Abstract
Objectives
Progranulin has been reported to have neuroprotective actions in cultured neurons. This study investigated the effect of recombinant rat progranulin on early brain injury after subarachnoid hemorrhage.
Design
Controlled in vivo laboratory study.
Setting
Animal research laboratory.
Subjects
Two hundred thirty adult male Sprague-Dawley rats weighing 280–320 g.
Interventions
Subarachnoid hemorrhage was induced in rats by endovascular perforation. Rat recombinant progranulin (1 and 3 ng) was administrated intracerebroventricularly at 1.5 hours after subarachnoid hemorrhage. Progranulin small interfering RNA was administrated by intracerebroventricularly at 1 day before subarachnoid hemorrhage induction. Subarachnoid hemorrhage grade, neurologic score, and brain water content were measured at 24 and 72 hours after subarachnoid hemorrhage. Neural apoptosis was evaluated by double immunofluorescence staining using terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick-end labeling and neuronal nuclei. For mechanistic study, the expression of progranulin, phosphorylated Akt, Akt, p-Erk, Erk, Bcl-2, and cleaved caspase-3 were analyzed by Western blot at 24 hours after subarachnoid hemorrhage. siRNA for sortilin 1 (a progranulin receptor) was used to intervene the downstream pathway.
Measurements and Main Results
The expression of progranulin decreased and reached the lowest point at 24 hours after subarachnoid hemorrhage. Administration of rat recombinant progranulin decreased brain water content and improved neurologic functions at both 24 and 72 hours after subarachnoid hemorrhage, while knockdown of endogenous progranulin aggravated neurologic deficits after subarachnoid hemorrhage. Rat recombinant progranulin treatment reduced neuronal apoptosis, while progranulin deficiency promoted neuronal apoptosis at 24 hours after subarachnoid hemorrhage. Rat recombinant progranulin promoted Akt activation, increased Bcl-2 level, but reduced caspase-3 level. Knockdown of progranulin binding factor sortilin 1 abolished the beneficial effects of rat recombinant progranulin at 24 hours after subarachnoid hemorrhage.
Conclusion
Rat recombinant progranulin alleviated neuronal death via sortilin 1-mediated and Akt-related antiapoptosis pathway. Rat recombinant progranulin may have potentials to ameliorate early brain injury for subarachnoid hemorrhage patients.
Keywords: Akt, early brain injury, progranulin, sortilin 1, subarachnoid hemorrhage
It has been estimated that 1–6% of the world population may harbor an intracranial aneurysm and that each year ~10/100,000 people suffer from an aneurysmal subarachnoid hemorrhage (SAH) (1, 2). Recently, early brain injury, starting immediately after aneurysm rupture to 72 hours, has been considered a primary target for treatment. Apoptosis of neuronal cells is believed to contribute to early brain injury after SAH (3, 4).
Progranulin (PGRN) is a 589-amino acid–secreted glycoprotein consisting of 7.5 repeats of a highly conserved 12-cysteinyl granulin motif in rodents (5, 6). In the periphery, PGRN involves in diverse pathophysiologies, such as wound healing, inflammation, tumorigenesis, and development (7, 8). In CNS, PGRN was found to be mainly expressed on neurons and PGRN expression in neurons increased as the cells mature (9). PGRN mutations are linked to frontotemporal lobar degeneration (FTLD), a form of disease characterized by severe neuronal loss in frontal and temporal brain regions of adult patients (10, 11). PGRN may function as a neurotrophic factor to enhance neuronal survival (12) because PGRN administration rescued cortical neurons from cell death induced by glutamate or oxidative stress in vitro (13). Furthermore, PGRN deficiency promoted neuron loss following toxin-induced injury (14).
