Abstract
Objective
Despite widespread use of proton pump inhibitors (PPIs), the incidence of esophageal adenocarcinoma (EAC) continues to rise. PPIs reduce reflux acidity, but only transiently inactivate gastric enzymes. Nonacid reflux, specifically nonacid pepsin, contributes to carcinogenesis in the larynx. Given the carcinogenic potential of pepsin and inefficacy of PPIs to prevent EAC, the presence and effect of pepsin in the esophagus should be investigated.
Methods
Normal and Barrett’s biopsies from eight Barrett’s esophagus patients were collected for pepsin analysis via Western blot and RT-PCR. Human esophageal cells cultured from healthy patients were treated with pepsin (0.01-1mg/ml; 1-20hours), acid (pH4) +/− pepsin (5minutes); real-time RT-PCR, ELISA and cell migration were assayed.
Results
Pepsin was detected in all eight Barrett’s, and four of eight adjacent normal specimens. Pepsinogen mRNA was observed in two Barrett’s, but not in normal adjacent samples. Pepsin induced PTSG2 (COX-2) and IL1β expression and cell migration in vitro.
Conclusions
Pepsin is synthesized by metaplastic, Barrett’s esophageal mucosa. Nonacid pepsin increases metrics of tumorigenicity in esophageal epithelial cells in vitro. These findings implicate refluxed and locally synthesized pepsin in development and progression of EAC and, in part, explain the inefficacy of PPIs in prevention of EAC.
Keywords: Barrett’s esophagus, adenocarcinoma of esophagus, pepsin, reflux, proton pump inhibitors, gastroesophageal reflux
INTRODUCTION
Esophageal adenocarcinoma (EAC) boasts the fastest rising incidence of any cancer in the United States.1 With an average 5-year survival rate of only 17% it ranks seventh among all cancers in mortality and is one of few cancers contributing to increasing death rates among males in the US.2 The mortality of EAC is attributed not only to its aggressive nature, but to failed screening indicators. Patient selection for endoscopic screening is currently based on severity of classical gastroesophageal reflux disease (GERD) symptoms (e.g. heartburn, regurgitation, or dysphagia). These criteria succeed in identifying only 5% of EAC patients.3 Widespread use of proton pump inhibitors (PPIs), which provide symptom relief without reducing reflux of other noxious gastric contents, may be responsible for the disparity between symptom severity and EAC. This class of antisecretory therapy is the third highest selling drug category in the United States, accounting for more than 113 million prescriptions annually, including many prescriptions for infants, and sales exceeding $14 billion. Up to 70 percent of PPI use is for unapproved indications and patients often continue therapy for extended durations without an end point.4-6 Yet, rather than having an advantageous effect on EAC, ever-growing PPI use is associated with a parallel rise in EAC incidence—an increase of more than 460% since the 1970’s when acid suppression medication became the world’s first billion dollar drug.7
In a recent study, endoscopic examination of 769 patients with GERD or laryngopharyngeal reflux (LPR) revealed a positive correlation between PPI use and increased odds for the presence of esophageal adenocarcinogenesis.8 Further, study subjects taking PPIs were more likely to have esophageal adenocarcinogenesis if they reported mild to no symptoms. A study of 9883 patients in Denmark similarly found that high-adherence and long-term use of PPIs was associated with significantly increased risk of adenocarcinoma or high-grade dysplasia.9 Antireflux surgery, however, which impedes reflux of all gastric contents, was associated with regression of Barrett esophagus and/or dysplasia.10 These findings not only highlight the potential danger of symptom-masking by PPIs, but suggest that nonacid components of refluxate could be primarily responsible for EAC progression. Bile salts and enzymes have been shown to be more injurious to esophageal mucosal surfaces in the higher pH refluxate characterisitic of patients on PPIs,11,12 and nonacid pepsin has been shown to promote tumorigenesis in models of weak or non- acid reflux that afflicts the upper airways.13-17 The role of pepsin, independent of acid, in EAC development and progression is relatively unknown. Here we aim to investigate the presence of esophageal pepsin in patients with the reflux-attributed metaplastic condition, Barrett’s esophagus (BE), and examine the potential of pepsin to drive inflammatory and carcinogenic changes in esophageal epithelial cells in vitro.
METHODS
Human Biopsy Specimens
For pepsin and pepsinogen analysis, intraoperative pinch biopsy specimens (1-2mm3) were obtained through upper endoscopy during elective general surgery procedures (gastric bypass, sleeve gastrectomy, incisional hernia, Nissen fundoplication) from patients with a previous histological diagnosis of Barrett’s esophagus; Medical College of Wisconsin (MCW) Institutional Review Board (IRB) PRO00006838. Biopsies were obtained from Barrett’s esophagus (BE) and neighboring normal tissue. BE and normal specimens were visually identified by the operating physician. Patients previously diagnosed with BE who underwent ablation and subsequently tested BE negative, were excluded.
For cell culture, intraoperative pinch biopsy specimens (1-2mm3) were harvested from the esophagus of patients with no prior history or preoperative symptoms of reflux (GERD-Health Related Quality of Life survey score of 0), and no indication of esophageal malignancy, undergoing surgery for elective general surgery procedures; MCW IRB PRO00004777.
Specimens were placed in saline for transport to the laboratory.
Human Esophageal Epithelial Primary Cell Culture
An in vitro cell culture model was created to facilitate investigation of cell and molecular inflammatory and carcinogenic changes following pepsin exposure. Esophageal epithelial (EE) cells were cultured from biopsy and epithelial phenotype confirmed as described previously.18 Cells of less than five passages were used for all experiments.
