Abstract
One consequence of modern cancer therapy is chemotherapy related cognitive dysfunction or “chemobrain”, the subjective experience of cognitive deficits at any point during or following chemotherapy. Chemobrain, a well-established clinical syndrome, has become an increasing concern because the number of long-term cancer survivors is growing dramatically. There is strong evidence that correlates changes in peripheral cytokines with the development of chemobrain in commonly used chemotherapeutic drugs for different types of cancer. However, the mechanisms by which these cytokines elicit change in the central nervous system are still unclear. In this review, we hypothesize that the administration of chemotherapy agents initiates a cascade of biological changes, with short-lived alterations in the cytokine milieu inducing persistent epigenetic alterations. These epigenetic changes lead to changes in gene expression, alterations in metabolic activity and neuronal transmission that are responsible for generating the subjective experience of cognition. This speculative but testable hypothesis should help to gain a comprehensive understanding of the mechanism underlying cognitive dysfunction in cancer patients. Such knowledge is critical to identify pharmaceutical targets with the potential to prevent and treat cancer-treatment related cognitive dysfunction and similar disorders.
Keywords: Cancer chemotherapy, Cognition dysfunction, Chemobrain, Cytokines and epigenetics
What is chemobrain?
The development of new chemotherapeutic agents and regimens for cancer therapy has led to a significantly reduced risk of recurrence and a higher survival rate in several types of cancer, particularly in breast cancer. This increase in cancer survivors, however, has led to an increased awareness of the chronic adverse effects of cancer chemotherapeutic agents, including undesirable effects on noncancerous cells secondary to the intended cytoxicity on cancer cells. One consequence of modern cancer therapy is post-chemotherapy related cognitive dysfunction, commonly referred to as “chemobrain” [1]. Cognitive dysfunction is the subjective experience when one has deficits in their cognitive function. Objectively measured cognitive deficits will be referred here as “cognitive impairment”. A significant number, estimated between 18% and 78% of breast cancer patients report dyscognition soon after initiating chemotherapy treatment [2,3]. While it is possible that cancer can cause cognitive dysfunction and impairment on its own [4–7], a defining feature of chemobrain is the onset of complaint after treatment initiation, with its corresponding assumption of causality. These symptoms are short-term in the majority of patients but have been reported to persist for months to years in ~35% of patients in disease-free remission [3,8]. The findings from the International Cognitive Workshop suggested that cancer-related cognitive dysfunction may be long-term and has been reported to last 5–10 years after treatments in the cancer survivors [9–11].
While chemobrain is not an uncommon clinical problem, it has been difficult to demonstrate clinically significant cognitive impairment. Repeated studies on the effects of chemotherapy have been unable to demonstrate cognitive impairment after treatment [12–20]. Studies that have shown cognitive impairment, both cross-sectional [21–24] and longitudinal [25–28], demonstrate that the impairment is modest, of unclear clinical significance, and correlates poorly with the severity of the subjective experience of chemobrain. Despite the paucity of evidence for cognitive impairment, patients with chemobrain consistently report clinically important cognitive dysfunction that impair their daily function, in particular in regards to attention, concentration, for-getfulness, word-finding, multi-tasking, and organization. The clinical presentation of chemobrain is notable for the discordance between the subjective experience of cognitive dysfunction and objective neuropsychiatric measurements [29].
This discordance between dyscognition and impairment been attributed to a variety of possible methodological causes, including problems with the subjective assessment of symptoms, methodological and sensitivity issues of modern cognitive testing, the difficulty of accurately defining both dyscognition and cognitive impairment [29]. To address these methodological issues, the International Cognition and Cancer Task Force (ICCTF) recommended 3 main tests with suggested clinical cut-points to determine cognitive impairment in patients with cancer and treatment [29,32]. The recommended tests, which measure learning and memory, processing speed, and executive function based on findings of the cognitive effects of chemotherapy on the frontal cortex, are Hopkins Verbal Learning Test-Revised (HVLT-R), Trail Making Test (TMT), and the Controlled Oral Word Association (COWA) of the Multilingual Aphasia Examination. These tests with the recommended standard deviation cut-points for assessment of cognitive impairment provide adequate sensitivity and psychometric properties to better measure cognitive impairment in patients with cancer and treatments [29,32]. However, it seems likely that the discordance between subjective dyscognition and objective impairment is a defining observation of the nature of chemobrain rather than simply being a measurement artifact.
Another important feature of chemobrain is its common, but not mandatory, relationship to several somatoform symptoms, in particular anxiety, depression and fatigue, and overall health-related decline [33]. The clinical picture of chemobrain is that of a patient developing a distressing and often disabling alteration in their subjective cognitive abilities that is difficult to objectively demonstrate and is temporally related to both the biological and psychological consequences of cancer chemotherapy.
Currently, chemobrain is hypothesized to be the result of neuronal injury with consequent inadequate repair, abnormal brain remodeling, and corresponding neuroendocrine-immunological changes [34]. Studies have described alterations in the blood–brain barrier that allow increased access of cytotoxic agents to vulnerable neurons. Neuroimaging studies suggest that structural and functional changes in the frontal cortex and related white matter tracts, which are implicated in executive and memory function, correlate with chemobrain. Alterations in these areas have been correlated with subjective and objective change in neurologic function [30,31,35], post-treatment volume loss [36,37], and partial recovery over time [4,37,38]. However, the methodology and small sample sizes of these neuroimaging studies do not demonstrate causality or neuronal injury [39]. Evidence to support that oxida-tive stress, neural repair, immunologic, and endocrine changes in chemobrain are severely limited. The essential questions underlying the validity of the hypotheses underlying the current chemobrain concept, that of direct causality and neuronal injury, are not answered by the scientific literature to date.
The state of the evidence for chemobrain strongly resembles that which is seen in fibromyalgia and chronic fatigue syndrome. Like chemobrain, patients with these illnesses experience subjective and clinically distressing dyscognition, with attention, concentration, forgetfulness, word-finding, multi-tasking, and organization being the most common complaints. Also like chemobrain, measurements of objective neuropsychologic function frequently fail to demonstrate impairment and what is seen in positive studies is of small clinical magnitude [40–42]. The increased recognition of cognitive symptoms in these disorders has led to their inclusion in diagnostic criteria [43,44]. These illnesses also draw support from neuroimaging studies that commonly show alterations in the structure and function of frontal cortical regions that are passingly similar to those documented in chemobrain [45]. Limited evidence of alterations in oxidative stress, neural repair, immunologic, and endocrine changes have also been reported [46]. Both of these illnesses have disputed causal triggers, such as trauma in fibromyalgia and infection in chronic fatigue syndrome, whose validity is also not answered by the scientific literature to date. The clinical and scientific experience of chemobrain is remarkably similar to the dyscognition reported in fibromyalgia and chronic fatigue syndrome. However, no comparative studies between these dyscognitive states have been performed to date. The implications of this observation are that specific chemotherapeutic-related neurologic injury is not required to create the somatic experience of chemobrain.
The discordance between the severity of subjective experience and that of objective impairment is the hallmark of somatoform illnesses, such as fibromyalgia and chronic fatigue syndrome. A somatoform view of chemobrain would consider it as an atypical yet predictable subjective experience that result from the normal functioning of the brain rather than from an injury. In this way, physiologic factors other than direct neurotoxicity from chemotherapeutic agents are the critical ones in establishing and maintaining chemobrain. Chemotherapy, or the psychological ramifications of cancer treatment, may simply be one of a variety of “triggers” that ultimately lead to dyscognition.