A potential role of PGRN in stroke has not been systematically investigated. For instance, PGRN-deficient hippocampal slices are hypersusceptible to microglial activating agents in oxygen glucose deprivation model (15), and PGRN expression decreases after middle cerebral artery occlusion and rat recombinant PGRN (r-PGRN) inhibits neutrophil infiltration (16). A direct neuroprotective effect of PGRN has not been established in either cerebral ischemia or brain hemorrhage animal models. Therefore, we investigated the neuroprotective effect of PGRN in a SAH rat model.
MATERIALS AND METHODS
Animals
All procedures were conducted following an institutionally approved protocol by the Institutional Animal Care and Use Committee at Loma Linda University and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Two hundred thirty adult Sprague-Dawley rats (280–320 g; Harlan, Indianapolis, IN) were housed in a light- and temperature-controlled environment with unlimited access to food and water.
SAH Model and Experimental Protocol
The endovascular perforation model of SAH in rats was performed as reported previously (17). Briefly, with 3% isoflurane anesthesia, a sharpened 4-0 monofilament nylon suture was inserted rostrally into the left internal carotid artery from the external carotid artery stump and perforated the bifurcation of the anterior and middle cerebral arteries. Sham-operated rats underwent the same procedures except that the suture was withdrawn without puncture. Animals were divided randomly into different groups before surgery. The protein expression of endogenous PGRN was detected at 3, 12, 24, 48, and 72 hours after SAH. Two dosages (1 and 3 ng) of r-PGRN (14) were administrated intracerebroventricularly at 1.5 hours after SAH. PGRN siRNA was injected by intracerebroventricularly at 1 day before SAH induction. SAH grade, neurologic score, and brain water content were measured at 24 and 72 hours after SAH. Neural apoptosis was evaluated by terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick-end labeling (TUNEL) staining at 24 hours after SAH. To study mechanisms, the expression of PGRN, phosphorylated Akt (p-Akt), Akt, p-Erk, Erk, Bcl-2, and cleaved caspase-3 (CC3) was analyzed by Western blot at 24 hours after SAH. Sortilin 1 (SORT1) siRNA was used to intervene the downstream pathway. r-PGRN (expressed in HEK 293 cells, signal peptide and rat PGRN [aa 1–602] are fused at the C-terminus to a FLAG-tag) was purchased from Sigma-Aldrich (St. Louis, MO). PGRN siRNA, SORT1 siRNA, and Scramble siRNA were purchased from OriGene Technologies (Rockville, MD).
Intracerebroventricular Drug Administration
Intracerebroventricular drug administration was performed as previously described (18). Briefly, rat was placed in a stereotaxic apparatus under 2.5% isoflurane anesthesia. The needle of a 10-μL Hamilton syringe (Microliter 701; Hamilton Company, Reno, NV) was inserted through a burr hole into the right lateral ventricle at the following coordinates relative to bregma: 1.5 mm posterior, 1.0 mm lateral, and 3.2 mm below the horizontal plane of the skull. r-PGRNs (1 ng/5 μL and 3 ng/5 μL) were injected at 1.5 hours after SAH induction by a pump at the rate of 0.5 μL/min, respectively. In order to enhance the gene silence efficiency, three different PGRN siRNAs were mixed: 1) 5′GCUGUCCUUCUAACAAUACCUGCTG 3′; 2) 5′GGGCUAACGACUAAAGAACUCCACA 3′; 3) 5′GGUUGGGAAUGUGGAAUGUGGUGCC 3′, three different SORT1 siRNAs were mixed: 1) 5′AGGACCUACACUAAAGAAAGCAGGG 3′; 2) 5′AGAUAGCACUCAGGAACAAAGGCTG 3′; 3) 5′GGGCUAUAAAGAGCAGUUCCUACGG 3′. And 500 pmol/3 μL PGRN siRNA, SORT1 siRNA, and scrambled siRNA were injected at 1 day before SAH induction by a pump at the rate of 0.5 μL/min. The needle was kept in place for an additional 15 minutes after total infusion. Finally, the incision was closed with sutures.
