Review on formyl-peptide receptors.
Keywords: leukocytes, trafficking, inflammation, immunity, cancer
Abstract
Formyl-peptide receptors are a family of 7 transmembrane domain, Gi-protein-coupled receptors that possess multiple functions in many pathophysiologic processes because of their expression in a variety of cell types and their capacity to interact with a variety of structurally diverse, chemotactic ligands. Accumulating evidence demonstrates that formyl-peptide receptors are critical mediators of myeloid cell trafficking in the sequential chemotaxis signal relays in microbial infection, inflammation, and immune responses. Formyl-peptide receptors are also involved in the development and progression of cancer. In addition, one of the formyl-peptide receptor family members, Fpr2, is expressed by normal mouse-colon epithelial cells, mediates cell responses to microbial chemotactic agonists, participates in mucosal development and repair, and protects against inflammation-associated tumorigenesis. These novel discoveries greatly expanded the current understanding of the role of formyl-peptide receptors in host defense and as potential molecular targets for the development of therapeutics.
Introduction
Leukocyte infiltration is a hallmark of inflammation, immune responses, and cancer and is critical for disease progression and resolution. Leukocyte trafficking and homing are mediated mainly by 2 families of GPCRs: 1 that recognizes chemokines, and 1 that recognizes classic chemoattractants derived from pathogens, damaged host tissues, and tumors [1–4]. FPRs belong to the family of classic chemoattractant GPCRs. Compared with other chemoattractant receptors, FPRs exhibit unique properties in the number of variants and the spectrum of ligands they interact with. The number and the sequences of genes coding for FPR members vary considerably among mammalian species. The human FPR family has 3 members, FPR1, FPR2, and FPR3 (formerly FPR, FPRL1, and FPRL2, respectively) [5–8]. The mouse FPR (mFPR or Fpr) gene family consists of at least 8 members [9]. mFPR1, now officially termed Fpr1, is considered the mouse ortholog of human FPR1, whereas Fpr2 is structurally and functionally most similar to human FPR2 [10]. The other 6 murine Fpr genes are expressed in leukocytes, but their encoded receptors remain unknown [8].
A prominent feature of FPR family members is their ligand diversity, which includes a variety of structurally diverse ligands [8, 11]. Therefore, FPRs (Fprs) are also considered as a class of PRRs that interact with either pathogen-associated chemotactic ligands (chemotactic PAMPs), or DAMPs. With the availability of genetically engineered mouse strains deficient in one or more Fprs, the critical roles of FPRs (Fprs) in diseases are increasingly being recognized [8]. These receptors are found to not only mediate leukocyte trafficking in disease states but also promote myeloid cell differentiation, colon epithelial homeostasis, and cancer progression. Therefore, a better understanding of the biologic significance of FPRs should have important clinical relevance. This review will focus on some recent developments in FPR studies. The readers are recommended to refer to other excellent reviews for more aspects of FPRs [6, 8, 9, 12].
THE PRR PROPERTIES OF FPRs AND IMPLICATIONS IN HOST DEFENSE
FPR1 and FPR2 [8, 9] were originally identified based on their capacity to recognize N-formyl peptides produced in nature by the degradation of either bacterial or host cell mitochondrial proteins, such as N-formyl peptides, which represent a major byproduct of bacterial and mitochondrial metabolism [13, 14]. As such, these peptides are not only ubiquitous in the context of inflammation and infection but are highly diverse structurally and functionally [9, 15]. These peptides act as danger signals, capable of alerting the immune system to elevated levels of cell death or to exposure to pathogenic bacteria. This is supported by the findings that both Fpr1- and Fpr2-deficient mice display increased susceptibility to microbial, such as Listeria monocytogenes infection [16, 17].
In a mouse septic-syndrome model, Fpr1 was critical in mediating neutrophil accumulation in response to circulating mitochondrial peptides [18], and in a liver sterile-injury model, it acted as an essential mediator of neutrophil accumulation, subsequent to chemokine GPCRs, in the necrotic center of the wound [19]. Consistent with its capacity to recognize bacterial chemotactic PAMPs, Fpr1 acts as a major participant in the host–commensal interaction during dysbiosis, as demonstrated in acute Toxoplasma gondii gastrointestinal infection of mice in which the control of commensal outgrowth was a highly coordinated process involving both the host response and microbial signals. Notably, neutrophil infiltration into the intestinal lumen results in the generation of organized, intraluminal structures that encapsulate commensals and limit their contact with the epithelium. Formation of these “luminal casts” depends on Fpr1 and, consequently, after infection, mice-deficient in Fpr1 display increased microbial translocation, poor commensal containment, and increased mortality [20].
One of the recent progresses in studies of FPRs is the identification of many host-derived agonist peptides as chemotactic DAMPs for FPRs, including SAA, Aβ42, LL-37, and a neutrophil granule protein, cathepsin G [21–26]. SAA is an acute-phase protein, which, by interacting with FPR2, converts neutrophils from a protumor to an antitumor phenotype [27, 28]. Aβ42 is a major causative factor of brain inflammation in Alzheimer disease [24, 29]. LL-37 and its mouse homolog CRAMP are antibacterial and also have alarmin activity. Recognition of LL-37 by FPR2 on tumor cells promotes angiogenesis by recruiting BM mesenchymal stem cells into the stroma of human ovarian cancer xenografts [30]. The biologic significance of cathepsin G as an FPR1 ligand remains unclear.
Another chemotactic DAMP, originally reported as an FPR1 ligand, Anx A1, was initially identified as an anti-inflammatory protein because of its capacity to retain neutrophils in blood vessels during inflammatory responses. However, Anx A1 was also found to increase the invasiveness of certain tumor cells by interacting with both FPR1 and FPR2 [31]. In addition, Anx A1 was shown as a major chemoattractant released by necrotic human GBM cells to activate FPR1 on live GBM cells [21]. Table 1 is an as-yet-incomplete list of important chemotactic PAMPs and DAMPs reported to date for FPRs (Fprs). The list also includes an FPR3 (FPRL2) ligand, F2L, which is a chemotactic peptide fragment derived from heme-binding protein that chemoattracts DCs [38], with a biologic role in vivo that is yet to be defined. Unlike FPR1 and FPR2, the expression of FPR3 is rather limited to monocytes and DCs and is highly phosphorylated and is more localized to small intracellular vesicles. This suggests that FPR3 rapidly internalizes after binding its ligands and, thereby, may serve as a “decoy” receptor [40, 41]. The mouse counterpart of FPR3 is not very clear at this point. Mouse neutrophils were chemoattracted by F2L. However, the cells from Fpr2 knockout mice totally lost chemotactic response to F2L, suggesting that mouse Fpr2 may function as both human FPR2 and FPR3 [39].
TABLE 1.
The ligand promiscuity of FPRs
| Source agonists | Diseases | FPR1 (Fpr1) | FPR2 (Fpr2) | FPR3 (Fpr2) | References |
|---|---|---|---|---|---|
| Bacteria | |||||
| fMLF (E.coli) | Infection | ++++ | ++ | [5, 9] H and M | |
| Listeria peptides | Infection | ++++ | +++ | [17, 32] M | |
| Helicobacter pylori (2–20) | Stomach cancer | +++ | ++ | [8, 9] H | |
| Host | |||||
| Mitochondrial PEP | Sepsis, injury | +++ | ++++ | [13, 14, 18] H and M | |
| LL37 (mouse CRAMP) | Host defense | +++ | [25] H, [26, 33] M | ||
| Cathepsin G | Host defense | +++ | [22] H | ||
| SAA | Inflammation | ++++ | [23] H, [28] M | ||
| Aβ42 | Alzheimer disease | ++++ | [24] H, [29] M | ||
| Prion protein 106–126 | Prion dis | +++ | [24] H | ||
| Anx A1 | Wound healing | ++ | +++ | ++ | [21, 31, 34] H, [35, 36] M |
| uPAR fragment | Coagulation | +++ | [37] H | ||
| F2L | DC migration | ++ | +++ | [38] H, [39] M |
FPRs recognize many pathogen- and host-derived chemotactic peptides associated with inflammation and cancer. +, relative potency of interaction; H, human FPRs; M, mouse Fprs; PEP, phosphoenolpyruvate; uPAR, urokinase plasminogen activator receptor.
Human FPR2 and its mouse counterpart Fpr2 have also been reported to interact with lipid-mediator LXA4 to either exert a proinflammatory or resolving activity in host responses in stress. The exact mechanistic basis for the dual role of LXA4 in host defense is not well established because the mediator has not been unanimously confirmed to be a bona fide FPR2 (Fpr2) agonist, and it has also been reported to interact with other cell surface or intracellular receptors, such as leukotriene receptors and aryl hydrocarbon receptor. Because of its unique physicochemical nature, some laboratories have not been able to verify LXA4 as an agonist for FPR2 or Fpr2 either for proinflammatory or anti-inflammatory activity. Therefore, despite the initial definition of FPR2 as ALX to indicate its identity as a LXA4 receptors, the controversy persists and should require a cooperative and rigorous effort to definitely confirm or disqualify LXA4 as an FPR2 (Fpr2) agonist.