We emphasize that viewing chemobrain as a somatoform illness does not undermine its clinical legitimacy or trivialize the patient suffering that comes with it. All human experiences are psychosomatic ones whose existence is dependent on discoverable physiological mechanisms that are potentially susceptible to therapeutic manipulation. Rather, accepting the possibility that chemobrain is related to that seen in somatoform illness provides a unique opportunity in examining the physiologic underpinnings of these illnesses. Do the biologic alterations that accompany the discrete, medically-induced physiologic stress of chemotherapy “trigger” long-term homeostatic change that is causally responsible for the somatoform experience of chemobrain? The current state of evidence is insufficient to answer this question; the answer would have important ramifications on the causality of all somatoform illness. Here, the authors take the position that such a trigger exists. We hypothesize that acute shifts in cytokines related to chemotherapy administration lead to epigenetic alterations. These epigenetic changes persist after the resolution of the chemotherapy-induced immunologic changes and are primarily responsible for creating and maintaining changes in neuroplasticity that underlie the somatoform experience of chemobrain.
2. The relation of alterations in cytokines to dyscognition
Although several candidate mechanisms have been hypothesized to explain chemobrain, the exact biological pathways remain unknown [3,47]. It is highly unlikely that a single biologic trigger is responsible for the dyscognition observed in cancer patients following chemotherapy. However, it seems likely that cognitive symptoms produced by cancers and cancer treatments may share a common final biological mechanism [3,48,49]. Studies from humans and animal models suggest that several cancer-related symptoms may involve the actions of cytokines. Cytokines, along with their systemic effects, have a role in cancer development, progression [50], and the commonly experienced adverse effects of chemotherapy, such as chemotherapy-induced peripheral neuropathy [48] and cognitive dysfunction [34,51]. Cancer patients who received immunotherapy of IL-2 or interferon-α (IFN-α) experienced dyscognition, depression and fatigue [52]. Cytokines have been demonstrated to induce both dyscognition and cognitive impairment (Table 1).
Table 1.
Cytokines/chemokines associated with chemotherapy-induced cognitive decline.
| Chemotherapy agents | Study design | Cytokine/chemokine changes | Results | References |
|---|---|---|---|---|
| Doxorubicin + cyclophosphamide + paclitaxel |
Cross-section study | ↑IL-6 (Log trans, breast cancer = 0.21, control = −0.28, p = 0.003) |
Reduced left hippocampal volume, reduced | Kesler et al. [30] |
| Cyclophosphamide + 5-fluorouracil and paclitaxel or methtrexate |
42 breast cancer patients and 35 healthy control |
↑TNF-α (Log Trans, Breast cancer = 0.93, Control = 0.56, p< 0.0001) |
Memory performance, and elevated IL-6 and TNFα in chemo group |
|
| 4.8 ± 3.4 years off-therapy | Hippocampal volume and cytokine levels showed significant (p < 0.05) omnibus F statistics |
Reduced in Hippocampal volume (cm3, mean (SD); left: breast cancer = 4.37 (0.40), control = 4.68 (0.49), p = 0.01; right: breast cancer = 4.36 (0.41), control = 4.61 (0.53), p = 0.07) |
||
|
Reduced memory performance (mean (SD), HVLT-R total recall: breast cancer = 49.3 (8.0), control = 57.1 (9.6) p = 0.03; HVLT-R delayed recall: breast cancer = 49.8 (6.4), control = 56.0 (8.1), p = 0.02; MMQ: breast cancer = 42.2 (11.2), control = 59.3 (7.4), p < 0.0001) |
||||
| Doxorubicin + cyclophosphamide + taxane; | Longitudinal study | Chemotherapy group: | Chemotherapy groups: | Pomykala et al. [62] |
| Epirubicin+ fluorouracil + cyclophosphamide |
23 breast cancer patients with chemotherapy and, 10 no chemotherapy Cytokines, FFDG PET/CT brain imaging at baseline and 1 year later |
|
|
|
| Doxorubicin + cyclophosphamide + taxane; | Cross-sectional study of cytokine genetic variations (SNPs), fatigue, depression and memory (Squire Memory) |
TNF-308 (p = 0.034) and IL6-174 (p = 0.037) independently associated with fatigue’ |
Women with more high-expression alleles across (TNF-308, IL6- 174, and ILB-511) reported higher levels of fatigue. Each high- expression allele increased the risk of more severe fatigue by 45%. Women with homozygous for the high expression of alleles of TNF-308 and IL6-174 reported the highest levels of fatigue. Marginally associated with memory complaints |
Bower et al. [63] |
| Epirubicin + fluorouracil + cyclophosphamide |
Questionnaire | The genetic risk index was also associated with depressive symptoms (p = 0.007) and memory complaints (p = .016). IL6-174 (p = 0.037) for depreesion |
||
| 171 breast cancer patients with chemotherapy <3 months |
IL6-174 GG(p = 0.089), TNF308 GG genotype (p = 0.082 for depression; p = 0.055 for memory |
|||
| AC/CAF (doxorubicin with cyclophosphamide, or cyclophosphamide plus fluorouracil) |
Cross-sectional study | ↑IL-6 (mean change 4.96 pg/mL) | Cycle 2: higher complain in heavy-headed, muddled throught, and forgetfulness |
Janelsins et al. [59] |
| 54 breast cancer patients | ↑IL-8 (mean change 1.19 pg/mL) | Cycle 4: all higher in heavy headedness, difficulty in thinking and concentration |
||
| ↑MCP-1 (mean change 48.80 pg/mL) MCP-1 correlation with concentration (r= −0.498), forgetfulness (r = −0.466) |
MCP-1 activates the BBB and causes neuroinflammation | |||
| ↓IL-6 (mean change 1.46 pg/mL) | Cycle 2: higher complain in difficulty thinking, concentration. | |||
| CMF (cyclophosphamide, methotrexate, fluorouracil) |
↓IL-8 (mean change 0.08 pg/mL) | Cycle 4: higher in muddled thought only. | ||
| ↓MCP-1 (mean change 15.81 pg/mL) Mean change = cycle 4-cycle2 |
Same forgetfulness complain in both AC/CAF and CMF | |||
| Lipodaunocin | Longitudinal study | ↑IL-1 (QOL r = −0.49) | Cognitive impairment, sickness behavior, “Fatigue, ;QOL, “sleep requirement, HPA stimulation |
Meyers et al. [65] |
| Cyclophosphamide/Topotecan ±Thalidomide |
54 AML/MDS patients with chemotherapy tested at baseline and 1 month later using HVLT, COWA, TMTA and FACT |
|||
| ↑IL-1RA (fatigue r = 0.52, interference r = 0.62, QOL r = −0.49) |
↑Fatigue, ↓QOL | |||
| ↑TNF-α (fatigue r = 0.41, interference r = 0.40, QOL r = −0.39) |
↑Fatigue, ↓QOL, ↓Executive function, memory deficits | |||
| ↑IL-6 (executive r = −0.36, fatigue r = 0.62, interference r = 0.60, QOL r = −0.42) |
BBB disturbance, ↓Executive function, stress modulation, | |||
| Fatigue↑, QOL↓ | ||||
| Join pain and Flu-like syndrome | ||||
| Ovarian cancer poor outcome; poor drug initial response | ||||
| ↑IL-8 (total recall r = 0.38, recognition r = 0.37) | Cognitive difficulty, join pain and Flu-like syndrome; memory↑ | |||
| Poor drug initial response | ||||
| Paclitaxel | Longitudinal study | ↑IL-10 (mean difference 1.3 pg/mL, p = 0.0021) | Strongly and positively correlated with joint pain (p = 0.003) | Pusztai et al. [57] |
| 90 breast cancer patients with chemotherapy |
↑IL-6 (mean difference 2.23 pg/mL, p = 0.018) | Strongly and positively correlated | ||
| ↑IL-8 (mean difference 2.7 pg/mL, p = 0.021) | with flu-like symptom (p = 0.008) | |||
| FAC (5-FU, adriamycin cyclophosphamide | ↓IL-8 (mean difference 3.15 pg/mL, p = 0.034) | |||
| Paclitaxel + Carboplatin | Longitudinal study | ↑IL-6 (control = 1.9 pg/mL, post-treatment = 21.3 pg/mL) | Poor treatment responses – paclitaxel resistance | Penson et al. [58] |
| 30 ovarian cancer patients with chemotherapy |
↑IL-8 (control = 46 pg/mL post-treatment = 193 pg/mL) | |||
| ↑MCP-1 (control = 1009 pg/mL, post treatment = 395 pg/ mL) |
Peritoneal MCP-1 markedly increased | |||
| Paclitaxel (T) or docetaxel (D) | Longitudinal study after 6 cycles | ↑IL-2 (control D = 84.33, T = 82.33; post treatment D = 132.87, T = 110.73, p < 0.0001) |
Docetaxel are more pronounced than those of paclitaxel | Tsavaris et al. [56] |
| 30 breast cancer patients with chemotherapy |
↑IFN-γ (control D = 57.00, T = 61.00; post treatment D = 103.07, T = 79.73, p < 0.0001) |
|||
| ↑IL-6 (control D = 57.00, T = 58.53; post treatment D = 106.13, T = 81.00, p < 0.0001) |
||||
| ↓IL-1 (control D = 121.27, T = 123.27; post treatment D = 90.40, T = 105.67, p < 0.0001) |
||||
| ↓TNF (control D = 113.27, T = 101.80; post treatment D = 79.33, T = 85.53, p < 0.0001) |
||||
| ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) |
Longitudinal study up of 12 months |
↓IL-1β→ TNF-α, TGF-β and PDGF | Chemoradiotherapy-induced lung toxicity | Villani et al. [60] |
↑, Increase; ↓, decrease; →, no change; r, Spearman r;. HVLT-R = Hopkins Verbal Learning Test Revised; MMQ = Multifactorial Memory Questionnaire.