SDS-PAGE/Western Blot
Presence of pepsin and pepsinogen protein in BE and neighboring normal tissue was assayed via SDS-PAGE/Western Blot. Pepsin protein observed in biopsies lacking pepsinogen mRNA and protein could be presumed to be of gastric origin, i.e. deposited during a reflux event. Pepsin observed in biopsies which contained pepsinogen mRNA and protein could be of local and/or gastric origin. Total protein was extracted from specimens as described.19 and quantified by Protein Assay (BioRad, Hercules, CA). 30 µg total protein was run by 10% SDS-PAGE alongside human pepsin 3b (positive control; from human gastric juice as described20; MCW IRB PRO00004759) and human pepsinogen I (negative control; Sigma-Aldrich, St. Louis, MO), transferred to PVDF membrane (GE Healthcare, Piscataway, NJ) and probed with a mouse monoclonal antibody developed against residues 296-311 of human pepsinogen A (SwissProt P00790) by Promab (Richmond, CA) with a limit of detection of 0.2ng pepsin 3b and 100ng pepsinogen I via SDS-PAGE/Western blot. Blots were incubated with peroxidase-conjugated secondary antibody (P0447/P0448, Dako, Copenhagen, Denmark) and Western Blotting Luminol Reagent (Santa Cruz Biotechnology, Santa Cruz, CA) and signal detected by x-ray film. All antibodies were diluted in phosphate buffered saline (PBS), 0.1% Tween-20, and 5% nonfat dry milk. Rabbit anti-pepsin antibody (sc-99081, Santa Cruz Biotechnology) with greater affinity for pepsinogen relative to pepsin was used for detection of pepsinogen protein.
Pepsinogen RT-PCR
RNA was extracted from esophageal biopsies using TRIZOL (Life Technologies), cleaned and DNAsed (RNeasy Mini Kit with DNase, Qiagen, Germantown, MD). Reverse transcription was performed on 250ng esophageal biopsy or gastric RNA (Agilent Technologies, Santa Clara, CA) using oligo d(T) primers (Superscript III Reverse Transcription kit, Life Technologies). Pepsinogen A was amplified (forward: ACCGTGGACAGCATCACCATG, reverse: TCTTCCTGGGAGGTGGCTG) with reaction conditions of 5 minutes at 95ºC, 30cycles of: 30 seconds at 94ºC, 30 seconds at 62ºC, 30 seconds at 72ºC, and 5 minutes at 72ºC. Hypoxanthine-guanine phosphoribosyltransferase 1 (HPRT1), was amplified as a positive control (forward: TGCTCGAGATGTGATGAAGG, reverse: CCTGACCAAGGAAAGCAAAG) with the following difference in reaction conditions (35cycles, 55ºC annealing). Primers spanned >100bp introns. Amplicon was separated on 2% agarose alongside 50-1000bp DNA Marker (Cambrex, East Rutherford, NJ).
Immunohistochemistry
Immunohistochemistry was used to confirm pepsin protein presence in BE and absence in neighboring normal tissue. Esophageal biopsies were fixed in formalin, paraffin-embedded, sectioned to 4um and mounted to glass slides. Following deparaffinizing, antigen retrieval was performed on PT Link (Dako) at 97°C for 20 minutes. Immunohistochemistry with mouse anti-pepsin antibody, peroxidase-conjugated secondary antibody (Dako), diaminobenzidine, and hematoxylin was performed on the Autostainer Plus using the EnVision™ FLEX High pH Detection Kit (Dako). Images were collected on a Nikon Eclipse Ti using NIS Elements software (Nikon, Tokyo, Japan).
IL1β ELISA
IL1β, a cytokine involved in chronic inflammation and cancer, was assayed in pepsin-treated and control EE cells to determine whether nonacid pepsin exposure could induce the IL1β cancer-related signaling pathway. EE cells were grown to 75% confluence and treated in duplicate with porcine pepsin (0.01mg/ml; Sigma-Aldrich) in normal growth media for one or twenty hours, or normal growth media without pepsin for 20 hours (control). Culture supernatants were collected and assayed in duplicate using Human IL-1 beta/IL-1F2 Quantikine ELISA Kit (R & D Systems, Minneapolis, MN). Student’s t-test was used to determine statistical significance.
PTGS2 (COX-2) Real time PCR
