Abstract
Background
The lung is a target organ for adverse health outcomes following exposure to arsenic. Several studies have reported a high prevalence of respiratory symptoms and diseases in subjects highly exposed to arsenic through drinking water, however, most studies to date has been performed in exposed adults, with little information on respiratory effects in children. The objective of the study was to evaluate the association between urinary levels of arsenic and its metabolites with lung function in children exposed in utero and in early childhood to high arsenic levels through drinking water.
Methods
A total of 358 healthy children were included in our study. Individual exposure was assessed based on urinary concentration of inorganic arsenic. Lung function was assessed by spirometry.
Results
Participants were exposed since pregnancy until early childhood to an average water As concentration of 152.13 μg/L. The mean urinary arsenic level registered in the studied subjects was 141.2 μg/L and only 16.7% had a urinary concentration below the national concern level. Forced vital capacity was significantly decreased in the studied population and it was negatively associated with the percent of inorganic arsenic. More than 57% of the subjects had a restrictive spirometric pattern. The urinary As level was higher in those children with restrictive lung patterns when compared with the levels registered in subjects with normal spirometric patterns.
Conclusion
Exposure to arsenic through drinking water during in utero and early life was associated with a decrease in FVC and with a restrictive spirometric pattern in the children evaluated.
Keywords: Arsenic, children, in utero exposure, lung function, spirometry
Introduction
Arsenic is a highly potent toxicant and carcinogen (IARC 2004). Worldwide, millions of individuals are exposed to arsenic in drinking water above the World Health Organization guideline value of 10 μg/L (Kinniburg and Smedley 2001; Nordstrom 2002; WHO 2004) Chronic arsenic exposure through drinking water has been correlated with increased incidence and mortality by cancers of the lung, skin, kidney, urinary bladder, and liver (Chiou et al., 1995; Hopenhayn-Rich et al., 1998). Chronic arsenic ingestion in drinking water also causes nonmalignant diseases. While arsenic can affect many tissues and organ systems, the lung seems particularly susceptible (NRC 2001). In fact, several studies have reported a high prevalence of respiratory symptoms in subjects highly exposed to arsenic through drinking water such as chronic cough, abnormal chest sound, shortness of breath (Mazumder et al., 2000), lower forced expiratory volume measured in 1 second (FEV1), and altered forced vital capacity (FVC) (von Ehrenstein et al., 2005). Additionally, De et al., (2004) reported that 57% of subjects chronically exposed to arsenic had respiratory symptoms with 53% having restrictive lung disease and 41% of the study participants having both obstructive and restrictive lung diseases. Mazumder et al., (2005) reported a 10-fold increased risk of chronic obstructive pulmonary disease (COPD), identified by high-resolution computed tomography. Among people with arsenical skin lesions increased mortality from COPD has been associated with exposure to high levels of arsenic in an As-endemic area of Chile (Smith et al., 2006). The majority of studies have investigated altered lung function and respiratory diseases in adults. Very few studies have correlated early-life arsenic exposure with alterations in lung function and respiratory disease in adults or children.
The first investigations into the health effects of early-life arsenic exposure came from the limited studies of children in Chile, which reported a high prevalence of respiratory illness among children with arsenical skin lesions compared with those without such lesions (Zaldivar 1980; Zaldivar and Ghani 1980). Among the more recent and relevant studies were those reported from Antofagasta, Chile. Residents of Antofagasta experienced a dramatic increase in concentrations of arsenic (> 500 ppb) in their drinking water from 1958 until remediation efforts began in the 1970's. In utero and early childhood exposure to these high levels resulted in increased mortality from lung cancer, bronchiectasis, and other chronic obstructive pulmonary disease in adults several decades after the exposures (Smith et al., 2006). In addition, these early life exposures resulted in decrements in lung function (Dauphine et al, 2011) and increased susceptibility to pulmonary infections in adults (Smith et al, 2011).
Animal and in vitro models have been used in attempts to determine the sites and the mechanisms of pulmonary developmental toxicity of inorganic arsenicals. Using a mouse model, in utero and early postnatal exposures to arsenic resulted in dose dependent increases in airway reactivity. These changes were irreversible and specific to exposures during lung development (Lantz et al., 2009). Alterations were correlated with protein and gene expression changes in extracellular matrix (collagen, elastin), resulting in increased smooth muscle mass around the small airways. These authors demonstrated that in utero and postnatal exposure to environmentally relevant levels of arsenic (50 or 100 ppb in water) can irreversibly alter pulmonary structure and function in adult mice.
Human and mouse models have clearly shown that early life exposures to arsenic can result in alterations in adult lung function and lung disease. However, at present no reports exist concerning the relationship of early life arsenic exposures and lung function in children. For this reason, the aim of this study was to evaluate the impact of in utero and early-life arsenic exposure through drinking water on lung function in children.
Material and Methods
Study population
Subjects were recruited by presentations to the parents at schools and by the distribution of flyers. More than 500 children were evaluated, however, only 358 children fulfill the inclusion criteria and they were included in our study. The participants were females and males aged 6-12 years residing in four rural communities in which the highest arsenic tap water levels have been detected in the last 20 years (104-360 ppb). These communities received groundwater through the local water supply and the high As levels detected in it is due to an over water extraction from the ground for crops. At present, water is obtained to a deep of 200-300 meters. These communities form part of the geographic area known as Comarca Lagunera, which is located in the north-central part of Mexico.
To aid in focusing on arsenic exposure that is related to in utero and/or post-natal exposure, we exclusively included only healthy volunteers who were conceived in the studied rural communities, whose mothers live their entire pregnancies in these communities and in who have remained as permanent residents of the same communities. Subjects who agreed to participate in the study were divided in four study groups (quartiles) according with their total urinary arsenic level (Group 1: <63 μg/l; Group 2: ≥63 <113 μg/l; Group 3: ≥113 <181 μg/l and Group 4: ≥181 μg/l).
Questionnaire application
Information was collected through in-person interviews and included socio-demographic variables (education, socioeconomic status, type of kitchen, type of fuel used for cooking), lifetime residential history, lifestyle factors (secondhand smoke defined as someone smoking regularly in the same room at home, and exercise), parent's occupational history, water source types (municipal tap water, bottled, other), current medications, medical history, and diet. Water consumption habits were ascertained through a standardized questionnaire.
