Summary
Background
Alcoholism exacts a toll on brain white matter microstructure, which has the potential of repair with sobriety. Diffusion tensor imaging (DTI) enables in vivo quantification of tissue constituents and localization of tracts potentially affected in alcoholism and its recovery. Extended longitudinal study of alcoholism's trajectory of effect on selective fiber bundles with sustained sobriety or decline with relapse, heretofore, has not been conducted.
Methods
Tract-based spatial statistics (TBSS) quantified white matter integrity throughout the brain in 47 alcoholics and 56 controls examined 2-5 times over 1-8 year intervals. Regions showing group differences were identified with a white matter atlas. For macrostructural comparison, corpus callosum and centrum semiovale volumes were measured on MRI.
Findings
TBSS identified a large cluster (threshold p<.001), where controls showed significant fractional anisotropy (FA) decline with aging and alcoholics had significantly lower FA than controls regardless of age. Over the examination interval, 27 alcoholics abstained, 10 relapsed into light drinking, and 10 relapsed into heavy drinking (>5kg/year). Despite abnormally low FA, age trajectories of the abstainers were positive and progressing toward normality, whereas those of the relapsers and controls were negative. Axial diffusivity (lower values indexing myelin integrity) was abnormally high in the total alcoholic group; however, the abstainers’ slopes paralleled those of controls, whereas the heavy-drinking relapsers’ slopes showed accelerated aging. Callosal genu and body microstructure but not macrostructure exhibited untoward alcoholism effects. Affected projection and association tracts had an anterior and superior distribution.
Interpretation
Return to heavy drinking resulted in accelerating microstructural white matter damage. Despite evidence for damage, alcoholics maintaining sobriety over extended periods showed improvement in brain fiber tract integrity reflective of fiber reorganization and myelin restoration, indicative of a neural mechanism explaining recovery.
Keywords: white matter, brain structure, longitudinal, diffusion tensor imaging, recovery, volume, normal aging
Introduction
Chronic alcoholism characteristically follows a dynamic course with periods of heavy drinking alternating with periods of partial or complete abstinence. Paralleling this course is decline or improvement in functioning and the condition of the brain, depending on myriad factors, including the length of sobriety and age at drinking or abstinence phase. Quantitative studies tracking alcoholism in the human condition must be naturalistic because of investigator inability to control access to alcohol over years. Thus, to date, even longitudinal studies on the effect of continued drinking compared with protracted sobriety in individuals with a history of alcohol dependence are relatively short-term, spanning weeks 1,2, months 3,4, and in only a few cases several years 5. The extent to which the brain continues to improve with prolonged sobriety or continues to decline with continued hazardous drinking has not been examined within the context of normal aging, essential for determining whether age is a relevant factor in recovery and relapse given the potential interaction of aging and alcoholism.
Postmortem studies report a predilection of brain white over gray matter to the neurotoxic effects of chronic alcohol dependence 6; for example, the corpus callosum becomes thin and atrophied 7,8. Callosal and other supratentorial white matter sustains demyelination 9, microtubule disruption 10, and axonal deletion, possibly arising from regional neuronal loss 7,11, which when present is selective to frontal cortex 12. Alcoholism-related white matter degeneration occurs in men and women 13 and is accelerated with aging 12.
Cross-sectional, in vivo neuroimaging studies comport with the postmortem findings in identifying white matter volume deficits and correlations between extent of white matter volume deficit and length of sobriety, suggestive of recovery 4,14,15. Controlled longitudinal studies report decreases in white matter volumes in alcoholics who had been sober for at least one month, but who then relapsed into drinking 16. Frontal white matter volume shrinkage from initial to 6-month followup correlated with the amount of alcohol consumed in the interval 3. Although volume fluctuation with drinking status offers useful leads for where to seek brain changes, MRI studies of white matter macrostructure provide little information about tissue quality and its constituents that might underlie volume change as is afforded with measures of microstructural tissue integrity. Further, in at least one study17, a measure of callosal microstructure but not macrostructure distinguished alcoholic women from controls.
Diffusion tensor imaging (DTI) has been instrumental in enabling the search for white matter microstructural constituents affected in alcoholism. The DTI metric of fractional anisotropy (FA) provides an index of the integrity of microstructural connectivity underlying brain circuitry. Components of the diffusion metric provide indices of axonal integrity, measured as longitudinal diffusivity (λL), and myelin integrity, measured as axial diffusivity (λT) 18. Cross-sectional DTI studies in human alcoholism report abnormally low FA or high diffusivity in commissural, association, and projection fiber tracts 19-21 with anterior and superior fibers more affected than inferior and posterior fibers 19,20. Axial diffusivity, the marker of myelin integrity, is typically sensitive to fiber tract degradation in alcoholics. One of the few longitudinal DTI studies revealed increased FA and decreased λT in abstainers relative to relapsers and to longitudinally-examined controls, who showed no measurable change over 13-16 months from baseline 22. A multi-modal imaging study examined alcoholics in a rehabilitation program after 1 week and 1 month of alcohol abstinence and found smoking to be a factor in FA recovery, although controls were not studied longitudinally 2. The present study examined controls and alcoholics upwards of 8 years.
White matter constituents and architecture, including cytoskeleton, microtubules, oligodendrocytes, and cell body extensions, which are capable of remodeling following injury, are candidate contributors to tissue volume recovery in alcoholism. Herein, we used tract-based spatial statistics (TBSS) to quantify white matter fiber integrity throughout the whole brain in alcoholics and controls examined two to five times over 1- to 8-year intervals. Trajectories of change occurring with age and with abstinence or relapse were modeled with linear mixed-effects statistics. The resulting DTI metrics of FA and diffusivity were also merged with a white matter atlas, which enabled spatial localization and quantification of pathology or improvement in selective, myelinated fiber systems. For comparison of macrostructural and microstructural sensitivity to detection of change, we measured the volume (i.e., macrostructure) of the corpus callosum and a large sample of the centrum semiovale using structural MRI, collected in the same session with DTI. Thus, we tested the following hypotheses: controls would exhibit age-related declines in white matter FA; alcoholics who continued drinking during the study would show steeper declines with aging than controls; and alcoholics who abstained from heavy drinking would show improvement in white matter FA but not necessarily white matter volume.