Gene expression of PTGS2, which is positively regulated by IL1β and associated with chronic inflammation, cancer, and EAC prognosis, was assayed in pepsin and/or acid -treated and control EE cells to determine whether nonacid pepsin exposure could induce PTGS2 expression, potentially as a result of activation of the IL1β cancer-related signaling pathway, and the degree to which it did relative to acid and acidified pepsin. EE cells were grown to 75% confluence and treated in five replicates with porcine pepsin (0.01mg/ml) in normal growth media (pH7.4) for one or twenty hours, growth media at pH4 with or without porcine pepsin (0.01mg/ml) for five minutes, or normal growth media without pepsin for 20 hours (control). Cells were rinsed with PBS. RNA was harvested using TRIZOL. 150ng RNA was reverse transcribed using Superscript VILO Synthesis Kit (Life Technologies) and PCR performed using Taqman gene expression assays (HPRT: Hs02800695_m1 and PTGS2 (COX-2): Hs00153133_m1) in a Viia7 real-time PCR instrument (Life Technologies). Ct values >35 were used for analysis. Ct values were normalized to the housekeeper (HPRT1). Replicated delta-Ct values within a plate were averaged. Averaged values from all genes were analyzed jointly in a mixed effects model with fixed group, gene, and group-by-gene interaction effects and random plate and sample effects. After finding a significant gene-by-group interaction (p<0.0001), slice F-tests were performed to compare groups within each gene, followed by Tukey’s HSD adjusted pairwise comparisons. Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
Cell Migration Assay
The scratch, or wound-healing, assay is a commonly used test of tumorigenicity which assesses the rate of repopulation of a cell-free area as a function of cell migration and proliferation. To determine the effect of nonacid pepsin, relative to acidified pepsin or acid, on wound healing as a measure of cell migration and proliferation EE cells were grown to 100% confluence on a 96-well Essen BioScience ImageLock Plate (Essen Bioscience, Ann Arbor, Michigan) at 37°Cwith 5% CO2. In twelve technical replicates, cells were either untreated or pretreated with growth media at pH4 with or without porcine pepsin (0.01mg/ml) for five minutes, rinsed with PBS and replaced in normal growth media. Consistent wounds were made using a WoundMaker (Essen Bioscience). Wells were rinsed with PBS and normal growth media or growth media with porcine pepsin (0.1 or 1mg/ml) was replaced for the duration of the assay. The plate was placed in an IncuCyte™ FLR incubator and live-cell imaging system (Essen Bioscience) for data collection at 2 hour time intervals over 24 hours. Essen Bioscience software returned the Relative Wound Density (RWD), corresponding to cell density within, relative to outside, the wound area, and Confluence corresponding to the cell confluence across the well. RWD collected over time was summarized for each well via the area under the curve (AUC) calculated by the trapezoidal rule. The AUC values were compared between groups using one-way ANOVA; pairwise comparisons were adjusted using Tukey’s method. Significance level was set to 5%, (two-sided comparisons). Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Pepsin detection in Barrett’s esophagus patient specimens
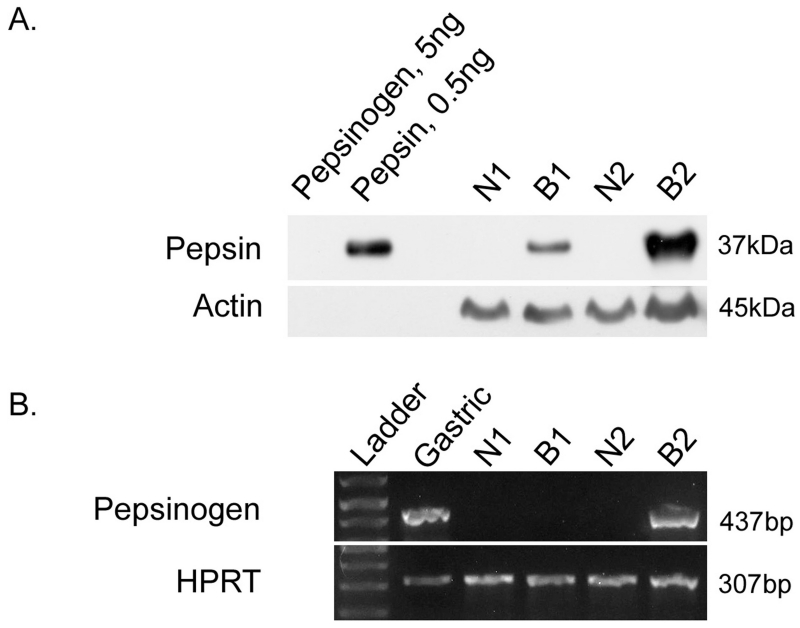
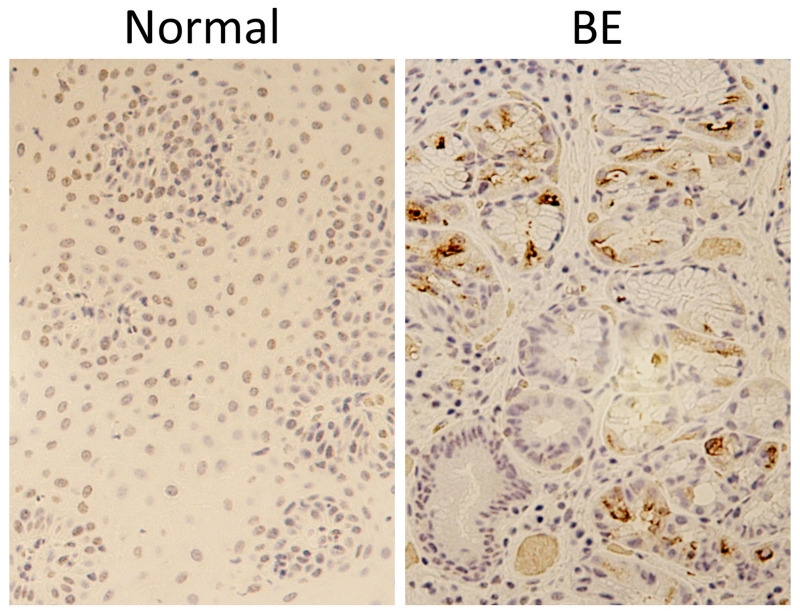
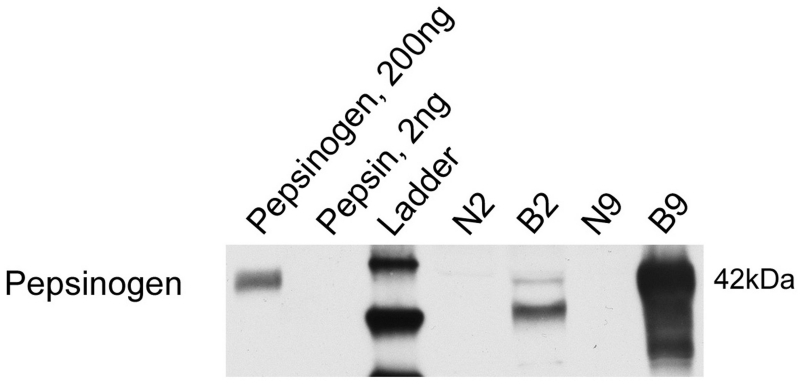
SDS-PAGE/Western blot analysis of BE and neighboring normal specimens demonstrated that pepsin protein was present in all eight Barrett’s specimens and in four of eight adjacent normal specimens analyzed (Table 1 and representative image, Figure 1A). Pepsin protein presence in BE specimens, and absence in a neighboring normal sample, was also demonstrated by immunohistochemistry (Figure 2). Pepsinogen A mRNA was observed in two Barrett’s specimens but was not observed in normal adjacent samples (Figure 1B). Pepsinogen protein was observed in pepsinogen mRNA-positive specimens whereas little to no pepsinogen was detected in neighboring normal tissue (Figure 3).
Table 1.
Pepsin protein and mRNA presence in Barrett’s and neighboring normal mucosa.
| Sample |
Pepsin
Protein |
Pepsinogen
mRNA |
|---|---|---|
|
| ||
| N1 | ND | ND |
| B1 | med | ND |
|
| ||
| N2 | ND | ND |
| B2 | med | present |
|
| ||
| N3 | ND | ND |
| B3 | med | ND |
|
| ||
| N4 | med | ND |
| B4 | high | ND |
|
| ||
| N5 | ND | ND |
| B5 | med | ND |
|
| ||
| N6 | low | ND |
| B6 | med | ND |
|
| ||
| N9 | low | ND |
| B9 | high | present |
|
| ||
| N10 | high | ND |
| B10 | med | ND |
N=Neighboring normal, B=Barrett’s epithelium
ND=not detected, med=medium
Figure 1. Pepsin protein and transcript were observed in Barrett’s epithelium.
