Structured Abstract
Purpose
The addition of bevacizumab, an anti-angiogenesis agent, to cytotoxic chemotherapy improves survival in patients with advanced non-squamous non-small cell lung cancers (nsNSCLCs). Regorafenib is an oral multi-targeted kinase inhibitor with potent anti-angiogenic activity that is approved for patients with advanced colorectal cancer and gastrointestinal stromal tumors. This phase I trial evaluated the safety, pharmacokinetics, and efficacy of regorafenib with cisplatin and pemetrexed for patients with advanced nsNSCLCs.
Methods
Chemotherapy-naïve patients with advanced nsNSCLCs were treated with regorafenib 60mg/day continuously and cisplatin 75mg/m2 plus pemetrexed 500mg/m2 once every three weeks for up to six cycles. Thereafter, regorafenib with or without pemetrexed could be continued as maintenance.
Results
Nine patients enrolled prior to premature termination of the study due to slow recruitment and a change in the development strategy of regorafenib by the study sponsor, partially due to slow enrollment. Five patients experienced at least one treatment-related Grade 3 adverse event. No grade 4–5 toxicity occurred. 5 of 9 (56%) patients had a partial response and the median progression-free survival was 7 months (range 1.5–15.1). Minor pharmacokinetic (PK) interactions between regorafenib and chemotherapy were observed.
Conclusions
Regorafenib had acceptable tolerability and minor PK interactions in combination with standard doses of cisplatin and pemetrexed in patients with advanced nsNSCLCs. Encouraging activity was appreciated in chemotherapy-naïve patients with advanced nsNSCLCs. However, the small number of patients treated limits conclusions that can be drawn from these results.
Keywords: regorafenib, non-small cell lung cancer (NSCLC), angiogenesis, chemotherapy
INTRODUCTION
Lung cancer continues to be the leading cause of cancer related mortality in the United States and worldwide (1). The majority of patients are diagnosed with advanced disease where platinum-based doublet chemotherapy remains the standard first line therapy (2). The median survival of patients with advanced non-squamous non-small cell lungs cancers (nsNSCLCs) treated with platinum-based doublet chemotherapy is approximately 10 months, with improvement to 12 months with the addition of the anti-angiogenesis agent, bevacizumab (3).
Regorafenib (BAY 73–4506; Bayer Pharma AG, Berlin, Germany) is a small molecule multi-kinase inhibitor with potent activity against multiple drivers of angiogenesis including VEGFR1–3, TIE2, FGFR1, PDGFR, as well as other oncogenic kinases such as RAF, KIT, and RET (4). Regorafenib (160mg/day for 21 of 28 days) was recently approved for monotherapy use in patients with refractory, advanced colorectal cancers (5) and gastrointestinal stromal tumors (6). In patients with advanced nsNSCLCs, regorafenib was previously evaluated as a single-agent in a continuous dose regimen in the expansion cohort of a dose-escalation phase I study (7). Of 23 patients were treated, seven (30.4%) experienced a treatment-related adverse event requiring reduction, interruption, or discontinuation of treatment; no Grade 4 or 5 toxicities were observed. In 18 patients evaluable for efficacy, 13 (76%) had stable disease for ≥ 6 weeks and 4 (22%) had stable disease for ≥12 weeks. One patient had progression-free survival of nearly 40 weeks and a tumor reduction of > 30% in two non-consecutive measurements.
We performed this Phase I trial (NCT01187615) to evaluate the safety, pharmacokinetics and preliminary activity of daily regorafenib administered with standard first-line cisplatin and pemetrexed chemotherapy for patients with advanced nsNSCLCs. After enrollment of nine patients, the trial was prematurely terminated due to slow recruitment and a change in developmental strategy by the sponsor, partly due to the slow enrollment. The results of these nine patients are presented here.
MATERIALS AND METHODS
Patients
Patients had pathologically confirmed stage IIIB or IV nsNSCLCs who had not previously received systemic therapy for advanced stage disease. Patients were required to have an ECOG Performance Status of 0–2 and adequate hematologic and organ function. Excluded patients were those with poor baseline hearing, brain metastases, history of seizures, radiographic evidence of major blood vessel involvement, history of arterial or venous thrombosis within the 6 months prior, major surgical procedures within the 4 weeks prior, or a history of hemoptysis within the 3 months prior (defined as 1 teaspoon or more of blood). This study was approved by the Institutional Review Boards of participating institutions and all patients signed informed consent.
Study design
This was a multi-center, open-label, phase I study. The primary objectives were to characterize the safety profile, examine potential pharmacokinetic interactions, and define the maximum tolerated dose (MTD) of regorafenib in combination with cisplatin and pemetrexed. The secondary end-points included evaluation of the objective response rate by RECIST v1.1 to study treatment. The study was intended to have two parts. In Part B, regorafenib was administered continuously (i.e. day 1–21 of each cycle). In Part A, regorafenib was to be administered in a sequential dosing with a 7-day wash out period before the next dose of chemotherapy (i.e. day 2–14 of each cycle, followed by a 7 day break). Ultimately, patients were only enrolled in Part B of this study and no patients were assigned to Part A prior to termination of this study. This study was approved by the Institutional Review Boards of participating institutions and all patients signed informed consent.
