Abstract
Leishmania donovani is an intracellular parasite that infects professional phagocytes and causes visceral leishmaniasis (VL). The immune response during VL has been extensively studied in the context of T-helper (Th)1 and Th2 responses. Immunity against this parasite is dependent on IFN-γ production and subsequent macrophage activation, and the Th2 response promotes granuloma formation. The cytokine IL-17A is associated with neutrophilic inflammation. Depletion of neutrophils during experimental VL results in enhanced parasitic loads. Furthermore, although patients resistant to VL showed enhanced levels of IL-17A in circulation, little is known about the role of IL-17A during VL infection. Here, we used IL-17A-deficient mice and IL-17A reporter mice to address the role of IL-17A during VL. IL-17A−/− mice were highly resistant to VL infection, showing decreased parasites in the liver and spleen. This unexpected phenotype was associated with enhanced IFN-γ production by T cells and decreased accumulation of neutrophils and monocytes, resulting in reduced number of granulomas. We also found γδ T and Th17 cells as the main IL-17A+ cells during VL infection. Our data reveal an unexpected role of IL-17A rendering susceptibility against L. donovani by regulating the IFN-γ response and promoting detrimental inflammation.—Terrazas, C., Varikuti, S., Kimble, J., Moretti, E., Boyaka, P. N., Satoskar, A. R. IL-17A promotes susceptibility during experimental visceral leishmaniasis caused by Leishmania donovani.
Keywords: granuloma, monocytes, neutrophils, γδ T cells, Th17
Leishmaniasis is transmitted by the bite of the infected sand fly of the genus Phlebotomus and Lutzomyia. Once inside the mammalian host, the parasite infects phagocytes such as neutrophils, dendritic cells, and macrophages. There are two main forms of manifestations in humans, cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL). These manifestations are dependent on the parasite species. CL is caused by Leishmania mexicana, Leishmania major, Leishmania braziliensis, and Leishmania amazonensis and the infection remains in the skin causing ulcers. VL, on the other hand, is caused by Leishmania donovani in Asia and Africa and Leishmania infantum/chagasi in South America, South Europe, Asia, and Africa. In VL, the infection spreads from the skin to the spleen, liver, and bone marrow causing hepatomegaly and splenomegaly and causes death if untreated (1). Despite the close genetic relationship between the parasites causing VL or CL, the protective and pathogenic immune response is quite different. This could be the result of parasite-specific factors and/or tissue-specific responses. In experimental models of leishmaniasis, important immunologic axes related with immunity or susceptibility have been established. IL-12-STAT4 and IFN-γ-iNOS pathways are important for parasite clearance in both CL and VL through the activation of the microbicidal function of the macrophage (2–7). On the other hand, the T-helper (Th)2 response is deleterious for CL, but plays an important role in granuloma formation and protection in VL (8–11). Besides the classic Th1 and Th2 paradigm, other cytokines produced by T cells may play an important role during leishmaniasis (12).
Among the potential microbicidal cytokines is the cytokine IL-17A. The IL-17 family consists of 6 members (IL-17A–F). The best studied is IL-17A, which is related to neutrophil recruitment and shares the receptor IL-17RA with IL-17E (IL-25), and IL-17F (13). The best-characterized role for IL-17A is mediating the pathogenic inflammation during experimental autoimmune encephalomyelitis (EAE) (14). Due to its inflammatory function, its role during microbial infections has also been investigated. For example, IL-17A mediates protection against Francisella tularensis, Mycobacterium tuberculosis, Staphylococcus aureus, and Candida albicans (15–18). The effector role of IL-17A is thought to mainly be mediated by the accumulation of neutrophils, the enhancement of proinflammatory cytokine secretion, and the increased production of antimicrobial peptides (13).