In cancer patients, there are multiple mechanisms that lead to alterations in the cytokine milieu. Cancer itself leads to increases in circulating cytokines and increased cytokine levels before treatment has been associated with cognitive decline in cancer patients [49]. Cytokines are also modulated by physical and psychological stress in both animals and humans [53]. Both acute and chronic stressors have been shown to increase circulating levels of IL-6 and INF-α [54]. Physical and psychological stressors that cancer patients experienced after diagnosis, chemotherapy and long-term follow-up are important factors that can lead to elevation in the circulating level of cytokines. The administration of chemotherapy also can alter cytokines as the medications induce tumor cell death and collateral tissue injury. Patients undergoing chemotherapy with taxanes or anthracycline containing regimen for breast, ovarian cancer, and Hodgkin’s disease have statistically significant increases in INF-α, IL-1β, IL-6, IL-8, IL-10, and MCP-1 [55–63]. Alterations of cytokines in human cancer appear to multifactorial in origin. In an animal model, adriamycin directly causes an increase in TNF-α peripherally, which was subsequently detected in the brain (hippocampus and cerebral cortex) although neither adriamycin nor its metabolites were found to readily cross the blood–brain-barrier BBB [64].
While the cytokine alterations in chemotherapy are not homogenous across patients, they do have a relationship with dyscognition. Multiple clinical studies have demonstrated that administration of a standard dose of chemotherapy causes increases in cytokine levels such as TNF-α, IL-6, IL-8, IL-10, and MCP-1in cancer patients and that these changes are more prominent in patients who experienced dyscognition (Table 1) [56– 59,65]. While the evidence correlating changes in peripheral cytokine levels to dyscognition in chemotherapy patients is strong, the mechanisms by which peripheral cytokines exert their cognitive effects are not. It is assumed that dyscognition is caused by neuronal alteration. However, most chemotherapeutic agents administered systemically do not cross the blood–brain-barrier (BBB). Cytokines in the brain are mainly derived by microglia, with smaller contributions from astrocytes, oligodendrocytes, and neurons, rather than peripheral sources [66]. It does not appear that chemotherapy alone can be implicated in creating central cytokine change. Rather than chemotherapy related cytokine changes acting directly on the brain, it has been postulated that there is communication between peripheral cytokines and cytokines inside the CNS. This model suggests that peripheral cytokines stimulate neu-ronal and supportive cells to release central cytokines, which then act to alter neuronal plasticity [67,68]. Recent studies show that there is significant bidirectional communication between the peripherally released cytokines and the cytokines in the brain through (1) active transport into the brain across the BBB [67,69], (2) passive crossing through the leaky regions in the BBB at circumventricular organs [70], (3) stimulation of central cytokine release by the peripheral cytokines through the local inflammatory network in the brain [71]. Cytokines have also been identified as the mediators which bridge the neuroendocrine and immune systems, with the potential to subsequently alter neural activity, neurotransmitter metabolism, neuronal and glial cell function, and neural repair/regeneration [34,67,72–74]. It seems feasible that peripheral cytokine changes can induce central cytokine changes, with subsequent alterations in neuronal function.
The mechanisms by which changes in central cytokines would induce alterations in cognitive dyscognition and impairment are entirely unknown. To date there is no scientific evidence to support any particular mechanism. However, the authors speculate that the cytokine alterations that occur after chemotherapy have an essential role in triggering a cascade of neurological events that lead to chemotherapy-induced cognitive decline [75]. Cytokine change may initiate alterations in neurotransmitter systems and neuronal integrity by inducing excitotoxic glutamate receptor-mediated damage, altering monoaminergic systems (5-HT, DA and NE), GABA, acetylcholine, neuropeptides, and nerve growth factors (BDNF), which are directly associated with cognitive function and neurodegenerative processes (Fig. 1). Chemotherapeutic injury may also lead to the over production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), with the consequence of producing further oxidative stress via the nitric oxide (NO) pathway [76–78]. (1) The activated glial cells produce and release local cytokines that was associated with an increase in induction of nitric oxide (NO) synthase [64,77]; (2) the overproduction of reactive oxygen species and oxidative stress derived from the NO pathway have been identified as the most frequent cause of DNA damage in neuronal cells [79,80]; and (3) this damage directly leads to cognitive decline and has been found in peripheral lymphocytes after chemotherapy in breast cancer patients [81,82]. Therefore, further characterization of cytokine expression after chemotherapy might yield new insight into the development of chemobrain that may lead to the development of effective prevention or treatment strategies.
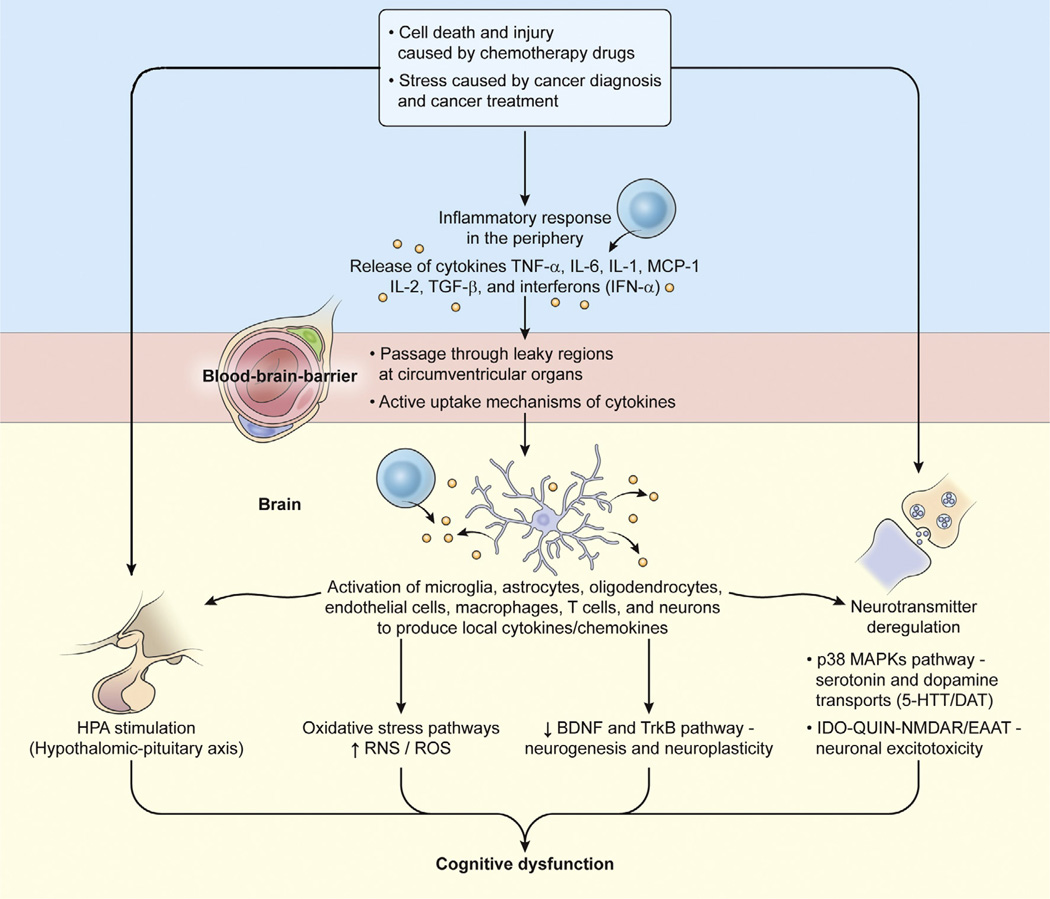
Fig. 1.