(A) Thirty micrograms (ug) esophageal biopsy lysate was analysed alongside human pepsin 3b and pepsinogen I (positive and negative controls, respectively) via SDS-PAGE/Western blot. Western blot was performed for both pepsin and actin (positive control). (B) RT-PCR was performed on Barrett’s specimens to investigate local pepsin synthesis in Barrett’s tissues. Human gastric cDNA template and hypoxanthine-guanine phosphoribosyltransferase 1 (HPRT) were used as a positive controls. Amplicon corresponding to pepsinogen A (437bp) was detected in human gastric tissue and one of the two Barrett’s esophagus samples. HPRT was detected in all samples (307bp).
Figure 2. Pepsin immunohistochemistry demonstrates pepsin in Barrett’s epithelium but not in neighboring normal control tissue.
Pepsin immunohistochemistry (brown) was performed on formalin-fixed, paraffin embedded biopsies from BE and neighboring normal tissue from a BE patient. Hematoxylin (purple) was used as counter-stain.
Figure 3. Pepsinogen protein was observed in pepsinogen mRNA-postivie BE specimens.
SDS-PAGE/Western blot was performed on 30ug lysate of biopsies from Barrett’s epithelium (B2, B9) and neighboring normal tissue (N2, N9) from patients whose BE specimens tested positive for pepsinogen mRNA. Pepsinogen protein was observed in BE specimens, whereas little to no pepsinogen was observed in neighboring normal tissue.
In vitro analyses: IL1β and PTGS2/COX-2 expression and cell migration
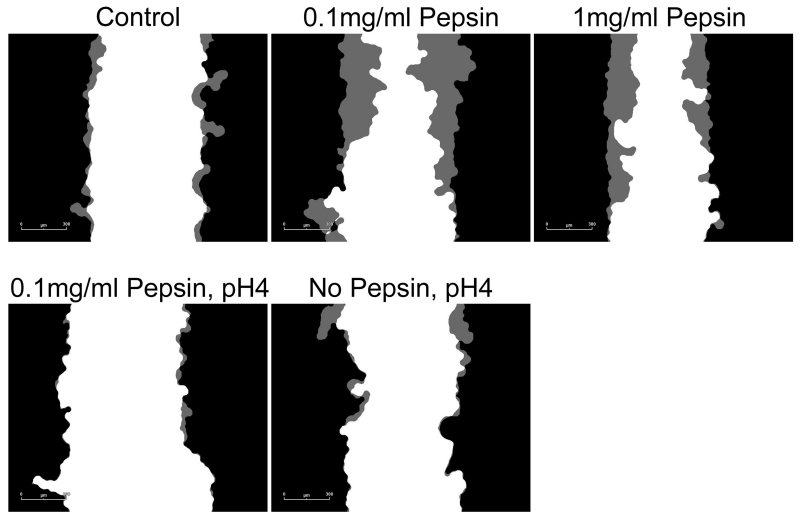
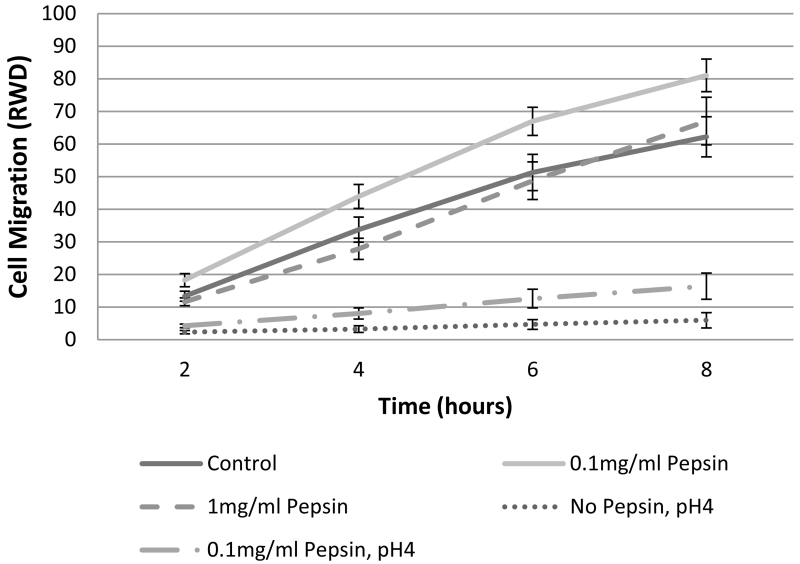
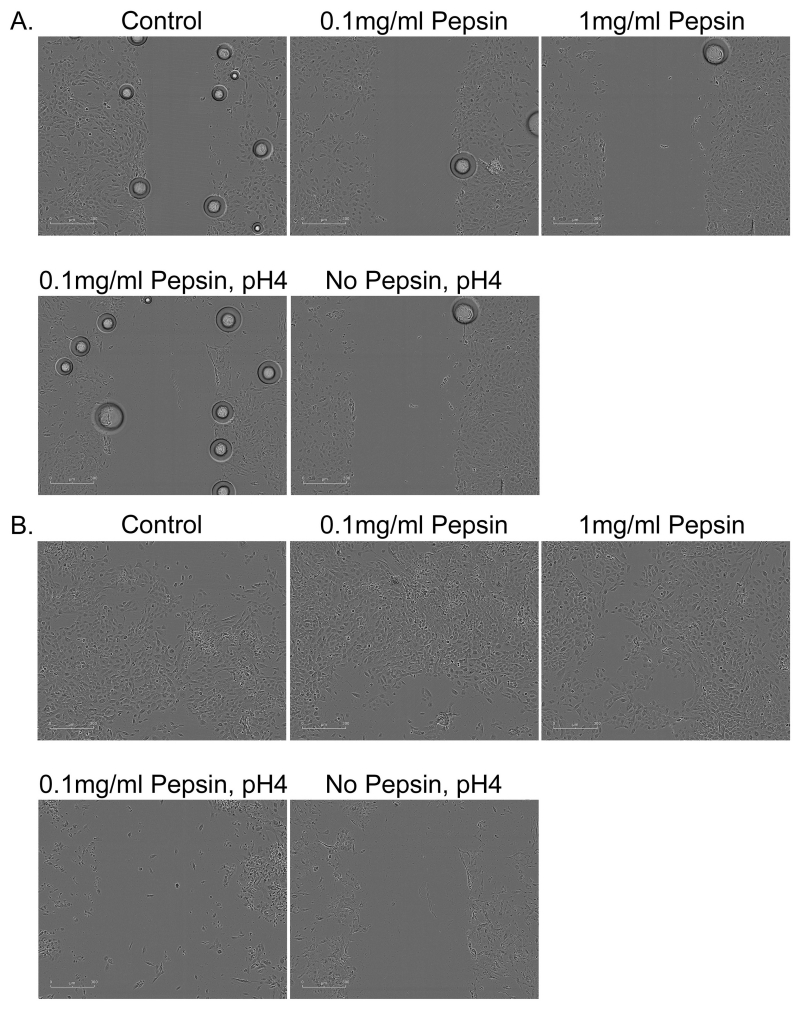
Concentrations of IL1β in cell culture supernatants of human esophageal primary cell cultures were 9.65 ± 1.26pg/ml in control or untreated cells, 19.18 ± 0.62pg/ml in cells treated with 0.1mg/ml pepsin for 1 hour, and 20.43 ± 0.54 in cells treated with 0.1mg/ml pepsin for 20 hours. Elevation in IL1β was statistically significant (p<0.05, Table 2). PTGS-2/COX-2 was elevated approximately 2-fold in all treatment conditions (p<0.05; Table 2). RWD metric of cell migration demonstrated a linear increase for approximately the first eight hours of observation in most conditions (Figure 4). Area under the curve analysis demonstrated that only 0.1mg/ml pepsin treatment induced a significant increase in RWD (Table 3 and 4). Pepsin treatment at 1mg/ml produced no change in RWD relative to control condition. Acid and combined acid and pepsin treatment significantly reduced RWD. Significant cell death was observed in acid and combined acid and pepsin treatment conditions (Table 5 and Figure 5), whereas cells in other conditions appeared healthy throughout the assay duration.