SAH Grade
The severity of SAH was blindly evaluated using the SAH grading scale at the time of euthanasia as previously reported. Seventeen rats with mild SAH (SAH grades ≤ 7 at 24 hr) were excluded from the study (19).
Neurologic Score
Neurologic score was evaluated at 1 hour before euthanized by a blinded observer according to the 18-point scoring system described by Garcia et al (20), with modifications.
Brain Water Content
Brain was removed at 24 or 72 hours after surgery and separated into left hemisphere, right hemisphere, cerebellum, and brain stem. Each part was weighed immediately after removal (wet weight) and once more after drying in 105°C for 72 hours. The percentage of water content was calculated as (wet weight – dry weight)/wet weight (21).
Immunofluorescence Staining
Double-fluorescence labeling was performed as previously described (17). Sections were incubated overnight at 4°C with rabbit anti-PGRN (Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-Iba1 (Santa Cruz Biotechnology), mouse anti-NeuN (EMD Millipore, Temecula, CA), and goat anti-glial fibrillary acidic protein (Santa Cruz Biotechnology) primary antibodies. Appropriate fluorescence dye—conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) were applied in the dark for 1 hour at 21°C. For negative controls, the primary antibodies were omitted, and the rest staining procedures were performed exactly in the same way. The sections were visualized by a fluorescence microscope. Photomicrographs were saved and merged by Image Pro Plus software (Olympus, Melville, NY). Double immunofluorescence staining was processed with anti-NeuN (1:400; Abcam, Cambridge, MA) and TUNEL (In situ Cell Death Detection Kit, Fluorescein; Roche, Mannheim, Germany). TUNEL-positive neurons were counted in a blinded manner. The extent of neuronal damage was evaluated by an apoptotic index, which was calculated as the average number of TUNEL-positive neurons in six sections per brain at ×400 magnification. The data were expressed as cells/mm2.
Western Blot
The left brain hemispheres (perforation side) were harvested and processed as previously described (22). Equal amounts of protein (50 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred onto nitrocellulose membranes. Membranes were incubated with the respective primary antibodies: rabbit polyclonal anti-PGRN (1:1,000) (Santa Cruz Biotechnology), rabbit polyclonal anti-CC3 (1:1,000) (Cell Signaling Technology, Danvers, MA), mouse monoclonal anti-extracellular signal-regulated kinase (ERK) 1/2 (1:1,000) (Cell Signaling Technology), mouse monoclonal antiphosphorylated ERK1/2 (1:1,000) (Cell Signaling Technology), rabbit polyclonal anti-Akt (1:1,000) (Cell Signaling Technology), rabbit polyclonal antiphosphorylated Akt (1:1,000) (Cell Signaling Technology), rabbit polyclonal anti-Bcl-2 (1:1,000) (Cell Signaling Technology), rabbit polyclonal anti-SORT1 (1:1,000) (Abcam), and goat polyclonal β-actin (1:6,000) (Santa Cruz Biotechnology). Immunoblots were processed with appropriate secondary antibodies (1:4,000; Santa Cruz Biotechnology) for 2 hours at room temperature. Bands were visualized after incubating the membranes with the ECL Plus chemiluminescence reagent kit (Amersham Bioscience, Arlington Heights, IL). The signal density was quantified using Image J (National Institutes of Health, Bethesda, MD). Results were exhibited as relative density ratio, normalized to the average value of the sham group.
Statistical Analysis
Data are expressed as a mean ± sd. All data were analyzed by oneway analysis of variance followed by Tukey post hoc test. p Value of less than 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism for Windows (GraphPad Software, La Jolla, CA).