Standardized questions were adapted to Spanish from the questionnaire used by the American Thoracic Society Division of Lung Disease (ATS-DLD 1978) to register the presence of respiratory symptoms. It included questions regarding frequent cough (defined as presence of cough on most days for 3 consecutive months or more during the year), chronic cough (defined as presence of cough for 3 consecutive months in 2 consecutive years), frequent phlegm (defined as bringing up phlegm on most days of month, for 3 consecutive months or more in a year), chronic phlegm (presence of phlegm for 3 consecutive months in 2 consecutive years), frequent wheezing (whistling sound heard on expiration within 2 years), chronic wheezing (whistling sounds heard on expiration more than 2 years), shortness of breath Grade I (shortness of breath, when hurrying on level ground or walking up a slight hill) and Grade II (dyspnea defined as walk slower than people of the same age on level ground because of breathlessness or has to stop for breathing when walking at own pace on level ground). Asthmatic volunteers confirmed by a doctor were not included in the study.
Written informed consent was obtained from each participant and from their parents to obtain biological samples, which were obtained at the time of interview. The study protocol was approved by the Ethics Committee of the School of Medicine at Torreon, University of Coahuila, Mexico.
Arsenic measurement in drinking water and urine
Drinking water samples were collected from the municipal well and analyzed for inorganic arsenic levels. No other contaminants in the drinking water were measured.
Individual exposure was assessed based on urinary concentration of the sum of inorganic arsenic. The urine samples were obtained during the late autumn and winter seasons to avoid the hottest seasons when there is much higher water consumption and children have increased outdoor activities. The in utero and childhood As exposure was calculated as the As tap water level mean registered in the studied communities during the period of 2000-20013. Urine samples were collected in polypropylene bottles and stored at −80°C until they were analyzed using the methodology described by U.S. Center for Disease Control (method 0161A/01 OD, 2004). Briefly, arsenic species in urine [AsV, AsIII, monomethylarsonic acid (MMAV), dimethylarsinic acid, (DMAV) and arsenobetaine] were separated by HPLC. Arsenic concentrations in water samples and urine were analyzed by inductively coupled plasma mass spectrometry utilizing the Standard Reference Water, SMR 1640 (NIST, Gaithersburg, MD, USA) and the freeze-dried Urine Reference Material for trace elements (Clinchek-control; RECIPE Chemicals instruments GmbH, Munich, Germany) for urine as quality control. The concentration of metabolites of inorganic arsenic in urine is a recognized biomarker of the exposure to inorganic arsenic (Vahter 2002). Additional exposure to DMA or to organic arsenic metabolized in the body to DMA, which usually is attributable to consumption of seafood such as bivalves and seaweeds, was considered minimal because such seafoods are essentially never eaten in this area.
Arsenic metabolism efficiency was calculated using the following formulas proposed by Del Razo et al., (1997) first methylation = MMAV/(AsV+AsIII); second methylation = DMAV/MMAV.
Lung function measurement
After height and weight were measured by interviewers, lung function was assessed according to American Thoracic Society guidelines (ATS 1995) using an EasyOne spirometer (NDD Medical Technologies, Zurich, Switzerland) in diagnostic mode for pulmonary function tests. The device was standardized to meet American Thoracic Society guidelines for lung function tests. The EasyOne has been used in a number of research studies in different countries and at a number of teaching hospitals in the United States (Menezes et al., 2005; NDD 2007; Perez-Padilla et al., 2006a). The instrument was calibrated each morning prior to data collection with a 3-L syringe (SensorMedics, USA).
The age, height, weight, sex and race predicted values of Forced Vital Capacity (FVC), forced expiratory volume in one second (FEV1) and their ratio (FEV1/FVC) were recorded in milliliters (ml) and percentage, respectively. For predicted values those of the Mexican-Americans in NHANES III (Hankinson et al., 1999). Predicted percentage of ≥ 80% for FVC and FEV1 and an FEV1/FVC ratio of ≥0.7 were considered as cut off values for lung function tests to be normal. These cut-offs are generally used internationally for categorizing lung volumes as normal or abnormal (Fletcher et al., 1959). Obstructive lung function was defined as having FEV1 <80% and FEV1/FVC <70% and restrictive lung function was defined as having FEV1 <80% and FEV1/FVC >70% (Celli and MacNee, 2004). The procedure was explained in detail to the participants who were allowed to practice until they felt comfortable. Pulmonary function was evaluated with the children seated, using a noseclip and a disposable mouthpiece. Exclusion criteria for spirometry included a positive answer for any of the following questions in the last three months: thoracic or abdominal surgery, heart problem, eye surgery and admission to hospital for any cardiac condition; pregnancy among the girls was also exclusion for spirometry.
Volunteers were instructed to take as deep a breath as possible and then blow as hard and long as possible into the spirometer. Following a demonstration and practice with the mouthpiece, participants performed tests in a sitting position with active coaching. The maneuver was repeated until the EasyOne indicated satisfactory results were achieved (e.g., FEV1 and FVC within 200 ml of previous values) or the participant chose to stop. Each subject's best trial (largest sum of FEV1 and FVC) was included in analyses. Spirometry data were reviewed by a pulmonologist.
Statistics
In the univariate analysis, independent and dependent variables were described according to their frequency and distribution measurements (arithmetic mean, range and standard deviations). In the bivariate analysis, the F test was used when the variable was divided into more than two categories, and we used Student's t test or the Mann Whitney test to compare different levels of arsenic when dichotomic variable categories were analyzed. This method permitted us to establish statistical differences among groups for each dependent variable. Linear regression models were used to assess crude or independent associations between subjects' variables with arsenic urine concentrations. Linear regression analysis was also performed separately for FEV1, FVC and FEV1/FVC ratio in order to determine the association of percentage predicted lung volumes with respiratory symptoms. Validity of the ATS respiratory questionnaire along with predictive values was also calculated for obstructive and restrictive lung patterns separately. In multiple analyses, we included all relevant variables identified in the bivariate model in addition to those possessing biological plausibility. The confounding variables included in the final analyses were gender, BMI, type of water consumed and mother's education level. All analyses were performed using the statistical software STATA 11.0 (Stata Corp., College Station, TX).