Methods
Participants and study design
Recruitment
The participants (47 alcoholics and 56 controls) were drawn from a longitudinal, 1.5T DTI database of 841 scans of subjects with a variety of conditions, and some had been included as part of a larger sample for cross-sectional analysis of baseline data 19,20. Alcoholic patients were recruited by referral from local inpatient and outpatient alcohol and drug treatment and detoxification centers (38/47 alcoholics) and by recruitment from the community, largely the greater Bay Area of northern California (9/47). Control participants were recruited by referral from patient participants, Internet posting, flyers, and word of mouth.
Clinical evaluation
Calibrated research clinical psychologists or a research nurse conducted a Structured Clinical Interview for DSM-IV (SCID) 23 to determine that participants met DSM-IV criteria for Alcohol Dependence. At study entry, 25 of 47 alcoholics met all 7 diagnostic criteria, 15 met 6 criteria, 2 met 5 criteria, 4 met 4 criteria, and 1 met 3 criteria; thus none met criteria for Alcohol Abuse. Alcohol and drug history were determined from self-reports obtained during structured clinical interview conducted by our trained and calibrated research staff; these reports comported with previous, independently obtained screening information and with clinical information provided, with permission from the participants, by staff from the recruitment treatment sites. In addition, breathalyzer testing was conducted at each visit.
Formal interview also served to exclude individuals who met lifetime criteria for schizophrenia or bipolar disorder; to identify volunteers meeting criteria for depressive or anxiety disorder; to confirm that prospective controls did not meet criteria for any Axis I disorder, including alcohol or other substance use or abuse disorders or neurological disease not related to alcohol use; and to confirm ability to undergo MRI. Of the 47 alcoholics, 13 had a lifetime history of major depressive disorder (MDD), but there was no significant difference in presence of MDD history between abstainers (7 of 27) and relapsers (6 of 20) (p=.758) in length of MDD remission (abstainers: median=90 weeks; range=4-602 weeks; relapsers: median=160 weeks; range=11-1480 weeks; p=.583). Five abstainers and four relapsers had a history of an anxiety disorder (p=.593).
DTI and MRI acquisition, processing, and quantification
Imaging data were collected on a 1.5T Signa Twin whole-body system with a quadrature head coil (General Electric Healthcare, Waukesha, WI). Image acquisition parameters, quantification, and processing methods for DTI (except TBSS) and MRI analyses are described in Supplemental Material.
Tract-Based Spatial Statistics (TBSS) procedure
The standard registration step of TBSS 24 was replaced with registration and reslicing to SRI24 atlas space coordinates 25. The initial mean FA image and FA skeleton were constructed from all 841 subjects in the laboratory database. The initial TBSS contrast group comparison comprised 374 scans from alcoholics and controls with one or more DTI scans: 101 alcoholics (68 men, 33 women) and 93 age-range matched controls (46 men, 47 women) ranging in age from 20-60 years at first scan. Permutation testing was performed with the FSL “TFCE Randomise” (Threshold-Free Cluster Enhancement) option with positive and negative alcoholic-control contrasts with de-meaned subjects’ age as a covariate and 1000 permutations. Statistical testing was performed with TBSS “corrp” (fully corrected for multiple comparisons across space) option, hereinafter referred to as “tfce_corrp” 24.
Statistical analysis
Dependent-variable trajectories of individual participants were calculated using the lmer function for linear mixed-effects modeling in the lme4 R statistical package [http://www.r-project.org/]. The lmer function also allowed for testing of nested random effects. To examine longitudinal trajectories of DTI metric change independent of age at data acquisition, we computed the trajectory slopes with the participant's age at acquisition entered into the model as the deviation from the mean age of the multiple DTIs available for each individual participant, which we refer to as “age-centered slope.” The degrees of freedom and p-values for the resulting t-values were produced from the ‘lmerTest’ package in R (version 2.0-6) and were based on Satterthwaite's approximation. Trajectory analyses examined DTI metrics using group and mean age as the fixed effects and centered age as the random effect: two-group comparisons of controls to all alcoholics; additional analyses tested pairs of alcoholic subgroups with each other or with controls.
Role of the funding source
This work was supported by grants from the U.S. National Institute on Alcohol Abuse and Alcoholism.
Results
A total of 283 DTI sessions were included in the longitudinal analysis of participants with two or more analyzable scans and ranging in age from 20-60 years at first scan (Table 1): 158 sessions on 56 individuals in the control group and 125 sessions on 47 individuals in the alcoholic group (Figure in Supplementary Information). Of the 47 alcoholics and 56 controls in this longitudinal analysis, 15 alcoholics and 11 controls had 3 MRIs; 5 alcoholics and 7 controls had 4 MRIs; and 2 alcoholics and 7 controls had 5 MRIs. The mean (standard deviation) time between first and last MRI was 2.76 (1.74) years (range=0.96-7.96 years) for alcoholics and 3.20 (2.27) years (range=0.51-8.24 years) for controls.
Table 1.