Dosing and administration
Regorafenib 60mg daily was administered continuously from Day 1 to Day 21, except for Cycle 1 when regorafenib dosing started on Day 2 in order to assess the pharmacokinetics of pemetrexed and cisplatin without concomitant regorafenib dosing. Alternative dosing schedules (e.g. 14 days on, 7 days off) and dose escalation of regorafenib were planned but did not occur due to premature termination of the study.
Pemetrexed 500 mg/m2 and Cisplatin 75 mg/m2 were administered on Day 1 of each cycle for up to six cycles, along with standard pre-medications and vitamin supplementation. After Cycle 6, regorafenib as single agent or in combination with pemetrexed was continued at the discretion of the investigator. Patients were allowed to continue treatment until progression, as defined by RECIST v1.1.
Statistical analyses
Safety
Safety analysis was preplanned; toxicity was monitored and graded using National Cancer Institute Common Toxicity Criteria, (CTCAE) version 3.0. The dose-limiting toxicities (DLTs) period was the first 21 days of treatment. Toxicity was considered a DLT only if it was felt to be related to regorafenib alone or if it was worse than expected from cytotoxic chemotherapy alone.
Pharmacokinetics
Plasma concentrations of regorafenib (BAY 73–4506) and its active metabolites, M-2 (BAY 75–7495), and M-5 (BAY 81–8752) (8), were determined at Bayer Pharma AG in Wuppertal, Germany after protein precipitation with acetonitrile/ammonium acetate buffer containing the internal standards followed by separation employing high-pressure liquid chromatography and tandem mass spectrometric detection (LC-MS/MS). Pemetrexed concentrations in plasma were determined at NorthEast Bioanalytical Labs, Hamden, CT. USA. Pemetrexed was determined in plasma after addition of an internal standard, protein precipitation with methanol (0.1% acetic acid) followed by separation employing high-pressure LC-MS/MS. Analyses of plasma samples for platinum were conducted at Nuvisan GmbH, Neu-Ulm, Germany. Platinum (from cisplatin) was determined in plasma (total) and plasma ultrafiltrate (unbound) after addition of an internal standard (lutetium) by ionization in an argon plasma and separation employing Inductively Coupled Plasma and Mass Spectrometric (ICP-MS) detection.
Plasma concentrations of regorafenib and its metabolites were evaluated on Cycle 1 Day 21 and Cycle 2 Day 1 at the following time points: pre-dose, 2, 4, 6, 8, 9, and 24 hours after the morning dose. Plasma concentrations of pemetrexed and total and unbound platinum concentrations were examined on Day 1 of Cycle 1 (pre-regorafenib) and Cycle 2 (following 21 days of regorafenib). Blood samples were collected before start of the pemetrexed infusion, and at 0.17 (end of pemetrexed infusion), 0.5, 1 (before start of cisplatin infusion), 1.5, 2, 2.5, 3 (end of cisplatin infusion), 4, 6, 8, 9, and 24 hours after start of the pemetrexed infusion.
Individual and geometric mean concentration versus time curves of all analytes were plotted by treatment. The derived PK parameters were AUC and Cmax of pemetrexed, AUC(0–8), AUC(0–24), AUC(0–-tlast) and Cmax of total platinum, and Cmax(md), AUC(0–24)md of regorafenib and metabolites (where “md” refers to multiple dosing). Based on these analyses, point estimates (least-squared-means) and exploratory 90% confidence intervals for the ratios “Cycle 2 / Cycle 1” of platinum, and pemetrexed and “Cycle 2, Day 1/ Cycle 1, Day 21” of regorafenib and metabolites were calculated.
Efficacy
Objective radiographic response was assessed using RECIST v1.1. Tumor measurements and evaluation of tumor response were performed at baseline and subsequently in Cycles 2, 4 and 6. Subsequent tumor evaluations were performed every 4 cycles. Progression-free survival was defined as the time of study enrollment until progression, as defined by RECIST v1.1, or death, whichever occurred first.
