incidence by taking mean of lag01 (PM2.5 averaged over the current day and the previous day), and up to mean lag05. We selected a lag according to the lowest Akaike Information Criterion (AIC) [28]. I(A) is an indicator variable, such that if A is true then I(A) = 1 and 0, otherwise; βk is the regression coefficient for year k and γ is the regression coefficient for weekday. To investigate if the effect of PM2.5 varies with age, stratified analysis at different age groups was also conducted, with age groups being classified as <5, 5–14, 15–24, 25–59, ≥60 years old. The statistical analysis was conducted using the mgcv package in R [36]. All statistical tests were two-sided and P-values with less than 0.05 were considered statistically significant.
Results
The distributions of daily ILI cases, daily PM2.5, daily temporal and humidity from January 1, 2008 to December 31, 2014 are depicted in Fig. 1. On average, there were 1763 ILI cases per day, 695 for age <5, 448 for age 5–14, 191 for age 15–25, 357 for age 25–29 and 71 for age 60+ (Table 1). Daily mean concentration of PM2.5 was 100.66 μg/m3, with standard deviation 80.86 μg/m3, which was more than four times higher than the WHO’s guideline of 25 μg/m3. The maximum PM2.5 reached at 568.6 μg/m3 on January 12, 2013, which was record breaking [22]. At the flu season (October-April), the number of ILI cases for all age groups, except for <5 years old, tends to be higher than the corresponding the number of ILI cases at the non-flu season (May-September). The median (Q1-Q3) for PM2.5 at flu season is 77.62 μg/m3 (44.50 μg/m3–131.00 μg/m3) and at the non-flu season is 83.26 μg/m3 (52.25 μg/m3–115.70 μg/m3). The temperature and humidity tend to be lower at the flu season as compared to the non-flu season with the mean (Q1-Q3) for temperature at flu season is 6.35 °C (-0.90 °C, 14.72 °C) and at the non-flu season is 25.60 °C (23.30 °C, 27.50 °C ); the mean (Q1-Q3) for humidity at flu season is 46 g/m3 (30 g/m3–63 g/m3) and at the non-flu season is 63 g/m3 (48 g/m3–73 g/m3) (Table 1).
Fig. 1.
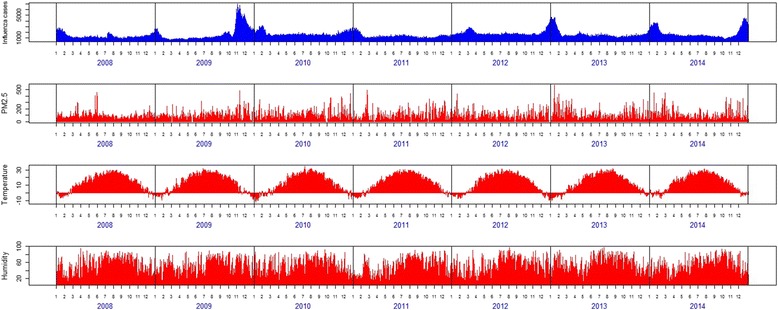
The time course of daily influenza cases, daily PM2.5, daily temperature and average humidity from January 1, 2008 to December 31, 2014
Table 1.
Summary statistics of daily ILI counts, PM2.5, and weather conditions in Beijing, China, during January 1, 2008 to December 31, 2014 (Q1, Q2 and Q3 denote the 25th, 50th and 75th percentile, respectively)
| Variable | Flu season (October-April) | Non-flu season (May-September) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Minimum | Q1 | Q2 | Q3 | Maximum | Mean ± SD | Minimum | Q1 | Q2 | Q3 | Maximum | |
| Daily ILI counts by age groups | ||||||||||||
| <5 | 695.45(227.78) | 270 | 529 | 673 | 815 | 1717 | 657.45(152.24) | 357 | 539 | 648 | 782 | 1088 |
| 5–14 | 448.38(250.29) | 124 | 311 | 389 | 505 | 2794 | 349.26(86.40) | 153 | 289 | 341 | 406 | 710 |
| 15–24 | 191.29(225.80) | 32 | 90 | 119 | 205 | 2097 | 104.44(25.47) | 44 | 86 | 103 | 120 | 197 |
| 25–59 | 356.89(294.82) | 79 | 179 | 246 | 429 | 1627 | 218.73(63.67) | 78 | 176 | 211 | 260 | 512 |
| 60+ | 71.24(53.97) | 10 | 40 | 54 | 83 | 381 | 51.52(22.86) | 16 | 38 | 48 | 62 | 447 |
| Fine airborne particulate matter (μg/m 3) | ||||||||||||
| PM 2.5 | 100.66(80.86) | 2.92 | 44.50 | 77.62 | 131.00 | 568.00 | 90.14(53.24) | 9.79 | 52.25 | 83.26 | 115.70 | 463.00 |
| Meteorological Measures | ||||||||||||
| Temperature | 7.13(8.87) | −12.50 | −0.90 | 6.35 | 14.72 | 26.60 | 25.09(3.36) | 11.20 | 23.30 | 25.60 | 27.50 | 34.50 |
| Humidity | 47.39(19.82) | 8 | 30 | 46 | 63 | 95 | 59.86(17.63) | 13 | 48 | 63 | 73 | 97 |
We compared the model fits for the Poisson, negative binomial and inverse Gaussian generalized additive models with log link functions when PM2.5 was lagged at single day or moving average ranging from 0 to 5 days prior to ILI reporting date. The detailed model comparison was presented in Table 2. Based on the Akaike’s Information Criterion (AIC) [28], the inverse Gaussian generalized additive model using the 2-day moving average of PM2.5 yielded the best fit. We further examined how well do the fitted values of response variable correspond to the observed data using the chi-square goodness of fit test. The results indicated that the inverse Gaussian generalized additive model with the 2-day moving average of PM2.5fitted the data very well (deviance = 0.102, df = 2463, p-value = 1), where the deviance measures the overall difference between the fitted values and the observed values of the response variable. The inverse Gaussian model provided a much better fit than the negative binomial regression (deviance =2525, df = 2464, p-value = 0.193); whereas the Poisson regression yielded a substantially larger deviance and failed to fit the data adequately (deviance = 358761, df = 2448, p-value < 0.001). Therefore, the inverse Gaussian generalized additive model was used in the subsequent analyses. All the covariates were statistically significant except for holiday weekend; therefore, it was removed from the subsequent analysis.