Table 2.
Pepsin-mediated elevation of IL1β and PTGS2 (COX-2) in esophageal cells in vitro.
| Fold Change* | p-value | |
|---|---|---|
|
|
||
| IL1β protein expression | ||
|
| ||
| 0.1mg/ml Pepsin (1hr) | 1.99 | 0.0211 |
| 0.1mg/ml Pepsin (20hr) | 2.12 | 0.0159 |
| PTGS2 (COX-2) gene expression | ||
|
| ||
| 0.1mg/ml Pepsin (1hr) | 2.06 | 0.0006 |
| 0.1mg/ml Pepsin (20hr) | 1.96 | 0.0018 |
| 0.1mg/ml Pepsin, pH4 (5min) | 1.98 | 0.0013 |
| No Pepsin, pH4 (5min) | 2.11 | 0.0003 |
Relative to Control
Figure 4. Esophageal cell migration in vitro is impeded by acid or acid/pepsin treatment, but enhanced by pepsin treatment.
Wound healing, or cell migration, was measured in confluent cultures of esophageal epithelial cells cultured from human biopsy via scratch assay. All pepsin alone (no acid) treatment conditions were initiated post-scratch and extended throughout the migration assay observation period; all pH4 treatments were limited to five minutes immediately prior to scratch because of the highly damaging nature of acid treatment. Cell migration into the area of the wound was monitored using an Essen Bioscience Live Cell Imaging System which provided (A) masks of the border of the initial wound (black) and cell migration progress (gray; 6 hours post-scratch shown, scale bar=300um), and (B) Relative Wound Density (RWD) as a metric of cell migration.
Table 3.
Tukey grouping of esophageal cell migration in vitro.
| Condition |
Area Under
Curve* |
Tukey
Group** |
|---|---|---|
| Control | 160.47 | B |
| 0.1mg/ml Pepsin | 210.07 | A |
| 1mg/ml Pepsin | 155.18 | B |
| 0.1mg/ml Pepsin, pH4 | 41.20 | C |
| No Pepsin, pH4 | 16.14 | C |
Based on Least Squares Means (α=0.05)
Conditions within same group are not signficantly different
Table 4.
Migration of esophageal cells following exposure to pepsin, acid or acid and pepsin in vitro.
| Condition |
Δ Area
Under Curve |
p-value |
|---|---|---|
| Relative to Control | ||
|
| ||
| 0.1mg/ml Pepsin | 49.60 | 0.0399 |
| 1mg/ml Pepsin | −5.29 | 0.9982 |
| 0.1mg/ml Pepsin, pH4 | −119.28 | <0.0001 |
| No Pepsin, pH4 | −144.34 | <0.0001 |
| Relative to 0.1mg/ml Pepsin | ||
|
| ||
| 1mg/ml Pepsin | −54.89 | 0.0161 |
| 0.1mg/ml Pepsin, pH4 | −168.88 | <0.0001 |
| No Pepsin, pH4 | −193.94 | <0.0001 |
| Relative to No Pepsin, pH4 | ||
|
| ||
| 0.1mg/ml Pepsin, pH4 | 25.05 | 0.6155 |
Table 5.