RESULTS
Patients
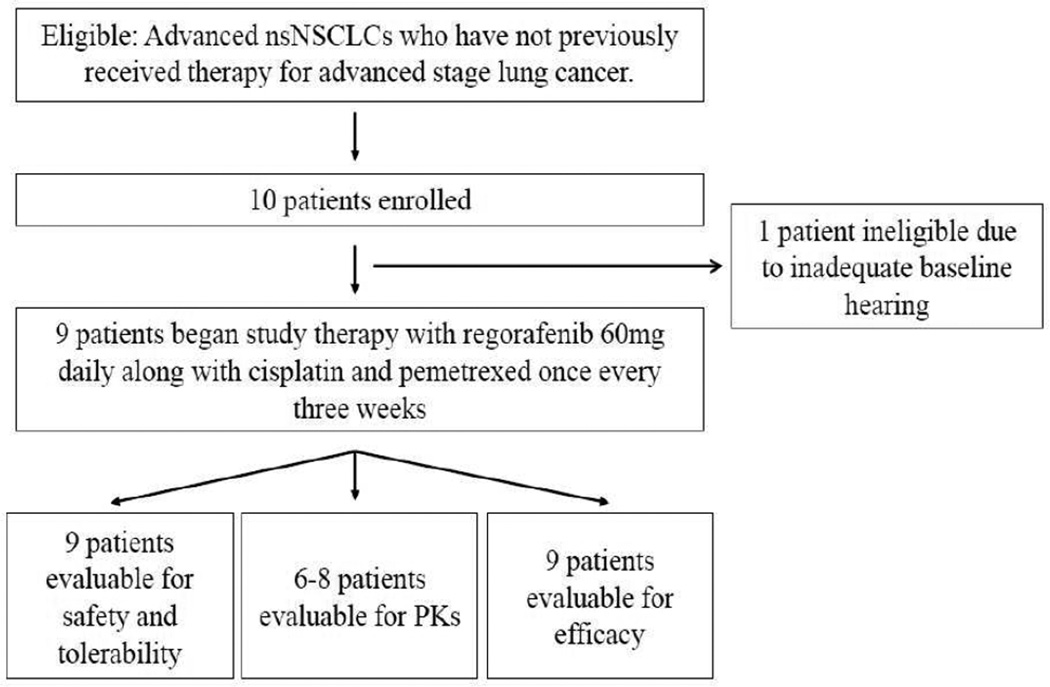
10 patients were enrolled in this study prior to its termination. One patient failed screening due to hearing impairment. Nine patients were treated with study therapy. The CONSORT diagram is shown in Figure 1. All patients had adenocarcinomas. In addition to the data planned for evaluation by the sponsor of this study, further tumor characteristics were determined as part of the routine evaluation at the treating institutions (see table 1): 3 patients had KRAS mutations; 1 patient had a exon 20 EGFR mutation; 5 patients were EGFR/KRAS WT. Four patients were male, five were female; the median age was 58 years (range 39–65); eight were current or former smokers with median of 27 pack-years; ECOG performance status was 0 (n=5) or 1 (n=4).
Figure 1.
Consort diagram - disposition of patients enrolled on study
Table 1.
Summary of treatment and outcomes to study treatment
| Molecular status |
Regorafenib dose reductions |
Pemetrexed dose reductions |
Cisplatin dose reductions |
Best response | PFS (months) | OS (months) | |
|---|---|---|---|---|---|---|---|
| Patient #1 | KRAS G12V | 1 | 1 | 1 | PR (−38%) | 7.6 | 42.4 |
| Patient #2 | KRAS G12V | 0 | 0 | 0 | PD (−44%#) | 1.5 | 13.1 |
| Patient #3 | NODI* | 0 | 0 | 0 | PR (−66%) | 15.1 | 82.1 |
| Patient #4 | NODI* | 0 | 0 | 0 | SD (−14%) | 4.5 | 24.4 |
| Patient #5 | KRAS G12A | 0 | 0 | 0 | PR (−44%) | 12.4 | 56.7 |
| Patient #6 | EGFR exon 20 | 0 | 0 | 0 | SD (−25%) | 2.6 | 15.7 |
| Patient #7 | NODI* | 0 | 0 | 0 | PR (−55%) | 6.7 | 34.7 |
| Patient #8 | NODI* | 1 | 1 | 1 | PR (−47%) | 6.6 | 31.4 |
| Patient #9 | NODI* | 1 | 1 | 0 | SD (−24%) | 7.0 | 32.6 |
NODI = “no oncogenic driver identified.
= patient had response in target lesions but growth in non-target lesions.
Treatment
The median treatment duration was 30 weeks (range 7–78 weeks). A summary of molecular features, need for dose reductions, and response outcomes are summarized in Table 1. Eight patients discontinued study therapy due to progressive disease and one patient stopped due toxicity.
Safety and tolerability
The treatment-related adverse events observed in this study are summarized in Table 2.
Table 2.