It is conceivable that with the progress of more rigorous studies, additional chemotactic PAMPs and DAMPs are likely to be added to the list to better explain the complexity of the nature of FPRs.
PARTICIPATION OF FPRs IN ORCHESTRATED LEUKOCYTE TRAFFICKING
It has been realized that both physiologic and pathologic trafficking of leukocytes in vivo is mediated by >1 sequentially expressed chemoattractant GPCRs on the cell surface regulated by differentiation or maturation stimulants in the microenvironment. Recent studies with genetically engineered mice in a variety of disease models have positioned FPRs (Fprs) in the process of leukocyte sensing of chemotactic cues, established either by chemotactic PAMPs or DAMPs, in bacterial infection, immune responses, and wound healing.
Fpr1/Fpr2–CXCR2-mediated neutrophil recruitment in the livers of mice infected with Listeria monocytogenes
Listeria species is an opportunistic pathogen that causes severe infections in immunocompromised individuals [42]. The incidence of listeriosis in human is low [43, 44], but the lethality rate is as high as 30% in infected patients [45, 46]. Listeria sp. enters a variety of mammalian cells in which the bacteria replicate and spread from one cell to the next to escape host immune surveillance [47–54]. The resistance to Listeria sp. infection is dependent on mobilization of immune responses, in particular neutrophils, into the infected site as the first and key step of host defense [50, 55, 56]. A classic paradigm defines one of the PRRs, TLR2, as a key mediator of host resistance by interacting with bacteria lipoprotein to directly promote the transcription of proinflammatory cytokines and chemokines through inflammasome pathways in immune cells to initiate host responses, in which chemokines CXCL1/2 are believed to be responsible for rapid neutrophil accumulation at the site of infection (Fig. 1) [57].
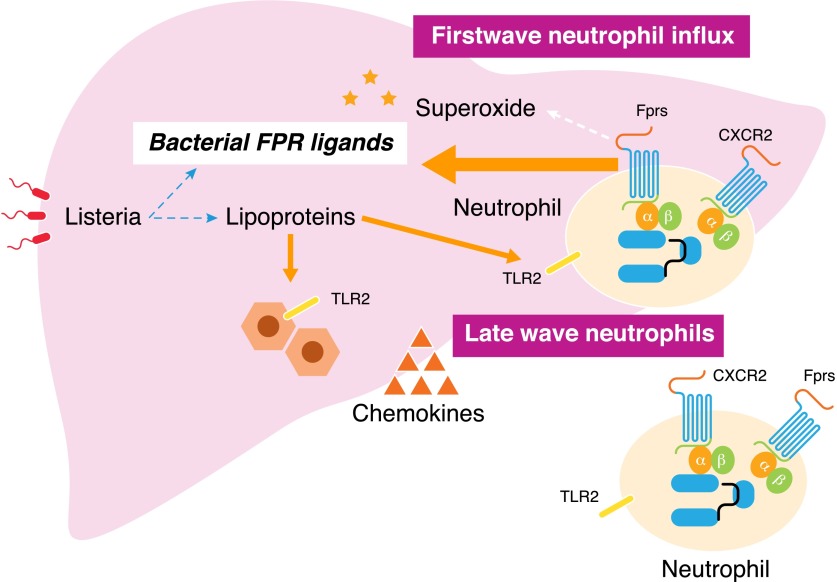
Figure 1. FPRs control the first wave neutrophil infiltration in Listeria-infected liver.
Both Fpr1 and Fpr2 in mice sense bacterial chemoattractants to directly mediate a rapid (within 30 min) neutrophil influx into Listeria-infected liver. Listerial lipoproteins activate TLR2 expressed by hepatocytes and leukocytes to enhance the production of CXCR2-specific chemokines to elicit a late wave (after 4 h) neutrophil recruitment in the liver. Neutrophils activated by bacterial FPR ligands produce superoxide critical for bacteria elimination.
It is, therefore, perplexing and surprising that deficiency in either Fpr1 or Fpr2 exacerbated the severity of Listeria sp. infection of mice [17]. A recent study aimed at elucidating the role of Fprs in neutrophil accumulation in Listeria infection reveals that neutrophil accumulation in the liver of WT mice with i.v. administration of Listeria initiates at as early as <30 min after infection and reaches its maximum at 4 h. In contrast, the neutrophil-specific chemokines CXCL1/2, which are implicated in the TLR2-mediated proinflammatory cascade [58–65] in listerial infection, are barely detectable in the liver 30 min after infection. The appearance of CXCL1/2 in the liver is detected starting at 4 h after infection, a time point far beyond the appearance of neutrophils in the liver. Interestingly, in Fpr-deficient mice, although the production of CXCL1/2 in Listeria-infected mouse liver showed kinetics and magnitude similar to that in WT mice, the early phase neutrophil accumulation is markedly reduced in either Fpr1- or Frp2-deficient mice and is almost completely absent in mice-deficient in both Fprs. Further studies show that Listeria produces chemotactic agonists for both Fpr1 and Fpr2, consistent with earlier findings that synthetic peptides based on the putative Listeria product sequences are potent neutrophil chemoattractants by interacting with both human and mouse FPRs [32]. Therefore, Fpr-deficient mice suffer from increased bacterial load in the liver and markedly reduced production of H2O2 by neutrophils in response to the bacteria, in association with compromised bacterial killing and markedly accelerated mortality.
Therefore, Fprs antecede CXC chemokines in sensing Listeria-derived chemotactic PAMPs to rapidly mediate neutrophil recruitment into the infected liver. This process should be important for bringing in many neutrophils to enable their responses to Listeria lipoproteins, potent PAMPs for TLR2. Thus, Fprs belong in the paradigm of host resistance to Listeria infection as one of the frontline sentinels responding to the invading pathogen (Fig. 1). However, whether this revised paradigm is applicable to infection by species other than Listeria is unclear. Considering the capacity of FPRs to recognize a broad range of chemotactic PAMPs (Table 1.), it is not surprising to note that recent studies have also implicated FPRs in infection models of pneumococcal meningitis [66] by recruiting early neutrophil infiltration in response to bacteria chemoattractants.
CCR2-Fpr2–CCR7 participates in the stepwise trafficking of DCs in allergic airway inflammation
DCs are critical in airway inflammatory responses [67, 68]. During viral infection, allergen challenge, or endotoxin inhalation, CD11b+ monocyte-derived DCs are rapidly recruited from the circulation into the airway. These cells are of monocytic origin [68, 69], which initiates and accounts for the severity of allergic airway inflammation. In mice, Ly6Chigh conventional monocytes are CX3CR1low, CCR2+, CD62L+, and CCR5−, and under inflammatory conditions, they differentiate into inflammatory DCs and acquire the capacity to prime T cell–mediated immune responses in draining lymph nodes [70]. In the lung, inflammatory stimuli, such as TLR PAMPs or exposure to environmental pollutants, trigger the production of chemokines that have been implicated in recruitment of inflammatory DCs in a CCR2-dependent manner [68, 69, 71, 72], via interaction with endogenous CCL2. However, other chemokine receptors have also been implicated in the recruitment of monocyte-derived inflammatory DCs into the lung, as evidenced by observations showing that CCR5- and CCR6-deficient mice have reduced cigarette smoke inhalation–induced airway inflammation [73, 74], suggesting a more complicated DC traffic pattern based on the context of the causes of the inflammatory syndrome. After trafficking of DCs precursors into the inflammatory airway, TLR agonist PAMPs or DAMPs rapidly down-regulate the function of chemokine GPCRs expressed on DCs [75], and further cell trafficking to the lymphatic tissues is mediated by then up-regulated CCR7 [76]. Thus, chemokine GPCR-mediated inflammatory DC trafficking has been accepted as the means by which DCs are recruited and directed to complete their journey from an innate arm to the adaptive arm of immune responses. Once DCs are mature and express CCR7, they can migrate from sites of innate inflammation to draining lymph nodes to stimulate T cells and to initiate adaptive immunity.