Results
Most of the socio-demographic, anthropometric characteristics, and life style variables of the participants were similar between studied groups, except the following variables: BMI was lower among subjects from Group 4; second hand smoking was lower in children form Group 3 and it was higher in volunteers from Group 4 (p<0.05) (Table 1). On the other hand, the frequency of consumption of tap water at home, at school and for cooking was significantly higher (p<0.05) in subjects from groups 3 and 4. The mean number of water glasses (300 ml) drank per day was 2.8 and no differences between groups were observed in terms of the amount of water ingested. Other possible sources of arsenic exposure including diet, agrochemicals, fuels, preservatives or others compounds containing arsenicals were negligible. A good correlation (0.6895) was found between As tap water level with the arsenic urinary level.
Table 1.
Socio-demographic, anthropometric characteristics, and life style of the participants. Results are shown as arithmetic mean and standard deviation.
| Group 1(n= 89) | Group 2 (n= 90) | Group 3 (n= 89) | Group (n= 90) | |
|---|---|---|---|---|
|
| ||||
| Age (years) | 8.8 1.8 | 9.2 1.88 | 9 1.80 | 8.7 1.64 |
|
| ||||
| Gender | ||||
| Female | 50 56.18% | 54 60.00% | 45 50.56% | 39 43.33% |
| Male | 39 43.82% | 36 40.00% | 44 49.44% | 51 56.67% |
|
| ||||
| Height (m) | 1.35 0.13 | 1.38 0.13 | 1.36 0.13 | 1.34 0.10 |
|
| ||||
| Weight (Kg) | 34.30 11.52 | 36.86 13.55 | 33.65 10.79 | 31.39 9.16 |
|
| ||||
| BMI** | 18.67 4.75 | 17.59 3.44 | 17.12 3.46 | 16.85 5.40* |
|
| ||||
| Schooling (years) | 3.63 1.78 | 3.79 1.88 | 3.57 1.71 | 3.26 1.53 |
|
| ||||
| Residency (years) | 8.33 1.95 | 8.55 2.23 | 8.43 1.90 | 8.22 1.81 |
|
| ||||
| Second hand smoking | ||||
| No | 66 74.15% | 62 80.00% | 75 84.26% | 55 61.11% |
| Yes | 23 25.84% | 18 20.00% | 14 15.73% | 35 38.88%* |
|
| ||||
| Father's occupation | ||||
| Agricultural worker | 23 27.71% | 18 22.78% | 18 22.78% | 25 30.86% |
| Use of agrochemicals | 15 16.85% | 12 13.33% | 12 13.48% | 15 16.66% |
|
| ||||
| Place of pregnancy | ||||
| Same community | 73 82.1% | 77 85.55% | 76 85.39% | 64 72.22% |
| Different community | 16 17.9% | 13 14.44% | 13 14.60% | 25 27.77% |
|
| ||||
| Use of folic acid during pregnancy | ||||
| No | 6 6.74% | 6 6.66% | 4 4.49 | 8 8.88% |
| Yes | 83 93.25% | 84 93.33% | 85 95.50% | 82 91.11% |
|
| ||||
| Smoking during pregnancy | ||||
| No | 88 98.87% | 84 100.00% | 88 98.87% | 90 100.00% |
| Yes | 1 1.12% | 0 | 1 1.12% | 0 |
|
| ||||
| Breastfeeding (months) | 9.97 7.77 | 9.94 9.27 | 9.47 10.55 | 8.94 9.47 |
|
| ||||
| Type of water used at home | ||||
| Purified | 50 56.17% | 46 51.11% | 36 40.44% | 28 31.11% |
| Tap water | 37 41.57% | 43 47.77% | 52 58.42%* | 59 65.55%* |
| Both | 2 2.24% | 3 3.33% | 2 2.24% | 3 3.33% |
|
| ||||
| Type of water used at school | ||||
| Purified | 13 14.60% | 15 16.66% | 12 13.48% | 3 3.33% |
| Tap water | 72 80.89% | 71 78.88% | 77 86.51% | 84 93.33%* |
| Both | 4 4.49% | 4 4.44% | 0 | 3 3.33% |
|
| ||||
| Type of water for cooking | ||||
| Purified | 2 2.24% | 16 17.77% | 14 15.73% | 7 7.77% |
| Tap water | 22 24.71% | 70 77.77%* | 72 80.89%* | 80 88.88%* |
| Both | 65 73.03% | 4 4.44% | 3 3.37% | 3 3.33% |
p<0.05: T test, Mann Whitney test or Chi2 (Group 1 compared with Groups 2-4)
BMI: calculated as: weight/height2
Total arsenic, methylated metabolites and inorganic As (iAs) levels were significantly higher (p<0.05) in subjects from groups 2-4 than in Group 1, and the %MMA was higher only in subjects from Group 4 (Table 2). Total arsenic urinary levels were positively associated with male gender (β 0.105; 95% CI 0.001, 0.20), and type of water used at home (β 0.153; 95% CI 0.05, 0.25) and negatively associated with BMI (β -0.014; 95% CI -0.0274, -0.0009) and with mother's schooling (β -0.013; 95% CI -0.0251, -0.001).
Table 2.
Total arsenic and its metabolites urinary levels in the studied population. Results are shown as arithmetic mean and standard deviation.