Well confluence at 30minute and 24hour post-acid treatment of esophageal cells in vitro.
| Condition |
Mean Well
Confluence |
Ratio* | p-value |
|---|---|---|---|
| Time = 30 minutes | |||
|
| |||
| Control | 51.76 | ||
| 0.1mg/ml Pepsin | 54.65 | 1.06 | 0.2239 |
| 1mg/ml Pepsin | 53.28 | 1.03 | 0.5114 |
| 0.1mg/ml Pepsin, pH4 | 40.55 | 0.78 | 0.0002 |
| No Pepsin, pH4 | 50.48 | 0.98 | 0.6339 |
| Time = 24 hours | |||
|
| |||
| Control | 79.11 | ||
| 0.1mg/ml Pepsin | 72.55 | 0.92 | 0.107124 |
| 1mg/ml Pepsin | 78.42 | 0.99 | 0.858228 |
| 0.1mg/ml Pepsin, pH4 | 39.58 | 0.50 | 4.25E-09 |
| No Pepsin, pH4 | 41.20 | 0.52 | 9.61E-10 |
Relative to Control
Figure 5. Migration assay 30 minutes and 24 hours following 5 minute acid or acid and pepsin exposure.
Esophageal cells (A) 30 minutes and (B) 24 hours after assay start. Cells were treated with pepsin alone throughout the duration of the assay; acid (pH4) treatment was limited to 5 minutes pretreatment prior to assay start at which time cells were rinsed and normal growth media was replaced. Bubbles observed in (A) are a normal product of media transfer that disappeared by subsequent image collection 2hours post-treatment.
DISCUSSION
The role of pepsin is typically considered secondary to acid in the pathophysiology of GERD, as evidenced by the exclusively acid-focused treatment paradigm of the last four decades. However, in the context of weak or non- acid reflux that afflicts the proximal airways, nonacid pepsin has been shown to activate inflammatory and carcinogenic signaling pathways, promote hyperproliferation, cell migration and anchorage-independent growth, and increase tumor volume in a hamster buccal pouch model.13-17, 21 Although the exact mechanism by which refluxed nonacid pepsin elicits such adverse effects is unknown, it has been shown that in a nonacid environment, pepsin’s enzymatic activity is neutralized allowing for interaction with a cell surface receptor, uptake via receptor-mediated endocytosis, and storage for up to 12 hours in intracellular vesicles that become increasingly acidic, generating conditions that would restore its proteolytic activity.15,22 Acid suppression pharmacotherapy does not reduce the frequency or number of reflux events, merely the acidity of such events,23 therefore the exposure of the esophagus to nonacid components of refluxate is not reduced in patients taking PPIs. In previous work, we have shown that esophageal epithelial cells exhibit receptor mediated endocytosis and intracellular storage of pepsin when exposed in a pH neutral environment.15 We did not, however, investigate whether endocytosed pepsin in esophageal cells produced inflammatory and carcinogenic effects similar to that observed in upper airway cells and tissues.
In the study described herein, pepsin was observed in the esophageal mucosa of patients with the reflux-associated preneoplastic condition, Barrett’s epithelium. Protein and mRNA corresponding to the pepsin precursor, pepsinogen, was observed in some Barrett’s epithelium specimens as well, revealing potential local production in addition to deposition of gastric pepsin following a reflux event. Pepsinogen mRNA was not observed in any neighboring normal specimens. These results corroborate previous studies demonstrating the presence of chief cells and pepsinogen mRNA and protein production in BE24-27 and the absence of pepsin protein in esophageal biopsies of pH confirmed reflux-free control subjects.28 Although historically it was concluded that local pepsin synthesis in Barrett’s epithelium did not pose a significant risk in terms of disease progression, advances made in the field over the last decade regarding the contribution of nonacid pepsin to inflammation and carcinogenesis strongly suggest otherwise.
To investigate the mechanisms by which nonacid pepsin might elicit esophageal epithelial cell damage, a primary culture of human esophageal epithelial cells was treated with nonacid pepsin and metrics of inflammation and carcinogenesis were examined. In this study, IL-1β protein expression, PTGS2 (COX-2) gene expression, and cell migration were elevated in cells treated with pepsin. The proinflammatory cytokine, IL-1β, is consistently upregulated in BE29 and induces spontaneous carcinogenesis of the esophagus in a transgenic mouse model. PTGS2/COX-2, which is positively regulated by IL-1β, is a prognostic marker of EAC30 and candidate target for medical therapy. COX-2 inhibitors have been shown to abrogate cell proliferation in EAC cells in vitro,31 reduce tumor incidence in animal models of EAC,32 and slow Barrett’s epithelium cell proliferation in a clinical trial.33 Interestingly, an epidemiologic study demonstrated that long-term PPI therapy has no advantageous effect on COX-2 expression34 suggesting that nonacid components of reflux may be responsible for continued dysregulation of COX-2 in patients taking PPIs.
In experiments described herein, PTGS2/COX-2 was elevated approximately 2-fold not only in cells treated with pepsin, but in cells treated with acid and combined acid and pepsin as well. In contrast, cell migration was enhanced by pepsin but impeded by brief exposure to acid or acid and pepsin. The difference in response may reflect the resting period of cells between acid treatment and assay: cells were harvested immediately post-treatment for PTGS2/COX-2 analysis whereas cell migration was measured starting two hours post-exposure. A significant reduction in cell number (relative to control) was observed in the combined acid and pepsin treated condition as soon as 30 minutes following treatment, becoming more extreme and evident in acid-only treated cells by 24 hours post-treatment. In previous work, we have found that even brief acid or acid and pepsin treatment incurs cell death in airway epithelial cells in vitro (unpublished) whereas pepsin treatment results in hyperproliferation and promotion of markers of inflammation and carcinogenesis. While other authors have reported an antiproliferative effect of acid on Barrett’s cancer cells and on Barrett’s mucosal explants, 35,36 this study is the first to show an antiproliferative effect of acid on normal esophageal cells. The implication of this finding, which is supported by the association of PPI use with EAC, is that nonacid reflux, and refluxed nonacid pepsin in particular, may present a greater threat of carcinogenesis than does acid.