Schematic representation of cytokine-mediated cascade associated with chemotherapy-induced cognitive dysfunction. Peripheral released cytokines can access the brain to initiate local release of cytokines (TNF-α, IL-1β, IL-6, and IFN-α) and chemokines (MCP-1) by following mechanisms: (1) passive crossing through the leaky regions in the blood–brain-barrier at circumventricular organs; (2) active transporting cross the blood–brain-barrier; (3) stimulating the HPA axis independently or synergistically by directly binding to the receptors expressed in the HPA axis or indirectly through affecting the secretion of ACTH from the hypothalamus, ACTH from the pituitary or glucocorticoids from the adrenal cortex; and (4) stimulation of endothelial cells and perivascular macrophages, monocytes and T cells in the brain to produce similar local cytokines/chemokines. To respond to the local released cytokines/chemokines, microglia, astrocytes, oligodendrocytes and neurons in the brain produce even more the similar types of cytokines/chemokines which in turn to influence neuronal neurotransmitters and integrity through (1) oxidative stress pathways to increase ROS and RNS (reactive oxygen and nitrogen species), which affect the synthesis of monoamines; (2) P38 MAPKs pathway to interfere with serotonin and dopamine transports (5-HTT/DAT) function; (3) affecting glutamate system by activation of IDO – QUIN – NMDAR/EAAT to lead to neuronal excitotoxicity; and finally (4) BDNF (brain derived neurotrophic factor) and TrkB pathway to negatively affect neurogenesis and neuroplasticity. All the pathways either working alone or synergistically contribute to the development of cognitive decline after chemotherapy exposure in cancer patients.
In summary, there is strong evidence that correlates changes in peripheral cytokines with the development of dyscognition in the setting of many if not all commonly used chemotherapeutic drugs for different types of cancer [64,76,78,83]. The changes are typically heterogenous, with small magnitude of change being seen in multiple cytokines simultaneously. However, the mechanisms by which these cytokines elicit change in the central nervous system are still unclear. We speculate that a variety of well-defined neuronal mechanisms enable peripheral cytokines to induce central cytokine changes, which trigger a subsequent cascade of neurological events as described below that lead to the experience of chemobrain [64,76–78,83].
3. Epigenetics and genetic changes associated with chemobrain
Nowadays, it is generally accepted that long-term cellular memory is mediated by epigenetic phenomena and global DNA methylation, which are associated with changes in the cytokine milieu [84]. Epigenetics encompasses an array of acquired and heritable modifications of DNA that regulate gene expression and function without altering the inherited DNA nucleotide sequence. Such modifications include DNA methylation and hydroxymethylation, histone modification, and non-coding RNA regulation [85]. Emerging studies indicate that epigenetic regulation of gene expression is involved in various brain-related disorders, such as addiction, depression, stress, and Alzheimer’s disease, that genetics alone cannot entirely explain [86–89]. Recent studies have indicated that epigenetics, in particular DNA methylation and histone acetylation, plays critical roles in brain development, memory formation, and more importantly, in regulation of learning and memory [90–93]. In patients with drug abuse recalcitrant to conventional therapy, long-lasting epigenetic modification has been demonstrated to occur in the limbic system that is prone to relapse [87,94]. Epigenetic alterations are essential mechanisms that enable external and internal environmental cues to interact with genes to creating long-lasting alterations in gene expression, with the potential to alter homeostatic function and subjective experience.
As detailed above, the various chemotherapy regimens used to treat cancer have a wide range of biologic effects. Despite the heterogeneity of chemotherapy and cancer treatment stresses, the cognitive difficulties that follow treatment are stereotypical. Also, the metabolic alterations induced by chemotherapy, such as alterations in cytokines, tend to be short-lived [95] but the experience of dyscognition is often chronic. This suggests that chemobrain dyscognition may have a variety of “triggers” that are able to alter a common upstream pathway responsible for perpetuating alterations in cognitive perception. It is reasonable to speculate that chemotherapy-induced reprogramming of the epigenome is the common pathway that leads to persistent dyscognition [51]. There is some early evidence to support the role of epigenetic change in chemobrain. Learning and memory impairments following CMF (cyclophosphamide, methotrexate, 5-fluorouracil) chemotherapy were found to be associated with increased histone H3 acetylation and decreased DHAC (Histone deacetylase) activity in the hippocampus in an animal model [96]. This chromatin remodeling leads to a decrease in neural cell proliferation in the hippocampus that might be the plausible mechanism in explaining persistent dyscognition after chemotherapy exposure as the DNA methylation induced by cytokines is transient and reversed in two weeks after cytokines are removed from the environment [97]. Acquired alterations in the methylation pattern have also been found in lymphocytes obtained from individuals experiencing psychosocial stress [98,99]. An association between the development and persistence of chemotherapy-induced neuropsychological disorders and epigenetic changes following chemotherapy treatment was recently shown in breast cancer patients [51]. In that study, the transient methylation for a subset of genes seen after chemotherapy only persisted in the patients who developed persistent neuropsychological symptoms. Further investigation into how cancer treatments induce epigenetic change seems warranted [100].
While the evidence is far from conclusive, the authors hypothesize that the administration of chemotherapy agents initiates a cascade of biological changes, with short-lived alterations in the cytokine milieu inducing persistent epigenetic alterations (Fig. 2). These epigenetic changes eventually lead to gene expression changes, altering metabolic activity and neuronal transmission that are responsible for generating the subjective experience of cognition. This proposed mechanism may also explain the inconsistencies observed from neuroimaging findings in patients with mild to moderate self-reported cognitive dysfunction. These neuroimaging inconsistencies may explain that persistent cognitive dysfunction is a subtle process triggered by cytokine dysregulation and epigenetic changes that arise from the damage in the brain caused by chemotherapy.