Table 2.
AIC scores for the Poisson, negative binomial and inverse Gaussian generalized additive model with log link function modeling the ILI incidence rate in association with PM2.5 interacting with flu season, while adjusting for daily temperature, humidity, month and year effects. The PM2.5 is lagged by 0, 1, 2, 3, 4, and 5 days prior to the ILI reporting date and lag01 (PM2.5 averaged over the current day and the previous day), lag 02 (PM2.5 averaged over the current day, the previous day and 2 days before the current day) and so on, up to mean lag05 (PM2.5 averaged over the past 6 days)
| lag | Inverse Gaussian | Negative binomial | Poisson |
|---|---|---|---|
| 0 | 36828 | 37244 | 381533 |
| 1 | 36842 | 37249 | 381169 |
| 2 | 36881 | 37284 | 385726 |
| 3 | 36913 | 37314 | 390007 |
| 4 | 36919 | 37320 | 390605 |
| 5 | 36912 | 37314 | 389934 |
| 01 | 36817 | 37237 | 381769 |
| 02 | 36818 | 37233 | 380303 |
| 03 | 36828 | 37243 | 381532 |
| 04 | 36840 | 37251 | 380915 |
| 05 | 36847 | 37256 | 381354 |
The bolded number indicates the smallest number in the table
The exposure–response relationships for PM2.5 (lag01) and ILI risk at the flu season (left panel of Fig. 2) suggested a very strong positive relationship between PM2.5 and ILI risk (p-value < 0.001), even after controlling for the weather conditions, seasonal and year trends. The estimated effect (slope) of PM2.5 was only marginal when PM2.5 was between 0 and about 70 and then increased sharply up until 200 and the trend tended to plateau between 200 and 300, which was followed by a sharp increase afterwards. We also observed a positive relationship between PM2.5 and ILI risk at the non-flu season (right panel of Fig. 2), but the effect was not statistically significant (p-value = 0.174), since all the pointwise 95 % confidence intervals covered zero and became very wide at higher values of PM2.5 because of limited data in this range (Table 1).
Fig. 2.
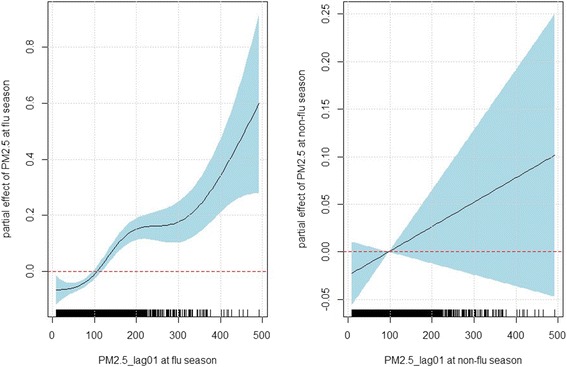
The panels display the estimated partial effect of 2-day moving average (current day to the previous day) of PM2.5 at the flu season (October-April) and non-flu season (May-September), based on the inverse Gaussian generalized additive model: The X-axis is the PM2.5 concentration (2-day moving average). The solid lines indicate the estimated log relative risk of ILI and the dashed lines indicate the corresponding 95 % confidence intervals
The ILI risk stayed high when temperature was below zero and then sharply decreased as temperature increased up until about 20 °C and then plateaued (p-value < 0.001), shown in the left panel of Fig. 3. The ILI risk also decreased with increased humidity (p-value < 0.001) (right panel of Fig. 3). Such inverse relationship between influenza risk and weather condition is consistent with other studies [40, 41], showing that wintertime cold temperatures increase respiratory morbidity and mortality, which is likely attributed to the fact that the virulence of influenza is expected to be stronger near zero than at subfreezing temperatures and a decrease in temperature makes airways more susceptible to the onset of respiratory infections [41].
Fig. 3.