Additional research is anticipated to address limitations of this study. First, BE specimens were identified as such only by visual inspection of the operating surgeon. Histochemical analysis of each biopsy by a qualified pathologist would be required to verify that the region used for molecular studies was indeed BE. Therefore, the absence of pepsinogen message in some BE specimens may be false negatives rather than an indication of differences between patients. Further, given the small study size, no useful correlation could be drawn between clinical measures of disease and levels of pepsin/pepsinogen protein or mRNA. In future studies, a larger population will be recruited from whom data such as pathological grading, pH-manometry testing, and prognostic indicators will be obtained. As not all Barrett’s epithelium progresses to adenocarcinoma, such in-depth study should help determine the utility of phenotypic traits, such as local pepsin production, as prognostic indicators.
CONCLUSION
The rapid and concomitant rise of EAC with PPI usage over the last 40 years clearly illustrates the failure of the current acid-targeting treatment paradigm to prevent reflux-attributed carcinogenesis and implicates nonacid reflux in development and progression of EAC. The study described herein demonstrates the presence of pepsin of both gastric and local origin in preneoplastic human esophageal mucosa and the capacity of nonacid pepsin to alter markers of inflammation and carcinogenesis in esophageal cells in vitro. These data may, in part, explain the risk of EAC associated with long-term PPI use and lend support for the use of more comprehensive strategies for prevention of reflux-attributed disease.
Acknowledgments
The authors would like to thank Aniko Szabo and Shi Zhao of the Medical College of Wisconsin (MCW) Division of Biostatistics for assistance with data analysis.
This study was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health Grant Number 8UL1TR000055, and by the MCW Department of Otolaryngology and Communication Sciences.
Footnotes
Declaration of Conflicting Interests
Matthew I Goldblatt, MD, is a speaker and consultant for GORE and Covidien. He is also a consultant and receives research funding from Davol. Jon Gould, MD, serves on the medical advisory board for Torax Medical.
REFERENCES
- 1.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376–86. doi: 10.1053/j.gastro.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y. Epidemiology of esophageal cancer. World Journal of Gastroenterology. 2013;19(34):5598–5606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122(1):26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 4.Choudhry MN, Soran H, Ziglam HM. Overuse and inappropriate prescribing of proton pump inhibitors in patients with Clostridium difficile-associated disease. QJM. 2008;101(6):445–448. doi: 10.1093/qjmed/hcn035. [DOI] [PubMed] [Google Scholar]
- 5.Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203–1209. doi: 10.1111/j.1365-2036.2005.02454.x. [DOI] [PubMed] [Google Scholar]
- 6.Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261–1266. doi: 10.1345/aph.1G703. [DOI] [PubMed] [Google Scholar]
- 7.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100(16):1184–7. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nason KS, Wichienkuer P, Awais O, Schuchert MJ, Luketich JD, O’Rourke RW, Hunter JG, Morris CD, Jobe BA. Gastroesophageal Reflux Disease Symptom Severity, Proton Pump Inhibitor Use, and Esophageal Carcinogenesis. Arch Surg. 2011;146(7):851–858. doi: 10.1001/archsurg.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hvid-Jensen F, Pedersen L, Funch-Jensen P, Drewes AM. Proton pump inhibitor use may not prevent high-grade dysplasia and oesophageal adenocarcinoma in Barrett’s oesophagus: a nationwide study of 9883 patients. Aliment Pharmacol Ther. 2014;39(9):984–91. doi: 10.1111/apt.12693. [DOI] [PubMed] [Google Scholar]
- 10.Hunter JG. Antireflux Surgery for Dysplastic Barrett’s Esophagus. World J Surg. 2015;39(3):595–6. doi: 10.1007/s00268-014-2817-3. [DOI] [PubMed] [Google Scholar]
- 11.Burnat G, Rau T, Elshimi E, Hahn EG, Konturek PC. Bile acids induce overexpression of homeobox gene CDX-2 and vascular endothelial growth factor (VEGF) in human Barrett’s esophageal mucosa and adenocarcinoma cell line. Scand J Gastroenterol. 2007;42(12):1460–1465. doi: 10.1080/00365520701452209. [DOI] [PubMed] [Google Scholar]
- 12.Kazumori H, Ishihara S, Rumi MAK, Kadowaki Y, Kinoshita Y. Bile acids directly augment caudal related homeobox gene Cdx2 expression in oesophageal keratinocytes in Barrett’s epithelium. Gut. 2006;55(1):16–25. doi: 10.1136/gut.2005.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly EA, Samuels TL, Johnston N. Chronic pepsin exposure promotes anchorage-independent growth and migration of a hypopharyngeal squamous cell line. Otolaryngol Head Neck Surg. 2014;150(4):618–24. doi: 10.1177/0194599813517862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston N, Yan JC, Hoekzema CR, Samuels TL, Stoner GD, Blumin JH, Bock JM. Pepsin promotes proliferation of laryngeal and pharyngeal epithelial cells. Laryngoscope. 2012;122(6):1317–25. doi: 10.1002/lary.23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston N, Wells CW, Samuels TL, Blumin JH. Rationale for targeting pepsin in the treatment of reflux disease. Ann Otol Rhinol Laryngol. 2010;119(8):547–58. doi: 10.1177/000348941011900808. [DOI] [PubMed] [Google Scholar]
- 16.Johnston N, Wells CW, Samuels TL, Blumin JH. Pepsin in nonacidic refluxate can damage hypopharyngeal epithelial cells. Ann Otol Rhinol Laryngol. 2009;118(9):677–85. doi: 10.1177/000348940911800913. [DOI] [PubMed] [Google Scholar]