Baseline demographics of the study groups: mean (SD) or frequency count
| Group Differences | Group Differences | ||||||
|---|---|---|---|---|---|---|---|
| Control | Alcoholic | χ2 or t-test (p≤.05) | Abstainers | Relapsers | χ2 or t-test (p≤.05) | ||
| Sex: M/F | 24/32 | 28/19 | 0.136 | 17/10 | 11/9 | 0.803 | |
| Age (yrs) | 43.0 (10.1) | 44.3 (9.2) | 0.513 | 43.6 (10.2) | 45.1 (7.7) | 0.593 | |
| Education (yrs) | 15.2 (2.3) | 13.4 (2.3) | 0.0001 | 13.5 (2.2) | 13.7 (2.0) | 0.79 | |
| n= | 56 | 47 | 27 | 20 | |||
| Handedness score (RH=14-30; LH=50-70) | 22.9 (11.9) | 28.7 (16.3) | 0.043 | 25.9 (14.4) | 32.4 (18.2) | 0.178 | |
| n= | 54 | 47 | 27 | 20 | |||
| Body mass index | 25.9 (5.3) | 26.5 (3.9) | 0.538 | 27.6 (4.1) | 25.1 (3.2) | 0.0283 | |
| n= | 56 | 47 | 27 | 20 | |||
| Socioeconomic status (lower score=higher statu | 31.1 (13.5) | 37.3 (13.9) | 0.028 | 34.8 (13.9) | 40.9 (13.5) | 0.144 | |
| n= | 50 | 46 | 27 | 19 | |||
| NART IQ | 111.6 (7.4) | 106.8 (8.2) | 0.004 | 106.3 (8.5) | 107.7 (8.0) | 0.607 | |
| n= | 53 | 43 | 26 | 17 | |||
| ALC onset age | — | 25.0 (8.9) | — | 24.3 (10.1) | 23.2 (7.4) | 0.721 | |
| n= | 47 | 21 | 17 | ||||
| Days sober before 1st DTI | — | 326.5 (550.9) | — | 365.5 (560.3) | 249.6 (520.8) | 0.474 | |
| n= | 45 | 27 | 20 | ||||
| Alc consumption before 1st DTI | 36.2 (55.8) | 932.2 (902.7) | 0.0001 | 742.8 (527.5) | 1368.4 (1247.9) | 0.036 | |
| n= | 54 | 47 | 23 | 18 | |||
| Alc consumption during DTI intervals | — | — | 0 | 28.7 | 0.0002 | ||
| 0 | 37.0 | ||||||
| n= | 27 | 20* | |||||
| Smoker (never/current or past) | 41/15 | 10/37 | 0.0001 | 9/18 | 1/19 | 0.047 | |
| Self-Defined Ethnicity | |||||||
| Caucasian | 36 | 29 | 0.031 | 17 | 11 | 0.857 | |
| African American | 10 | 17 | 9 | 8 | |||
| Other | 10 | 1 | 1 | 1 |
The median value of the 10 relapsers with an interim scan quantitative alcohol history was used as the estimated value for the alcoholic without such a history.
By SCID interview at initial visit, 30 (63.8%) of the 47 alcoholics met DSM-IV-TR criteria for abuse or dependence of at least one street drug over their lifetime. The most common drug of abuse was cocaine (76.7%), followed by cannabis (43.3%), and amphetamines (36.7%). All participants with drug abuse/dependence were in remission, except one man who met criteria for current cannabis dependence; excluding this participant, remission ranged from 7-1546 weeks (mean = 325.5 ± 391.9 weeks; median = 121.5 weeks).
Self-reported drinking histories between the first and last DTI were available for 46 of the 47 alcoholics; the one subject without quantitative drinking history did provide relapse information. Alcohol consumption/year was computed over the observation interval, yielding a trimodal distribution of alcoholics: 27 were totally abstinent; 10 were light-drinking relapsers and reported an average of 2.1 kg/year (0.7 - 4.1 kg), with 5 drinking at alcohol abuse levels and 5 drinking at alcohol dependence over the interval; and 10 were heavy-drinking relapsers with greater than 5 kg alcohol consumed per year, reporting an average of 17.3 kg/year (7.6 - 28.8 kg) and all met criteria for Alcohol Dependence over the interval. The light and heavy drinking groups did not differ in number of criteria met for Alcohol Dependence at study entry (p=.740). Of the 10 heavy-drinking relapsers, 6 had past drug abuse/dependence history; of the 10 light-drinking relapsers, 7 had past drug abuse/dependence history and one of these 10 also had current cannabis dependence. Of the 27 abstinent alcoholics, 17 had a past drug abuse/dependence history. One abstainer, five light-drinking relapsers, and one heavy-drinking relapser resumed drug consumption between scans.
TBSS detection of normal aging effects
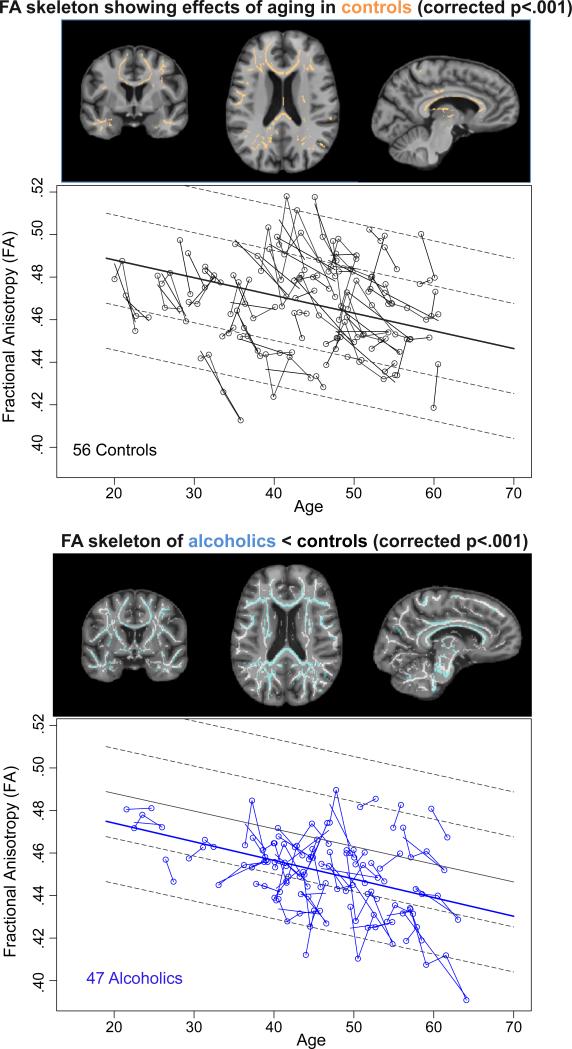
A TBSS testing of aging effects in the control group revealed a large cluster (34,990mm3) of significant FA decline with age (tfce_corrp <.05) (Figure 1, top). Therefore, the alcoholic-control group TBSS comparisons used age as a covariate. The resulting TBSS contrast analysis of the entire alcoholic vs. control group (374 scans) produced a single large, widespread cluster (55,958 voxels at 1mm3, i.e., 55.958cc) with a tfce_corrp<.001 for alcoholic FA less than control FA (Figure 1, bottom), and none in the opposite direction.
Figure 1.
Top pair: Images displaying the FA white matter skeleton of the controls showing significant effects of aging in the controls (corrected p<.001) overlaid in orange on coronal (left), axial (middle), and sagittal (right) SPGR images. The data plots display individual slopes of controls’ data at each DTI (black), where each participant's values are connected over time and the age-centered slope of each participant is overlaid on the longitudinal data points. The solid regression line is the mean and the dotted lines are 1 and 2 standard deviations from the mean of the controls.