| Group 1 (n= 89) | Group 2 (n= 90) | Group 3 (n= 89) | Group 4 (n= 90) | |
|---|---|---|---|---|
| Total As (μg/L) | 41.08 ±14.4 | 84.95 ±14.19** | 143.75 ±19.4** | 294.01 ±122.7** |
| AsIII | 3.98 ±2.92 | 9.30 ±4.1** | 17.42 ±9.0** | 39.13 ±23.0** |
| AsV | 9.77 ±14.2 | 9.30 ±18.1 | 6.91 ±12.7* | 11.51 ±22.8* |
| MMA | 5.16 ±2.4 | 10.79 ±4.0** | 19.63 ±6.1** | 40.74 ±21.6** |
| DMA | 22.11 ±15.3 | 53.32 ±20.3** | 96.21 ±21.5** | 189.90 ±86.0** |
| First methylation | 0.96 ±0.8 | 0.83 ±0.3 | 0.94 ±.3 | 0.93 ±0.3 |
| Second methylation | 4.66 ±3.3 | 5.40 ±2.4 | 5.36 ±1.8 | 5.05 ±1.6 |
| iAs | 13.75 ±14.2 | 18.45 ±16.9** | 23.95 ±15.0** | 51.60 ±31.9** |
| %iAs | 32.85 ±32.0 | 21.93 ±20.2 | 16.73 ±10.9 | 17.16 ±8.6 |
| %MMA | 12.53 ±3.6 | 12.67 ±3.9 | 13.61 ±3.6 | 13.74 ±3.3** |
| %DMA | 53.37 ±31.1 | 62.69 ±21.4 | 67.04 ±13.0 | 64.88 ±12.7 |
p <0.05
p< 0.001: Ttest or Mann Whitney test (Group 1 compared with Groups 2-4)
The three most frequently recorded respiratory symptoms in the studied population were frequent phlegm (9.77%), frequent cough (9.77%), and frequent wheezing (5.86%) (Table 3). Less than 8.2% of children referred chronic phlegm and cough. Most of the respiratory complaints and number of respiratory diseases were slightly more frequent but not different (p>0.05) in subjects from group 3 (Table 3). In addition to these symptoms, respiratory diseases were investigated and bronchiolitis was the most frequent disease registered among participants (11.4%), and it was slightly more frequent in children from groups 2 and 4 when compared with the recorded in children from Group 1 (3.49% vs 11.9 and16.19.5%, respectively) (p<0.05) (data not shown). Other diseases such as, asthmatic bronchiolitis, pneumonia, whooping cough, and asthma were infrequent (<4%).
Table 3.
Frequency of respiratory symptoms in the children studied. Results are shown as frequency and percentage.
| Group 1 (n= 89) | Group 2 (n= 90) | Group 3 (n= 89) | Group 4 (n= 90) | |
|---|---|---|---|---|
|
| ||||
| Frequent cough | ||||
| No | 84 94.38 | 81 90.00 | 78 87.64 | 78 86.66 |
| Yes | 5 5.61 | 9 10.00 | 11 12.35 | 12 13.33 |
|
| ||||
| Chronic cough | ||||
| No | 85 95.50 | 87 96.66 | 87 97.75 | 85 94.44 |
| Yes | 4 4.49 | 3 3.33 | 2 2.24 | 5 5.55 |
|
| ||||
| Frequent phlegm | ||||
| No | 82 92.13 | 77 85.55 | 84 94.38 | 80 88.88 |
| Yes | 7 7.86 | 13 14.44 | 5 5.61 | 10 11.11 |
|
| ||||
| Chronic phlegm | ||||
| No | 86 96.62 | 84 93.33 | 87 97.75 | 86 95.55 |
| Yes | 3 3.37 | 6 6.66 | 2 2.24 | 4 4.44 |
|
| ||||
| Frequent wheezing | ||||
| No | 83 93.25 | 83 92.22 | 87 97.75 | 84 93.33 |
| Yes | 6 6.74 | 7 7.77 | 2 2.24 | 6 6.66 |
|
| ||||
| Chronic wheezing | ||||
| No | 86 92.62 | 88 97.77 | 87 97.75 | 88 97.77 |
| Yes | 3 3.37 | 2 2.22 | 2 2.24 | 2 2.22 |
|
| ||||
| Shortness of breath grade I | ||||
| No | 89 100 | 90 100 | 89 100 | 90 100 |
| Yes | 0 | 0 | 0 | 0 |
|
| ||||
| Number of respiratory diseases per year | ||||
| 0 | 4 4.49 | 2 2.22 | 4 4.49 | 4 4.44 |
| 1-3 | 69 77.52 | 72 80.00 | 68 76.40 | 69 76.66 |
| 4-7 | 15 16.85 | 16 17.78 | 18 20.22 | 15 16.66 |
| 8 -10 | 1 1.12 | 0 | 0 | 2 2.22 |
p <0.05
p< 0.001: Ttest or Mann Whitney test (Group 1 compared with Groups 2-4)
On the basis of quality assessment of spirometry reports, 9 entries were excluded from the analysis. Forty two percent of total volunteers had a normal spirometric evaluation; meanwhile, the percent of normal spirometries was significantly higher in group 1 (48%) than in groups 3 and 4 (34% and 38%). Most of the evaluated children had a decrement in the residuals of FEV1 (59.8 %) and FVC (73.4%). The percent predicted FEV1 and FVC mean values in the population was negative (Figure 1); and the FEV1 and FVC deficits were significantly bigger (p<0.02) in volunteers from group 3 and 4, respectively (Table 4), meanwhile, the FVC residual was lower only in subjects from Group 3 (p<0.01) (Table 4). FVC was the parameter most affected in the studied population and it was negatively associated with AsV (β -0.003; 95% CI -0.006, -0.001) and with percent of inorganic As (%iAs) (β -0.003; 95% CI -0.005, -0.008), and positively associated with DMA (β 0.002; 95% CI 0.006, 0.005) and with secondary methylation (β 0.026; 95% CI 0.006, 0.689). In the adjusted multivariate analysis, only %iAs (β -0.003; 95% CI -0.006, -0.001) and DMA (β 0.003; 95% CI 0.001, 0.005) maintained their association with FVC.
Figure 1.
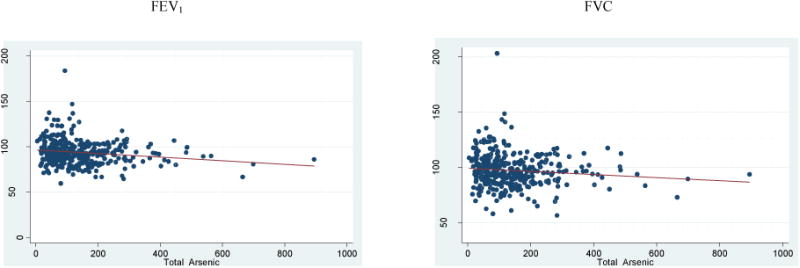
Relationship between percent of predicted FEV1 and FVC with total arsenic urinary levels.
Table 4.