RESULTS
No significant changes of the physiological variables (body temperature, blood gases, and body weight) were observed between different experimental groups (data not shown). For SAH animal model, filament puncture induced extensive bleeding, which was particularly pronounced on the left side, around the Circle of Willis and along the ventral brain stem (Supplemental Fig. 1A, Supplemental Digital Content 1, http://links.lww.com/CCM/B322). At 24 hours post hemorrhage, the SAH grading was 12.8 ± 2.1 out of a possible 18 in the SAH groups (n = 12) (Supplemental Fig. 1B, Supplemental Digital Content 1, http://links.lww.com/CCM/B322).
Expression of PGRN After SAH
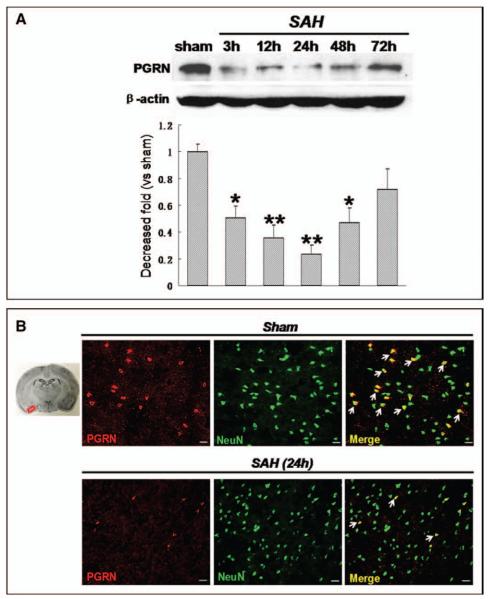
The results of Western blot for PGRN protein expression in cerebral cortex showed that PGRN was highly expressed in sham animals and started to decrease at 3 hours after SAH with a lowest peak at 24 hours after SAH (Fig. 1A). The morphological studies further confirmed that the expression of PGRN was mainly in neurons (Fig. 1B) while almost negatively expressed neither in astrocytes or microglias in cerebral cortex (Supplemental Fig. 2, Supplemental Digital Content 1, http://links.lww.com/CCM/B322). At 24 hours after SAH, the number of PGRN positive neurons dramatically decreased compared with that of sham group (Fig. 1B).
Figure 1.
Time course of progranulin (PGRN) expression after subarachnoid hemorrhage (SAH) induction. A, PGRN highly expressed in sham animals, which started to decrease at 3 hr after SAH with its lowest peak at 24 hr after SAH. B, PGRN expression in neurons from cortex obviously decreased at 24 hr after SAH. n = 6 for each group. *p < 0.05, **p < 0.01 versus sham. Scale bar = 50 μm.
Effect of r-PGRN on Brain Edema and Neurologic Function After SAH
Two dosages of r-PGRN (1.0 ng/5 μL and 3.0 ng/5 μL) were administrated intracerebroventricularly at 1.5 hours after SAH. Neurologic score and brain water content were measured. As indicated in Figure 2A, neurobehavioral function impairment was evident in SAH group compared with sham group at both 24 and 72 hours after SAH (p < 0.05, n = 6). Post-SAH administration of high dosage r-PGRN significantly ameliorated neurobehavioral deficits at 24 hours after SAH (p < 0.05 vs SAH + vehicle, n = 6). Accordingly, brain water content significantly increased in the left and right hemisphere at both 24 and 72 hours after SAH (p < 0.05 vs sham, n = 6) (Fig. 2B). High dose of r-PGRN treatment significantly decreased the water content of the left hemisphere at both 24 and 72 hours (p < 0.05 vs SAH + vehicle, n = 6) (Fig. 2B).
Figure 2.
Rat recombinant progranulin (r-PGRN) decreased brain water content and improved neurologic functions at 24 and 72 hr after subarachnoid hemorrhage (SAH). A, High dosage r-PGRN improved the neurobehavioral deficits at 24 hr and 72 hr after SAH. B, High dosage r-PGRN decreased brain water content at 24 and 72 hr after SAH. n = 6 for each group. *p < 0.05 versus sham; #p < 0.05 versus SAH + vehicle.