Bottom pair: Mean study FA skeleton (white) and alcoholic < control difference (blue) overlaid on coronal (left), axial (middle), and sagittal (right) SPGR images (FA of alcoholics < controls, p<.001). The data plots display individual slopes of alcoholics’ data at each DTI (blue); the solid regression line is the mean and the dotted lines are 1 and 2 standard deviations from the mean of the controls. Note that the regression of the alcoholics is about 0.75 SD lower than that of the controls.
Longitudinal analyses of the TBSS cluster of white matter microstructure measured with DTI
For longitudinal analyses of subjects with two or more scans, identified clusters were conservatively thresholded at tfce_corrp <.001 and applied to each subject's skeleton (i.e., the skeleton values defined by the location of the cluster) for all observations, producing a mean cluster FA for each subject. The mean value of λT for the cluster voxels for each individual for each observation time was also tested for group differences.
Differences between the 56 controls and all 47 alcoholics with multiple scans are presented first, followed by three-group statistics comparing 56 controls, 27 complete abstainers, and 20 relapsers (comprising the 10 light and 10 heavy drinkers).
FA trajectories
Regardless of age, the alcoholics had lower FA than controls (t=−4.48, df=100.6, p=.00001). Both groups showed significant declines in FA with aging (t=−5.84, df=21.4, p=.00001), but the aging slope of the total group of alcoholics was not significantly different from that of the controls (t=.107, df=35.0, p=.294) (Figure 1, bottom).
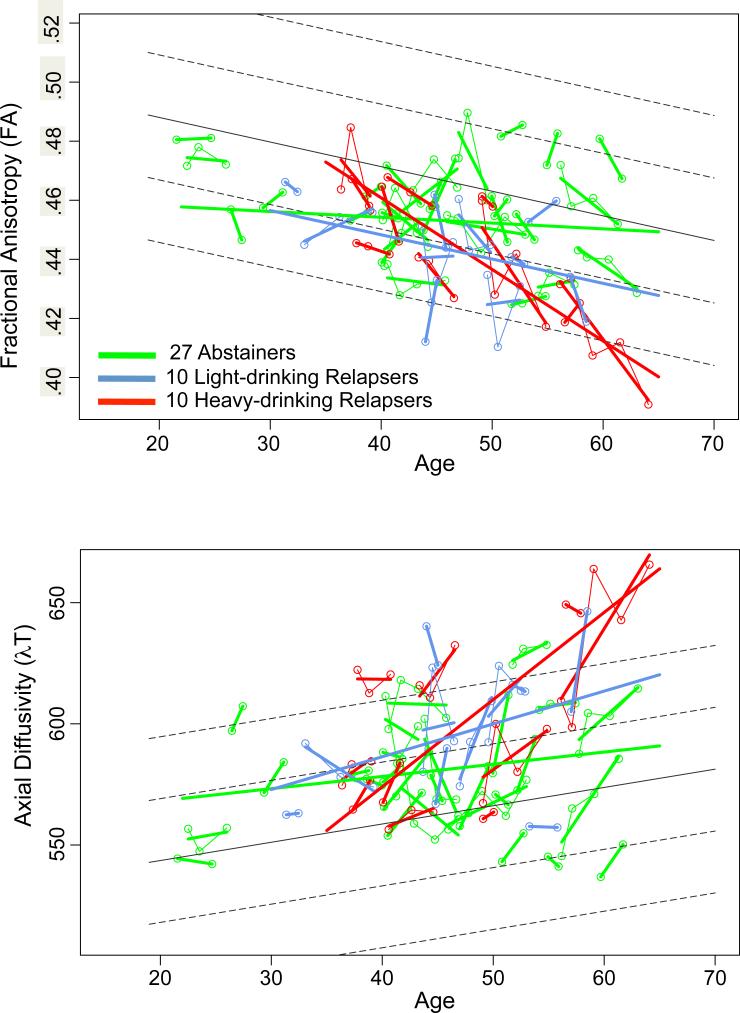
In the three-way comparison of abstainers, all relapsers, and controls (Figure 2, top), both the abstainers (t=-2.609, df=99.70, p=.0105) and the relapsers (t=−4.998, df=99.70, p=.00001) had lower FA than controls. Although these two alcoholic subgroup had aging trajectories significantly different from controls, the slopes were in opposite directions: like the controls, the relapsers’ slope was negative, evidence for accelerated aging, whereas the abstainers’ slope was positive and different from that of the controls (t=2.138, df=175.7, p=.0339).
Figure 2.
Plots of individual multifocal cluster FA (top) and λT (bottom) by age for each alcoholic group: 27 abstainers (green), 10 light-drinking relapsers (blue), and 10 heavy-drinking relapsers (red). Each participant's values are connected over time and the age-centered slope of each participant is overlaid on the longitudinal data points. The solid gray regression line is the expected volume by age regression based on the controls; dotted lines are ±1 and 2 S.D.
λT trajectories
Together, the controls and alcoholics showed an aging effect with higher λT with advancing age (t=3.370, df=21.9, p=.0028). Further, both the abstainers (t=2.679, df=98.96, p=.0086) and the light+heavy drinking relapsers (t=5.225, df=98.96, p=.00001) had higher λT than controls. An accelerated aging effect showed only a trend (t=1.917, df=25.92, p=.0663), which was explained by the slopes differences between the heavy-drinking relapsers and abstainers (t=2.970, df=162.965, p=.0034) (Figure 2, bottom).