However, the model of chemokine GPCRs alone in DC trafficking in allergic airway inflammation is inadequate and has recently had to incorporate one of the FPRs, Fpr2, as an indispensible link in the chain of events. This has been shown by greatly reduced severity of OVA-elicited allergic airway inflammation in Fpr2-deficient mice [77], in association with a marked reduction of infiltration of Ly6C+ monocyte-derived inflammatory DCs in the small airways and a subsequent lack of DCs in the T cell zones in the draining mediastinal lymph nodes. These observations raise the possibility that Fpr2 might be an active participant in the sequential chemoattractant signal relay required for the trafficking of Ly6C+ monocyte-derived inflammatory DCs in the inflamed lung. In fact, the role of CCR2, Fpr2, and CCR7 in DC trafficking is tightly orchestrated from circulation via airways to the lymph nodes [33]. This updated DC trafficking model illustrates the necessity for CCR2 to mobilize Ly6Chigh monocytic DC precursors from the BM into the circulation [78, 79], where the cells undergo extravasation into the perivascular regions of inflamed lung and become immature DCs upon exposure to DAMPs present in the airway. The immature DCs lose the expression of functional CCR2, but gain high-level expression of Fpr2, which guides the cells into the peribronchiolar regions in response to a host-derived chemotactic DAMP—CRAMP [33]—which not only forms a chemotactic gradient cue to mediate DC trafficking but also is capable of promoting DC maturation stimulated by TLR ligand PAMPs [80]. A shift of the chemoattractant GPCR expression occurs again as DCs mature; the Fpr2 is down-regulated, but the DC homing chemokine GPCR, CCR7, is highly elevated and enables the matured DCs to be directed into lymphatic organs. Thus, DC trafficking in the inflammatory airway consists of a fine-tuned functional relay of chemoattractant GPCRs on the cell surface, progressing from CCR2, with Fpr2 as an intermediate, and CCR7 as the final player to complete the last segment of homing (Fig. 2). These discoveries, therefore, are the basis for a modified model of DC trafficking in airway inflammation in which CCR2, Fpr2, and CCR7 sequentially respond to their respective endogenous ligands resulting in the initiation, amplification, and resolution of the host responses. Despite the high homology of Fpr1 with Fpr2, Fpr1 does not share with Fpr2 the endogenous chemotactic DAMP peptide CRAMP; therefore, its role in allergic airway inflammation remains unclear and requires further investigation.
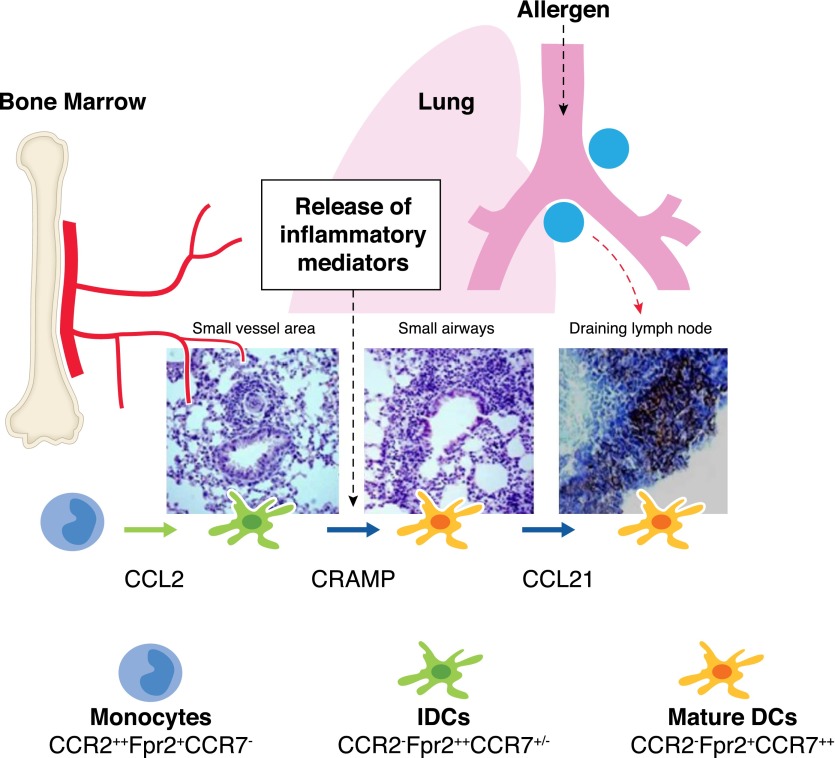
Figure 2. Sequential sensing of chemotactic cues in the tissue by Fpr2 and chemokine GPCRs by inflammatory DCs in allergic airway inflammation.
The chemokine receptor CCR2 mobilizes inflammatory DC precursors from the circulation to the perivascular region of the inflamed lung in response to the cognate ligand CCL2. Once in the perivascular regions, DCs express high levels of Fpr2 with down-regulation of CCR2, presumably in response to environmental inflammatory stimuli, such as PRR ligands and cytokines. Fpr2 then mediates DC trafficking to the peribronchiolar areas in response to the endogenous Fpr2 ligand CRAMP, which is increased in the lung during inflammatory responses. In the peribronchiolar areas, inflammatory DCs undergo full maturation in association with the expression of high levels of the chemokine GPCR CCR7 for further homing into the draining lymph nodes to elicit adaptive immune responses.
In addition to sequential chemoattractant GPCR expression induced by microenvironmental stimulants, DC trafficking in vivo may use multiple models that also include the potential involvement of GPCR heterologous desensitization, in which activation of one GPCR may cause a temporary unresponsiveness in another GPCR, despite its normal-level expression on the cell surface and the presence of its cognate ligand or ligands [81, 82]. It is, therefore, important to study the pattern of GPCR requirement for DC trafficking in individual disease states to avoid generalization of a single model. In this context, use of mice-deficient in single or multiple receptors should be most helpful, but caution is needed concerning whether deletion of one GPCR gene may affect the expression and function of another GPCR.
Cooperation of Fpr2 with other GPCRs in wound healing
Neutrophil recruitment from blood to extravascular sites of sterile or infectious tissue injury is a hallmark of early innate immune responses, and the molecular events leading to cell exit from the bloodstream have not been well-defined [67, 68]. Once outside the vessel, individual neutrophils often show strikingly coordinated chemotaxis and cluster formation, simulating the swarming behavior of insects [69–76, 83]. A 2-photon, intravital microscopy study recently revealed the molecular nature of the players that mediate neutrophil responses on the levels of a single cell and a population within the complex space of inflamed skin tissue in a mouse ear. This defines a critical role for intercellular signal networking among neutrophils mediated by LTB4, which rapidly amplifies acute inflammatory responses to local cell death in laser beam–inflicted wounds, resulting in an enhanced radius of highly directed, interstitial neutrophil recruitment. Integrin receptors are dispensable for long-distance cell migration [84] but have a role in maintaining dense cellular clusters when neutrophil congregates rearrange the collagen-fiber network in the dermis to form a collagen-free zone at the wound center. In this newly formed environment, integrins in concert with neutrophil-derived LTB4 and other chemoattractants, presumably CRAMP and CXCL2 that use Fpr2 and CXCR2 promote local neutrophil accumulation while forming a tight wound seal whose borders cease growing in concert with late recruitment of monocytes and macrophages at the edge of the displaced collagen fibers. These observations identify the factors that contribute to neutrophil swarming in the extravascular space of damaged skin tissue and reveal how local events are propagated over large distances. Consequently, sequential signaling mediated by chemoattractant GPCRs—Ltb4r1, Fpr2, and CXCR2—coordinates an organized neutrophil swarming that isolates the injured site from surrounding viable tissue [85], which may be important for the initiation of a healing process.
In another skin-wound healing model, Fprs seem to act as the first player in the chemotaxis signal relay, resulting in rapid neutrophil infiltration [86]. The study shows that, in normal mice, neutrophils infiltrate the dermis in the wound before the production of neutrophil-specific CXC chemokines by the injured tissue. In contrast to normal mice, the early neutrophil infiltration is markedly reduced in mice-deficient in both Fprs in association with delayed wound closure. The critical role of Fprs in wound healing is based on the findings that skin wound tissues contain chemotactic DAMPs (Table 1.) that chemoattract FPR expressing neutrophils. Therefore, Fprs are critical for normal healing of the sterile skin wound by mediating neutrophil infiltration [86].
Overall, these observations suggest the complexity of the contribution of FPRs (Fprs) to the healing of wounds at different anatomic sites and that careful consideration is required to target these receptors to either accelerate the healing or to prevent excessive inflammatory responses, which may cause greater damage to vital organs [87].
THE ROLE OF FPR2 IN THE MAINTENANCE OF COLON MUCOSAL HOMEOSTASIS AND PROTECTION AGAINST INFLAMMATION-ASSOCIATED TUMORIGENESIS
Although FPRs were first identified on phagocytic leukocytes, these receptors are also expressed by many nonhematopoietic cells, including intestinal epithelial cells and have important roles in colon mucosal homeostasis, inflammatory responses, and tumorigenesis.
UC is associated with an elevated risk for colorectal cancer [88]. It is believed that the chronic inflammatory process associated with UC is responsible for the neoplastic transformation of the intestinal epithelium [89]. Proinflammatory cytokines and chemokines, as well as matrix-degrading enzymes, growth factors, and reactive oxygen species present in the tissue microenvironment of UC, enhance epithelial cell proliferation, abnormal cell turnover, leukocyte infiltration, and angiogenesis, culminating in mutagenesis and tumor formation [90, 91]. The association of UC with cancer involves inflammation of the submucosa of the colon, induced by direct contact with the intestinal microorganisms that promote tumor growth in the overlaying epithelium [92]. Therefore, the capacity of the intestinal epithelial layer to cope with the intestinal microbiota is important not only for limiting inflammation but also for preventing tumorigenesis in the colon.