Knockdown of Endogenous PGRN Aggravated Neurologic Deficits After SAH
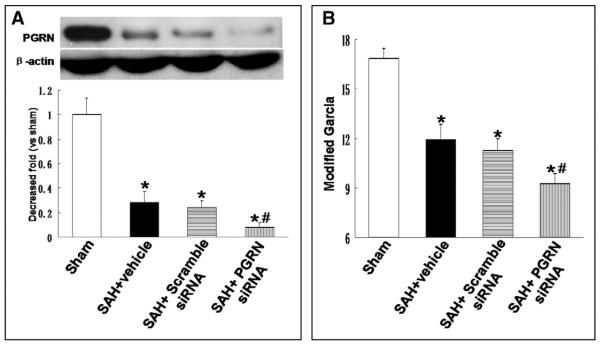
PGRN siRNA was administrated by intracerebroventricularly at 24 hours before SAH induction to efficiently knock down the protein. Neurologic score evaluation was also performed at 24 hours after SAH. As shown in Figure 3A, PGRN expression decreased at 24 hours after SAH. PGRN expression continued to decrease after PGRN siRNA administration, compared with that from vehicle and scramble siRNA group at 24 hours after SAH (p < 0.05; n = 6). Knockdown of endogenous PGRN significantly aggravated neurologic deficits at 24 hours after SAH (p < 0.05 vs SAH + scramble siRNA; n = 6 (Fig. 3B).
Figure 3.
Knockdown of endogenous progranulin (PGRN) aggravated neurologic deficits after subarachnoid hemorrhage (SAH). A, PGRN expression decreased at 24 hr after SAH and continued to decrease after PGRN siRNA administration. B, Knockdown of endogenous PGRN significantly aggravated neurologic deficits at 24 hr after SAH. n = 6 for each group. *p < 0.05 versus sham; #p < 0.05 versus SAH + scramble siRNA.
r-PGRN Treatment and PGRN Deficiency on Neuronal Apoptosis After SAH
NeuN/TUNEL double immunofluorescence staining was performed to evaluate neuronal apoptosis. The total number of TUNEL and NeuN double-stained cells significantly increased in vehicle and scramble siRNA groups at 24 hours after SAH (p < 0.05 vs sham; n = 4 (Fig. 4). TUNEL-positive neurons were reduced after treatment of high dose of r-PGRN at 24 hours after SAH (p < 0.05 vs SAH + vehicle; n = 4) but increased after administration of PGRN siRNA (p < 0.05 vs SAH + scramble siRNA; n = 4).
Figure 4.
Rat recombinant progranulin (r-PGRN) treatment reduced neuronal apoptosis and progranulin (PGRN) deficiency promoted neuronal apoptosis at 24 hr after subarachnoid hemorrhage (SAH). A and B, Total number of terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick-end labeling (TUNEL) and NeuN double-stained cells significantly increased in vehicle and scramble siRNA group at 24 hr after SAH. However, it was reduced by high dosage r-PGRN treatment but increased to even higher level after administration of PGRN siRNA. n = 4 for each group. *p < 0.05 versus sham; #p < 0.05 versus SAH + vehicle; &p < 0.05 versus SAH + scramble siRNA. Scale bar = 50 μm.
PGRN on Akt, Bcl-2, and CC-3 Levels After SAH
As shown in Figure 5A–C, the expression of p-Akt and Bcl-2 but not p-Erk significantly decreased at 24 hours after SAH (p < 0.05 vs sham, n = 6), whereas administration of high-dose r-PGRN reversed the decline of both p-Akt and Bcl-2 expression (p < 0.05 vs SAH + vehicle; n = 6). The protein expression of CC3 significantly increased at 24 hours after SAH, while administration of r-PGRN reduced its expression (p < 0.05 vs SAH + vehicle; n = 6) (Fig. 5D). As shown in Figure 5, A and C, PGRN siRNA aggravated the decrease of both p-Akt and Bcl-2 expression (p < 0.05 vs SAH + scramble siRNA; n = 6). The expression of CC3 increased to an even higher level at 24 hours after SAH in PGRN siRNA group (p < 0.05 vs SAH + scramble siRNA; n = 6 (Fig. 5D).