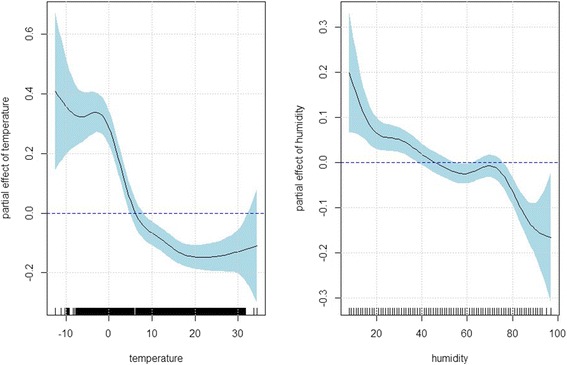
The panels display the estimated partial effect of temperature and humidity based on the inverse Gaussian generalized additive model: The x-axis tick labels in the panels represent the observed values temperature and humidity, respectively. The solid lines indicate the estimated log relative risk of ILI and the dashed lines indicate the corresponding 95 % confidence intervals
The left panel of Fig. 4 indicated that the ILI risk reached the peak during January and then decreased sharply until mid of February followed by a rapid upward trend during early spring and then increased steadily for the rest of the year. The steep drop might be due to large migration out of the city during the Spring festival, which is a traditional festival for family reunion in China. On average, the relative risk of ILI tended to be higher in years 2009, 2011 and 2012 as compared to 2008, 2013 and 2014 and year 2011 had the lowest ILI risk, shown in the right panel of Fig. 4.
Fig. 4.
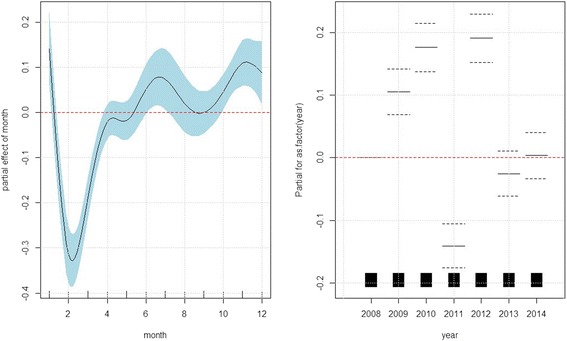
The panels display the estimated partial effect for month and year, based on the inverse Gaussian generalized additive model: The solid lines indicate the estimated log relative risk of ILI and the dashed lines indicate the corresponding 95 % confidence intervals. For the effect of year, year 2008 is set as baseline
We also investigated the effect of PM2.5 by flu season across different age groups. PM2.5 had almost no effect on influenza incidence across all age groups at the non-flu season (shown in the bottom panels in Fig. 5), which is consistent with the findings from the overall analysis previously stated. By contrast, at the flu season, the effect (slope) of PM2.5 was not significantly different from zero when PM2.5 was below about 70 μg/m3; whereas when PM2.5 was beyond 70 μg/m3, the effect of PM2.5 had an increasing gradient as PM2.5 increased. Such pattern was clearly more substantial in the middle aged groups and tended to be most pronounced for age group 25–59 (shown in the top panels of Fig. 5). In general, PM2.5 had the greatest effect sizes for adults (age 25–59), followed closely by young adults (age 15–24), and then elderly (age 60+) and school age children (age 5–14) and the PM2.5 has the least pronounced effect for the children under 5 years of age (age <5). To further compare the effect of PM2.5 across different age groups, Fig. 6 displayed the effect of PM2.5 with PM2.5 being set as 100 μg/m3 to 500 μg/m3 at an increment of 50 μg/m3. The graph demonstrated that log relative risk of ILI was not substantially different across all age groups when PM2.5 was below 250 μg/m3; whereas when PM2.5 was around 300 μg/m3, the log relative risk of ILI at age 25–59 remained to be highest compared with other age groups, followed closely by the age groups 15–24 and 60+. The width of the confidence intervals became larger as PM2.5 increased, due to the limited observations at the extremely large values of PM2.5.
Fig. 5.
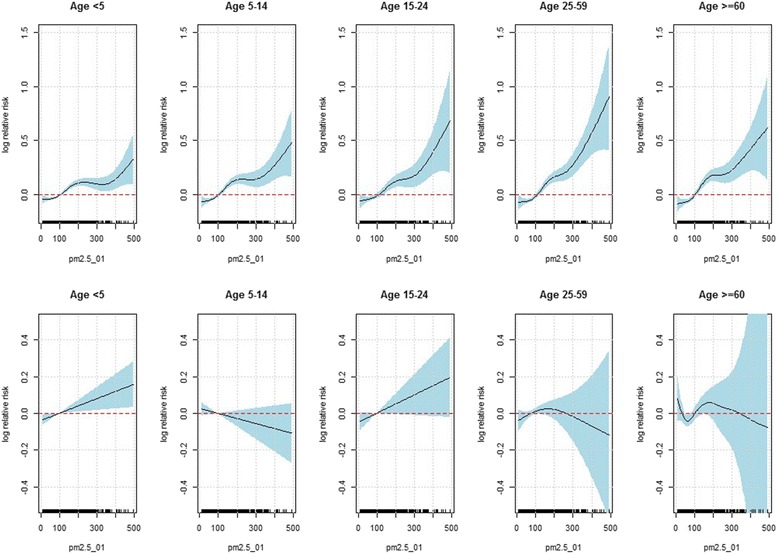
Estimated partial effect of PM2.5 based on the stratified analysis for each age group at the flu season (top panels) and non-flu season (bottom panels), based on the inverse Gaussian generalized additive model: The X-axis is the PM2.5 concentration (2-day moving average). The solid lines indicate the estimated log relative risk of ILI and the dashed lines indicate the corresponding 95 % confidence intervals
Fig. 6.