Regions comprising the TBSS cluster
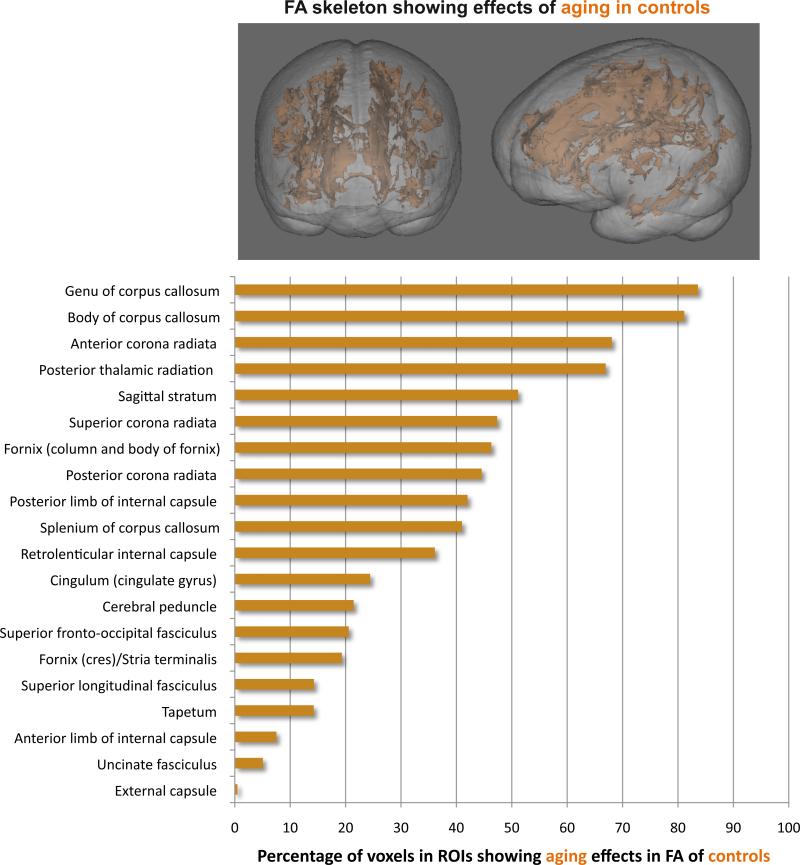
The TBSS FA cluster showing significant normal aging effects in the controls alone was segmented with the white matter atlas 26 for anatomical localization of aging effects. The data were expressed as the percent of significant FA cluster voxels for each of the 27, atlas-defined, regions of interest (ROIs) (see Supplementary Information). Of the 27 ROIs, controls exhibited significant aging effects in 20 ROIs, with the percentage of significant voxels per ROI ranging from 0.49% in the external capsule to 83.6% in the genu (Figure 3).
Figure 3.
Top: 3D rendering of the FA skeleton (orange) overlaid on an SPGR volume image showing significant effects of aging in the controls. Bottom: Percentage of significant voxels in each atlas-defined region of interest of the multifocal FA cluster showing an aging effect among controls.
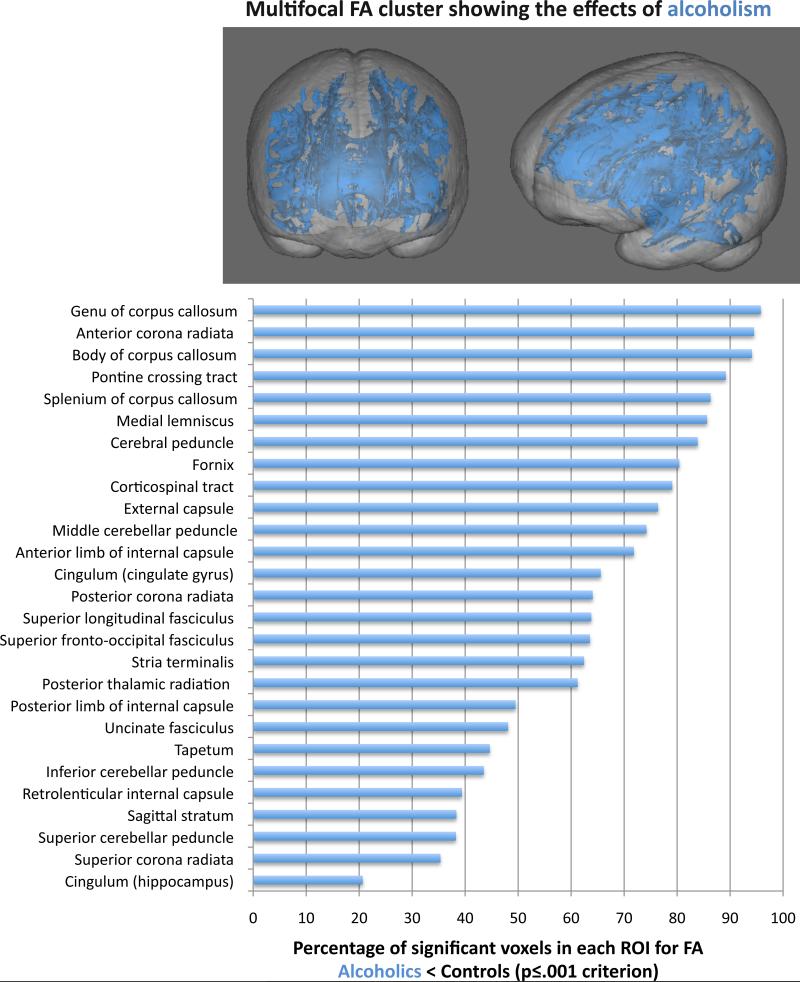
The alcoholic<control group FA difference cluster was also segmented for anatomical localization of the differences, and the data were expressed as the percent of significant FA cluster voxels for each ROI. Percentage of voxels in an ROI meeting criterion for alcoholic<control group differences ranged from 20.7% in the hippocampal cingulum to 95.8% in the genu (Figure 4).
Figure 4.
Top: 3D rendering of the FA skeleton (blue) overlaid on an SPGR volume image showing significant differences in alcoholics relative to controls. Bottom: Percentage of significant voxels in each atlas-defined region of interest of the multifocal FA cluster showing an alcoholism effect.