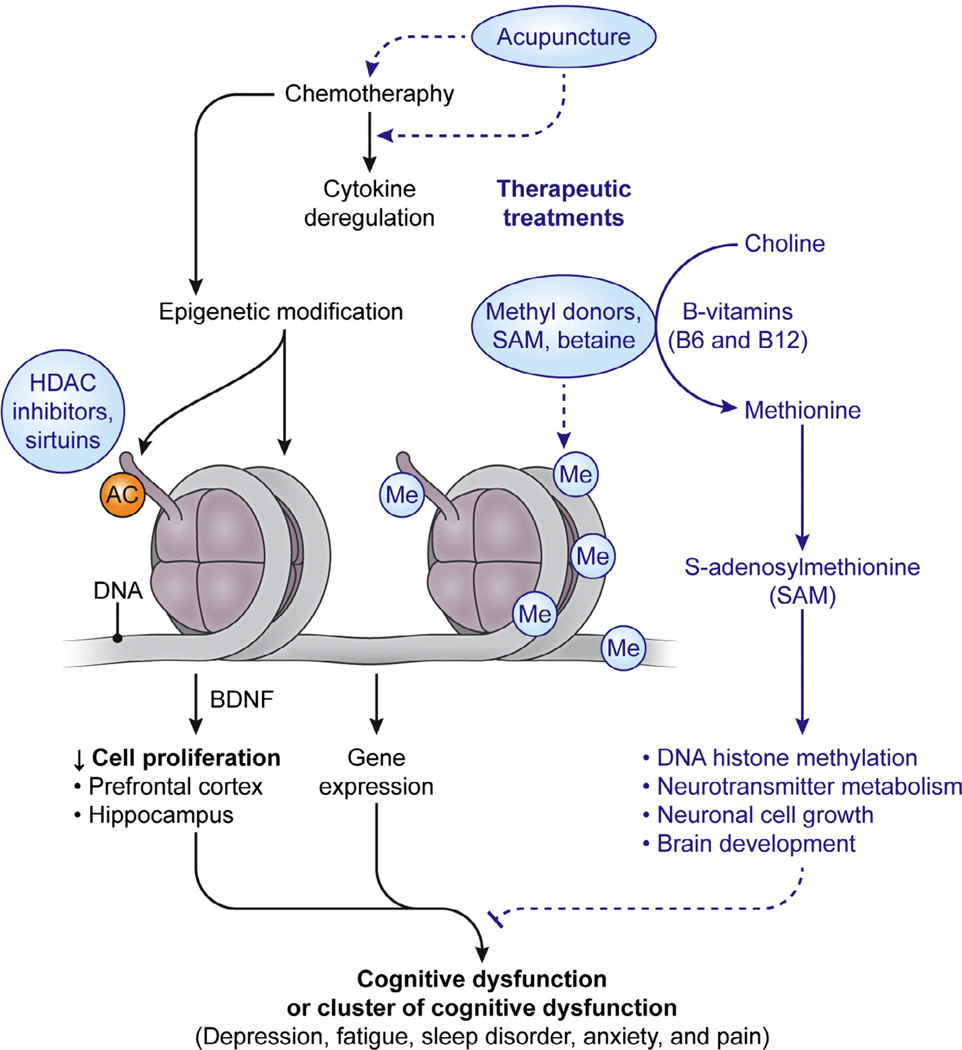
Fig. 2.
Schematic illustration of epigenetic modulation and epigenetic therapeutic approaches for chemotherapy-induced cognitive dysfunction. Chemotherapy induced reprogramming of the epigenome, DNA methylation and histone modification, may be the plausible common pathway leading to persistent cognitive dysfunction after exposure of chemotherapeutic agents. Chemotherapeutic agents cause cytokine deregulation and may also directly induce epigenetic changes through DNA methylation and histone modification. Each of the factors, alone or synergistically, leads to changes in gene expression and cell proliferation in the brain, particularly in the hippocampal and prefrontal cortical areas, which may eventually lead to the manifestation of persistent cognitive dysfunction after chemotherapy. Therefore, therapeutic intervention (1) by tipping the balance of pro- and anti-inflammatory cytokines by acupuncture, (2) modification of DNA methylation by SAM and betaine, or histone acetylation by sirtuins, or (3) increasing neurotrophic factors, BDNF, level in the brain, might prove to be the therapeutic intervention of the future in psychoneurological symptoms induced by chemotherapy exposure.
4. Can “chemobrain” be properly studied, prevented or corrected?
The causal model above postulates that the act of treating cancer evokes a heterogeneous, but clinically relevant, significant alteration of the extracellular cytokine milieu. This abrupt immunologic change acts as a trigger that ushers in a series of functional alterations in the genome, from = which the symptoms of cognitive dysfunction stem.
It is clearly evident that cancer treatment causes people to develop profound alterations in their subjective experience of their own cognition. Modern cancer treatment is complex, with important physiologic, psychological, and sociocultural dimensions. However, cancer-related dyscognition appears to be a distinct phenomenon with the clear onset and extent following chemotherapy, which provides an opportunity to conduct pre-clinical and clinical trials to prevent or reduce the cognitive toxicities of chemotherapy. There is good evidence that cancer treatment causes a “bump” in cytokine levels as early as in the 24 h after treatment [58]. This “bump” is best characterized as a seemingly small but statistically significant increase in the concentration of several pro-inflammatory cytokines induced by chemotherapy administration. Despite the current evidentiary weaknesses of the cytokine hypothesis, it has several strengths that make it attractive to consider. There is powerful evidence that demonstrates that cytokine release is a very early response to environmental change and enables downstream physiologic changes in organisms. Conversely, epigenetic changes appear to occur towards the end of responses to environmental change and can persist indefinitely. Cytokines do appear to be a reasonable agent to induce upstream changes in genetic expression. Furthermore, similar cytokine abnormalities have been observed in other cognitive disorders, suggesting that a variety of environmental stimuli may trigger a similar chain of events that lead to cognitive dysfunction. However, the greatest strengths of this hypothesis are that it is both testable with modern laboratory techniques and meets Karl Popper’s falsification requirements. Current observation strongly suggests that the administration of cancer treatment causes cognitive dysfunction. It is possible to ethically design experiments in cancer patients where individuals with normal cognition are given a discrete physiologic stress that will frequently induce dyscognition. Such an approach is not possible in somatoform disorders, in which causality is less clear and potential experimental stressors (such as infection and injury) are not ethical to administer to otherwise healthy individuals. Whereas it is possible to watch asymptomatic individuals develop chemobrain after treatment, it is typically impossible to do such in other dyscognitive disorders. Illnesses such as fibromyalgia and chronic fatigue syndrome are typically recognized after the clinical symptoms are already present and attempts to understand the contribution of discrete triggering events are subject to recall bias. In this way, chemobrain represents a causal model for dyscognition in which it is possible to observe the physiologic changes that occur as an individual develops dyscognition, in particular changes in cytokines and epigenetics.
Further, the cytokine hypothesis suggests a range of potential therapeutic targets. One potential approach would be to prevent the acute change in cytokines related to cancer treatment from occurring. Agents that inhibit cytokine activity, such as monoclonal antibodies and small molecular inhibitors, may confer benefit either alone or as an adjuvant treatment to chemotherapy-induced cognitive decline in cancer patients. TNF-α antagonists (etanercept and infliximab) have been shown to inhibit fatigue and improve depressive symptoms in patients with advanced cancer [101,102]. P2×7 antagonist that inhibits IL-1b release has been shown to reduce depressive-like profiles [103] and neuropathic pain [104] in animal models. Specific p38 MAPK and NF-κB inhibitors that block inflammatory signaling transduction have generated great interest from their use in the treatment of cytokine-induced depressive behavior and antidepressant-like effects in animal models. Anti-inflammatory cytokines, IL-10, IL-4 and minocycline may also have the potential therapeutic effects on chemotherapy-induced cognitive decline by inhibition of proin-flammatory cytokine release through modulation of the caspase pathways. Even acupuncture may have therapeutic potential considering its effects on suppressing proinflammatory cytokines, TNF-α, IL-1β, IL-6, and IL-10 [105,106]. Acupuncture has been often used to alleviate the side effects of cancer treatment, including pain, nausea, hot flashes, fatigue, anxiety/mood disorders, and sleep disturbance [107]. A series of interesting studies suggest a therapeutic role in dyscognition, for example, acupuncture improved cognitive function of patients with mild cognitive impairment (MCI) and various dementia [108–112], with clinical improvement correlating with alterations in functional connectivity and resting state activity of particular brain regions [110,112]. Such approaches to the prevention of cancer-therapy dyscognition are reasonable, currently feasible, and scientifically testable.