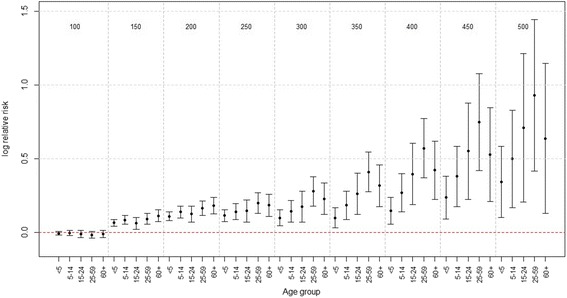
The log relative risk of ILI in association with PM2.5 when PM2.5 was set as 100 μg/m 3 to 500 μg/m 3 at an increment of 50 μg/m 3, by age groups, when all the other covariates were held at their mean levels
Discussion
This study is one of the few to investigate the association between PM2.5 and ILI. The results of the present study are based on one of the most extensive data sets used thus far in Beijing, China to assess the impact of PM2.5 on daily influenza. We provided evidence for the first time, to our knowledge, that PM2.5 is non-linearly associated with daily ILI risk based on an inverse Gaussian generalized additive model, after adjusting for the weather conditions, seasonal and year trend. We have assessed the potential lagged effect of PM2.5 on ILI risk and our study suggested that PM2.5 averaged over the current day and the previous day had the greatest predictive power.
Apart from the statistical evidence of PM exposure enhancing the risk of respiratory viral illness, there has been increasing evidence to support major hypotheses on the biological mechanism underlying this relationship [42–45]. Transmission of viruses via airborne routes is influenced by droplet suspended in the air and the droplet size determines whether the particle will quickly settle to an environmental surface or remain airborne long enough to be inhaled into the respiratory tract of a susceptible host. For example, a study has shown that airborne viruses may be transported by dust storms, which contains many PM [46]. In fact, PM is small enough to suspend in the air for long periods of time, which may provide “condensation nuclei” to which virus droplets attach [45]. Many studies have also reported that PM induces both airway epithelial damage and barrier dysfunction, which could result in a temporary immunosuppressive pulmonary microenvironment [42–45].
Further, the differential effect of PM2.5 at the flu season as compared with the non-flu season advances our further understanding of the biological mechanisms of the influenza transmission. Our findings indicated that the ILI risk increased progressively with increased PM2.5 at the flu season; whereas the effect of PM2.5 was not significant at the non-flu season. We speculate that at the flu season, the amount of viral load was sufficiently high and also the hosts were more susceptible through body cooling and/or drying of the respiratory tract. By contrast, at the non-flu season, even when PM2.5 was high, the hosts were not as susceptible to virus infection, might partly because the warmer temperature and higher humidity govern the hosts to be resistant to virus infection leading to the overall lower amount of viral load suspended in the air. As such, both the amount of viral load and pollutant emits as the viral agent could play crucial roles simultaneously determining how PM2.5 facilitates the influenza virus transmission.
The other novel contribution of our study is that we assessed the effect of PM2.5 on influenza risk by age groups. Our findings were consistent with other studies. For instance, a number of studies have concluded that PM2.5 components such as elemental carbon, organic carbon, nitrates, and sulfate were associated with higher proportions of respiratory conditions such as pneumonia, asthma, and bronchitis, based on the hospital admissions for respiratory conditions among children [47–49], in healthy or susceptible and occupational adults [50]. The finding was also consistent with previous studies on elderly populations reporting associations between PM2.5levels and hospitalizations for respiratory diseases, including respiratory tract infections, chronic obstructive pulmonary disease, and pneumonia [51, 52]. A number of research have been conducted to identify if there are certain subpopulations are particularly susceptible to PM10 and some research has shown that the effect of PM10 did not vary with age [53]; however, few studies have been conducted to evaluate if and how the effect of PM2.5 differs across different age groups. Our results indicated that at the flu season, PM2.5 was strongly associated with ILI risk across all age groups (p-value < 0.001), but PM2.5 had the greatest effect sizes for adults (age 25–59), followed by young adults (age 15–24), and then elderly (age 60+) and school age children (age 5–14) and the PM2.5 has the least pronounced effect for the children under 5 years of age (age <5). The difference in effect sizes across the age groups may be attributable to the exposure differences to the outdoor environments, such as home, schools, workplaces, vehicles, etc. Individuals aged between 25 and 59 or 15–24, who commute to work or school in personal vehicles or public transportation on roadways, are those mostly likely to be exposed to a substantial portion of their daily dose of air pollution during commuting activities [54, 55]. The high exposure to the outdoor pollutants for those people might partially explain why the effect of PM2.5 among those people is almost consistently more pronounced compared with other age groups. Interestingly, the number of ILI cases for the groups of individuals between 25 and 59 or 15–24 are markedly lower compared with the under 5 years of age group (Table 1). Similarly, the number of ILI cases for the elderly population is the lowest across all age groups, whereas the impact of PM2.5 is almost as high as the young adults and occupational adults. We hypothesize that the elderly people are mostly likely to suffer from chronic diseases, such as asthma, chronic obstructive pulmonary disease (COPD), diabetes, and cardiovascular disease, which may determine their susceptibility to the short-term exposure to elevated levels of air pollution [44, 56]. Such hypothesis is supported by toxicology experiments. For example, researchers have exposed normal and compromised rats to concentrated ambient particles drawn from the outside air in Boston. After three days of exposure to concentrated ambient particles (6 h per day at 228 to 288 μg/m3), mortality was 37 % among rats with induced chronic bronchitis, 19 % among rats with monocrotyline-induced inflammation, and 0 % among normal rats [6]. The elderly with pre-existing chronic respiratory diseases are therefore particularly venerable, since PM can increase oxidative stress, aggravate background inflammation and transient declines in lung function, leading to acute exacerbation of respiratory symptoms [42, 44, 57]. The effect of PM2.5 for the under 5 year of age group, albeit weaker, is interesting. The number of ILI cases for the under 5 years of age group is the highest among all the age groups (Table 1), since their lungs are still developing ability to fight off bacterial and viral infections, so they are more susceptible to influenza viruses. Youngsters are therefore urged to stay indoor during flu season to reduce their exposures to influenza viruses. Nevertheless, the effect of PM2.5 is still significantly associated with ILI for the children of age under 5, so exposure to outdoor pollutants must occur indoors for the association to be plausible. Ventilation modifies the ability of ambient particles to penetrate indoors. The fraction of the fine particles penetrate indoors as shown to range between 0.3 and 1.0 depending on home ventilation rates [58]. As such, lower exposure to PM2.5 for the children who most of the time staying indoors may lead to the reduced effect of PM2.5 on ILI incidence.