FPR1 has been shown to localize along the lateral membrane of crypt epithelial cells in the human colon. Activation of FPR1 by bacterial fMLF promotes epithelial growth and restoration of the mucosal integrity [93]. In mice, some chemotactic PAMPs and DAMPs, such as Anx A1, fMLF, and viable Lactobacillus rhamnosus GG, stimulate Fpr1 on intestinal epithelial cells, and this leads to the generation of reactive oxygen species via enterocyte NADPH oxidase 1, resulting in rapid phosphorylation of focal adhesion kinase and ERK/MAPK [34]. Activated FPR1 also mediates the migration and proliferation of enterocytes adjacent to colonic wounds [94]. Intestinal crypts of the Fpr1-deficient mice contain increased number of proliferating epithelial cells and show slower migration along the crypt-villus axis, despite their normal intestinal tissue architecture, suggesting that Fpr1 may be important in maintaining the homeostasis of the intestinal epithelia with yet unclear mechanistic basis [93]. In addition, in the mouse colon, commensal bacterial lysates and fMLF activate ERK/MAPK pathway in an FPR-dependent manner [95], without clear identity of the receptor subtypes that respond to commensal products.
To elucidate the identity of individual Fprs involved in commensal bacteria interaction in the colon, a recent study [96] using Fpr single- or double-deficient mice revealed that Fpr2, rather than Fpr1, appears to have a more prominent role in maintaining the normal growth of colonic epithelial cells. Fpr2 is found to be expressed on the apical and lateral membranes of mouse colon-crypt epithelial cells and mediates fMLF-dependent epithelial-cell proliferation and renewal. Moreover, colonic epithelial cells in Fpr2-deficient mice displayed defects in commensal bacterium-dependent homeostasis, shown by the absence of responses to fMLF stimulation, shortened colon crypts, reduced acute inflammatory responses to dextran sulfate sodium challenge, delayed mucosal restoration after chronic injury, and increased azoxymethane-induced tumorigenesis. In contrast to Fpr2, although Fpr1 also mediates fMLF-stimulated colon epithelia chemotaxis and activation, the crypt length in the colon of Fpr1-deficient mice is normal when compared with that of the WT mice. In addition, the colon of Fpr1 and Fpr2 double-deficient mice showed shortened crypts comparable to that observed in Fpr2 single-deficient mice, suggesting a phenocopy of the Fpr2 single deficiency. Furthermore, bacteria RNA sequencing suggests “dysbiosis” in the colon of Fpr2-deficient mice, with increased abundance of microbiome. These results confirm a critical role of Fpr2 in the homeostasis, inflammation, and epithelial repair processes in the colon, potentially mediated by interaction with microbiome products. These observations, consistent with the critical role of Fprs in Listeria resistance, confirm the important position of FPRs in the coevolution of mammals with microbiome, which is mutually beneficial in the homeostatic conditions but may err once the balance of the commensals vs. host is disrupted.
THE DUAL ROLE OF FPRs IN CANCER PROGRESSION
Under physiologic conditions, FPRs expressed by normal cells are essential for host defense against microbial infection, as well as for control of inflammation, immune responses, and epithelial homeostasis. However, malignant cells also express FPRs and respond to bacteria or endogenous agonists. For instance, in human GC cells, aberrantly expressed FPRs, upon activation, mediates epithelial–mesenchymal transition, proliferation, migration, and cell resistance to apoptosis [97]. However, it is intriguing that, in xenograft experiments, GC cells with silenced FPR1 formed more rapidly growing tumors in immune-compromised mice. Mechanistic studies showed that tumors formed by GC cells with silenced FPR1 contain higher vascular density, suggesting FPR1 may promote the production of antiangiogenic factors by GC cells, thus reducing the blood supply to the tumor.
The promoting activity of FPR1 in human glioma and other tumors
In contrast to observations with GC cells, in some circumstances, an opposing effect of FPRs was observed malignant tumors. In human glioma, FPR1 was found to be selectively expressed by cells of the more highly malignant GBM [98]. By interacting with ligand DAMPs (Table 1.) released into the tumor microenvironment, FPR1 transactivated the receptor for EGF, and the 2 receptors cooperated to enhance the survival, invasiveness, and production of angiogenic factors by GBM cells [98–102]. The contribution of FPR1 to GBM progression was demonstrated in studies showing that small interfering RNA targeting of FPR1 markedly reduced the tumorigenic capacity of GBM cells in nude mice. Moreover, FPR1 may be involved in the establishment of GBM, because the receptor is expressed by CD133/Nestin+ glioma stem-like cells [103], which, upon implantation in nude mice, form more rapidly growing tumors and produce higher levels of the angiogenic factors as a consequence of FPR1 activation. More recently, Anx A1 released by necrotic GBM cells was identified as an activator of FPR1 expressed by live GBM cells which, in turn, exacerbated their malignant phenotype. These studies established a paracrine/autocrine FPR1/Anx A1 axis in the GBM microenvironment that provides critical signals for tumor progression [15] (Fig. 3). The clinical relevance of FPR1 in GBM was shown by the observation that, in human surgically resected glioma specimens, FPR1 and Anx A1 are both expressed by more highly malignant tumors with an inverse correlation with patient survival [21, 98].
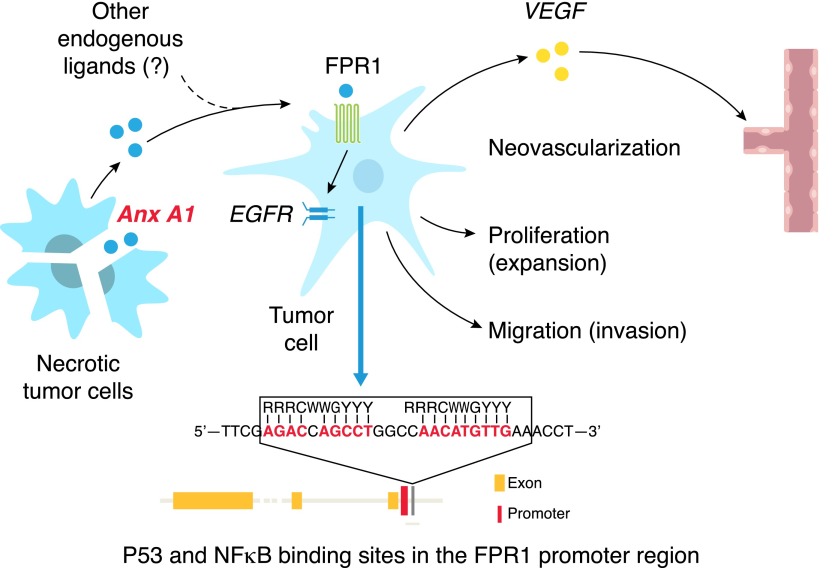
Figure 3. FPR1 promotes the progression of human glioblastoma.
FPR1 is selectively expressed by highly malignant human glioblastoma cells. The transcription of FPR1 in glioblastoma cells is promoted by overactivated NF-κB, which competitively binds to the FPR1 promoter. Demethylation of P53 increases its competitive binding to the FPR1 promoter and promotes the differentiation of glioblastoma cells accompanied by reduced expression of FPR1. Upon activation by the ligand Anx A1 released by necrotic tumor cells, FPR1 mediates glioblastoma cell chemotaxis, invasion, and production of proangiogenic factors, such as vascular endothelial cell growth factor and CXCL8. FPR1 in glioblastoma cells also transactivates the receptor for EGF via a G-protein–SRC kinase pathway, and 2 receptors cooperate to exacerbate the malignant phenotype of the tumor cells.
FPR1 and 2 are also expressed by human breast cancer cells, and activation with Anx A1 enhances tumor cell proliferation [104]. In addition, consistent with findings in human GBM, human liver cancer cells express FPR1, which promotes the chemotaxis, invasion, proliferation, and production of angiogenic factors by cancer cells. Silencing FPR1 markedly reduced the tumorigenic capacity of human liver cancer cells in immunocompromised mice. These observations suggest that FPRs may be used by a variety of malignant tumors to exacerbate their progression. In this context, FPRs in malignant tumors may be considered plausible targets in designing novel therapeutics. As a support of this notion, treatment of mice bearing human GBM with an FPR1 antagonist, CHIPS was shown to prolong mouse survival [105].
M1 macrophage polarization supported by Fpr2
Despite their potential tumor-promoting activities in a number of cancer cells, Fpr2 in macrophages has been shown to sustain M1 polarization to benefit antitumor host defense by down-regulating the content of TAMs, a hallmark of cancer-associated inflammation [106]. TAMs are believed to be recruited by chemokines, and the cells can either impede tumor growth by producing cytotoxic mediators or promote tumor progression by producing growth-inducing and angiogenic factors [107, 108]. Blood-derived monocytes enter tumor tissues and differentiate into macrophages followed by further development into M1 or M2 polarized subtypes, which differ in their patterns of cytokine secretion and function [106]. The “classically activated” M1 macrophages contribute to tumor rejection through type I cytokine production and antigen presentation [108, 109]. The “alternatively activated” M2 macrophages enhance angiogenesis and tissue remodeling through type II cytokines [110, 111]. In >80% of cancers, TAMs mostly exhibit an M2 phenotype [112]. Tumor- and stroma-produced mediators, including a variety of chemokines, promote the recruitment and activation of TAMs, which contribute to tumor cell proliferation, migration, angiogenesis, and metastasis [113].