Figure 5.
Progranulin (PGRN) promoted Akt activation, increased Bcl-2 level, and reduced cleaved caspase-3 (CC3) level at 24 hr following subarachnoid hemorrhage (SAH). Changes of phosphorylated Akt (p-Akt) (A), p-Erk (B), Bcl-2 (C), and CC3 (D) expression in different animal groups. n = 6 for each group. *p < 0.05 versus sham; #p < 0.05 versus SAH + vehicle; &p < 0.05 versus SAH + scramble siRNA.
Knockdown of SORT1 Abolished the Effects of r-PGRN After SAH
SORT1 was identified as a high affinity and specific binding factor of PGRN, which mediating the endocytosis and delivery of PGRN (23, 24). SORT1 siRNA was administrated intracerebroventricularly at 24 hours before SAH induction. As indicated in Figure 6A, SORT1 siRNA could efficiently knockdown the protein. r-PGRN treatment increased p-Akt and Bcl-2, and decreased CC3 compared with vehicle group (p < 0.05; n = 6) (Fig. 6B–D). SORT1 siRNA remarkably abolished the up-regulation of p-Akt and Bcl-2 and down-regulation of CC3 after r-PGRN administration (p < 0.05 vs SAH + r-PGRN + scramble siRNA; n = 6) (Fig. 6B–D). Additionally, the improvement of neurologic deficit by r-PGRN was also reversed by SORT1 siRNA (p < 0.05 vs SAH + r-PGRN + scramble siRNA; n = 6) (Supplemental Fig. 3, Supplemental Digital Content 1, http://links.lww.com/CCM/B322).
Figure 6.
Knockdown of sortilin 1 (SORT1) abolished the beneficial effects of rat recombinant progranulin (r-PGRN) at 24 hr after subarachnoid hemorrhage (SAH). A, SORT1 siRNA efficiently knocked down the protein expression. Changes of phosphorylated Akt (p-Akt) (B), Bcl-2 (C), and cleaved caspase-3 (CC3) (D) expression at 24 hr after SAH in different groups. n = 6 for each group. *p < 0.05 versus SAH + vehicle; #p < 0.05 versus SAH + scramble siRNA.
DISCUSSION
Recent studies reported that neuronal apoptosis was involved in the pathogenesis of early brain injury and anti-apoptosis treatment may be beneficial in experimental or clinical SAH (3, 4). The results from this study indicated that PGRN exerted an important neuroprotective role after SAH. PGRN deficiency aggravated neuronal apoptosis and neurologic deficits, whereas r-PGRN treatment remarkably decreased neuronal apoptosis and improved neurologic function after SAH. rPGRN promoted Akt activation, increased Bcl-2, and reduced caspase-3 level at 24 hours after SAH. Furthermore, knockdown SORT1 by siRNA abolished the antiapoptosis effects of PGRN.
PGRN, a glycosylated protein secreted by a variety of cells, participates in diverse pathophysiologies, such as wound healing, inflammation, tumorigenesis, and development (7, 8). The potential role of PGRN in CNS especially after stroke began to attract attention recently (15, 16). After developmental stage, PGRN is expressed in specific neuronal populations, such as cortical and hippocampal pyramidal neurons (9). Null mutations of PGRN gene was considered as a cause of FTLD (10, 11), whereas PGRN levels reduced in the cerebrospinal fluid of FTLD patients with a PGRN mutation, and exogenous PGRN promoted neuronal survival and enhanced neurite outgrowth in cultured neurons. Furthermore, it has been reported that PGRN can rescue cortical neurons from cell death induced by glutamate or oxidative stress (13). In the present study, we observed that the expression of PGRN decreased and reached the lowest point at 24 hours after SAH, consistent with the observation in a cerebral ischemia model (16). More importantly, r-PGRN treatment significantly decreased neuronal apoptosis and improved neurologic function after SAH, while PGRN deficiency aggravated neuronal apoptosis and neurologic deficits. All of these results supported a neuroprotective role of PGRN in the brain.