There are several limitations to our study. First, the PM2.5 data was based on only one monitoring site. Although it can reflect the exposure of the PM2.5 in Beijing in general, the PM2.5 varied from location to location. Future studies with multiple surveillance data are warranted, which could more accurately reflect the exposure. The ILI cases might be also underreported, since not all the patients visit the surveillance hospitals in the network and the people with mild influenza symptoms tend to visit the local physician or stay at home. In addition, misclassification of the influenza may occur, since the ILI symptoms are very similar to other respiratory diseases. Our current study suggested that PM2.5, as a specific component of the mixture of air pollutants is significantly associated with ILI. However, different pollution sources can be variably associated with different outcomes interactively and simultaneously and no single pollution source can be attributed to an outcome. A number of studies demonstrated that PM effect remained robust, after controlling the effects of other pollutants, such as the carbon monoxide (CO), sulfur dioxide (SO2), Nitrogen dioxide (NO2), or ozone (O3) [14, 20, 21, 59–62]. A recent systematic review was also conducted based on thirty-three time-series and case-crossover studies in the Chinese population reporting mortality effects of acute exposure to six air quality criteria pollutants including PM10, PM2.5, SO2, NO2, O3, and CO [63]. Their study suggested per 10 μg/m3 increases in PM10 and PM2.5, the summary risks of excess death increased 0.32 % (95 % CI: 0.23, 0.40) and 0.51 % (95 % CI: 0.30, 0.73) in respiratory mortality, after controlling for other pollutants. Although most studies consistently suggested the robust association of PM2.5 and adverse health outcomes, we recognize other pollutants could be responsible for the adverse health outcomes. Therefore, interpretation our current study should be cautious and replication of the study with more comprehensive exposure data is needed. Furthermore, the effects of PM2.5 could be a function of its complex chemical components and composition [64–66]. Therefore, further investigations to advance our understanding of the chemical constituents and sources of PM2.5 are warranted for designing effective emission control policies [67].
In addition, future estimates can be performed for different gender groups, provided that the population size for such groups can be retrieved or estimated. There are other potential sources of heterogeneity, e.g. ones having to do with the geographic distribution of participants and their underlying health conditions. Last, the present study is based on Beijing, and more extensive studies are warranted to ascertain how generalizable our results are to other regions. Given the gap in knowledge, our results provided a good starting point and a priori hypothesis for further studies. Further laboratory studies are in great need to understand the plausible mechanisms underlying the association; as well as longitudinal studies to confirm the causal relationship between exposure to PM2.5 and influenza onset.
Conclusions
Our novel findings suggested that residents in Beijing should be considered at increased risk of ILI during highly polluted days at the flu season (October-April). Such associations are not confounded by long-term time trends, or by weather conditions, all of which were properly controlled in the generalized additive model. While weather is an important predictor of ILI, there was no evidence in these analyses that the PM2.5 associations were confounded by weather. Furthermore, the effect of PM2.5 was strongly associated with ILI risk across all age groups, but the effect was the most pronounced among adults (age 25–59), followed by young adults (age 15–24), school children (age 5–14) and the elderly (age 60+) and the effect of PM2.5 was the least pronounced for the children under 5 years of age (age < 5). These findings provided an improved understanding of interplay of PM2.5 and influenza viruses at the flu season and how the effect of PM2.5 on ILI risk differed by age groups, which are of great importance in order to enhance the accuracy of surveillance systems, to have more precise predictions on influenza epidemics and pandemics in the future to help both environmental policy-making and public health preparedness.
Acknowledgments
The authors are grateful for insightful comments and suggestions from the reviewers. We also thank all the participants for their co-operation in the data collection. This study was supported by Beijing Municipal Science & Technology Commission (No.Z 131100005613048) and the Capital Health Research and Development of Special (2014-1-1011) and Natural Science and Engineering Research Council of Canada Discovery Grant (RGPIN 436102-2013).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CF, JL, WS, YZ and QW have made contributions to conception and design, acquisition of data, and analysis and interpretation of data; CF, JL and WS have been involved in drafting the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Contributor Information
Cindy Feng, Email: cindy.feng@usask.ca.
Jian Li, Email: jli8@tulane.edu.
Wenjie Sun, Email: wsun3@tulane.edu.
Yi Zhang, Email: zps347@163.com.
Quanyi Wang, Email: bjcdcxm@126.com.