Summary of treatment-related adverse events (TRAEs) by Common Terminology Criteria for Adverse Events v3.0
| Adverse events | Common (>20%) TRAEs of all grades and other TRAEs of interest(*) |
Grade 3 TRAEs (No grade 4–5 toxicities were observed) |
TRAEs prompting interruption/discontinuation of study therapy |
|---|---|---|---|
| Any/patient | 9 (100.0) | 5 (55.6) | 5 (55.6) |
| Constitutional symptoms | |||
| Fatigue | 8 (88.9) | 2 (22.2) | 1 (11.1) |
| Gastrointestinal | |||
| Anorexia | 4 (44.4) | ||
| Constipation | 5 (55.6) | ||
| Diarrhea | 5 (55.6) | 2 (22.2) | 1 (11.1) |
| Dry mouth | 2 (22.2) | ||
| Esophagitis | 2 (22.2) | ||
| Heartburn | 2 (22.2) | ||
| Mucositis | 4 (44.4) | ||
| Nausea | 7 (77.8) | ||
| Taste Alteration | 5 (55.6) | ||
| Vomiting | 2 (22.2) | ||
| Neurology | |||
| Dizziness | 6 (66.7) | ||
| Neuropathy: motor | 2 (22.2) | ||
| Neuropathy: sensory | 4 (44.4) | ||
| Pain | |||
| Any | 7 (77.8) | 2 (22.2) | |
| Pain, Back | 4 (44.4) | ||
| Pain, Chest wall | 2 (22.2) | 1 (11.1) | 1 (11.1) |
| Pain, Head/headache | 4 (44.4) | 1 (11.1) | |
| Pain, Joint | 2 (22.2) | ||
| Pain, Muscle | 2 (22.2) | ||
| Pain, Stomach | 0 | 1 (11.1) | |
| Pulmonary / upper respiratory | |||
| Cough | 4 (44.4) | 1 (11.1) | |
| Dyspnea | 4 (44.4) | 2 (22.2) | |
| Hiccoughs | 2 (22.2) | ||
| Voice changes | 3 (33.3) | ||
| Hemoptysis | 1 (11.1) | 1 (11.1) | |
| Cardiac general | |||
| Hypertension | 6 (66.7) | 1 (11.1) | |
| Dermatology / skin | |||
| Alopecia | 3 (33.3) | ||
| Nail changes | 2 (22.2) | ||
| Rash/desquamation | 4 (44.4) | ||
| Hand-foot syndrome | 1 (11.1) | ||
| Hemorrhage / bleeding | |||
| Epistaxis | 3 (33.3) | ||
| Infection | |||
| Infection | 2 (22.2) | 2 (22.2) | |
| Lymphatics | |||
| Edema: Limb | 3 (33.3) | ||
| Allergy / immunology | |||
| Rhinitis | 2 (22.2) | ||
| Auditory / hearing | |||
| Tinnitus | 2 (22.2) | ||
| Musculoskeletal | |||
| Weakness | 2 (22.2) | 1 (11.1) | 1 (11.1) |
| Ocular | |||
| Watery eye | 3 (33.3) | ||
| Renal | |||
| Urinary frequency | 3 (33.3) | ||
| Vascular | |||
| Thrombosis/embolism | 1 (11.1) | ||
| Laboratory | |||
| ↑ Amylase/Lipase | 4 (44.4) | 1 (11.1) | 1 (11.1) |
| ↑ INR | 1 (11.1) | 1 (11.1) | 1 (11.1) |
| ↑ Creatinine | 2 (22.2) | 1 (11.1) | |
| ↑ AST/ALT | 6 (66.7) | ||
| ↑ Potassium | 4 (44.4) | ||
| ↑ Glucose | 9 (100) | ||
| ↓ Albumin | 2 (22.2) | ||
| ↓ Sodium | 5 (55.6) | ||
| ↓ Hemoglobin | 9 (100) | ||
| ↓ Neutrophils/leuokocytes | 2 (22.2) | ||
| ↓ Platelets | 5 (55.6) |
All patients had at least one treatment-related adverse event (TRAE). The most common TRAEs of any grade were fatigue (88.9%), nausea (77.8%), dizziness and hypertension (66.7% each), constipation, diarrhea, taste alteration, and rash (55.6%, each).
Five patients (56%) experienced at least one Grade 3 TRAE, including dyspnea (33.3%), fatigue, diarrhea, and pain (22.2% each). No treatment-related Grade 4 or 5 toxicities were observed. One patient discontinued study treatment due to grade 2 hemoptysis. No deaths occurred during this study or within 30 days of study drug administration.
Three patients had an asymptomatic change in QT duration greater than 60 msec compared to baseline, although one patient had prolongation noted before treatment began on Cycle 1, Day 1. Ondansetron, which has been associated with a prolonged QT duration (9–11), was taken by all three patients.
Pharmacokinetic analyses
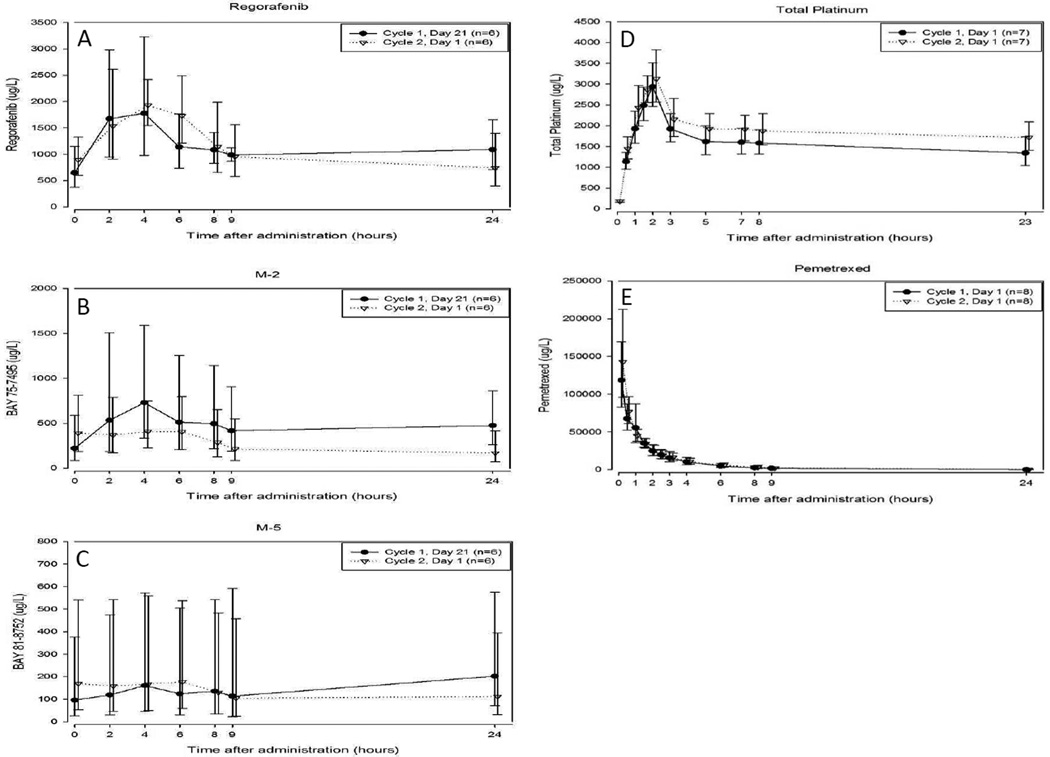
No differences were seen in the mean concentrations of regorafenib and M-5 when regorafenib was given concurrently with chemotherapy (Cycle 2, Day 1) compared to when regorafenib was taken alone (Cycle 1, Day 21), although there was a slight decrease in mean M-2 concentrations (Figures 2A–2C).