BDNF and its receptor tropomyosin-related kinase receptor type B (TRKB) play a potential role in the pathogenesis of neurological and neuropsychological disorders [113]. Epigenetic or pharmacological enhancement of BDNF–trkB signaling restores was reported to reverse the aging-related cognitive decline [114]. BDNF polymorphisms are associated with impaired memory and cognition, along with reduced hippocampal activation as measured by fMRI [115–117]. Age-related BDNF declines have been reported to be associated with declines in hippocampal volume and spatial memory in the elderly [118]. Low BDNF is associated with cognitive impairment in patients with schizophrenia [119] and Alzheimer’s disease [120–122]. Significantly decreased blood serum BDNF levels have been detected in patients with cognitive impairment due to obstructive sleep apnoea/hypopnoea syndrome [123]. Given its potent effects on neuronal function and survival in various cell systems in the CNS, BDNF has been evaluated in patients with various neurology cal disorders, including amyotrophic lateral sclerosis (ALS), peripheral neuropathy, Parkinson’s disease and Alzheimer’s disease [113]. However, delivery of BDNF remains a substantial challenge for clinical trials because it is a moderately sized and charged protein and only minimal amount of BDNF administrated peripherally crosses the BBB to reach neurons in the brain. Acupuncture has been reported to increase neurotrophic factors [124] and the levels of nerve growth factors in the brain by altering the permeability of the BBB [125]. In rats, electric acupuncture enhanced motor recovery after cerebral infarction that was associated with increased expression of BDNF in the brain.
With cytokines acting as a trigger to upstream changes, anti-cytokine therapies may have little therapeutic effect once upstream mechanisms responsible for dyscognition have been established, given that the most clinically available anti-cytokine antibodies are not readily to penetrate the blood–brain barrier. Antibody concentrations in the brain are typically about a thousand times lower than in the blood. Therefore, to better prevent development of cognitive dysfunction, anti-cytokine therapies would be best used by blocking cytokine production or inhibiting cytokine release in the peripheral prior to triggering the consequent events in the CNS [64,77]. However, epigenetic changes are dynamic and the pathological changes caused by epigenetic modifications can be reversed prior to the development of permanent symptoms by targeting enzymes or other factors that control or maintain the epigenetic status [126]. Treatments that seek to reverse casual epigenetic modifications have the potential to be effective. Such treatments are still in their infancy. S-adenosyl methionine (SAM) is an important methyl group donor required for proper DNA methylation and has been used to treat memory and cognitive symptoms in depressed patients [127,128]. Betaine, another methyl donor, has been shown to improve memory in mice memory impairment induced by lipopolysaccharide [129]. Histone deacetylases (HDACs) inhibitors can also alter epigenetic modifications, which have been studied in memory and cognition [130]. In a mouse model, administration of crebinostat, a HDAC inhibitor, improves memory [131,132]. Sirtuins, a class III HDAC inhibitors found in red grape skin and wine resveratrol have been found to improve cognitive function in mice [133] and are currently under phase II clinical trial (ADAS-Cog, ClinicalTrials.gov; NCT01504854, 2013).
In summary, cognitive dysfunction remains a common and debilitating effect of cancer treatment, with no effective prevention and treatment, although a variety of pharmacologic and non-pharmacological strategies have been investigated. We present a speculative but testable hypothesis of how cognitive dysfunction may occur following chemotherapy. Unlike other dyscognitive illnesses, it is both scientifically and ethically feasible to study the onset of “chemobrain” by administering a major physiologic stress and observing the biological ramifications. It should be possible to gain a comprehensive understanding of the mechanism underlying cognitive dysfunction in cancer patients. Such knowledge is critical to identifying methods to both prevent and treat cancer-treatment dyscognition and potentially other dyscognitive disorders.
Acknowledgments
The authors are gratefully acknowledge Alan Hoofring and Tyler Ethan from the NIH Medical Arts for their help with the illustrations. The authors are also gratefully acknowledge Steven E. Zhang from Stanford School of Medicine for his critical reading and grammar checking of the whole manuscript. This work was supported by Project Funding (1ZIANR000014-05) to XMW, NINR intramural program, NIH and 2008 NIH Bench-to-Bedside Program Awards to XMW.
References
- 1.Berger A, Shuster JL, Von Roenn JH. Principles and practice of palliative care and supportive oncology. 4th. xvii. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 2.Cull A, et al. What do cancer patients mean when they complain of concentration and memory problems? Br J Cancer. 1996;74(10):1674–1679. doi: 10.1038/bjc.1996.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30(30):3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahles TA, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110(1):143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wefel JS, et al. ‘Chemobrain’ in breast carcinoma?: A prologue. Cancer. 2004;101(3):466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 6.Wefel JS, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100(11):2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 7.Wefel JS, et al. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116(14):3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 8.Silberfarb PM. Chemotherapy and cognitive defects in cancer patients. Annu Rev Med. 1983;34:35–46. doi: 10.1146/annurev.me.34.020183.000343. [DOI] [PubMed] [Google Scholar]
- 9.de Ruiter MB, et al. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32(8):1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heflin LH, et al. Cancer as a risk factor for long-term cognitive deficits and dementia. J Natl Cancer Inst. 2005;97(11):854–856. doi: 10.1093/jnci/dji137. [DOI] [PubMed] [Google Scholar]
- 11.Vardy J, et al. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19(4):623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- 12.Debess J, et al. Cognitive function after adjuvant treatment for early breast cancer: a population-based longitudinal study. Breast Cancer Res Treat. 2010;121(1):91–100. doi: 10.1007/s10549-010-0756-8. [DOI] [PubMed] [Google Scholar]
- 13.Donovan KA, et al. Cognitive functioning after adjuvant chemotherapy and/or radiotherapy for early-stage breast carcinoma. Cancer. 2005;104(11):2499–2507. doi: 10.1002/cncr.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galica J, et al. The impact of chemotherapy-induced cognitive impairment on the psychosocial adjustment of patients with nonmetastatic colorectal cancer. Clin J Oncol Nurs. 2012;16(2):163–169. doi: 10.1188/12.CJON.163-169. [DOI] [PubMed] [Google Scholar]
- 15.Hermelink K, et al. Two different sides of ‘chemobrain’: determinants and nondeterminants of self-perceived cognitive dysfunction in a prospective, randomized, multicenter study. Psychooncology. 2010;19(12):1321–1328. doi: 10.1002/pon.1695. [DOI] [PubMed] [Google Scholar]
- 16.Kurita K, et al. Long-term cognitive impairment in older adult twins discordant for gynecologic cancer treatment. J Gerontol A Biol Sci Med Sci. 2011;66(12):1343–1349. doi: 10.1093/gerona/glr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tager FA, et al. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: a controlled longitudinal study. Breast Cancer Res Treat. 2010;123(1):25–34. doi: 10.1007/s10549-009-0606-8. [DOI] [PubMed] [Google Scholar]
- 18.Jansen CE, et al. Chemotherapy-induced cognitive impairment in women with breast cancer: a critique of the literature. Oncol Nurs Forum. 2005;32(2):329–342. doi: 10.1188/05.ONF.329-342. [DOI] [PubMed] [Google Scholar]
- 19.Ahles TA, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20(2):485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 20.van Dam FS, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90(3):210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 21.Mehnert A, et al. The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue and health related quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Educ Couns. 2007;66(1):108–118. doi: 10.1016/j.pec.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Poppelreuter M, et al. Cognitive dysfunction and subjective complaints of cancer patients a cross-sectional study in a cancer rehabilitation centre. Eur J Cancer. 2004;40(1):43–49. doi: 10.1016/j.ejca.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Downie FP, et al. Cognitive function, fatigue, and menopausal symptoms in breast cancer patients receiving adjuvant chemotherapy: evaluation with patient interview after formal assessment. Psychooncology. 2006;15(10):921–930. doi: 10.1002/pon.1035. [DOI] [PubMed] [Google Scholar]