The potential mechanisms underlying the neuroprotective effect of PGRN was investigated previously in cell cultures. Extracellular PGRN stimulated phosphorylation/activation of the neuronal mitogen-activated protein kinase/extracellular-regulated protein kinase kinase/ERK and phosphatidylinositol-3 kinase/Akt cell survival pathways and rescued cortical neurons from cell death induced by glutamate or oxidative stress (13). In this study, we observed that administration of r-PGRN after SAH significantly enhanced the expression of p-Akt accompanied by the increase of Bcl-2 expression and reduction of CC3 expression. Knockdown of PGRN by siRNA decreased Akt expression and aggravated CC3 activation. Based on these data, it is likely that r-PGRN enhanced Akt-related cell survival and antiapoptosis effects in neurons after SAH. In addition, SORT1 has recently been identified as a high affinity and specific binding factor of PGRN and mediating the endocytosis and delivery of PGRN (23, 24). We used SORT1 siRNA as an intervention to explore its role in the effect of r-PGRN treatment. SORT1 siRNA remarkably abolished the effect of r-PGRN on up-regulation of p-Akt, Bcl-2, and down-regulation of CC3. Furthermore, the improvement of neurologic function by r-PGRN was also reversed by SORT1 siRNA. Taken together, it is likely that the neuroprotective effect of r-PGRN after SAH may be mediated by SORT1 and involved Akt pathways (more details shown in Supplemental Fig. 4, Supplemental Digital Content 1, http://links.lww.com/CCM/B322).
There are some limitations in this study. First, we cannot exclude the possibility that anti-inflammation may play a role in the neuroprotective effect of PGRN (13, 14). In addition, it has recently been reported that PGRN deficiency leads to major alterations in blood-brain barrier (BBB) structure and function that predispose PGRN-knockout mice to BBB breakdown and increased ischemic brain injury, implying that PGRN also has an important vasoprotective effect (25). Thus, the link between different protective effects of PGRN needs to be considered in future studies.
In conclusion, we suggest that PGRN plays a neuroprotective role after SAH that r-PGRN may have potentials to reduce early brain injury via SORT1-mediated and Akt-related anti-apoptosis pathways.
Acknowledgments
Drs. Li, He, Junjia Tang, Chen, Jiping Tang, and Feng received support for article research from the National Institutes of Health (NIH) and the Natural Science Foundation of China (NSFC). Their institutions received grant support from the NIH and the NSFC. Drs. Li and Feng received support from Chinese Natural Science Foundation (81471214, 81070931, and 81220108009). Dr. Zhang received support for article research from the NIH (NS081740 and NS082184). His institution received grant support from the NIH.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Biller J, Godersky JC, Adams HP., Jr Management of aneurysmal subarachnoid hemorrhage. Stroke. 1988;19:1300–1305. doi: 10.1161/01.str.19.10.1300. [DOI] [PubMed] [Google Scholar]
- 2.Schievink WI, Riedinger M, Jhutty TK, et al. Racial disparities in subarachnoid hemorrhage mortality: Los Angeles County, California, 1985-1998. Neuroepidemiology. 2004;23:299–305. doi: 10.1159/000080096. [DOI] [PubMed] [Google Scholar]
- 3.Sehba FA, Hou J, Pluta RM, et al. The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol. 2012;97:14–37. doi: 10.1016/j.pneurobio.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Feng H, Sherchan P, et al. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol. 2014;115:64–91. doi: 10.1016/j.pneurobio.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhandari V, Bateman A. Structure and chromosomal location of the human granulin gene. Biochem Biophys Res Commun. 1992;188:57–63. doi: 10.1016/0006-291x(92)92349-3. [DOI] [PubMed] [Google Scholar]