Residual spirometric parameters*, spirometric pattern and severity of pulmonary restriction in the studied population. Results are shown as arithmetic mean and standard deviation.
| Spirometric variable | Group 1 (n= 87) | Group 2 (n= 88) | Group 3 (n= 88) | Group 4 (n= 86) |
|---|---|---|---|---|
| Percent predicted FEV1 | 98.97 14.1 | 99.20 18.1 | 95.81 14.5** | 95.41 12.5 |
| Percent predicted FVC | 96.19 12.9 | 96.24 16.0 | 91.33 13.9 | 90.82 10.7** |
| FEV1 residual* (mL) | -63.86 30.5 | -54.93 35.5 | -87.14 38.0 | -77.12 37.6 |
| FVC residual* (mL) | -120.86 29.8 | -115.64 36.0 | -190.39 42.7** | -167.27 38.1 |
| FEV1/FVC (%) | 0.91 0.05 | 0.91 0.05 | 0.92 0.05 | 0.92 0.05 |
| Spirometric pattern | ||||
| Normal | 42 48.28% | 43 48.86% | 30 34.09% | 33 38.37% |
| Restrictive | 45 51.72% | 45 51.14% | 58 65.91% | 53 61.63% |
| Severity of pulmonary restriction (% of predicted FVC) | ||||
| Low (≥70) | 45 51.72% | 44 48.88% | 57 64.72% | 48 55.81% |
| Moderate (≥60 <70) | 0 | 0 | 1 1.19% | 5 5.82% |
| Moderate to severe (≥50 <60) | 0 | 1 2.26% | 0 | 0 |
| Severe (≥34 <50) | 0 | 0 | 0 | 0 |
| Grave (<34) | 0 | 0 | 0 | 0 |
Residual = obtained minus predicted.
Ttest, Mann Whitney or Chi2 < 0.02 (Group 1 compared with Groups 2-4)
The results of FEV1 and FVC show that a total of 199 subjects (57%) had a restrictive spirometric pattern and no more than 2% had an obstructive pattern. From the children with a restrictive pattern, more than 96% had a low severity grade. No differences in the frequency in the five types of severity pulmonary restriction were found between groups, except in the frequency of moderate severity, which was higher among volunteers from Group 4 (Table 4).
Urinary arsenic concentration was significantly higher in those children with a restrictive spirometric pattern when compared with subjects with a normal pattern. In volunteers with moderate pulmonary restrictive patterns, the urinary arsenic level was twice the mean recorded in the overall studied population (291.2 vs 141.2 μg/L) (Table 5).
Table 5.
Total arsenic urinary levels and spirometric patterns. Results are shown as arithmetic mean and standard deviation.
| Spirometric Pattern | Severity of Pulmonary Restriction | ||||
|---|---|---|---|---|---|
| Normal (n=148) | Restrictive (n=199) | P* | Low restriction (n=192) | Moderate restriction (n=6) | |
| Total Arsenic (μg/L) | 128.1 ± 108.0 | 149.9 ± 119.1* | 0.02 | 145.9 ± 114.4 | 291.2 ± 192.5 |
Mann Whitney test (Normal compared with restrictive).
Discussion
To our knowledge, this is the first study evaluating the association between arsenic exposures through drinking water and lung function in children. It is widely known that the main source and route of arsenic exposure in humans is through drinking water.
In the present study, participants were exposed since pregnancy until early childhood to an average water As concentration of 152.13 ± 49.35 μg/L (range 104-275 μg/L). The mean urinary arsenic level registered in the overall studied subjects was 141.2 μg/L. From all children evaluated, only 16.7% had a urinary concentration below the national concern level (<50 μg/L). The proportions of urinary As metabolites found in our study population were consistent with the relative distribution in other populations exposed to arsenic (range of 10–30% iAs, 10–20% MMA, and 60–80% DMA) (Hopenhayn-Rich et al., 1998). In addition, the methylation pattern seen in our study is similar to what has been reported in other Mexican children studies (Del Razo et al., 1994). The pattern is slightly different from what has been reported in Belgian (Buchet et al., 1988) and North-American children (Kalman et al., 1990). Differences in metabolic profiles between these ethnic groups may be due mainly to the dissimilar As exposure, genetics, and diet.
Guifan et al., (2006) reported different proportion of As metabolites between children and adults depending on the level of exposure. These authors found that the concentrations of all As metabolites were higher in children than in adults when exposed to 20-90 μg/l, but lower in children than in adults in a 160 μg/L exposed group. In our work, most of the arsenic metabolites were higher (p<0.05) in the most exposed groups and the %MMA was more than 8 times greater when in Group 4 when compared with Group 1 (p=0.003). This may partially explain our results showing a decrement in lung function, because high proportion of iAs and of MMA in urine has been associated with increased risk of a number of different health effects in adults (Tseng 2007) and monomethylation of arsenic produces a toxic metabolite (Bing et al., 2001; Nesnow et al., 2002). While gender has been associated with differences in As metabolism, there were not any differences between males and females in this age group. According with the recollected data, the main source of the As levels in the children studied is the ingestion and use of tap water; other As sources such as diet, environmental pollution or other compounds containing arsenicals (agrochemicals, preservatives, fuels) were negligible. Another possibility to explain that levels is that they are carriers of As metabolic enzymes polymorphisms.
Evidence suggests that arsenic exposure in drinking water has been associated with an increase in respiratory symptoms or diseases in exposed adults. Sensitive windows of exposure may exist during development that can affect adult function. Exposures to high levels of arsenic may be particularly harmful. During pregnancy in utero transplacental transfer of arsenic occurs in humans (Concha et al., 1998) which can lead to functional changes via altered fetal programming (Heindel 2007; Vahter 2008). In addition, early-life or childhood exposures can alter lung structure and function because of interference with rapid organogenesis and differences in children's water intake, metabolism, and detoxification (Landrigan et al., 2004). These alterations may contribute to the increased susceptibility of developing chronic respiratory disease in arsenic exposed human populations (Ramsey et al., 2013). In our study, 81% of the children were conceived in the studied rural communities and 19% in other close rural communities in which the As water levels must be similar because they receive the tap water from the same water supply system; 97% of them have lived their entire life in the same location. Therefore, the children in our study have been exposed chronically in utero and during their early childhood life to high As levels. In order to completely demonstrated that in utero exposure produced effects on lung development or if the effects are due to chronic arsenic exposure to the growing child, additional studies will be needed, including a cohort that has moved into the study areas with children borne elsewhere.