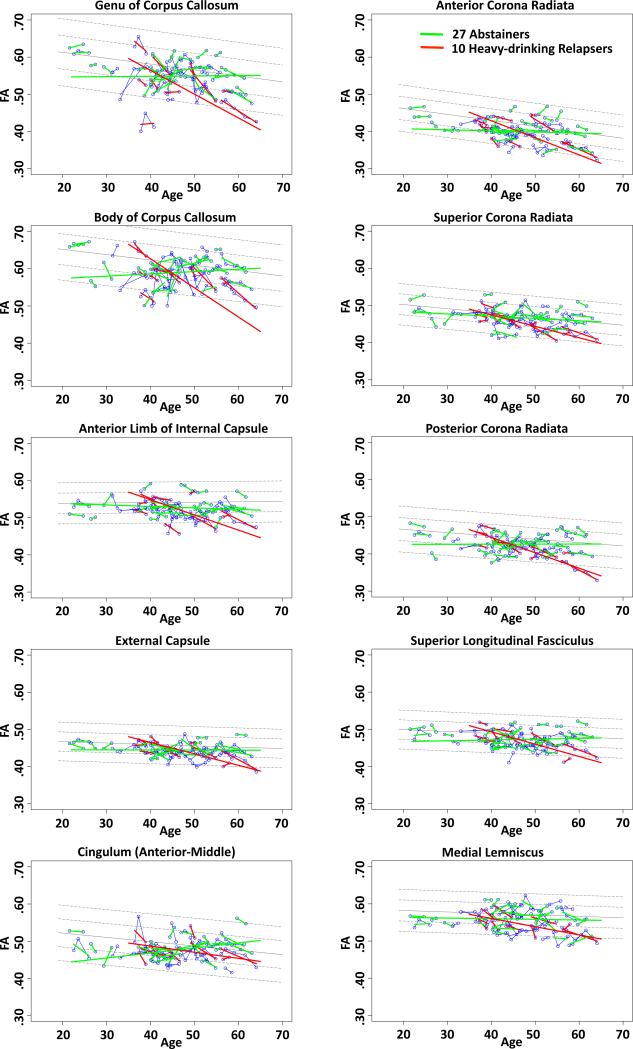
Given that the most prominent difference among the alcoholic subgroups was between the total abstainers and high-drinking relapsers, we limited the comparisons of the individual ROIs to these extreme groups. Within the alcoholics, 10 ROIs in the TBSS cluster were identified as having significantly steeper slopes in the heavy-drinking relapsers than the abstainers (FDR corrected), indicating accelerated decline in FA with aging (Table in Supplementary Information, Figure 5). Group differences in slopes included 2 midline regions (genu and body) and 8 bilateral regions (cingulum; anterior, superior, posterior corona radiata; medial lemniscus; internal and external capsules; superior longitudinal fasciculus). No differences between the abstainers and relapsers in λT met FDR significance, nor did differences based on group level irrespective of slope.
Figure 5.
Regional FA trajectories showing significant differences among abstainers (green), light-drinking relapsers (blue), or heavy-drinking relapsers (red), whose values are plotted on the age regressions of the controls (solid gray line=mean, dotted lines are ±1 and 2 S.D.).
Effect of illicit drug abuse/dependence on white matter microstructural trajectories
A two-group t-test, examining FA of the multifocal cluster between the 30 alcoholics with a past history of drug abuse/dependence and the 17 without a drug history, yielded no significant difference (t(45)=0.604, p=.55). Analysis of variance examining drug history effects in the abstainers compared with the 20 relapsers yielded only a significant alcohol-by-drug interaction (F(1,43)=10.36, p=.0025), where the slopes of the abstainers without a drug history were not different from those of the relapsers with a drug history (t(21)=.4001, p=.693).
White matter macrostructural trajectories based on volumetric MRI measures
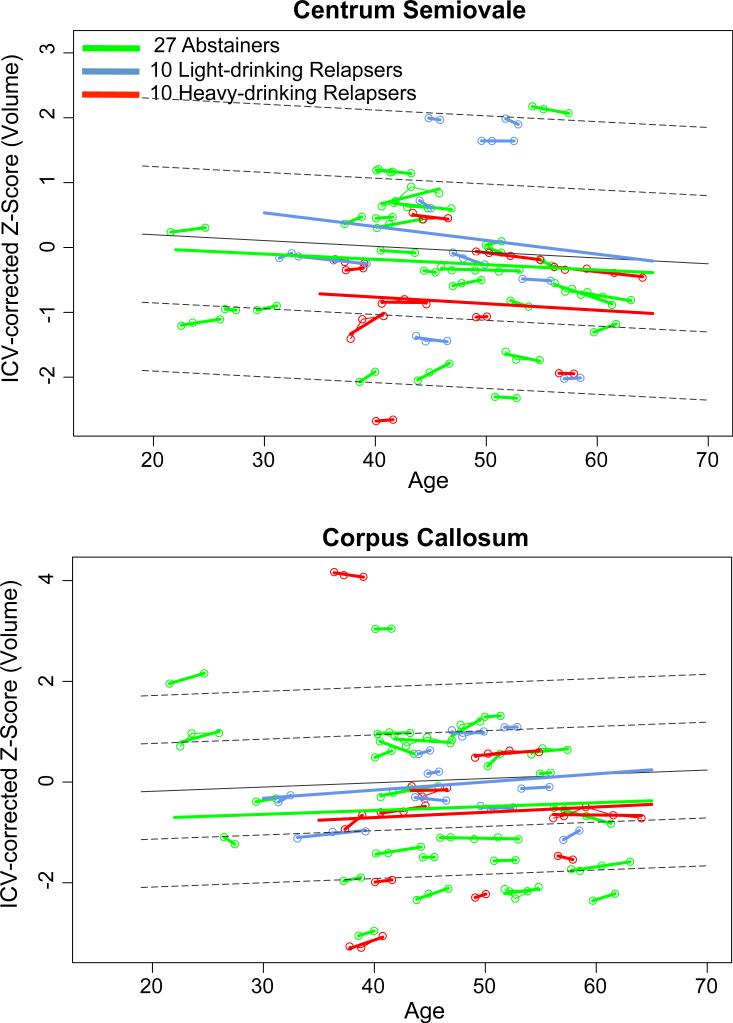
Trajectories of volumes of the corpus callosum and centrum semiovale were tested over age. For the corpus callosum, neither group effects nor trajectory differences were detected between the total group of alcoholics and controls or between abstainers and relapsers. Although groups did not differ significantly in volume trajectories of the centrum semiovale, the abstainers showed a greater positive slope than the relapsers and controls (t=2.171, p<.05) (Figure 6).
Figure 6.
Regional white matter volume trajectories of the abstainers (green), light-drinking relapsers (blue), or heavy-drinking relapsers (red), whose values are plotted on the age regressions of the controls (solid gray line=mean, dotted lines are ±1 and 2 S.D.).