- 6.Bhandari V, Palfree RG, Bateman A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci U S A. 1992;89:1715–1719. doi: 10.1073/pnas.89.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bateman A, Bennett HP. Granulins: The structure and function of an emerging family of growth factors. J Endocrinol. 1998;158:145–151. doi: 10.1677/joe.0.1580145. [DOI] [PubMed] [Google Scholar]
- 8.Eriksen JL, Mackenzie IR. Progranulin: Normal function and role in neurodegeneration. J Neurochem. 2008;104:287–297. doi: 10.1111/j.1471-4159.2007.04968.x. [DOI] [PubMed] [Google Scholar]
- 9.Petkau TL, Neal SJ, Orban PC, et al. Progranulin expression in the developing and adult murine brain. J Comp Neurol. 2010;518:3931–3947. doi: 10.1002/cne.22430. [DOI] [PubMed] [Google Scholar]
- 10.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 11.Cruts M, Gijselinck I, van der Zee J, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 12.Van Damme P, Van Hoecke A, Lambrechts D, et al. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol. 2008;181:37–41. doi: 10.1083/jcb.200712039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Xilouri M, Bruban J, et al. Extracellular progranulin protects cortical neurons from toxic insults by activating survival signaling. Neurobiol Aging. 2011;32:2326.e5–2326.16. doi: 10.1016/j.neurobiolaging.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens LH, Zhang J, Barmada SJ, et al. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J Clin Invest. 2012;122:3955–3959. doi: 10.1172/JCI63113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin F, Banerjee R, Thomas B, et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med. 2010;207:117–128. doi: 10.1084/jem.20091568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egashira Y, Suzuki Y, Azuma Y, et al. The growth factor progranulin attenuates neuronal injury induced by cerebral ischemia-reperfusion through the suppression of neutrophil recruitment. J Neuroinflammation. 2013;10:105. doi: 10.1186/1742-2094-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan J, Manaenko A, Chen S, et al. Role of SCH79797 in maintaining vascular integrity in rat model of subarachnoid hemorrhage. Stroke. 2013;44:1410–1417. doi: 10.1161/STROKEAHA.113.678474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Ma Q, Krafft PR, et al. P2X7R/cryopyrin inflammasome axis inhibition reduces neuroinflammation after SAH. Neurobiol Dis. 2013;58:296–307. doi: 10.1016/j.nbd.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugawara T, Ayer R, Jadhav V, et al. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia JH, Wagner S, Liu KF, et al. Neurologic deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa Y, Suzuki H, Altay O, et al. Preservation of tropomyosin-related kinase B (TrkB) signaling by sodium orthovanadate attenuates early brain injury after subarachnoid hemorrhage in rats. Stroke. 2011;42:477–483. doi: 10.1161/STROKEAHA.110.597344. [DOI] [PubMed] [Google Scholar]
- 22.Hu Q, Ma Q, Zhan Y, et al. Isoflurane enhanced hemorrhagic transformation by impairing antioxidant enzymes in hyperglycemic rats with middle cerebral artery occlusion. Stroke. 2011;42:1750–1756. doi: 10.1161/STROKEAHA.110.603142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu F, Padukkavidana T, Vægter CB, et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68:654–667. doi: 10.1016/j.neuron.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee WC, Almeida S, Prudencio M, et al. Targeted manipulation of the sortilin-progranulin axis rescues progranulin haploinsufficiency. Hum Mol Genet. 2014;23:1467–1478. doi: 10.1093/hmg/ddt534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackman K, Kahles T, Lane D, et al. Progranulin deficiency promotes post-ischemic blood-brain barrier disruption. J Neurosci. 2013;33:19579–19589. doi: 10.1523/JNEUROSCI.4318-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]