Figure 2.
Summary of pharmacokinetics of regorafinib, platinum, and pemetrexed. A–C: Geometric mean concentrations versus time profile of (A) regorafenib and its metabolites (B) M-2 and (C) M-5 following a 60mg dose of regorafenib administered without (Cycle 1, Day 21) or with (Cycle 2, Day 1) cisplatin and pemetrexed. D–E: Geometric mean concentration versus time profile of (D) total platinum and (E) pemetrexed without (Cycle 1, Day 1) or with (Cycle 2, Day 1) regorafenib.
Mean AUC(0–24)md and Cmax,md of regorafenib and M-5 were comparable on both days. For M-2, mean AUC(0–24)md and Cmax,md were 48% and 38% lower, respectively, following the pemetrexed and cisplatin treatment. High variability in AUC and Cmax is to be noted for both M-2 (coefficient of variation (CV) >75%) and M-5 (CV>150%) (Table 3A).
Table 3.
Pharmacokinetic parameters for (A) regorafenib, M-2 and M-5 following regorafenib alone (C1 D21) and following administration of cisplatin and pemetrexed (C2 D1), and (B) pemetrexed and total platinum following chemotherapy alone (Cycle 1 Day 1) or after 21 days of regorafenib (C2 D1)
| 3A | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Regorafenib (n = 6) | M-2 (n = 6) | M-5 (n = 6) | |||||||
| C1 D21 (Mean/%CV) |
C2 D1 (Mean/%CV) |
Ratio (90% CI) |
C1 D21 (Mean/%CV) |
C2 D1 (Mean/%CV) |
Ratio (90% CI) |
C1 D21 (Mean/%CV) |
C2 D1 (Mean/%CV) |
Ratio (90% CI) |
|
| AUC(0–24)md (mg·h/L) |
28.8/28.0 | 26.7/36.3 | 0.93 (0.77–1.12) |
12.4/76.0 | 6.44/80.5 | 0.52 (0.44–0.61) |
3.82/173 | 3.21/169 | 0.84 (0.67–1.06) |
| Cmax,md (mg/L) |
2.11/52.1 | 2.16/27.1 | 1.03 (0.70–1.51) |
0.85/83.9 | 0.526/80.2 | 0.62 (0.46–0.83) |
0.23/156 | 0.23/184 | 0.97 (0.78–1.20) |
| tmax,mda (hours) |
4.0 (2–24a) | 4.0 (2–6.25a) | - | 4.0 (2–24a) | 5.0 (0–6.25a) | - | 16.0 (4–24a) | 5.0(0–6.25a) | - |
| 3B | ||||||
|---|---|---|---|---|---|---|
| Pemetrexed (n = 8) | Platinum (n = 7) | |||||
| Parameter | C1 D1 (Mean/%CV) |
C2 D1 (Mean/%CV) |
Ratio (90% CI) |
C1 D1 (Mean/%CV) |
C2 D1 (Mean/%CV) |
Ratio (90% CI) |
| AUC# (mg·h/L) |
180/30.2 | 190/21.7 | 1.05 (0.96–1.16) |
37.7/21.1b | 46.7/18.1c | 1.17 (1.14–1.20) |
| Cmax (mg/L) |
132/25.9 | 157/17.4 | 1.20 (1.05–1.35) |
2.94/17.9 | 3.37/13.3 | 1.15 (1.05–1.25) |
| tmaxa (hours) |
0.18 (0.17–1.0a) | 0.17 (0.17–0.67a) | 2.00 (2.00–2.17a) | 2.00 (1.00–2.17a) | ||
AUC(0–24)md = area under the curve from 0 to 24 hours after multiple dosing; Cmax,md = maximum observed drug concentration after multiple dosing; CV = coefficient of variation; Ratio = C2D1:C1D21; tmax = time of maximum observed concentration;
Median and range (minimum – maximum)
AUC(0–infinity) given for pemetrexed, AUC(0–24) given for platinum; Cmax = maximum observed drug concentration; CV = coefficient of variation; tmax = time of maximum observed concentration; Ratio = C2D1:C1D1;
Median and range (minimum – maximum) given;
n=6;
n=5
There was no difference in mean pemetrexed concentration on Cycle 1, Day 1 (before regorafenib began) compared to Cycle 2, Day 1 (after regorafenib had been given for the preceding 20 days). The mean concentrations of total platinum in the terminal portion of the curve appeared to be slightly higher following a regorafenib treatment cycle (Figures 2D–2E).