Experimental and clinical studies have shown that MCP-1 (also known as CCL2) is most frequently expressed by tumor cells, and its concentration is correlated with the level of TAMs in tumors [112, 114]. In a LLC implantation model, Fpr2-deficient mice bearing subcutaneously implanted LLC cells exhibited significantly shortened survival than did normal mice because of more rapidly growing tumors. In contrast, in transgenic mice over-expressing Fpr2, subcutaneously implanted LLC tumors grew more slowly than those in WT littermates. Investigation of tumor tissues revealed more TAMs in tumors grown in Fpr2-deficient mice. Macrophages derived from Fpr2-deficient mice also showed a more-potent chemotactic response to LLC-derived supernatant, which could be neutralized by an anti-CCL2 antibody, indicating CCL2 as a major chemoattractant for TAMs in LLC tumor. The increased chemotaxis of Fpr2-deficient mouse macrophages in response to LLC supernatant was due to their higher expression of CCR4, a chemokine GPCR that is also recognized by the ligand CCL2 [115, 116]. Interestingly, treatment with Fpr2 antagonists increased the chemotactic response of WT mouse macrophages to CCL2, in association with increased expression of CCR4; LLC tumor cell supernatant and Fpr2 agonists were capable of polarizing WT macrophages toward an M1 phenotype [117]. Therefore, Fpr2 appears to increase host defense against implanted LLC by favoring macrophages with an M1 phenotype with more-potent antitumor activities (Fig. 4). However, a caveat may exist in generalizing this LLC model because not all tumors produce copious levels of CCL2, and the nature of LLC-derived Fpr2 agonist DAMPs is unknown. More studies are required to elucidate the precise mechanisms by which Fpr2 promotes macrophages polarization into an M1 subtype to limit tumor growth and whether this property of Fpr2 could be exploited to develop antitumor drugs.
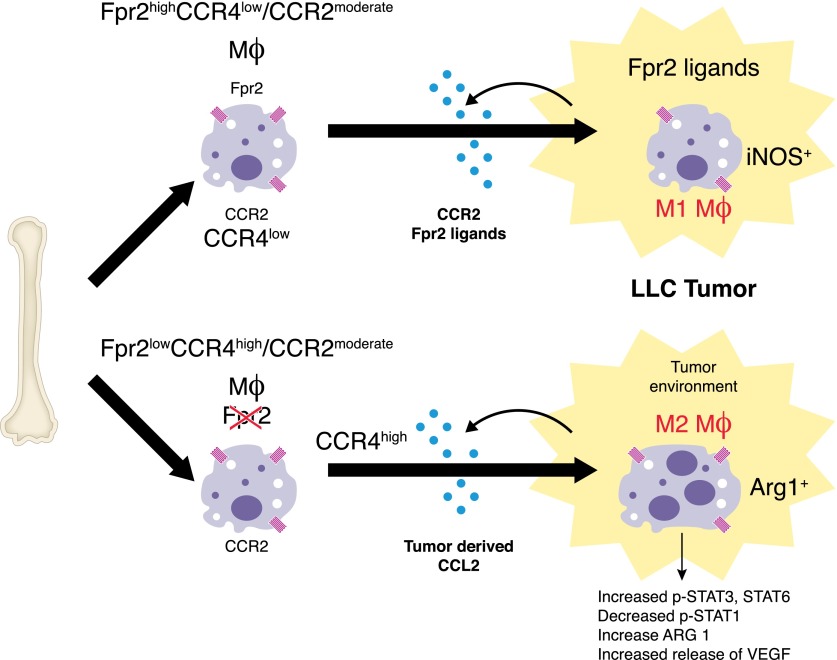
Figure 4. Fpr2 promotes antitumor host defense.
Mouse macrophages expressing both CCR2 and Fpr2 infiltrate transplanted LLC where macrophages undergo M1 polarization in response to tumor-derived Fpr2 ligands. Macrophages-deficient in Fpr2 express high levels of CCR4, which synergizes with CCR2 to recruit TAM in response to tumor-derived CCL2, followed by polarization into M2 cells in response to an as-yet-undefined tumor microenvironment factor.
CONCLUDING REMARKS
FPRs, as a subfamily of classic chemoattractant GPCRs, have one of the most diverse collections of ligands, and they are expressed by a great variety of cell types, including cancer cells. Recent studies have shown an essential role of FPRs in critical pathophysiologic conditions, not only in leukocyte trafficking but also in epithelial homeostasis and tumorigenesis. One of most notable features of FPRs in these pathophysiologic processes is that they act as key players in the sequential sensing of chemoattractant gradients by inflammatory cells, in particular neutrophils and DCs, in a tissue microenvironment that dynamically regulates the expression of chemoattractant GPCRs to guide the cells to their destination where efficient host responses are mounted. Another important, paradigm-changing discovery is the role of Fpr2 in protecting colon mucosal homeostasis and inflammation and antitumor defense by interaction with commensal products. These novel findings greatly expanded the scope of FPR biology whose pathophysiologic relevance had remained obscure for decades since their discovery. However, much more remains to be learned, with rigorous exploration of the participation of FPRs in more disease models, which, fortunately, has become increasingly feasible with the generation of genetically engineered mice with altered expression of FPR genes. It is thus plausible that future studies focusing on the regulation, signal transduction, and structural basis for ligand recognition, and importantly, on participation in pathophysiologic processes in humans, should yield novel therapeutic targets for diseases.
AUTHORSHIP
L.L., K.C., T.Y., S.S., J.Z., X.B. and J.M.W contributed to the composition and editing of the manuscript.
ACKNOWLEDGMENTS
This project was funded in part with federal funds from the U.S. National Institutes of Health (NIH) National Cancer Institute (NCI), under Contract No. HHSN261200800001E, and also in part by the Intramural Research Program of NCI, NIH. Additional sources of support were The Starting Foundation for New Teachers of Shanghai Jiao Tong University, Shanghai, China (Grant 14X100040016 to L.L.); the National Natural Science Foundation of China (Grant 81470073 to L.L., Grant 31170861 to S.S., Grant 81101771 to Y.X., Grant 81473127 to J.Z., and Grant 81230062 to X.B.). The authors thank Dr. J. J. Oppenheim for critically reviewing the manuscript, Ms. C. Lamb and Ms. S. Livingstone for secretarial assistance.