In our study, more than 93% mothers received folic acid during pregnancy, which could diminish the effects of arsenic during development due to its antioxidant properties (Mukherjee et al., 2006) and by donation of methyl groups (Friso et al., 2002). However, the percentage of mothers taking folic acid was similar in the studied groups indicating that this would not account for the observed arsenic-induced differences between the groups.
In our study, the questionnaire did not show any significant differences in the prevalence of respiratory symptoms or diseases between groups. Respiratory symptoms and diseases were not associated with As urinary levels or its metabolites. These data are in agreement with those reported by Dauphine et al., (2011) who found no evidence of associations between arsenic levels and chronic cough, phlegm and chronic bronchitis in adults that had been exposed to high levels during lung development. A limitation of our study was that respiratory symptoms and diseases were assessed by questionnaire, which may be affected by recall bias, leading to either over- or underestimation of the association.
However, using spirometry measurements, a decrease of 5% and 8% of predicted percent of FEV1 and FVC was observed, respectively. The percent of predicted FEV1 and FVC were significantly lower in the most exposed groups (3 and 4, respectively). Dauphine et al., (2011) described decreases of 4.6% in FEV1 and 2.7% in FVC values in adults who were exposed to arsenic concentrations of 50 – 250 μg/L during lung development. Higher decreases in these parameters (11.5% and 12.2%) were seen with exposures greater than 800 μg/L. When we compared the results of FEV1 and FVC residuals between our groups, we found a significant decrease in both parameters in Group 3, and an inverse relationship between total urinary As levels with spirometric parameters. The reduction of FVC residual was 1.38 times higher (p<0.05) in group 4, however, in the multivariate analysis, only FVC was negatively associated with %iAs and positively associated with %DMA. In contrast with our results, several authors (De et al., 2004; Parvez et al., 2008; Mazumder et al., 2000; 2005; Milton et al., 2002, 2003; von Ehrenstein et al., 2005) have reported an inverse association between urinary As levels not only for FCV, but also for FEV1, and FEV1/FVC ratio. These data was obtained in adults, some with visible arsenical lesions, a fact that may bias the assessment of pulmonary function. The decreases in FEV1 and FVC registered in children suggest a restrictive spirometric pattern. von Ehrenstein et al., (2005) found equivalent patterns in exposed adults who had a restrictive (e.g., lung fibrotic or neuromuscular) pattern. De et al., (2004) reported in their study, that 53% of exposed subjects had restrictive lung disease. The restrictive pattern in our study was more frequently observed in the most exposed group of children (61.6-65.9%) and it was positively associated with As urinary levels. From children with the restrictive pattern, 96.51% had pulmonary restriction grade 1 (low), while, one (1.19%) and 5 children (5.82%) from groups 3 and 4, respectively, had pulmonary restriction grade 2 (moderate) and one child (2.26%) from group 2 having a grade 4 (moderate to severe). In children with restriction grade 2, urinary As levels were almost twice that recorded for children with restriction grade 1.
Our data suggest that As should be considered a potentially harmful risk factor for decrement in lung function during infancy and it may contribute to the increased susceptibility for chronic respiratory diseases later in life. Several investigators have used lung development in mice to model what could occur during human developmental. While lung development in the mouse occurs much more rapidly, the stages of lung development are similar in both species. Mice are born with less well developed lungs than humans but bot nice and humans go through a rapid postnatal alveolar development after birth. In the case of humans this continues until the ages of 8-12 years old, while in mice this process is almost complete in 28 day old animals. Thus comparison of mice at 28 days old is equivalent to approximately a 10 year old human lung.
Restrictive lung disease is associated with diseases of the parenchyma of the lung or of the respiratory muscles. Previously, arsenic has been shown to alter extracellular matrix protein production (Lantz et al., 2009) and to inhibit wound repair (Olsen et al., 2008). Alterations in connective tissue could result in inappropriate distal lung development, especially alveolar septation that occurs from birth to around 10 years of age in humans. Development of fibrosis is accompanied by restrictive lung disease. In addition, inhibition of wound repair was due to inappropriate migration and differentiation of epithelial cells. Inhibition of these processes during distal airway and parenchymal development could reduce the numbers of cells producing surfactants, leading to restrictive lung disease.
Although the mechanism of As-induced respiratory illness is not fully understood, several studies have shown that a large amount of As is deposited and stored in the lung, especially in the epithelium (Gerhardsson et al., 1988; Saady et al., 1989). In rabbits, arsenic has been shown to accumulate in the lung more than in any other organ except the liver and kidney (Bertolero et al., 1981; Marafante et al., 1981). Other animal studies show that DMA is retained longer in the lungs than in other tissues (Kenyon et al., 2008) and it has been proposed that the levels of arsenic in the fetal lung would be about 1% of the administered arsenic, mostly in the form of dimethylarsenic (Devesa et al.,2006). Several authors have suggested that the mechanism of action of As in the lungs is that it can enhance tissue inflammation (De et al., 2004; Nemery 1990), inducing respiratory function impairment by oxidative stress (Hays et al., 2006; Lantz and Hays, 2006) or by producing or increasing pulmonary fibrosis (Nemery 1990, von Ehrenstein et al., 2005). Increased inflammatory responses have been reported in infants born to arsenic exposed mothers (Fry et al., 2007) and arsenic alters markers of inflammation (soluble receptor for advanced glycation end products, matrix metalloproteinase – 9, tissue inhibitor of matrix metalloproteinase 1) in adults exposed to 20 μg/L arsenic.
In conclusion, arsenic significantly reduces FVC in children in a dose dependent manner and significantly increases the percentage of children with pulmonary restriction grade 1. Decreases in FVC were associated with %iAs in the urine. Similar results are seen in adults that have been exposed to arsenic during lung development, even when arsenic levels are subsequently reduced. Control of exposure to arsenic during critical early life lung development may be important for reducing arsenic-induced decrements in pulmonary function.
Acknowledgments
Sponsors: This work was supported in part by the University of Coahuila and by the Superfund National Institute of Environmental Health Sciences (grant number ES-04940).