The mean AUC of pemetrexed was comparable on both days, although Cmax was slightly increased after regorafenib. A slight increase in mean AUC(0–24) and Cmax of total platinum following the Cycle 2 dose of cisplatin was also observed. The magnitude of the effect was less than 25%, as evidenced by the upper bound of the 90% CI. Overall, the exposures of pemetrexed and total platinum were not substantially altered by prior treatment with regorafenib for 21 days at a dose of 60 mg/day (Table 3B).
Efficacy
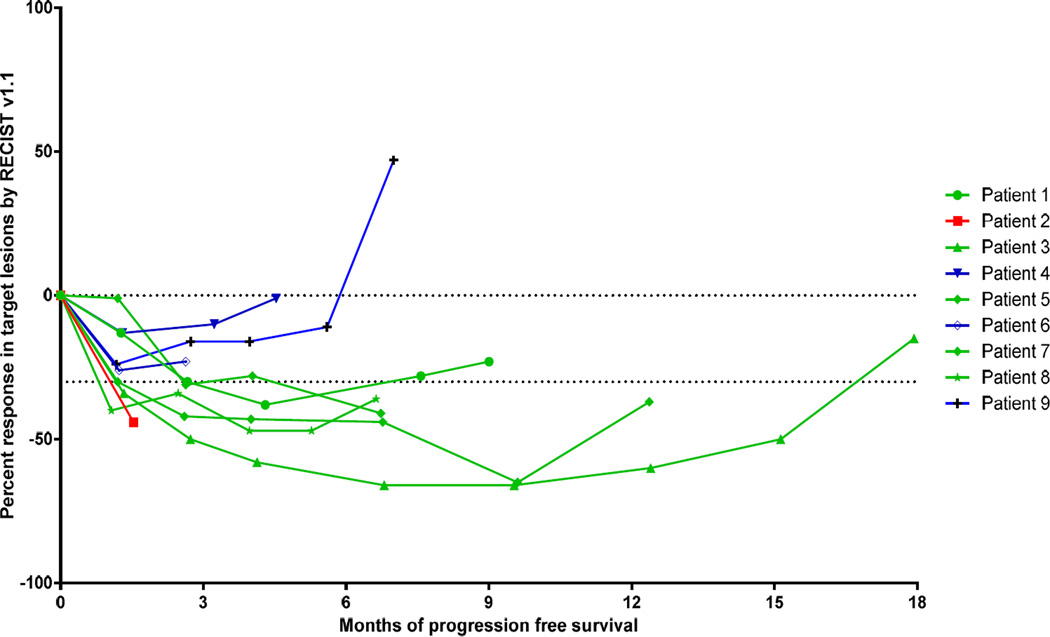
Five patients of nine (55.6%) achieved an objective partial response by RECIST v1.1. The median progression-free survival in this cohort was 7 months (range 1.5–15.1). Figure 3 depicts the depth and duration of responses to therapy that were observed. No patients died during this study.
Figure 3.
Spider curve of radiographic response to therapy per RECIST v1.1. Patients with partial response are noted in green, stable disease in blue, and progressive disease in red.
DISCUSSION
This is the first study to explore the safety and pharmacokinetics of regorafenib when administered in combination with standard platinum-doublet chemotherapy for the treatment of patients with advanced nsNSCLCs. Although this study did not complete its planned enrollment, the preliminary results indicate that regorafenib given continuously with cytotoxic chemotherapy has manageable safety and a minor impact on the pharmacokinetics of either regorafenib or chemotherapy.
Expected toxicities from regorafenib, including hypertension (12) and hand-foot syndrome (13) occurred at similar rates as in previous studies of regorafenib (5–7). The safety profile of regorafenib, cisplatin, and pemetrexed in this study was similar to that seen in other studies of cisplatin and pemetrexed alone (14) and studies of chemotherapy with bevacizumab (3, 15, 16).
Administration of pemetrexed and cisplatin immediately prior to regorafenib resulted in minor alterations in regorafenib pharmacokinetics. The mean AUCs of regorafenib and the active metabolite M-5 were not influenced, whereas active metabolite M-2 was on average 48% lower following pemetrexed and cisplatin dosing. The mechanism of a specific reduction in M-2 is not known; neither pemetrexed nor cisplatin are known to be inhibitors or inducers of CYP3A4 or UGT1A9, which are the main enzymes involved in regorafenib metabolism. High variability and other concomitant medications such as antiemetics may have contributed to this observation. The clinical relevance of the decrease in M-2 mean AUC is unknown but is unlikely to be clinically significant.