- 24.Shilling V, et al. The effects of adjuvant chemotherapy on cognition in women with breast cancer-preliminary results of an observational longitudinal study. Breast. 2005;14(2):142–150. doi: 10.1016/j.breast.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Reid-Arndt SA, Hsieh C, Perry MC. Neuropsychological functioning and quality of life during the first year after completing chemotherapy for breast cancer. Psychooncology. 2010;19(5):535–544. doi: 10.1002/pon.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fliessbach K, et al. Neuropsychological outcome after chemotherapy for primary CNS lymphoma: a prospective study. Neurology. 2005;64(7):1184–1188. doi: 10.1212/01.WNL.0000156350.49336.E2. [DOI] [PubMed] [Google Scholar]
- 27.Weis J, Poppelreuter M, Bartsch HH. Cognitive deficits as long-term side-effects of adjuvant therapy in breast cancer patients: ‘subjective’ complaints and ‘objective’ neuropsychological test results. Psychooncology. 2009;18(7):775–782. doi: 10.1002/pon.1472. [DOI] [PubMed] [Google Scholar]
- 28.Poppelreuter M, Weis J, Bartsch HH. Effects of specific neuropsychological training programs for breast cancer patients after adjuvant chemotherapy. J Psychosoc Oncol. 2009;27(2):274–296. doi: 10.1080/07347330902776044. [DOI] [PubMed] [Google Scholar]
- 29.Hutchinson AD, et al. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38(7):926–934. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Bruno J, Hosseini SM, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48(3):329–338. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deprez S, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;32(3):480–493. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wefel JS, et al. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 33.Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care. 2013;2(3):231–238. doi: 10.1136/bmjspcare-2011-000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald BC, et al. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30(20):2500–2508. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inagaki M, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109(1):146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 37.Deprez S, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30(3):274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 38.de Ruiter MB, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;33(12):2971–2983. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Button KS, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 40.Walitt B, et al. Automated neuropsychiatric measurements of information processing in fibromyalgia. Rheumatol Int. 2008;28(6):561–566. doi: 10.1007/s00296-007-0487-2. [DOI] [PubMed] [Google Scholar]
- 41.Glass JM. Review of cognitive dysfunction in fibromyalgia: a convergence on working memory and attentional control impairments. Rheum Dis Clin North Am. 2009;35(2):299–311. doi: 10.1016/j.rdc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Ocon AJ. Caught in the thickness of brain fog: exploring the cognitive symptoms of chronic fatigue syndrome. Front Physiol. 2013;4:63. doi: 10.3389/fphys.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfe F, et al. The American college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 44.Holmes GP, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1988;108(3):387–389. doi: 10.7326/0003-4819-108-3-387. [DOI] [PubMed] [Google Scholar]
- 45.Nebel MB, Gracely RH. Neuroimaging of fibromyalgia. Rheum Dis Clin North Am. 2009;35(2):313–327. doi: 10.1016/j.rdc.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. J Pain. 2009;10(8):777–791. doi: 10.1016/j.jpain.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janelsins MC, et al. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Semin Oncol. 2011;38(3):431–438. doi: 10.1053/j.seminoncol.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang XM, et al. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012;59(1):3–9. doi: 10.1016/j.cyto.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cleeland CS, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 50.Mantovani A, Pierotti MA. Cancer and inflammation: a complex relationship. Cancer Lett. 2008;267(2):180–181. doi: 10.1016/j.canlet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Lyon D, et al. Potential epigenetic mechanism(s) associated with the persistence of psychoneurological symptoms in women receiving chemotherapy for breast cancer: a hypothesis. Biol Res Nurs. 2013 doi: 10.1177/1099800413483545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trask PC, et al. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18(11):2316–2326. doi: 10.1200/JCO.2000.18.11.2316. [DOI] [PubMed] [Google Scholar]
- 53.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 54.Gibb J, Al-Yawer F, Anisman H. Synergistic and antagonistic actions of acute or chronic social stressors and an endotoxin challenge vary over time following the challenge. Brain Behav Immun. 2013;28:149–158. doi: 10.1016/j.bbi.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med. 2001;63(3):376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Tsavaris N, et al. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87(1):21–27. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pusztai L, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25(3):94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Penson RT, et al. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer. 2000;10(1):33–41. doi: 10.1046/j.1525-1438.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 59.Janelsins MC, et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Support Care Cancer. 2012;20(4):831–839. doi: 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villani F, et al. Serum cytokine in response to chemoradiotherapy for Hodgkin’s disease. Tumori. 2008;94(6):803–808. doi: 10.1177/030089160809400605. [DOI] [PubMed] [Google Scholar]
- 61.Kesler S, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(Suppl):S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pomykala KL, et al. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013;7(4):511–523. doi: 10.1007/s11682-013-9243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bower JE, et al. Cytokine genetic variations and fatigue among patients with breast cancer. J Clin Oncol. 2013;31(13):1656–1661. doi: 10.1200/JCO.2012.46.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tangpong J, et al. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol Dis. 2006;23(1):127–139. doi: 10.1016/j.nbd.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 65.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104(4):788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 66.Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. 2000;157(5):683–694. doi: 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- 67.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition-the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50(12):2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 68.Kelley KW. A new look for brain, behavior, and immunity. Brain Behav Immun. 2003;17(1):1–2. doi: 10.1016/s0889-1591(02)00053-3. [DOI] [PubMed] [Google Scholar]
- 69.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37(1):26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 70.Pan W, Kastin AJ. Interactions of cytokines with the blood-brain barrier: implications for feeding. Curr Pharm Des. 2003;9(10):827–831. doi: 10.2174/1381612033455332. [DOI] [PubMed] [Google Scholar]
- 71.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246C:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raison CL, Miller AH. Depression in cancer: new developments regarding diagnosis and treatment. Biol Psychiatry. 2003;54(3):283–294. doi: 10.1016/s0006-3223(03)00413-x. [DOI] [PubMed] [Google Scholar]
- 73.Bower JE, et al. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 74.Greenberg DB, et al. Treatment-related fatigue and serum interleukin-1 levels in patients during external beam irradiation for prostate cancer. J Pain Symptom Manage. 1993;8(4):196–200. doi: 10.1016/0885-3924(93)90127-h. [DOI] [PubMed] [Google Scholar]
- 75.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Y, et al. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv. 2007;7(3):147–156. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- 77.Tangpong J, et al. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: insight into the mechanism of chemobrain. J Neurochem. 2007;100(1):191–201. doi: 10.1111/j.1471-4159.2006.04179.x. [DOI] [PubMed] [Google Scholar]
- 78.Aluise CD, et al. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-alpha release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic Biol Med. 2011;50(11):1630–1638. doi: 10.1016/j.freeradbiomed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Caldecott KW. DNA single-strand breaks and neurodegeneration. DNA Repair (Amst) 2004;3(8–9):875–882. doi: 10.1016/j.dnarep.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 80.Abner CW, McKinnon PJ. The DNA double-strand break response in the nervous system. DNA Repair (Amst) 2004;3(8–9):1141–1147. doi: 10.1016/j.dnarep.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Blasiak J, et al. DNA damage and repair in type 2 diabetes mellitus. Mutat Res. 2004;554(1–2):297–304. doi: 10.1016/j.mrfmmm.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 82.Nadin SB, et al. DNA damage and repair in peripheral blood lymphocytes from healthy individuals and cancer patients: a pilot study on the implications in the clinical response to chemotherapy. Cancer Lett. 2006;239(1):84–97. doi: 10.1016/j.canlet.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 83.Aluise CD, et al. Chemo brain (chemo fog) as a potential side effect of doxorubicin administration: role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Adv Exp Med Biol. 2010;678:147–156. doi: 10.1007/978-1-4419-6306-2_19. [DOI] [PubMed] [Google Scholar]