Glossary
- Aβ42
amyloid β peptide 42
- Anx A1
annexin 1
- BM
bone marrow
- CCL
chemokine (C-C motif) ligand
- CCR
C-C chemokine receptor type
- CHIPS
chemotaxis inhibitory protein of Staphylococcus aureus
- CRAMP
cathelin-related antimicrobial peptide
- CX3CR
chemokine (C-X3-C motif) receptor
- CXCL
chemokine (C-X-C motif) ligand
- CXCR
C-X-C chemokine receptor type
- DAMP
damage-associated molecular pattern
- DC
dendritic cell
- EGF
epidermal growth factor
- F2L
formyl-peptide receptor (FPR)-like (FPRL)-2 ligand
- FPR
formyl-peptide receptor
- FPRL
formyl-peptide receptor-like
- GBM
glioblastoma multiforme
- GC
gastric cancer
- GPCR
Gi–protein-coupled receptors
- LL-37
human cathelicidin
- LLC
Lewis lung cancer
- LTB4
lipid chemoattractant leukotriene B4
- LXA4
lipoxin A4
- OVA
ovalbumin
- PAMP
pathogen-associated molecular pattern
- PRR
pattern recognition receptor
- SAA
serum amyloid A
- TAM
tumor-associated macrophage
- UC
ulcerative colitis
- WT
wild type
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Wang J. M., Deng X., Gong W., Su S. (1998) Chemokines and their role in tumor growth and metastasis. J. Immunol. Methods 220, 1–17. [DOI] [PubMed] [Google Scholar]
- 2.Kristiansen K. (2004) Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol. Ther. 103, 21–80. [DOI] [PubMed] [Google Scholar]
- 3.Griffith J. W., Sokol C. L., Luster A. D. (2014) Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu. Rev. Immunol. 32, 659–702. [DOI] [PubMed] [Google Scholar]
- 4.Lira S. A., Furtado G. C. (2012) The biology of chemokines and their receptors. Immunol. Res. 54, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye R. D., Cavanagh S. L., Quehenberger O., Prossnitz E. R., Cochrane C. G. (1992) Isolation of a cDNA that encodes a novel granulocyte N-formyl peptide receptor. Biochem. Biophys. Res. Commun. 184, 582–589. [DOI] [PubMed] [Google Scholar]
- 6.Murphy P. M., Ozçelik T., Kenney R. T., Tiffany H. L., McDermott D., Francke U. (1992) A structural homologue of the N-formyl peptide receptor: characterization and chromosome mapping of a peptide chemoattractant receptor family. J. Biol. Chem. 267, 7637–7643. [PubMed] [Google Scholar]
- 7.Bao L., Gerard N. P., Eddy R. L. Jr, Shows T. B., Gerard C. (1992) Mapping of genes for the human C5a receptor (C5AR), human FMLP receptor (FPR), and two FMLP receptor homologue orphan receptors (FPRH1, FPRH2) to chromosome 19. Genomics 13, 437–440. [DOI] [PubMed] [Google Scholar]
- 8.Le Y., Murphy P. M., Wang J. M. (2002) Formyl-peptide receptors revisited. Trends Immunol. 23, 541–548. [DOI] [PubMed] [Google Scholar]
- 9.Ye R. D., Boulay F., Wang J. M., Dahlgren C., Gerard C., Parmentier M., Serhan C. N., Murphy P. M. (2009) International Union of Basic and Clinical Pharmacology, LXXIII: Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartt J. K., Barish G., Murphy P. M., Gao J. L. (1999) N-formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, a second mouse neutrophil FPR. J. Exp. Med. 190, 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cattaneo F., Parisi M., Ammendola R. (2013) Distinct signaling cascades elicited by different formyl peptide receptor 2 (FPR2) agonists. Int. J. Mol. Sci. 14, 7193–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy P. M. (1994) The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 12, 593–633. [DOI] [PubMed] [Google Scholar]
- 13.Chadwick V. S., Mellor D. M., Myers D. B., Selden A. C., Keshavarzian A., Broom M. F., Hobson C. H. (1988) Production of peptides inducing chemotaxis and lysosomal enzyme release in human neutrophils by intestinal bacteria in vitro and in vivo. Scand. J. Gastroenterol. 23, 121–128. [DOI] [PubMed] [Google Scholar]
- 14.Schiffmann E., Corcoran B. A., Wahl S. M. (1975) N-formylmethionyl peptides as chemoattractants for leucocytes. Proc. Natl. Acad. Sci. U. S. A. 72, 1059–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marasco W. A., Phan S. H., Krutzsch H., Showell H. J., Feltner D. E., Nairn R., Becker E. L., Ward P. A. (1984) Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J. Biol. Chem. 259, 5430–5439. [PubMed] [Google Scholar]
- 16.Gao J. L., Lee E. J., Murphy P. M. (1999) Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J. Exp. Med. 189, 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu M., Chen K., Yoshimura T., Liu Y., Gong W., Wang A., Gao J. L., Murphy P. M., Wang J. M. (2012) Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci. Rep. 2, 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., Hauser C. J. (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald B., Pittman K., Menezes G. B., Hirota S. A., Slaba I., Waterhouse C. C., Beck P. L., Muruve D. A., Kubes P. (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366. [DOI] [PubMed] [Google Scholar]
- 20.Molloy M. J., Grainger J. R., Bouladoux N., Hand T. W., Koo L. Y., Naik S., Quinones M., Dzutsev A. K., Gao J. L., Trinchieri G., Murphy P. M., Belkaid Y. (2013) Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe 14, 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y., Liu Y., Yao X., Ping Y., Jiang T., Liu Q., Xu S., Huang J., Mou H., Gong W., Chen K., Bian X., Wang J. M. (2011) Annexin 1 released by necrotic human glioblastoma cells stimulates tumor cell growth through the formyl peptide receptor 1 [published correction in Am. J. Pathol. (2011), 179, 2674]. Am. J. Pathol. 179, 1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun R., Iribarren P., Zhang N., Zhou Y., Gong W., Cho E. H., Lockett S., Chertov O., Bednar F., Rogers T. J., Oppenheim J. J., Wang J. M. (2004) Identification of neutrophil granule protein cathepsin G as a novel chemotactic agonist for the G protein-coupled formyl peptide receptor. J. Immunol. 173, 428–436. [DOI] [PubMed] [Google Scholar]
- 23.Su S. B., Gong W., Gao J. L., Shen W., Murphy P. M., Oppenheim J. J., Wang J. M. (1999) A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J. Exp. Med. 189, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Y., Gong W., Tiffany H. L., Tumanov A., Nedospasov S., Shen W., Dunlop N. M., Gao J. L., Murphy P. M., Oppenheim J. J., Wang J. M. (2001) Amyloid β42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J. Neurosci. 21, RC123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Yang, Chen Q., Schmidt A. P., Anderson G. M., Wang J. M., Wooters J., Oppenheim J. J., Chertov O. (2000) LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192, 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurosaka K., Chen Q., Yarovinsky F., Oppenheim J. J., Yang D. (2005) Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J. Immunol. 174, 6257–6265. [DOI] [PubMed] [Google Scholar]
- 27.De Santo C., Arscott R., Booth S., Karydis I., Jones M., Asher R., Salio M., Middleton M., Cerundolo V. (2010) Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat. Immunol. 11, 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang T. S., Wang J. M., Murphy P. M., Gao J. L. (2000) Serum amyloid A is a chemotactic agonist at FPR2, a low-affinity N-formylpeptide receptor on mouse neutrophils. Biochem. Biophys. Res. Commun. 270, 331–335. [DOI] [PubMed] [Google Scholar]
- 29.Cui Y. H., Le Y., Gong W., Proost P., Van Damme J., Murphy W. J., Wang J. M. (2002) Bacterial lipopolysaccharide selectively up-regulates the function of the chemotactic peptide receptor formyl peptide receptor 2 in murine microglial cells. J. Immunol. 168, 434–442. [DOI] [PubMed] [Google Scholar]
- 30.Coffelt S. B., Marini F. C., Watson K., Zwezdaryk K. J., Dembinski J. L., LaMarca H. L., Tomchuck S. L., Honer zu Bentrup K., Danka E. S., Henkle S. L., Scandurro A. B. (2009) The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc. Natl. Acad. Sci. U. S. A. 106, 3806–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babbin B. A., Lee W. Y., Parkos C. A., Winfree L. M., Akyildiz A., Perretti M., Nusrat A. (2006) Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J. Biol. Chem. 281, 19588–19599. [DOI] [PubMed] [Google Scholar]
- 32.Southgate E. L., He R. L., Gao J. L., Murphy P. M., Nanamori M., Ye R. D. (2008) Identification of formyl peptides from Listeria monocytogenes and Staphylococcus aureus as potent chemoattractants for mouse neutrophils. J. Immunol. 181, 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K., Liu M., Liu Y., Wang C., Yoshimura T., Gong W., Le Y., Tessarollo L., Wang J. M. (2013) Signal relay by CC chemokine receptor 2 (CCR2) and formylpeptide receptor 2 (Fpr2) in the recruitment of monocyte-derived dendritic cells in allergic airway inflammation. J. Biol. Chem. 288, 16262–16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leoni G., Alam A., Neumann P. A., Lambeth J. D., Cheng G., McCoy J., Hilgarth R. S., Kundu K., Murthy N., Kusters D., Reutelingsperger C., Perretti M., Parkos C. A., Neish A. S., Nusrat A. (2013) Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J. Clin. Invest. 123, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buss N. A., Gavins F. N., Cover P. O., Terron A., Buckingham J. C. (2015) Targeting the annexin 1-formyl peptide receptor 2/ALX pathway affords protection against bacterial LPS-induced pathologic changes in the murine adrenal cortex. FASEB J. 29, 2930–2942. [DOI] [PubMed] [Google Scholar]