- 17.Pearson JP, Parikh S, Orlando RC, Johnston N, Allen J, Tinling SP, Johnston N, Belafsky P, Arevalo LF, Sharma N, Castell DO, Fox M, Harding SM, Morice AH, Watson MG, Shields MD, Bateman N, McCallion WA, van Wijk MP, Wenzl TG, Karkos PD, Belafsky PC. Review article: reflux and its consequences--the laryngeal, pulmonary and oesophageal manifestations. Conference held in conjunction with the 9th International Symposium on Human Pepsin (ISHP) Kingston-upon-Hull, UK, 21-23 April 2010. Aliment Pharmacol Ther. 2011;33(Suppl 1):1–71. doi: 10.1111/j.1365-2036.2011.04581.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee SH, Samuels T, Bock JM, Blumin JH, Johnston N. Establishment of an immortalized laryngeal posterior commissure cell line as a tool for reflux research. Laryngoscope. 2015;125(2):E73–7. doi: 10.1002/lary.24952. doi: 10.1002/lary.24952. [DOI] [PubMed] [Google Scholar]
- 19.Axford SE, Sharp N, Ross PE, et al. Cell biology of laryngeal epithelial defenses in health and disease: preliminary studies. Ann Otol Rhinol Laryngol. 2001;110:1099–1108. doi: 10.1177/000348940111001203. [DOI] [PubMed] [Google Scholar]
- 20.Crapko M, Kerschner JE, Syring M, et al. Role of extra-esophageal reflux in chronic otitis media with effusion. Laryngoscope. 2007;117:1419–1423. doi: 10.1097/MLG.0b013e318064f177. [DOI] [PubMed] [Google Scholar]
- 21.Samuels TL, Johnston N. Pepsin as a causal agent of inflammation during nonacidic reflux. Otolaryngol Head Neck Surg. 2009;141(5):559–63. doi: 10.1016/j.otohns.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Johnston N, Wells CW, Blumin JH, Toohill RJ, Merati AL. Receptor-mediated uptake of pepsin by laryngeal epithelial cells. Ann Otol Rhinol Laryngol. 2007;116(12):934–8. doi: 10.1177/000348940711601211. [DOI] [PubMed] [Google Scholar]
- 23.Vela MF, Camacho-Lobato L, Srinivasan R, Tutuian R, Katz PO, Castell DO. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology. 2001;120(7):1599–1606. doi: 10.1053/gast.2001.24840. [DOI] [PubMed] [Google Scholar]
- 24.Pals G, Eriksson AW, Pronk JC, Frants RR, Klinkenberg-Knol EC, Bosma A, Westerveld BD, Taggart RT, Samloff IM, Meuwissen SG. Differential expression of pepsinogen isozymogens in a patient with Barrett esophagus. Clin Genet. 1988;34(2):90–7. doi: 10.1111/j.1399-0004.1988.tb02842.x. [DOI] [PubMed] [Google Scholar]
- 25.Mangla J. Acid and pepsin production by Barrett’s epithelium: role of radionuclide imaging in diagnosis. In: Spechler SJ, Goyal RK, editors. Barrett’s Esophagus: Pathophysiology, Diagnosis and Management. Elsevier; New York: 1985. pp. 49–57. [Google Scholar]
- 26.Kahrilas P. What is the cell line giving rise to the columnar mucosa? The Esophageal Mucosa: Epidemiology. In: Giuli Robert, Tytgat Guido N.J., DeMeester Tom R., Galmiche Jean-Paul., editors. Proceedings from the World Organization for Specialized Studies on Diseases of the Esophagus. 2004. p. 186. [Google Scholar]
- 27.Antoniolo D. Are parietal and chief cells present in Barrett’s esophagus, and are such cells functional? The Esophageal Mucosa: Mucosa and Histology. In: Giuli Robert, Tytgat Guido N.J., DeMeester Tom R., Galmiche Jean-Paul., editors. Proceedings from the World Organization for Specialized Studies on Diseases of the Esophagus. 2004. p. 208. [Google Scholar]
- 28.Johnston N, Dettmar PW, Lively MO, Postma GN, Belafsky PC, Birchall M, Koufman JA. Effect of pepsin on laryngeal stress protein (Sep70, Sep53, and Hsp70) response: role in laryngopharyngeal reflux disease. Ann Otol Rhinol Laryngol. 2006;115(1):47–58. doi: 10.1177/000348940611500108. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald RC, Abdalla S, Onwuegbusi BA, Sirieix P, Saeed IT, Burnham WR, et al. Inflammatory gradient in Barrett’s oesophagus: implications for disease complications. Gut. 2002;51:316–22. doi: 10.1136/gut.51.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M, Huang J, Zhu Z, Zhang J, Li K. Systematic review and meta-analysis of tumor biomarkers in predicting prognosis in esophageal cancer. BMC Cancer. 2013;13:539. doi: 10.1186/1471-2407-13-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souza RF, Shewmake K, Beer DG, Cryer B, Spechler SJ. Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res. 2000;60(20):5767–72. [PubMed] [Google Scholar]
- 32.Oyama K, Fujimura T, Ninomiya I, Miyashita T, Kinami S, Fushida S, Ohta T, Koichi M. A COX-2 inhibitor prevents the esophageal inflammation-metaplasia-adenocarcinoma sequence in rats. Carcinogenesis. 2005;26(3):565–70. doi: 10.1093/carcin/bgh340. [DOI] [PubMed] [Google Scholar]
- 33.Kaur BS, Khamnehei N, Iravani M, Namburu SS, Lin O, Triadafilopoulos G. Rofecoxib inhibits cyclooxygenase 2 expression and activity and reduces cell proliferation in Barrett’s esophagus. Gastroenterology. 2002;123(1):60–7. doi: 10.1053/gast.2002.34244. [DOI] [PubMed] [Google Scholar]
- 34.Lao-Sirieix P1, Roy A, Worrall C, Vowler SL, Gardiner S, Fitzgerald RC. Effect of acid suppression on molecular predictors for esophageal cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(2):288–93. doi: 10.1158/1055-9965.EPI-05-0528. [DOI] [PubMed] [Google Scholar]
- 35.Feagins LA, Zhang HY, Hormi-Carver K, Quinones MH, Thomas D, Zhang X, Terada LS, Spechler SJ, Ramirez RD, Souza RF. Acid has antiproliferative effects in nonneoplastic Barrett’s epithelial cells. Am J Gastroenterol. 2007;102(1):10–20. doi: 10.1111/j.1572-0241.2006.01005.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang HY, Zhang X, Hormi-Carver K, Feagins LA, Spechler SJ, Souza RF. In non-neoplastic Barrett’s epithelial cells, acid exerts early antiproliferative effects through activation of the Chk2 pathway. Cancer Res. 2007;67(18):8580–7. doi: 10.1158/0008-5472.CAN-07-2023. [DOI] [PubMed] [Google Scholar]