The exposure of pemetrexed and total platinum was not substantially altered by prior treatment with regorafenib for 21 days at a dose of 60 mg/day. A slight increase in mean AUC(0–24) of total platinum (<25% increase) was observed following regorafenib administration. One prior study has described the pharmacokinetic impact of regorafenib on chemotherapy (17). The results in this study were similar, with a slight increase in the total oxaliplatin AUC after treatment with regorafenib.
Although the small size of this study limits conclusions that can be drawn, the safety and efficacy seen in this trial are encouraging. Five of 9 (55.6%) patients had an objective response, whereas objective response to standard first-line chemotherapy for patients with nsNSCLCs is typically 30–35% (3, 14, 15). Of note, regorafenib is known to have effect against oncogenic kinases such as RAF and RET (4). None of the patients in this cohort were known to have alterations in these kinases, although partial responses were seen in 2 of 3 patients with KRAS mutations.
In contrast to this study, previous combinations of anti-angiogenic tyrosine kinase inhibitors with chemotherapy have been characterized by poor tolerability and minimal activity. Sorafenib has been combined with chemotherapy in several studies of patients with advanced NSCLCs but toxicity and lack of improvement in survival have limited the development of this combination (18, 19). Similarly, the addition of sunitinib to cisplatin and pemetrexed resulted in substantial myelosuppression and no objective responses were seen in eight patients with NSCLCs, although it was not reported whether or not these patients had previously been treated with chemotherapy (20). Although it is uncertain what specific properties differentiate regorafenib from other multi-targeted kinase inhibitors such as sorafenib and sunitinib, the responses to regorafenib seen in patients previously treated with sorafenib (21) and sunitinib (6) suggest a degree of non-overlapping mechanism of action. Regorafenib is particularly similar structurally to sorafenib (22–24), but regorafenib has increased activity against targets such as VEGFR-2, PDGFR-β, FGFR-1, and c-Kit (24), unique inhibition of TIE-2, and ~3 times greater potency in human xenograft models (4, 22). It is possible these mechanisms may underlie the preliminarily promising results in this study with regorafenib in combination with chemotherapy, but careful comparative studies are needed to identify and deconvolute the distinct targets relevant to response to regorafenib and other multi-targeted kinase inhibitors.
The major limitation of this report is the small number of evaluable patients. The safety, pharmacokinetic, and efficacy results therefore need to be interpreted with caution. It should also be noted that the dose of regorafenib in this trial (60mg daily continuously) differs from the approved dosing regimen of regorafenib monotherapy in patients with colorectal cancers and gastrointestinal stromal tumors (160mg/day for 21 days, followed by 7 day treatment break) (5, 6).
Our study describes the promising but preliminary safety, pharmacokinetics, and efficacy of regorafenib in combination with cisplatin and pemetrexed in patients with non-squamous NSCLC. This study was terminated prematurely, however the overall development of regorafenib in a variety of cancers remains robust. Building upon reassuring pharmacokinetic data, regorafenib is being examined in combination with other platinum-based chemotherapy for esophagogastric cancers (NCT01913639) and colon cancer (NCT01298570), To further examine the potential role of regorafenib in patients with NSCLCs and other solid cancers, phase I studies are ongoing to examine other combinations of regorafenib with cetuximab (NCT01973868) or refametinib (NCT02168777). Pivotal studies of regorafenib are ongoing including phase III studies in hepatocellular carcinoma (NCT01774344) along with earlier phase studies in biliary cancers (NCT02115542, NCT02053376), pancreas cancers (NCT02080260), and sarcomas (NCT01900743, NCT02048722). We believe the findings from our study will be useful for the understanding of the effects of regorafenib in patients with cancers and inform the application of regorafenib in future clinical trials.
CLINICAL PRACTICE POINTS.
Regorafenib is a small molecule multi-targeted kinase inhibitor with potent activity against multiple drivers of angiogenesis and other oncogenic kinases that was recently approved for treatment of patients with refractory, advanced colorectal cancers and gastrointestinal stromal tumors.
This Phase I trial evaluated the safety, pharmacokinetics, and preliminary activity of regorafenib administered with standard first-line cisplatin and pemetrexed chemotherapy for patients with advanced nsNSCLCs. Nine patients were treated prior to the early closure of the trial.
Regorafenib had acceptable tolerability and minor PK interactions in combination with standard doses of cisplatin and pemetrexed. Encouraging activity was appreciated in chemotherapy-naïve patients with advanced nsNSCLCs.