References
- 1.Polichetti G, Cocco S, Spinali A, Trimarco V, Nunziata A. Effects of particulate matter (PM(10), PM(2.5) and PM(1)) on the cardiovascular system. Toxicology. 2009;261(1-2):1–8. doi: 10.1016/j.tox.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 2.Janssen NA, Fischer P, Marra M, Ameling C, Cassee FR. Short-term effects of PM2.5, PM10 and PM2.5-10 on daily mortality in The Netherlands. Sci Total Environ. 2013;463–464:20–26. doi: 10.1016/j.scitotenv.2013.05.062. [DOI] [PubMed] [Google Scholar]
- 3.Brunekreef B, Holgate ST. Air pollution and health. Lancet (London, England) 2002;360(9341):1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 4.Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect. 2005;113(8):934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Perspect. 2000;108(10):941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz J, Dockery DW, Neas LM. Is daily mortality associated specifically with fine particles? J Air Waste Manag Assoc. 1996;46(10):927–939. doi: 10.1080/10473289.1996.10467528. [DOI] [PubMed] [Google Scholar]
- 7.Polichetti G, Capone D, Grigoropoulos K, Tarantino G, Nunziata A, Gentile A. Effects of ambient air pollution on birth outcomes: an overview. Crit Rev Environ Sci Technol. 2013;43(7):752–774. doi: 10.1080/10643389.2011.627011. [DOI] [Google Scholar]
- 8.Atkinson RW, Fuller GW, Anderson HR, Harrison RM, Armstrong B. Urban ambient particle metrics and health: a time-series analysis. Epidemiology. 2010;21(4):501–511. doi: 10.1097/EDE.0b013e3181debc88. [DOI] [PubMed] [Google Scholar]
- 9.Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, Hoffmann B, Fischer P, Nieuwenhuijsen MJ, Brunekreef B, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14(9):813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 10.Chan TL, Lippmann M. Experimental measurements and empirical modelling of the regional deposition of inhaled particles in humans. Am Ind Hyg Assoc J. 1980;41(6):399–409. doi: 10.1080/15298668091424942. [DOI] [PubMed] [Google Scholar]
- 11.Qiu H, Yu IT, Tian L, Wang X, Tse LA, Tam W, Wong TW. Effects of coarse particulate matter on emergency hospital admissions for respiratory diseases: a time-series analysis in Hong Kong. Environ Health Perspect. 2012;120(4):572–576. doi: 10.1289/ehp.1104002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan AJ, Johnston SL. Air pollution and infection in respiratory illness. Br Med Bull. 2003;68:95–112. doi: 10.1093/bmb/ldg022. [DOI] [PubMed] [Google Scholar]
- 13.Maté T, Guaita R, Pichiule M, Linares C, Díaz J. Short-term effect of fine particulate matter (PM2.5) on daily mortality due to diseases of the circulatory system in Madrid (Spain) Sci Total Environ. 2010;408(23):5750–5757. doi: 10.1016/j.scitotenv.2010.07.083. [DOI] [PubMed] [Google Scholar]
- 14.Naess O, Nafstad P, Aamodt G, Claussen B, Rosland P. Relation between concentration of air pollution and cause-specific mortality: four-year exposures to nitrogen dioxide and particulate matter pollutants in 470 neighborhoods in Oslo, Norway. Am J Epidemiol. 2007;165(4):435–443. doi: 10.1093/aje/kwk016. [DOI] [PubMed] [Google Scholar]
- 15.Guaita R, Pichiule M, Mate T, Linares C, Diaz J. Short-term impact of particulate matter (PM(2.5)) on respiratory mortality in Madrid. Int J Environ Health Res. 2011;21(4):260–274. doi: 10.1080/09603123.2010.544033. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez E, Linares C, Rodriguez LF, Bleda MJ, Diaz J. Short-term impact of particulate matter (PM2.5) on daily mortality among the over-75 age group in Madrid (Spain) Sci Total Environ. 2009;407(21):5486–5492. doi: 10.1016/j.scitotenv.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 17.Gong P, Liang S, Carlton EJ, Jiang Q, Wu J, Wang L, Remais JV. Urbanisation and health in China. Lancet. 2012;379(9818):843-52. [DOI] [PMC free article] [PubMed]
- 18.Gao M, Guttikunda SK, Carmichael GR, Wang Y, Liu Z, Stanier CO, Saide PE, Yu M. Health impacts and economic losses assessment of the 2013 severe haze event in Beijing area. Sci Total Environ. 2015;511c:553–561. doi: 10.1016/j.scitotenv.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Han L, Zhou W, Li W, Li L. Impact of urbanization level on urban air quality: a case of fine particles (PM(2.5)) in Chinese cities. Environ Pollut (Barking, Essex : 1987) 2014;194:163–170. doi: 10.1016/j.envpol.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Wong CM, Ma S, Hedley AJ, Lam TH. Effect of air pollution on daily mortality in Hong Kong. Environ Health Perspect. 2001;109(4):335–340. doi: 10.1289/ehp.01109335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong CM, Yang L, Thach TQ, Chau PY, Chan KP, Thomas GN, Lam TH, Wong TW, Hedley AJ, Peiris JS. Modification by influenza on health effects of air pollution in Hong Kong. Environ Health Perspect. 2009;117(2):248–253. doi: 10.1289/ehp.11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang Y, Fang L, Pan H, Zhang K, Kan H, Brook JR, Sun Q. PM2.5 in Beijing - temporal pattern and its association with influenza. Environ Health. 2014;13:102. doi: 10.1186/1476-069X-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pope CA, 3rd, Dockery DW. Acute health effects of PM10 pollution on symptomatic and asymptomatic children. Am Rev Respir Dis. 1992;145(5):1123–1128. doi: 10.1164/ajrccm/145.5.1123. [DOI] [PubMed] [Google Scholar]
- 24.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3(10):1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaffer FL, Soergel ME, Straube DC. Survival of airborne influenza virus: effects of propagating host, relative humidity, and composition of spray fluids. Arch Virol. 1976;51(4):263–273. doi: 10.1007/BF01317930. [DOI] [PubMed] [Google Scholar]
- 26.Yang P, Thompson MG, Ma C, Shi W, Wu S, Zhang D, Wang Q. Influenza vaccine effectiveness against medically-attended influenza illness during the 2012-2013 season in Beijing, China. Vaccine. 2014;32(41):5285–5289. doi: 10.1016/j.vaccine.2014.07.083. [DOI] [PubMed] [Google Scholar]
- 27.Yang P, Duan W, Lv M, Shi W, Peng X, Wang X, Lu Y, Liang H, Seale H, Pang X, et al. Review of an influenza surveillance system, Beijing, People’s Republic of China. Emerg Infect Dis. 2009;15(10):1603–1608. doi: 10.3201/eid1510.081040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akaike H. Information theory and an extension of the maximum likelihood principle. In: Parzen E, Tanabe K, Kitagawa G, editors. Selected papers of Hirotugu Akaike. New York: Springer; 1998. pp. 199–213. [Google Scholar]
- 29.Agresti A. Categorical Data Analysis. NJ: Wiley & Sons; 2012.