- 84.Stenvinkel P, et al. Impact of inflammation on epigenetic DNA methylation - a novel risk factor for cardiovascular disease? J Intern Med. 2007;261(5):488–499. doi: 10.1111/j.1365-2796.2007.01777.x. [DOI] [PubMed] [Google Scholar]
- 85.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 86.Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233(1):102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 87.Maze I, Nestler EJ. The epigenetic landscape of addiction. Ann N Y Acad Sci. 2011;1216:99–113. doi: 10.1111/j.1749-6632.2010.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mifsud KR, et al. Epigenetic mechanisms in stress and adaptation. Brain Behav Immun. 2011;25(7):1305–1315. doi: 10.1016/j.bbi.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 89.Adwan L, Zawia NH. Epigenetics: a novel therapeutic approach for the treatment of Alzheimer’s disease. Pharmacol Ther. 2013;139(1):41–50. doi: 10.1016/j.pharmthera.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levenson JM, et al. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281(23):15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- 91.Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89(4):599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Molfese DL. Advancing neuroscience through epigenetics: molecular mechanisms of learning and memory. Dev Neuropsychol. 2011;36(7):810–827. doi: 10.1080/87565641.2011.606395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sultan FA, Day JJ. Epigenetic mechanisms in memory and synaptic function. Epigenomics. 2011;3(2):157–181. doi: 10.2217/epi.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bilinski P, et al. Epigenetic regulation in drug addiction. Ann Agric Environ Med. 2012;19(3):491–496. [PubMed] [Google Scholar]
- 95.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 96.Briones TL, Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011;12:124. doi: 10.1186/1471-2202-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Whitaker JW, et al. An imprinted rheumatoid arthritis methylome signature reflects pathogenic phenotype. Genome Med. 2013;5(4):40. doi: 10.1186/gm444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mathews HL, et al. Epigenetic patterns associated with the immune dysregulation that accompanies psychosocial distress. Brain Behav Immun. 2011;25(5):830–839. doi: 10.1016/j.bbi.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toyokawa S, et al. How does the social environment ‘get into the mind’? Epigenetics at the intersection of social and psychiatric epidemiology. Soc Sci Med. 2012;74(1):67–74. doi: 10.1016/j.socscimed.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ai S, et al. DNA methylation as a biomarker for neuropsychiatric diseases. Int J Neurosci. 2012;122(4):165–176. doi: 10.3109/00207454.2011.637654. [DOI] [PubMed] [Google Scholar]
- 101.Monk JP, et al. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006;24(12):1852–1859. doi: 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- 102.Raison CL, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perez-Medrano A, et al. Discovery and biological evaluation of novel cyanoguanidine P2X(7) antagonists with analgesic activity in a rat model of neuropathic pain. J Med Chem. 2009;52(10):3366–3376. doi: 10.1021/jm8015848. [DOI] [PubMed] [Google Scholar]
- 105.Shiue HS, et al. DNA microarray analysis of the effect on inflammation in patients treated with acupuncture for allergic rhinitis. J Altern Complement Med. 2008;14(6):689–698. doi: 10.1089/acm.2007.0669. [DOI] [PubMed] [Google Scholar]
- 106.Zhang ZJ, Wang XM, McAlonan GM. Neural acupuncture unit: a new concept for interpreting effects and mechanisms of acupuncture. Evid Based Complement Alternat Med. 2012;2012:429412. doi: 10.1155/2012/429412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia MK, et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. J Clin Oncol. 2013;31(7):952–960. doi: 10.1200/JCO.2012.43.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chou P, Chu H, Lin JG. Effects of electroacupuncture treatment on impaired cognition and quality of life in Taiwanese stroke patients. J Altern Complement Med. 2009;15(10):1067–1073. [PubMed] [Google Scholar]
- 109.Deng QS, Fang ZC, Yin Y. Ionic mechanism of acupuncture on improvement of learning and memory in aged mammals. Am J Chin Med. 1995;23(1):1–9. doi: 10.1142/S0192415X9500002X. [DOI] [PubMed] [Google Scholar]
- 110.Feng Y, et al. FMRI connectivity analysis of acupuncture effects on the whole brain network in mild cognitive impairment patients. Magn Reson Imaging. 2012;30(5):672–682. doi: 10.1016/j.mri.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 111.Zhao L, et al. Electroacupuncture on the head points for improving gnosia in patients with vascular dementia. J Tradit Chin Med. 2009;29(1):29–34. doi: 10.1016/s0254-6272(09)60027-3. [DOI] [PubMed] [Google Scholar]
- 112.Wang Z, et al. Effect of acupuncture in mild cognitive impairment and Alzheimer disease: a functional MRI study. PLoS One. 2012;7(8):e42730. doi: 10.1371/journal.pone.0042730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10(3):209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 114.Zeng Y, et al. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci. 2011;31(49):17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 115.Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 116.Dempster E, et al. Association between BDNF val66 met genotype and episodic memory. Am J Med Genet B Neuropsychiatr Genet. 2005;v134B;(1):73–75. doi: 10.1002/ajmg.b.30150. [DOI] [PubMed] [Google Scholar]
- 117.Hariri AR, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23(17):6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Erickson KI, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30(15):5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang XY, et al. Cognitive and serum BDNF correlates of BDNF Val66Met gene polymorphism in patients with schizophrenia and normal controls. Hum Genet. 2012;131(7):1187–1195. doi: 10.1007/s00439-012-1150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Connor B, et al. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Brain Res Mol Brain Res. 1997;49(1–2):71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- 121.Narisawa-Saito M, et al. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimer’s disease. Neuroreport. 1996;7(18):2925–2928. doi: 10.1097/00001756-199611250-00024. [DOI] [PubMed] [Google Scholar]
- 122.Hock CH, et al. Alterations in neurotrophins and neurotrophin receptors in Alzheimer’s disease. J Neural Transm Suppl. 2000;59:171–174. doi: 10.1007/978-3-7091-6781-6_19. [DOI] [PubMed] [Google Scholar]
- 123.Wang WH, et al. Relationship between brain-derived neurotrophic factor and cognitive function of obstructive sleep apnea/hypopnea syndrome patients. Asian Pac J Trop Med. 2012;5(11):906–910. doi: 10.1016/S1995-7645(12)60169-2. [DOI] [PubMed] [Google Scholar]
- 124.Kim MW, et al. Electroacupuncture enhances motor recovery performance with brain-derived neurotrophic factor expression in rats with cerebral infarction. Acupunct Med. 2012;30(3):222–226. doi: 10.1136/acupmed-2011-010126. [DOI] [PubMed] [Google Scholar]
- 125.Lin XM, et al. Effect of electroacupuncture on the permeability of blood-brain barrier for nerve growth factor and its relevance to PKC pathway in cerebral ischemia rats. Zhen Ci Yan Jiu. 2009;34(2):110–113. [PubMed] [Google Scholar]
- 126.Feinberg AP. Epigenetics at the epicenter of modern medicine. JAMA. 2008;299(11):1345–1350. doi: 10.1001/jama.299.11.1345. [DOI] [PubMed] [Google Scholar]
- 127.Oliveira AM, Hemstedt TJ, Bading H. Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities. Nat Neurosci. 2012;15(8):1111–1113. doi: 10.1038/nn.3151. [DOI] [PubMed] [Google Scholar]
- 128.Levkovitz Y, et al. Effects of S-adenosylmethionine augmentation of serotonin-reuptake inhibitor antidepressants on cognitive symptoms of major depressive disorder. J Affect Disord. 2012;136(3):1174–1178. doi: 10.1016/j.jad.2011.04.059. [DOI] [PubMed] [Google Scholar]
- 129.Miwa M, et al. Effects of betaine on lipopolysaccharide-induced memory impairment in mice and the involvement of GABA transporter 2. J Neuroinflammation. 2011;8:153. doi: 10.1186/1742-2094-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Graff J, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483(7388):222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fass DM, et al. Crebinostat: a novel cognitive enhancer that inhibits histone deacetylase activity and modulates chromatin-mediated neuroplasticity. Neuropharmacology. 2013;64:81–96. doi: 10.1016/j.neuropharm.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kilgore M, et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2010;35(4):870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huber K, Superti-Furga G. After the grape rush: sirtuins as epigenetic drug targets in neurodegenerative disorders. Bioorg Med Chem. 2011;19(12):3616–3624. doi: 10.1016/j.bmc.2011.01.018. [DOI] [PubMed] [Google Scholar]