Acknowledgments
Sponsored by BayerHealthCare Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This trial is registered at clinicaltrials.gov as NCT01187615.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013, CA. A Cancer Journal for Clinicians. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC lung cancer staging project: Proposals for the revision of he TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. Journal of Thoracic Oncology. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. The New England Journal of Medicine. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. International Journal of Cancer Journal. 2011;129(1):245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 5.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kies MS, Blumenschein GR, Christensen O, Lin T, Tolcher A. Phase I study of regorafenib (BAY 73–4506), an inhibitor of oncogenic and angiogenic kinases, administered continuously in patients with advanced refractory non-small cell lung cancer. Journal of Clinical Oncology. 2010;28:15s. 2010 (suppl; abstr 7585) [Google Scholar]
- 8.Strumberg D, Scheulen ME, Schultheis B, Richly H, Frost A, Buchert M, et al. Regorafenib (BAY 73–4506) in advanced colorectal cancer: a phase I study. British Journal of Cancer. 2012;106(11):1722–1727. doi: 10.1038/bjc.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hesketh P, Navari R, Grote T, Gralla R, Hainsworth J, Kris M, et al. Double-blind, randomized comparison of the antiemetic efficacy of intravenous dolasetron mesylate and intravenous ondansetron in the prevention of acute cisplatin-induced emesis in patients with cancer. Dolasetron Comparative Chemotherapy-induced Emesis Prevention Group. Journal of Clinical Oncology. 1996;14(8):2242–2249. doi: 10.1200/JCO.1996.14.8.2242. [DOI] [PubMed] [Google Scholar]
- 10.Lofters WS, Pater JL, Zee B, Dempsey E, Walde D, Moquin JP, et al. Phase III double-blind comparison of dolasetron mesylate and ondansetron and an evaluation of the additive role of dexamethasone in the prevention of acute and delayed nausea and vomiting due to moderately emetogenic chemotherapy. Journal of Clinical Oncology. 1997;15(8):2966–2973. doi: 10.1200/JCO.1997.15.8.2966. [DOI] [PubMed] [Google Scholar]
- 11.Kris MG, Grunberg SM, Gralla RJ, Baltzer L, Zaretsky SA, Lifsey D, et al. Dose-ranging evaluation of the serotonin antagonist dolasetron mesylate in patients receiving high-dose cisplatin. Journal of Clinical Oncology. 1994;12(5):1045–1049. doi: 10.1200/JCO.1994.12.5.1045. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Xu J, Nie W, Huang G, Tang J, Guan X. Risk of hypertension with regorafenib in cancer patients: a systematic review and meta-analysis. European Journal of Clinical Pharmacology. 2013 doi: 10.1007/s00228-013-1598-1. [DOI] [PubMed] [Google Scholar]
- 13.Belum VR, Wu S, Lacouture ME. Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: a meta-analysis. Investigational New Drugs. 2013;31(4):1078–1086. doi: 10.1007/s10637-013-9977-0. [DOI] [PubMed] [Google Scholar]
- 14.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. Journal of Clinical Oncology. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 15.Patel JD, Socinski MA, Garon EB, Reynolds CH, Spigel DR, Olsen MR, et al. PointBreak: A Randomized Phase III Study of Pemetrexed Plus Carboplatin and Bevacizumab Followed by Maintenance Pemetrexed and Bevacizumab Versus Paclitaxel Plus Carboplatin and Bevacizumab Followed by Maintenance Bevacizumab in Patients With Stage IIIB or IV Nonsquamous Non-Small-Cell Lung Cancer. Journal of Clinical Oncology. 2013;31(34):4349–4357. doi: 10.1200/JCO.2012.47.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. Journal of Clinical Oncology. 2009;27(8):1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 17.Schultheis B, Folprecht G, Kuhlmann J, Ehrenberg R, Hacker UT, Kohne CH, et al. Regorafenib in combination with FOLFOX or FOLFIRI as first- or second-line treatment of colorectal cancer: results of a multicenter, phase Ib study. Annals of Oncology. 2013;24(6):1560–1567. doi: 10.1093/annonc/mdt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies JM, Dhruva NS, Walko CM, Socinski MA, Bernard S, Hayes DN, et al. A phase I trial of sorafenib combined with cisplatin/etoposide or carboplatin/pemetrexed in refractory solid tumor patients. Lung Cancer. 2011;71(2):151–155. doi: 10.1016/j.lungcan.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scagliotti G, Novello S, von Pawel J, Reck M, Pereira JR, Thomas M, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. Journal of Clinical Oncology. 2010;28(11):1835–1842. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 20.Camidge DR, Blais N, Jonker DJ, Soulieres D, Doebele RC, Ruiz-Garcia A, et al. Sunitinib combined with pemetrexed and cisplatin: results of a phase I dose-escalation and pharmacokinetic study in patients with advanced solid malignancies, with an expanded cohort in non-small cell lung cancer and mesothelioma. Cancer Chemotherapy and Pharmacology. 2013;71(2):307–319. doi: 10.1007/s00280-012-2008-6. [DOI] [PubMed] [Google Scholar]
- 21.Bruix J, Tak WY, Gasbarrini A, Santoro A, Colombo M, Lim HY, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. European Journal of Cancer. 2013;49(16):3412–3419. doi: 10.1016/j.ejca.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Research. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 23.Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nature Biotechnology. 2005;23(3):329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 24.Strumberg D, Schultheis B. Regorafenib for cancer. Expert Opinion on Investigational Drugs. 2012;21(6):879–889. doi: 10.1517/13543784.2012.684752. [DOI] [PubMed] [Google Scholar]