- 30.Cox DR. Some remarks on overdispersion. Biometrika. 1983;70(1):269–274. doi: 10.1093/biomet/70.1.269. [DOI] [Google Scholar]
- 31.Johnson NL, Kemp AW, Kotz S. Univariate discrete distributions. 3. Hoboken, NJ: Wiley; 2005. [Google Scholar]
- 32.Tweedie MCK. Statistical properties of inverse Gaussian distributions. II. 1957. pp. 696–705. [Google Scholar]
- 33.Chhikara RS, Folks JL. Estimation of the inverse Gaussian distribution function. J Am Stat Assoc. 1974;69(345):250–254. doi: 10.1080/01621459.1974.10480165. [DOI] [Google Scholar]
- 34.Folks JL, Chhikara RS. The inverse Gaussian distribution and its statistical application--a review. J R Stat Soc Ser B Methodol. 1978;40(3):263–289. [Google Scholar]
- 35.Nelder JA, Wedderburn RWM. Generalized linear models. J R Stat Soc Ser A (General) 1972;135(3):370–384. doi: 10.2307/2344614. [DOI] [Google Scholar]
- 36.Wood S. Generalized additive models: an introduction with R. Boca Raton, Florida: Chapman and Hall/CRC; 2006.
- 37.Banks RB. Growth and diffusion phenomena: mathematical frameworks and applications. Berlin: Springer; 1994. [Google Scholar]
- 38.Gruebler A, Nakicenovic N. Diffusion of technologies and social behavior. Berlin: Springer; 1991. [Google Scholar]
- 39.Marchetti C, Meyer PS, Ausubel JH. Human population dynamics revisited with the logistic model: how much can be modeled and predicted? Technol Forecast Soc Change. 1996;52(1):1–30. doi: 10.1016/0040-1625(96)00001-7. [DOI] [PubMed] [Google Scholar]
- 40.van Noort SP, Aguas R, Ballesteros S, Gomes MG. The role of weather on the relation between influenza and influenza-like illness. J Theor Biol. 2012;298:131–137. doi: 10.1016/j.jtbi.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 41.Jaakkola K, Saukkoriipi A, Jokelainen J, Juvonen R, Kauppila J, Vainio O, Ziegler T, Ronkko E, Jaakkola JJ, Ikaheimo TM. Decline in temperature and humidity increases the occurrence of influenza in cold climate. Environ Health. 2014;13(1):22. doi: 10.1186/1476-069X-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donaldson K, Tran CL. Inflammation caused by particles and fibers. Inhal Toxicol. 2002;14(1):5–27. doi: 10.1080/089583701753338613. [DOI] [PubMed] [Google Scholar]
- 43.Li XY, Gilmour PS, Donaldson K, MacNee W. Free radical activity and pro-inflammatory effects of particulate air pollution (PM10) in vivo and in vitro. Thorax. 1996;51(12):1216–1222. doi: 10.1136/thx.51.12.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim CS, Kang TC. Comparative measurement of lung deposition of inhaled fine particles in normal subjects and patients with obstructive airway disease. Am J Respir Crit Care Med. 1997;155(3):899–905. doi: 10.1164/ajrccm.155.3.9117024. [DOI] [PubMed] [Google Scholar]
- 45.Lee GI, Saravia J, You D, Shrestha B, Jaligama S, Hebert VY, Dugas TR, Cormier SA. Exposure to combustion generated environmentally persistent free radicals enhances severity of influenza virus infection. Part Fibre Toxicol. 2014;11:57. doi: 10.1186/s12989-014-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen PS, Tsai FT, Lin CK, Yang CY, Chan CC, Young CY, Lee CH. Ambient influenza and avian influenza virus during dust storm days and background days. Environ Health Perspect. 2010;118(9):1211–1216. doi: 10.1289/ehp.0901782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostro B, Roth L, Malig B, Marty M. The effects of fine particle components on respiratory hospital admissions in children. Environ Health Perspect. 2009;117(3):475–480. doi: 10.1289/ehp.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yap P-S, Gilbreath S, Garcia C, Jareen N, Goodrich B. The influence of socioeconomic markers on the association between fine particulate matter and hospital admissions for respiratory conditions among children. Am J Public Health. 2013;103(4):695–702. doi: 10.2105/AJPH.2012.300945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oftedal B, Brunekreef B, Nystad W, Madsen C, Walker SE, Nafstad P. Residential outdoor air pollution and lung function in schoolchildren. Epidemiology. 2008;19(1):129–137. doi: 10.1097/EDE.0b013e31815c0827. [DOI] [PubMed] [Google Scholar]