Discussion
Longitudinal analysis of whole brain white matter microstructure revealed a large, widespread cluster of fiber bundles showing declining FA with aging over a 1- to 8-year interval in controls and alcoholics irrespective of drinking status. Although age trajectories of these two groups were essentially parallel, the FA of these alcoholics was significantly lower than that of the controls. When drinking status over the study interval was considered, the relapsing alcoholics showed continued worsening, whereas the totally abstaining alcoholics showed improvement in DTI indices of fiber integrity. Specifically, the FA trajectories of the relapsers exhibited accelerated aging relative to controls, whereas the trajectories of the abstainers was in the direction of improvement toward normality. The diffusion measures were generally complementary to the FA patterns and indicative of continuing myelin damage in the relapsers and repair in the abstainers.
These longitudinal findings indicate that only selective fiber systems showed an alcoholism-aging interaction and that accelerated aging, observed in FA and axial diffusivity, was confined to relapsers. Atlas-identified fiber tracts showing significantly steeper aging FA slopes especially in heavy-drinking relapsers than abstainers included anterior commissural tracts (genu and body), projection fibers (corona radiata, external capsule, internal capsule anterior limb), and association fibers (superior longitudinal fasciculus). The changes in callosal genu and body are consistent with observations in the one other controlled longitudinal study, which followed participants over 13 to 16 months 22, and would not have been recognized if drinking status had been ignored.
The difference in DTI microstructural measures and MRI macrostructural measures in detecting longitudinal change comports with our earlier cross-sectional study of alcoholic women demonstrating disruption of white matter fiber integrity using DTI not detected with volume measures using MRI 17. DTI was instrumental in identifying disrupted tissue quality and principal constituents likely affected. Myelin and myelinated axons are amenable to repair with normal aging 27 and white matter diseases 28. In models of high alcohol exposure, axonal remodeling can occur 29. In vivo DTI has revealed profound genu and fimbria-fornix FA deficits in a rodent alcohol-binge model that was rapidly reversible following several alcohol-free days30, providing credence to the assertion that DTI metrics are sensitive to alcoholism-related changes in white matter fiber microstructure.
Speculations on molecular mechanisms of affected myelin in alcoholism include consideration of DTI metrics in animal models of stroke. Abnormal diffusion in affected areas of white matter undetected with macrostructural imaging corresponds to down-regulation of microtubule-associated protein 2 (MAP2) expression. Analogous to these findings are those in human brain tissue of alcoholics that express a constellation of abnormal gene expression associated with metabolism, cell survival, and energy production; those associated with MAP2 are essential for highly-ordered myelin 31. Further, greater MAP2 protein expression occurs in callosal genu than splenium or frontal white matter in alcoholics 32, implicates the local effect of alcoholism on fiber tracts.
The strengths of this controlled, longitudinal study are accompanied by several limitations. The relapser subgroup was smaller than the totally abstaining group, possibly owing to the non-clinic, recruitment base of this study. Nonetheless, the results were adequately robust to identify accelerated aging in relapsers not occurring in abstainers. Further, although illicit drug use occurred in the alcoholics, it was not detected as a significant moderating factor in any of the quantitative analyses. Cigarette smoking was a common co-addiction in the alcoholics, occurring in all but one of 20 relapsers but in 9 of 18 abstainers. In this sample, history of smoking was confounded with alcoholism history and relapse, precluding differentiating the influence of drinking from smoking on white matter microstructural health e.g., 33,34. Even longitudinal study when initiated after the onset of hazardous drinking does not have a premorbid measure of the brain's condition but in followup can track brain structural changes through the course of alcoholism.
In conclusion, return to heavy drinking interacted with normal aging to accelerate microstructural white matter damage in selective fiber systems. On the positive side, despite evidence for damage, alcoholics who maintained sobriety over extended periods showed improvement in brain white matter integrity, reflective of fiber reorganization and myelin restoration, indicative of a neural mechanism explaining recovery and perhaps enhancing chances for sustaining sobriety.
RESEARCH IN CONTEXT
Systematic review
We searched PubMed for articles published in English for all available years up to May 2014, with the initial search terms “alcoholism, DTI, longitudinal, human, adult” and subsequent search terms, including, for example, “alcohol,” “white matter,” “recovery,” “sobriety,” “relapse.” A number of papers have been published using structural MRI to measure regional white matter volumes in alcoholics over days, weeks, or a few years following abstinence or relapse into drinking, only two longitudinal studies were identified that used DTI to measure regional white matter microstructure in recovering alcoholics. One DTI study revealed increased FA and decreased axial diffusivity (λT) in 15 abstainers relative to 15 controls at two time points: after 2 weeks and after 13-16 months of abstinence 22. Another study used multi-modal imaging to examine alcoholics in a rehabilitation program after 1 week and 1 month of alcohol abstinence and found smoking to be a factor in FA recovery, although controls, whether smoking or not, were not studied longitudinally 2. No DTI study to date had examined large groups of alcoholics and controls longitudinally for an extended period (1 to 8 years), had tracked subgroups of alcoholics as they relapsed into drinking or remained sober, or had examined the data in the context of normal aging.
Interpretation
Heretofore, some but not all neuroimaging studies of human alcoholics identified either enlargement of regional white matter volume with sustained sobriety or volume decline with drinking relapse. Such differences when measured in contrast with potential longitudinal change in low-to-no drinking, non-alcoholic adults provided support for the possibility that brain structure can show apparent improvement in adults with chronic alcoholism when they achieve and maintain sobriety. Given that DTI has been more sensitive than structural volumetric MRI in detecting the effects of alcoholism in cross-sectional study, remaining in question was whether in vivo measurement of the microstructure of damaged white matter could be more sensitive in detecting recovery with abstinence or further degradation with continued hazardous drinking. Our study provides initial, quantitative data for decline in FA, an index of disorganization of microstructural constituents of white matter fiber tracts, and increasing λT, an index of myelin degradation, with normal aging in the controls, accelerated aging in relapsed alcoholics, and improvement toward normality in abstinent alcoholics. Thus, the clinical relevance of these findings is two-fold: 1) despite evidence for damage, alcoholics who maintained sobriety over extended periods showed improvement in brain white matter integrity, reflective of fiber reorganization and myelin restoration, whereas relapsers showed further degradation of fiber tracts; 2) the patterns of change revealed through the DTI metrics are indicative of selective neural mechanisms focused on the health of myelin explaining recovery and perhaps enhancing chances for sustaining sobriety.