- 36.Dalli J., Jones C. P., Cavalcanti D. M., Farsky S. H., Perretti M., Rankin S. M. (2012) Annexin A1 regulates neutrophil clearance by macrophages in the mouse bone marrow. FASEB J. 26, 387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Resnati M., Pallavicini I., Wang J. M., Oppenheim J., Serhan C. N., Romano M., Blasi F. (2002) The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc. Natl. Acad. Sci. U. S. A. 99, 1359–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Migeotte I., Riboldi E., Franssen J. D., Grégoire F., Loison C., Wittamer V., Detheux M., Robberecht P., Costagliola S., Vassart G., Sozzani S., Parmentier M., Communi D. (2005) Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J. Exp. Med. 201, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao J. L., Guillabert A., Hu J., Le Y., Urizar E., Seligman E., Fang K. J., Yuan X., Imbault V., Communi D., Wang J. M., Parmentier M., Murphy P. M., Migeotte I. (2007) F2L, a peptide derived from heme-binding protein, chemoattracts mouse neutrophils by specifically activating Fpr2, the low-affinity N-formylpeptide receptor. J. Immunol. 178, 1450–1456. [DOI] [PubMed] [Google Scholar]
- 40.Rabiet M. J., Macari L., Dahlgren C., Boulay F. (2011) N-formyl peptide receptor 3 (FPR3) departs from the homologous FPR2/ALX receptor with regard to the major processes governing chemoattractant receptor regulation, expression at the cell surface, and phosphorylation. J. Biol. Chem. 286, 26718–26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorward D. A., Lucas C. D., Chapman G. B., Haslett C., Dhaliwal K., Rossi A. G. (2015) The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am. J. Pathol. 185, 1172–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stavru F., Archambaud C., Cossart P. (2011) Cell biology and immunology of Listeria monocytogenes infections: novel insights. Immunol. Rev. 240, 160–184. [DOI] [PubMed] [Google Scholar]
- 43.Kasper S., Huhulescu S., Auer B., Heller I., Karner F., Würzner R., Wagner M., Allerberger F. (2009) Epidemiology of listeriosis in Austria. Wien. Klin. Wochenschr. 121, 113–119. [DOI] [PubMed] [Google Scholar]
- 44.Goulet V., Hedberg C., Le Monnier A., de Valk H. (2008) Increasing incidence of listeriosis in France and other European countries. Emerg. Infect. Dis. 14, 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson R. (2009) Listeriosis remains a cause for concern in Europe. BMJ 338, b319. [DOI] [PubMed] [Google Scholar]
- 46.Allerberger F., Wagner M. (2010) Listeriosis: a resurgent foodborne infection. Clin. Microbiol. Infect. 16, 16–23. [DOI] [PubMed] [Google Scholar]
- 47.Tilney L. G., Portnoy D. A. (1989) Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109, 1597–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mengaud J., Ohayon H., Gounon P., Mege R-M, Cossart P. (1996) E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84, 923–932. [DOI] [PubMed] [Google Scholar]
- 49.Yu W. L., Dan H., Lin M. (2008) InlA and InlC2 of Listeria monocytogenes serotype 4b are two internalin proteins eliciting humoral immune responses common to listerial infection of various host species. Curr. Microbiol. 56, 505–509. [DOI] [PubMed] [Google Scholar]
- 50.Zenewicz L. A., Shen H. (2007) Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect. 9, 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonazzi M., Lecuit M., Cossart P. (2009) Listeria monocytogenes internalin and E-cadherin: from bench to bedside. Cold Spring Harb. Perspect. Biol. 1, a003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cossart P., Toledo-Arana A. (2008) Listeria monocytogenes, a unique model in infection biology: an overview. Microbes Infect. 10, 1041–1050. [DOI] [PubMed] [Google Scholar]
- 53.Hamon M., Bierne H., Cossart P. (2006) Listeria monocytogenes: a multifaceted model. Nat. Rev. Microbiol. 4, 423–434. [DOI] [PubMed] [Google Scholar]
- 54.Drevets D. A., Bronze M. S. (2008) Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol. Med. Microbiol. 53, 151–165. [DOI] [PubMed] [Google Scholar]
- 55.Pamer E. G. (2004) Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4, 812–823. [DOI] [PubMed] [Google Scholar]
- 56.Cousens L. P., Wing E. J. (2000) Innate defenses in the liver during Listeria infection. Immunol. Rev. 174, 150–159. [DOI] [PubMed] [Google Scholar]
- 57.Torres D., Barrier M., Bihl F., Quesniaux V. J., Maillet I., Akira S., Ryffel B., Erard F. (2004) Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect. Immun. 72, 2131–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chapman R. W., Minnicozzi M., Celly C. S., Phillips J. E., Kung T. T., Hipkin R. W., Fan X., Rindgen D., Deno G., Bond R., Gonsiorek W., Billah M. M., Fine J. S., Hey J. A. (2007) A novel, orally active CXCR1/2 receptor antagonist, Sch527123, inhibits neutrophil recruitment, mucus production, and goblet cell hyperplasia in animal models of pulmonary inflammation. J. Pharmacol. Exp. Ther. 322, 486–493. [DOI] [PubMed] [Google Scholar]
- 59.Rose J. J., Foley J. F., Murphy P. M., Venkatesan S. (2004) On the mechanism and significance of ligand-induced internalization of human neutrophil chemokine receptors CXCR1 and CXCR2. J. Biol. Chem. 279, 24372–24386. [DOI] [PubMed] [Google Scholar]
- 60.Cunha T. M., Barsante M. M., Guerrero A. T., Verri W. A. Jr., Ferreira S. H., Coelho F. M., Bertini R., Di Giacinto C., Allegretti M., Cunha F. Q., Teixeira M. M. (2008) Treatment with DF 2162, a non-competitive allosteric inhibitor of CXCR1/2, diminishes neutrophil influx and inflammatory hypernociception in mice. Br. J. Pharmacol. 154, 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reichel C. A., Khandoga A., Anders H. J., Schlöndorff D., Luckow B., Krombach F. (2006) Chemokine receptors Ccr1, Ccr2, and Ccr5 mediate neutrophil migration to postischemic tissue. J. Leukoc. Biol. 79, 114–122. [DOI] [PubMed] [Google Scholar]
- 62.Chintakuntlawar A. V., Chodosh J. (2009) Chemokine CXCL1/KC and its receptor CXCR2 are responsible for neutrophil chemotaxis in adenoviral keratitis. J. Interferon Cytokine Res. 29, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L., Ran L., Garcia G. E., Wang X. H., Han S., Du J., Mitch W. E. (2009) Chemokine CXCL16 regulates neutrophil and macrophage infiltration into injured muscle, promoting muscle regeneration. Am. J. Pathol. 175, 2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burdon P. C., Martin C., Rankin S. M. (2005) The CXC chemokine MIP-2 stimulates neutrophil mobilization from the rat bone marrow in a CD49d-dependent manner. Blood 105, 2543–2548. [DOI] [PubMed] [Google Scholar]
- 65.Monson K. M., Dowlatshahi S., Crockett E. T. (2007) CXC-chemokine regulation and neutrophil trafficking in hepatic ischemia-reperfusion injury in P-selectin/ICAM-1–deficient mice. J. Inflamm. (Lond.) 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oldekamp S., Pscheidl S., Kress E., Soehnlein O., Jansen S., Pufe T., Wang J. M., Tauber S. C., Brandenburg L. O. (2014) Lack of formyl peptide receptor 1 and 2 leads to more severe inflammation and higher mortality in mice with of pneumococcal meningitis. Immunology 143, 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nathan C. (2002) Points of control in inflammation. Nature 420, 846–852. [DOI] [PubMed] [Google Scholar]
- 68.Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689. [DOI] [PubMed] [Google Scholar]
- 69.Chtanova T., Schaeffer M., Han S. J., van Dooren G. G., Nollmann M., Herzmark P., Chan S. W., Satija H., Camfield K., Aaron H., Striepen B., Robey E. A. (2008) Dynamics of neutrophil migration in lymph nodes during infection. Immunity 29, 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peters N. C., Egen J. G., Secundino N., Debrabant A., Kimblin N., Kamhawi S., Lawyer P., Fay M. P., Germain R. N., Sacks D. (2008) In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321, 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruns S., Kniemeyer O., Hasenberg M., Aimanianda V., Nietzsche S., Thywissen A., Jeron A., Latgé J. P., Brakhage A. A., Gunzer M. (2010) Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 6, e1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yipp B. G., Petri B., Salina D., Jenne C. N., Scott B. N., Zbytnuik L. D., Pittman K., Asaduzzaman M., Wu K., Meijndert H. C., Malawista S. E., de Boisfleury Chevance A., Zhang K., Conly J., Kubes P. (2012) Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18, 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liese J., Rooijakkers S. H., van Strijp J. A., Novick R. P., Dustin M. L. (2013) Intravital two-photon microscopy of host-pathogen interactions in a mouse model of Staphylococcus aureus skin abscess formation. Cell. Microbiol. 15, 891–909. [DOI] [PubMed] [Google Scholar]