References
- Bertolero F, Marafante E, Rade JE, Pietra R, Sabbioni E. Biotransformation and intracellular binding of arsenic in tissues of rabbits after intraperitoneal administration of 74As labeled arsenite. Toxicology. 1981;20(1):35–44. doi: 10.1016/0300-483x(81)90103-7. [DOI] [PubMed] [Google Scholar]
- Bing L, Jingbo P, Guifan S. Monomethylarsinic acid: a more toxic immediate metabolites of inorganic As metabolism. Chin J Endemiol. 2001;20:219–221. [Google Scholar]
- Buchet JP, Lauwerys R. Role of thiols in the in-vitro methylation of inorganic arsenic by rat liver cytosol. Biochem Pharmacol. 1988;37:3149–3153. doi: 10.1016/0006-2952(88)90313-9. [DOI] [PubMed] [Google Scholar]
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- CDC. Urine arsenic speciation HPLCICPDRCMS. Laboratory Procedure Manual. 2004 Method 0161A/01 OD. [Google Scholar]
- Concha G, Vogler G, Nermell B, Vahter M. Low-level arsenic excretion in breast milk of native Andean women exposed to high levels of arsenic in the drinking water. Int Arch Occup Environ Health. 1998;71(1):42–46. doi: 10.1007/s004200050248. [DOI] [PubMed] [Google Scholar]
- Chiou HY, Hsueh YM, Liaw KF, Horng SF, Chiang MH, Pu YS, et al. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res. 1995;55:1296–1300. [PubMed] [Google Scholar]
- Dartmouth Trace Element Analysis. Core: Lab Methods. http://www.dartmouth.edu/∼toxmetal/assets/pdf/teamethods.pdf.
- Dauphine DC, Ferreccio C, Guntur S, Yuan Y, Hammond SK, Balmes J, Smith AH, Steinmaus C. Lung function in adults following in utero and childhood exposure to arsenic in drinking water: preliminary findings. Int Arch Occup Environ Health. 2011;84:591–600. doi: 10.1007/s00420-010-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De BK, Majumdar D, Sen S, Guru S, Kundu S. Pulmonary involvement in chronic arsenic poisoning from drinking contaminated ground-water. J Assoc Physicians India. 2004;52:395–400. [PubMed] [Google Scholar]
- Del Razo LM, Hernandez JL, Garcia-Vargas GG, Ostrosky-Wegman P, Cortinas de Nava C, Cebrian ME. Urinary excretion of arsenic species in a human population chronically exposed to arsenic via drinking water: a pilot study. In: Chappell WR, Abernathy CO, Cothern CR, editors. Arsenic Exposure and Health. Northwood, England: Science and Technology Letters; 1994. pp. 91–100. [Google Scholar]
- Del Razo LM, Garcia-Vargas GG, Vargas H, Albores A, Gonsebatt ME, et al. Altered profile of urinary arsenic metabolites in adults with chronic arsenicism. A pilot study. Arch Toxicol. 1997;71:211–217. doi: 10.1007/s002040050378. [DOI] [PubMed] [Google Scholar]
- Devesa V, Adair BM, Liu J, Waalkes MP, et al. Arsenicals in maternal and fetal mouse tissues after gestational exposure to arsenite. Toxicology. 2006;224:147–155. doi: 10.1016/j.tox.2006.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;1:258–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friso S, Choi SW. Gene-nutrient interactions and DNA methylation. J Nutrition. 2002;132:2382S–2387S. doi: 10.1093/jn/132.8.2382S. [DOI] [PubMed] [Google Scholar]
- Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, Bhattacharya S, Kandjanapa K, Soontararuks S, Nookabkaew S, Mahidol C, Ruchirawat M, Samson LD. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS Genet. 2007;11:e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardsson L, Brune D, Nordberg GF, Wester PO. Multielemental assay of tissues of deceased smelter workers and controls. Sci Total Environ. 1988;74:97–110. doi: 10.1016/0048-9697(88)90131-3. [DOI] [PubMed] [Google Scholar]
- Guifan Sun, Yuanyuan Xu, Xin Li, Yaping Jin, Bing Li, Xiance Sun. Urinary arsenic metabolites in children and adults exposed to arsenic in drinking water in Inner Mongolia, China. Environ Health Perspect. 2006;115:648–652. doi: 10.1289/ehp.9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Heindel JJ. Role of exposure to environmental chemicals in the developmental basis of disease and dysfunction. Reprod Toxicol. 2007;23(3):257–259. doi: 10.1016/j.reprotox.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Smith AH. Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba, Argentina. Int J Epidemiol. 1998;27:561–569. doi: 10.1093/ije/27.4.561. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) IARC Monograph. Vol. 84. IARC; Lyon: 2004. Some drinking-water disinfectants and contaminants, including arsenic. [PMC free article] [PubMed] [Google Scholar]
- Kenyon EM, Hughes MF, Adair BM, Highfill JH, Crecelius EA, Clewell HJ, Yager JW. Tissue distribution and urinary excretion of inorganic arsenic and its methylated metabolites in C57BL6 mice following subchronic exposure to arsenate in drinking water. Toxicol Appl Pharmacol. 2008;232:448–455. doi: 10.1016/j.taap.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Kalman DA, Hughes J, van Belle G, Burbacher T, Bolgiano D, Coble K, Mottet NK, Polissar L. The effect of variable environmental arsenic contamination on urinary concentrations of arsenic species. Environ Health Perspect. 1990;89:145–151. doi: 10.1289/ehp.9089145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinniburg DG, Smedley PL. Summary. Vol. 1. Nottingham, UK: British Geological Survey; 2001. Arsenic contamination of ground water in bangladesh. Available: http://www.bgs.ac.uk/arsenic/bphase2/Reports/Vol1Summary.pdf. [Google Scholar]
- Landrigan PJ, Kimmel CA, Correa A, Eskenazi B. Children's health and the environment: public health issues and challenges for risk assessment. Environ Health Perspect. 2004;112:257–265. doi: 10.1289/ehp.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz RC, Chaua Binh, Sarihana Priyanka, Wittenbd Mark L, Pivnioukae Vadim I, Chena Guan Jie. In utero and postnatal exposure to arsenic alters pulmonary structure and function. Toxicol Appl Pharmacol. 2009;235(1):105–113. doi: 10.1016/j.taap.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marafante E, Rade J, Sabbioni E, Bertolero F, Foa V. Intracellular interaction and metabolic fate of arsenite in the rabbit. Clin Toxicol. 1981;18(11):1335–1341. doi: 10.3109/00099308109035074. [DOI] [PubMed] [Google Scholar]