During CL caused by L. major, IL-17A increases the pathology by recruiting neutrophils into the lesion site (19, 20). The specific role for IL-17A during VL remains to be investigated. However, some reports have suggested a protective role of IL-17A during VL, especially because depletion of neutrophils during the infection worsens the disease (21). In agreement with this hypothesis, patients resistant to VL had increased circulating levels of IL-17 (22). These findings open the question of whether IL-17A could be a protective cytokine during VL. Here we investigated the role of IL-17A during experimental VL by using IL-17A reporter and IL-17A-deficient mice. Strikingly, we found a remarkable ability of IL-17A-deficient mice to control L. donovani infection in the liver and spleen. These data demonstrate for the first time a detrimental role for IL-17A during L. donovani infection. Our data add the cytokine IL-17A to the complex immunologic network regulating immunity against L. donovani.
MATERIALS AND METHODS
Animals and L. donovani infection
C57BL/6 mice (6 to 8 wk old) were purchased from Harlan Laboratories (Indianapolis, IN, USA). IL-17A−/− mice were donated by Dr. Yoichiro Iwakura. IL-17A green fluorescent protein (GFP) reporter mice were purchased from The Jackson Laboratory, Bar Harbor, ME, USA Mice were maintained and bred at The Ohio State University animal facility according to animal protocols and University Laboratory Animal Resources regulations. L. donovani (Ldv82) amastigotes were recovered from the spleen of an infected Syrian hamster. In some experiments, mice were infected L. donovani expressing DsRedgenerated as reported before (23). Groups of 3–5 mice were intravenously injected with 1 × 107 amastigotes and were euthanized using a CO2 chamber at 15 and 30 d postinfection (dpi). For IL-17A recombinant treatment, mice were intraperitoneally injected with 10 µg of rIL-17A (Biolegend, San Diego, CA, USA) as reported elsewhere (24) and euthanized at 30 dpi. Parasitic loads were evaluated as L. donovani units (LDU) by counting the number of parasites per 1000 cell nuclei in Giemsa-stained smears (Sigma-Aldrich, St. Louis, MO, USA). The resulting number was then multiplied by the tissue weight as reported previously (3).
Flow cytometry
Mice were euthanized at 15 or 30 dpi, and single-cell suspensions were obtained by mechanical disruption of the spleens and livers through 70 µm cell strainer (BD Biosciences, San Jose, CA, USA). Nonparenchymal cells from the livers were obtained by Percoll centrifugation. Surface staining was carried out by blocking the unspecific antibody binding with normal mouse serum (Jackson ImmunoResearch, West Grove, PA, USA) for 15 min. Then cocktail antibody was added to the cells following incubation in the dark at 4°C for 20 min. After surface staining, the cells were fixed with formaldehyde for 10 min at room temperature. For intracellular staining, the cells were washed two times with permeabilization buffer (Biolegend). Finally, the cells were incubated in the presence of anti IFN-γ (BD Biosciences) for 45 min at room temperature. After 2 washes with permeabilization buffer, the cells were resuspended in PBS and analyzed by flow cytometry. NK, B, and T cells were identified by using anti-NK1.1, anti-CD19, anti-CD3, anti-CD8, anti-CD4 (Biolegend) and γδ δ T-cell receptor (TCR) (BD Biosciences). Identification of IL-17A-expressing cells was carried out in IL-17A-GFP reporter mice by detecting GFP in different cell populations. Neutrophils and monocytes were identified with the antibodies anti-Ly6C, Ly6G, and CD11b (Biolegend). Flow cytometry was performed by using BD FACS Calibur (BD Biosciences). Flowcytometry data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Cytokine detection
Spleen cells were plated at the concentration of 0.5 × 106 cells per well (in 96 well plates) in RPMI medium (supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin, 1% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 0.1% β-mercaptoethanol, purchased from Life Technologies (Grand Island, NY, USA). After 72 h of stimulation with L. donovani antigen (20 µg/ml), cell supernatants were recovered and stored at −20°C. Concentrations of IL-4, IL-6, IL-10, IL-13, IL-17A, and IFN-γ were determined by ELISA sandwich by using capture and detection antibodies purchased from Biolegend. For intracellular cytokine detection, splenocytes were stimulated for 18 h with Leishmania antigen. Ionomycin and phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) were added during the last 6 h in the presence of Brefeldin A (Biolegend), then intracellular staining was carried out as described above.