Supplementary Material
Acknowledgement
The authors would like to thank the many research assistants who were diligent in scheduling and data collection over the years and note in particular Crystal Caldwell for her exceptional recruitment efforts and Priya Asok for her care in collection and oversight of clinical laboratory data.
Funding
This work was funded by the U.S. National Institute on Alcohol Abuse and Alcoholism (AA012388, AA017168, AA005965, AA013521-INIA).
Footnotes
Contributors
AP and EVS participated in all aspects of the study, data analysis, and manuscript writing. AP, KMP, and TR conducted the DTI and MRI analyses. MJR, SAS, and WC oversaw data scoring and management. NMZ oversaw DTI and MRI data acquisition and management. MJR and SAS participated in subject recruitment and screening. All authors participated in aspects of manuscript writing and its review.
Conflicts of interest
None of the authors has any conflicts of interest with the work presented and have no financial disclosures relevant to this work.
References
- 1.van Eijk J, Demirakca T, Frischknecht U, Hermann D, Mann K, Ende G. Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcoholism, clinical and experimental research. 2013;37(1):67–74. doi: 10.1111/j.1530-0277.2012.01853.x. [DOI] [PubMed] [Google Scholar]
- 2.Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ. Cerebral white matter recovery in abstinent alcoholics--a multimodality magnetic resonance study. Brain. 2010;133(Pt 4):1043–53. doi: 10.1093/brain/awp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segobin SH, Chetelat G, Le Berre AP, et al. Relationship between brain volumetric changes and interim drinking at six months in alcohol-dependent patients. Alcoholism, clinical and experimental research. 2014;38(3):739–48. doi: 10.1111/acer.12300. [DOI] [PubMed] [Google Scholar]
- 4.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34(3):879–87. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55(10):905–12. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- 6.Harper C, Dixon G, Sheedy D, Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(6):951–61. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- 7.de la Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta neuropathologica. 2014;127(1):71–90. doi: 10.1007/s00401-013-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarnowska-Dziduszko E, Bertrand E, Szpak G. Morphological changes in the corpus callosum in chronic alcoholism. Folia Neuropathol. 1995;33(1):25–9. [PubMed] [Google Scholar]
- 9.Lewohl J, Wang L, Miles M, Zhang L, Dodd P, Harris R. Gene expression in human alcoholism: Microarray analysis of frontal cortex. Alcoholism: Clinical and Experimental Research. 2000;24:1873–82. [PubMed] [Google Scholar]
- 10.Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J Neurochem. 2002;81(4):802–13. doi: 10.1046/j.1471-4159.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- 11.Harper CG, Kril JJ. Neuropathological changes in alcoholics. In: Hunt WA, Nixon SJ, editors. Alcohol Induced Brain Damage: NIAAA Research Monograph No 22. National Institute of Health; Rockville, MD: 1993. pp. 39–69. [Google Scholar]
- 12.Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79(4):983–98. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- 13.Harper CG, Smith NA, Kril JJ. The effects of alcohol on the female brain - A neuropathological study. Alcohol Alcohol. 1990;25(5):445–8. [PubMed] [Google Scholar]
- 14.Ruiz SM, Oscar-Berman M, Sawyer KS, Valmas MM, Urban T, Harris GJ. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcoholism, clinical and experimental research. 2013;37(1):110–22. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monnig MA, Tonigan JS, Yeo RA, Thoma RJ, McCrady BS. White matter volume in alcohol use disorders: a meta-analysis. Addiction biology. 2013;18(3):581–92. doi: 10.1111/j.1369-1600.2012.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19(5):1177–91. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 17.Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage. 2002;15:708–18. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- 18.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26(1):132–40. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65(8):680–90. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfefferbaum A, Rosenbloom MJ, Fama R, Sassoon SA, Sullivan EV. Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: Selective relations to dissociable functions. Alcohol Clin Exp Res. 2010;34(7):1201–11. doi: 10.1111/j.1530-0277.2010.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagga D, Sharma A, Kumari A, et al. Decreased white matter integrity in fronto-occipital fasciculus bundles: relation to visual information processing in alcohol-dependent subjects. Alcohol (Fayetteville, NY. 2014;48(1):43–53. doi: 10.1016/j.alcohol.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Alhassoon OM, Sorg SF, Taylor MJ, et al. Callosal White Matter Microstructural Recovery in Abstinent Alcoholics: A Longitudinal Diffusion Tensor Imaging Study. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1998. [Google Scholar]
- 24.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 25.Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A. The SRI24 multi-channel atlas of normal adult human brain structure. Hum Brain Mapp. 2010;31(5):798–819. doi: 10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori S, Wakana S, Nagae-Poetscher LM, Van Zijl PMC. An Atlas of Human White Matter. Elsevier B.V.; Amsterdam, the Netherlands: 2005. [Google Scholar]
- 27.Peters A, Sethares C. Is there remyelination during aging of the primate central nervous system? J Comp Neurol. 2003;460(2):238–54. doi: 10.1002/cne.10639. [DOI] [PubMed] [Google Scholar]
- 28.Miller RH, Fyffe-Maricich SL. Restoring the balance between disease and repair in multiple sclerosis: insights from mouse models. Dis Model Mech. 2010;3(9-10):535–9. doi: 10.1242/dmm.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pentney RJ. Remodeling of neuronal dendritic networks with aging and alcohol. Alcohol Alcohol. Supplement. 1991;1:393–7. [PubMed] [Google Scholar]
- 30.Pfefferbaum A, Zahr NM, Mayer D, Rohlfing T, Sullivan EV. Rapid plasticity in select fiber tracts of the rat with alcohol bringe and recovery. NeuroImage in revision [Google Scholar]
- 31.Liu J, Lewohl JM, Harris RA, et al. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006;31(7):1574–82. doi: 10.1038/sj.npp.1300947. [DOI] [PubMed] [Google Scholar]
- 32.Kashem MA, Harper C, Matsumoto I. Differential protein expression in the corpus callosum (genu) of human alcoholics. Neurochem Int. 2008;53(1-2):1–11. doi: 10.1016/j.neuint.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Hudkins M, O'Neill J, Tobias MC, Bartzokis G, London ED. Cigarette smoking and white matter microstructure. Psychopharmacology. 2012;221(2):285–95. doi: 10.1007/s00213-011-2621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. NeuroImage. 2011;54(1):42–8. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.