- Mazumder DN, Haque R, Ghosh N, De BK, Santra A, Chakraborti D, et al. Arsenic in drinking water and the prevalence of respiratory effects in West Bengal, India. Int J Epidemiol. 2000;29:1047–1052. doi: 10.1093/ije/29.6.1047. [DOI] [PubMed] [Google Scholar]
- Mazumder DN, Steinmus C, Bhattacharaya P, von Ehrenstein OS, Ghosh N, Gotway M, et al. Bronchitis in persons with skin lesions resulting from arsenic in drinking water. Epidemiology. 2005;16:760–765. doi: 10.1097/01.ede.0000181637.10978.e6. [DOI] [PubMed] [Google Scholar]
- Menezes AM, Perez-Padilla R, Jardim JR, Muiño A, Lopez MV, Valdivia G, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- Milton AH, Hasan Z, Rahman A, Rahman M. Non-cancer effects of chronic arsenicosis in Bangladesh: preliminary results. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2003;38:301–305. doi: 10.1081/ese-120016896. [DOI] [PubMed] [Google Scholar]
- Milton AH, Rahman M. Respiratory effects and arsenic contaminated well water in Bangladesh. Int J Environ Health Res. 2002;12:175–179. doi: 10.1080/09603120220129346. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Das D, Mukherjee M, Das AS, Mitra C. Synergistic effect of folic acid and vitamin B12 in ameliorating arsenic-induced oxidative damage in pancreatic tissue of rat. J Nutr Biochem. 2006;17(5):319–327. doi: 10.1016/j.jnutbio.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Nemery B. Metal toxicity and the respiratory tract. Eur Respir J. 1990;3:202–219. [PubMed] [Google Scholar]
- Nesnow S, Roop BC, Lambert G, Kadiiska M, Mason RP, Cullen WR, Mass MJ. DNA damage induced by methylated trivalent arsenicals is mediated by reactive oxygen species. Chem Res Toxicol. 2002;15:1627–1634. doi: 10.1021/tx025598y. [DOI] [PubMed] [Google Scholar]
- NDD Medical Technologies. Reference List of Institutions and Companies using EasyOne. 2007 Available: http://www.ndd.ch/Downloads/reference_list_users.pdf.
- Nordstrom DK. Public health. Worldwide occurrences of arsenic in ground water. Science. 2002;296(5576):2143–2145. doi: 10.1126/science.1072375. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) Arsenic in drinking water 2001 update. National Academy Press; Washington, USA: 2001. [Google Scholar]
- Olsen CE, Liquori AE, Zong Y, Lantz RC, Burgess JL, Boitano S. Arsenic upregulates MMP-9 and inhibits wound repair in human airway epithelial cells. AM J Physiol Lung Cell Mol Physiol. 2008;295:L263–L302. doi: 10.1152/ajplung.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez F, Chen Y, Brandt-Rauf PW, Bernard A, Dumont X, Slavkovich V, Argos M, D'Armiento J, Foronjy R, Hasan MR, Eunus HE, Graziano JH, Ahsan H. Nonmalignant respiratory effects of chronic arsenic exposure from drinking water among never-smokers in Bangladesh. Environ Health Perspect. 2008;116(2):190–5. doi: 10.1289/ehp.9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Padilla R, Vazquez-Garcia JC, Marquez MN, Jardim JR, Pertuze J, Lisboa C, et al. The long-term stability of portable spirometers used in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care. 2006a;51:1167–1771. [PubMed] [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Aposhian HV. Monomethylarsonous acid (MMAIII) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol. 2000;163:203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Ramsey KA, Larcombe AN, Sly PD, Zosky GR. In utero exposure to low dose arsenic via drinking water impairs early life lung mechanics in mice. BMC Pharmacol Toxicol. 2013;14(1):13. doi: 10.1186/2050-6511-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saady JJ, Blanke RV, Poklis A. Estimation of the body burden of arsenic in a child fatally poisoned by arsenite weedkiller. J Anal Toxicol. 1989;13:310–312. doi: 10.1093/jat/13.5.310. [DOI] [PubMed] [Google Scholar]
- Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull WHO. 2000;78:1093–1103. [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Liaw J, Ferreccio C, Steinmaus C. Evidence from chile that arsenic in drinking water may increase mortality from pulmonary tuberculosis. Am J Epidemiol. 2011;173:414–20. doi: 10.1093/aje/kwq383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CH. Arsenic methylation, urinary arsenic metabolites and human diseases: current perspective. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25(1):1–22. doi: 10.1080/10590500701201695. [DOI] [PubMed] [Google Scholar]
- Vahter M. Late effects of early exposure to arsenic. Basic Clin Pharmacol Toxicol. 2008;102(2):204–211. doi: 10.1111/j.1742-7843.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- von Ehrenstein OS, Mazumder DN, Yuan Y, Samanta S, Balmes J, Sil A, et al. Decrements in lung function related to arsenic in drinking water in West Bengal, India. Am J Epidemiol. 2005;162:533–541. doi: 10.1093/aje/kwi236. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Recommendations. 3rd. Vol. 1. Geneva: WHO; 2004. Guidelines for Drinking-Water Quality. Available: http://www.who.int/water_sanitation_health/dwq/GDWQ2004web.pdf. [Google Scholar]
- Zaldivar R, Ghai GL. Clinical epidemiological studies on endemic chronic arsenic poisoning in children and adults, including observations on children with high- and low-intake of dietary arsenic. Zentralbl Bakteriol. 1980;170:409–421. [PubMed] [Google Scholar]
- Zaldivar R. A morbid condition involving cardio-vascular, broncho-pulmonary, digestive and neural lesions in children and young adults after dietary arsenic exposure. Zentralbl Bakteriol. 1980;170:44–56. [PubMed] [Google Scholar]