Histology
Tissues were preserved in formalin and then included in paraffin. Tissue sections (0.5 µm) were stained with hematoxylin and eosin (Thermo Scientific, Waltham, MA, USA). Granuloma development was evaluated by a certified pathologist as reported before (6). For confocal microscopy, tissue was stored in O.C.T. (Sakura Finetek, Torrance, CA, USA), and 0.5 µm sections were incubated with blocking solution (anti-mouse serum) followed by incubation with anti-neutrophil elastase (Santa Cruz Biotechnologies, Dallas, TX, USA) and Ly6C Alexa fluor 647 (Biolegend). Secondary antibodies anti goat Alexa fluor 488 (Thermo Scientific) were used to detect neutrophil elastase. Images were acquired using the confocal microscope LSM700 and analyzed in Zen software (Carl Zeiss, Jena, Germany.
Statistical analysis
Groups of 3–5 mice were used for each experiment. Student’s t test was performed in GraphPad software (La Jolla, CA, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
IL-17A response is mediated by unconventional T cells and CD4 Th cells during L. donovani infection
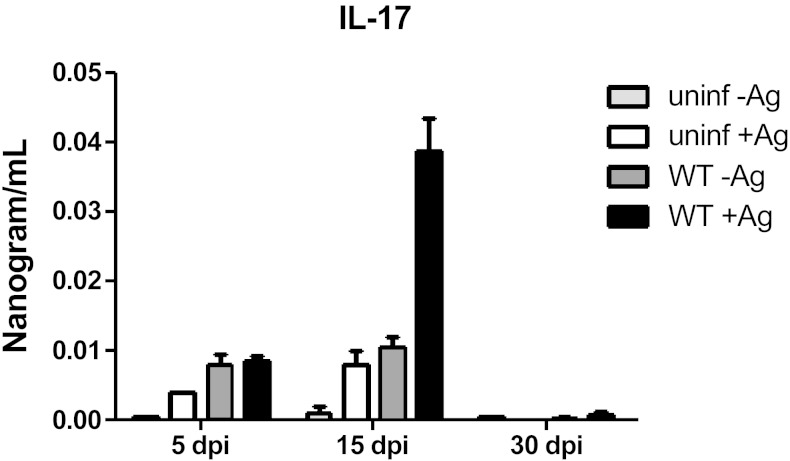
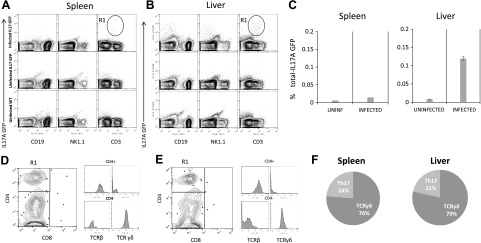
The production of IL-17A during human VL has been observed and correlated with protection (22). However, there is no information about the kinetics of IL-17A expression during experimental VL caused by L. donovani. To evaluate the production of IL-17A during experimental L. donovani infection, we first investigated the levels of IL-17A in supernatants of infected splenocytes restimulated or not with L. donovani antigen at different times. We found increased levels of IL-17A in the supernatants of restimulated infected splenocytes at 15 dpi. At 30 dpi, IL-17A production was still detectable but at reduced levels (Fig. 1A). IL-17A is produced by different cells depending on the pathology. For example, IL-17A is produced by CD4+ T cells during experimental autoimmune encephalomyelitis (EAE) and by B cells during Trypanosoma cruzi infection (14, 25). To identify IL-17A producer cells during VL, we infected IL-17A transcriptional reporter mice with L. donovani and investigated IL-17A expression in different immune cells by flow cytometry. We focused on 15 dpi because that was the time with enhanced IL-17A response in the spleen (Fig. 1). IL-17A-expressing cells were found in both the liver and spleen of L. donovani-infected mice; in contrast, IL-17A-positive cells were scarce under basal conditions in the organs analyzed (Fig. 2A). Interestingly, the liver presented a higher frequency of IL-17A-expressing cells when compared with the spleen (Fig. 2B). In both organs, CD3+ cells represented the majority of GFP-expressing cells. In contrast, B cells and NK cells did not express IL-17A (Fig. 2A). Further analysis of the CD3+ IL17A+ cells revealed 2 main populations expressing IL-17A. Interestingly, CD3+CD4−CD8− TCRβ− cells represented the majority of IL-17A-positive cells in both organs (Fig. 2C). The second population of IL-17A-positive cells was CD4+ TCRβ+, thus regular Th cells (Fig. 2D, E). Because γδ T cells commonly express CD3 but not CD4, CD8, or TCRβ, we investigated γδ TCR expression in the IL-17+ population. The CD3+CD4−CD8−IL-17A+ cells were γδ-positive and represented the majority of cells expressing IL-17A in both the spleen and liver (Fig. 2D–F).