- 50.Mu L, Deng F, Tian L, Li Y, Swanson M, Ying J, Browne RW, Rittenhouse-Olson K, Zhang JJ, Zhang ZF, et al. Peak expiratory flow, breath rate and blood pressure in adults with changes in particulate matter air pollution during the Beijing Olympics: a panel study. Environ Res. 2014;133:4–11. doi: 10.1016/j.envres.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delfino RJ, Murphy-Moulton AM, Becklake MR. Emergency room visits for respiratory illnesses among the elderly in Montreal: association with low level ozone exposure. Environ Res. 1998;76(2):67–77. doi: 10.1006/enrs.1997.3794. [DOI] [PubMed] [Google Scholar]
- 53.Zanobetti A, Schwartz J, Gold D. Are there sensitive subgroups for the effects of airborne particles? Environ Health Perspect. 2000;108(9):841–845. doi: 10.1289/ehp.00108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Nazelle A, Nieuwenhuijsen MJ, Anto JM, Brauer M, Briggs D, Braun-Fahrlander C, Cavill N, Cooper AR, Desqueyroux H, Fruin S, et al. Improving health through policies that promote active travel: a review of evidence to support integrated health impact assessment. Environ Int. 2011;37(4):766–777. doi: 10.1016/j.envint.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Zuurbier M, Hoek G, Oldenwening M, Lenters V, Meliefste K, van den Hazel P, Brunekreef B. Commuters’ exposure to particulate matter air pollution is affected by mode of transport, fuel type, and route. Environ Health Perspect. 2010;118(6):783–789. doi: 10.1289/ehp.0901622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simoni M, Baldacci S, Maio S, Cerrai S, Sarno G, Viegi G. Adverse effects of outdoor pollution in the elderly. J Thorac Dis. 2015;7(1):34–45. doi: 10.3978/j.issn.2072-1439.2014.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacNee W, Donaldson K. Mechanism of lung injury caused by PM10 and ultrafine particles with special reference to COPD. Eur Respir J Suppl. 2003;40:47s–51s. doi: 10.1183/09031936.03.00403203. [DOI] [PubMed] [Google Scholar]
- 58.Nieuwenhuijsen MJ. Exposure assessment in environmental epidemiology: Oxford, United Kingdom: Oxford University Press; 2015.
- 59.Yang Y, Li R, Li W, Wang M, Cao Y, Wu Z, Xu Q. The association between ambient air pollution and daily mortality in Beijing after the 2008 olympics: a time series study. PLoS One. 2013;8(10):e76759. doi: 10.1371/journal.pone.0076759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersen ZJ, Wahlin P, Raaschou-Nielsen O, Scheike T, Loft S. Ambient particle source apportionment and daily hospital admissions among children and elderly in Copenhagen. J Expo Sci Environ Epidemiol. 2007;17(7):625–636. doi: 10.1038/sj.jes.7500546. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz J. Is the association of airborne particles with daily deaths confounded by gaseous air pollutants? An approach to control by matching. Environ Health Perspect. 2004;112(5):557–561. doi: 10.1289/ehp.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Linares C, Díaz J. Short-term effect of concentrations of fine particulate matter on hospital admissions due to cardiovascular and respiratory causes among the over-75 age group in Madrid. Spain Public Health. 2010;124(1):28–36. doi: 10.1016/j.puhe.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Shang Y, Sun Z, Cao J, Wang X, Zhong L, Bi X, Li H, Liu W, Zhu T, Huang W. Systematic review of Chinese studies of short-term exposure to air pollution and daily mortality. Environ Int. 2013;54:100–111. doi: 10.1016/j.envint.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Ye B, Ji X, Yang H, Yao X, Chan CK, Cadle SH, Chan T, Mulawa PA. Concentration and chemical composition of PM2.5 in Shanghai for a 1-year period. Atmos Environ. 2003;37(4):499–510. doi: 10.1016/S1352-2310(02)00918-4. [DOI] [Google Scholar]
- 65.Sun Y, Zhuang G, Wang Y, Han L, Guo J, Dan M, Zhang W, Wang Z, Hao Z. The air-borne particulate pollution in Beijing—concentration, composition, distribution and sources. Atmos Environ. 2004;38(35):5991–6004. doi: 10.1016/j.atmosenv.2004.07.009. [DOI] [Google Scholar]
- 66.Duan FK, He KB, Ma YL, Yang FM, Yu XC, Cadle SH, Chan T, Mulawa PA. Concentration and chemical characteristics of PM2.5 in Beijing, China: 2001–2002. Sci Total Environ. 2006;355(1–3):264–275. doi: 10.1016/j.scitotenv.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 67.He K, Yang F, Ma Y, Zhang Q, Yao X, Chan CK, Cadle S, Chan T, Mulawa P. The characteristics of PM2.5 in Beijing, China. Atmos Environ. 2001;35(29):4959–4970. doi: 10.1016/S1352-2310(01)00301-6. [DOI] [Google Scholar]