- 74.Harvie E. A., Green J. M., Neely M. N., Huttenlocher A. (2013) Innate immune response to Streptococcus iniae infection in zebrafish larvae. Infect. Immun. 81, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kreisel D., Nava R. G., Li W., Zinselmeyer B. H., Wang B., Lai J., Pless R., Gelman A. E., Krupnick A. S., Miller M. J. (2010) In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc. Natl. Acad. Sci. U. S. A. 107, 18073–18078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakasone E. S., Askautrud H. A., Kees T., Park J. H., Plaks V., Ewald A. J., Fein M., Rasch M. G., Tan Y. X., Qiu J., Park J., Sinha P., Bissell M. J., Frengen E., Werb Z., Egeblad M. (2012) Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 21, 488–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen K., Le Y., Liu Y., Gong W., Ying G., Huang J., Yoshimura T., Tessarollo L., Wang J. M. (2010) A critical role for the g protein-coupled receptor mFPR2 in airway inflammation and immune responses. J. Immunol. 184, 3331–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Provoost S., Maes T., Joos G. F., Tournoy K. G. (2012) Monocyte-derived dendritic cell recruitment and allergic TH2 responses after exposure to diesel particles are CCR2 dependent. J. Allergy Clin. Immunol. 129, 483–491. [DOI] [PubMed] [Google Scholar]
- 79.Serbina N. V., Pamer E. G. (2006) Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317. [DOI] [PubMed] [Google Scholar]
- 80.Chen K., Xiang Y., Huang J., Gong W., Yoshimura T., Jiang Q., Tessarollo L., Le Y., Wang J. M. (2014) The formylpeptide receptor 2 (Fpr2) and its endogenous ligand cathelin-related antimicrobial peptide (CRAMP) promote dendritic cell maturation. J. Biol. Chem. 289, 17553–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Richardson R. M., Ali H., Tomhave E. D., Haribabu B., Snyderman R. (1995) Cross-desensitization of chemoattractant receptors occurs at multiple levels: evidence for a role for inhibition of phospholipase C activity. J. Biol. Chem. 270, 27829–27833. [DOI] [PubMed] [Google Scholar]
- 82.Caux C., Vanbervliet B., Massacrier C., Ait-Yahia S., Vaure C., Chemin K., Dieu-Nosjean M. C., Vicari A. (2002) Regulation of dendritic cell recruitment by chemokines. Transplantation 73(1, Suppl)S7–S11. [DOI] [PubMed] [Google Scholar]
- 83.Ng L. G., Qin J. S., Roediger B., Wang Y., Jain R., Cavanagh L. L., Smith A. L., Jones C. A., de Veer M., Grimbaldeston M. A., Meeusen E. N., Weninger W. (2011) Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J. Invest. Dermatol. 131, 2058–2068. [DOI] [PubMed] [Google Scholar]
- 84.Lämmermann T., Bader B. L., Monkley S. J., Worbs T., Wedlich-Söldner R., Hirsch K., Keller M., Förster R., Critchley D. R., Fässler R., Sixt M. (2008) Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55. [DOI] [PubMed] [Google Scholar]
- 85.Lämmermann T., Afonso P. V., Angermann B. R., Wang J. M., Kastenmüller W., Parent C. A., Germain R. N. (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu M., Chen K., Yoshimura T., Liu Y., Gong W., Le Y., Gao J. L., Zhao J., Wang J. M., Wang A. (2014) Formylpeptide receptors mediate rapid neutrophil mobilization to accelerate wound healing. PLoS One 9, e90613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu X., Ma B., Malik A. B., Tang H., Yang T., Sun B., Wang G., Minshall R. D., Li Y., Zhao Y., Ye R. D., Xu J. (2012) Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat. Immunol. 13, 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clevers H. (2004) At the crossroads of inflammation and cancer. Cell 118, 671–674. [DOI] [PubMed] [Google Scholar]
- 89.Balkwill F., Mantovani A. (2001) Inflammation and cancer: back to Virchow? Lancet 357, 539–545. [DOI] [PubMed] [Google Scholar]
- 90.Coussens L. M., Werb Z. (2002) Inflammation and cancer. Nature 420, 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coussens L. M., Tinkle C. L., Hanahan D., Werb Z. (2000) MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao H., Gulen M. F., Qin J., Yao J., Bulek K., Kish D., Altuntas C. Z., Wald D., Ma C., Zhou H., Tuohy V. K., Fairchild R. L., de la Motte C., Cua D., Vallance B. A., Li X. (2007) The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity 26, 461–475. [DOI] [PubMed] [Google Scholar]
- 93.Babbin B. A., Jesaitis A. J., Ivanov A. I., Kelly D., Laukoetter M., Nava P., Parkos C. A., Nusrat A. (2007) Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J. Immunol. 179, 8112–8121. [DOI] [PubMed] [Google Scholar]
- 94.Alam A., Leoni G., Wentworth C. C., Kwal J. M., Wu H., Ardita C. S., Swanson P. A., Lambeth J. D., Jones R. M., Nusrat A., Neish A. S. (2014) Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 7, 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wentworth C. C., Jones R. M., Kwon Y. M., Nusrat A., Neish A. S. (2010) Commensal-epithelial signaling mediated via formyl peptide receptors. Am. J. Pathol. 177, 2782–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen K., Liu M., Liu Y., Yoshimura T., Shen W., Le Y., Durum S., Gong W., Wang C., Gao J. L., Murphy P. M., Wang J. M. (2013) Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J. Clin. Invest. 123, 1694–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prevete N., Liotti F., Visciano C., Marone G., Melillo R. M., de Paulis A. (2015) The formyl peptide receptor 1 exerts a tumor suppressor function in human gastric cancer by inhibiting angiogenesis [published online ahead of print September 29, 2014]. Oncogene 34, 3826–3838. [DOI] [PubMed] [Google Scholar]
- 98.Zhou Y., Bian X., Le Y., Gong W., Hu J., Zhang X., Wang L., Iribarren P., Salcedo R., Howard O. M., Farrar W., Wang J. M. (2005) Formylpeptide receptor FPR and the rapid growth of malignant human gliomas. J. Natl. Cancer Inst. 97, 823–835. [DOI] [PubMed] [Google Scholar]
- 99.Yao X. H., Ping Y. F., Chen J. H., Chen D. L., Xu C. P., Zheng J., Wang J. M., Bian X. W. (2008) Production of angiogenic factors by human glioblastoma cells following activation of the G-protein coupled formylpeptide receptor FPR. J. Neurooncol. 86, 47–53. [DOI] [PubMed] [Google Scholar]
- 100.Huang J., Hu J., Bian X., Chen K., Gong W., Dunlop N. M., Howard O. M., Wang J. M. (2007) Transactivation of the epidermal growth factor receptor by formylpeptide receptor exacerbates the malignant behavior of human glioblastoma cells. Cancer Res. 67, 5906–5913. [DOI] [PubMed] [Google Scholar]
- 101.Huang J., Chen K., Chen J., Gong W., Dunlop N. M., Howard O. M., Gao Y., Bian X. W., Wang J. M. (2010) The G-protein-coupled formylpeptide receptor FPR confers a more invasive phenotype on human glioblastoma cells. Br. J. Cancer 102, 1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang J., Chen K., Huang J., Gong W., Dunlop N. M., Howard O. M., Bian X., Gao Y., Wang J. M. (2009) Regulation of the leucocyte chemoattractant receptor FPR in glioblastoma cells by cell differentiation. Carcinogenesis 30, 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yao X. H., Ping Y. F., Chen J. H., Xu C. P., Chen D. L., Zhang R., Wang J. M., Bian X. W. (2008) Glioblastoma stem cells produce vascular endothelial growth factor by activation of a G-protein coupled formylpeptide receptor FPR. J. Pathol. 215, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khau T., Langenbach S. Y., Schuliga M., Harris T., Johnstone C. N., Anderson R. L., Stewart A. G. (2011) Annexin-1 signals mitogen-stimulated breast tumor cell proliferation by activation of the formyl peptide receptors (FPRs) 1 and 2. FASEB J. 25, 483–496. [DOI] [PubMed] [Google Scholar]
- 105.Boer J. C., Domanska U. M., Timmer-Bosscha H., Boer I. G., de Haas C. J., Joseph J. V., Kruyt F. A., de Vries E. G., den Dunnen W. F., van Strijp J. A., Walenkamp A. M. (2013) Inhibition of formyl peptide receptor in high-grade astrocytoma by chemotaxis inhibitory protein of S. aureus. Br. J. Cancer 108, 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vakkila J., Lotze M. T. (2004) Inflammation and necrosis promote tumour growth. Nat. Rev. Immunol. 4, 641–648. [DOI] [PubMed] [Google Scholar]
- 107.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555. [DOI] [PubMed] [Google Scholar]
- 108.Joyce J. A., Pollard J. W. (2009) Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Talmadge J. E., Donkor M., Scholar E. (2007) Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 26, 373–400. [DOI] [PubMed] [Google Scholar]
- 110.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686. [DOI] [PubMed] [Google Scholar]
- 111.Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pollard J. W. (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 4, 71–78. [DOI] [PubMed] [Google Scholar]
- 113.Jin G., Kawsar H. I., Hirsch S. A., Zeng C., Jia X., Feng Z., Ghosh S. K., Zheng Q. Y., Zhou A., McIntyre T. M., Weinberg A. (2010) An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS One 5, e10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qian B. Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L. R., Kaiser E. A., Snyder L. A., Pollard J. W. (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yadav A., Saini V., Arora S. (2010) MCP-1: chemoattractant with a role beyond immunity—a review. Clin. Chim. Acta 411, 1570–1579. [DOI] [PubMed] [Google Scholar]
- 116.Frossard J. L., Lenglet S., Montecucco F., Steffens S., Galan K., Pelli G., Spahr L., Mach F., Hadengue A. (2011) Role of CCL-2, CCR-2 and CCR-4 in cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. J. Clin. Pathol. 64, 387–393. [DOI] [PubMed] [Google Scholar]
- 117.Liu Y., Chen K., Wang C., Gong W., Yoshimura T., Liu M., Wang J. M. (2013) Cell surface receptor FPR2 promotes antitumor host defense by limiting M2 polarization of macrophages. Cancer Res. 73, 550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]