Figure 1.
IL-17A production during visceral leishmaniasis. C57BL/6 mice were infected intravenously with 1 × 107 L. donovani amastigotes. At 5, 15, or 30 dpi, the mice were euthanized. Spleen cultures were stimulated with 20 µg/ml of L. donovani soluble antigen, after 72 h IL-17A was detected in the supernatants by ELISA. Data representative of 2 independent experiments (n = 3–4). *P < 0.05.
Figure 2.
IL-17A is mainly expressed by γδ and CD4+ T cells during L. donovani infection. A, B) IL-17A genetic expression reporter mice were infected with 1 × 107 L. donovani amastigotes. After 15 d of infection, spleen (A) and liver (B) were obtained, and the expression of IL-17A was investigated among immune subsets of B cells (CD19+), NK cells (NK1.1+), and T cells (CD3+). Uninfected IL-17A GFP mice and uninfected WT mice were used as controls to determine IL-17A-positive cells. C) Frequencies of IL-17A GFP+ cells were analyzed in total splenocytes or nonparenchymal liver cells. D, E) CD3+ IL17A+ cells were gated in A and B as R1 and CD4 and CD8 expression was analyzed by flow cytometry in the spleen (D) or liver (E). Histograms represent the expression of TCRβ or TCRγδ in CD4+ or CD4-/low subsets gated in the dot plot, showing that CD4+ cells expressed TCRβ, whereas CD4−/low expressed TCRγδ. F) Frequency of T cells expressing IL-17A based on their phenotypic characterization by flow cytometry. Data represent 3 independent experiments (n = 3).
IL-17A-deficient mice are resistant to L. donovani infection
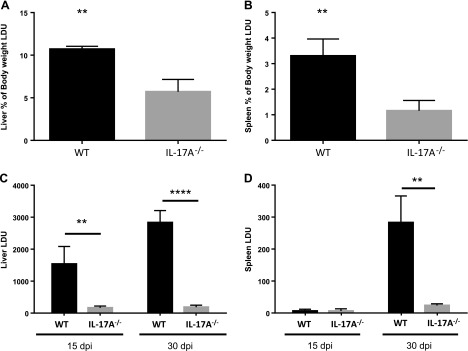
To investigate the importance of IL-17A during experimental VL, we intravenously infected IL-17A-deficient mice with L. donovani amastigotes and compared the course of the infection with wild-type (WT) mice. WT mice showed splenomegaly and hepatomegaly, the main pathologies associated with VL; however, these pathologies were reduced in IL-17A−/− mice at 30 dpi (Fig. 3A, B). Contrary to our expectations, the livers of IL-17A−/− mice showed strikingly lower LDU compared with their WT counterparts at 15 dpi. At 30 dpi, the parasitic loads in the livers of WT mice were augmented, whereas IL-17A−/− mice continued to have low parasitic loads (Fig. 3C). As expected, the parasitic loads in the spleens accumulated slower than in the livers of WT mice, with the parasitic loads being similar between WT and IL-17A−/− mice at 15 dpi. However, at 30 dpi, WT mice showed significantly higher parasitic loads than IL-17A-deficient mice (Fig. 3D), demonstrating that absence of IL-17A production regulates L. donovani burden in both the liver and spleen.
Figure 3.
IL-17A-deficient mice are highly resistant against L. donovani infection. IL-17A-deficient mice were intravenously infected with 1 × 107 L. donovani amastigotes. After 15 or 30 d, the mice were euthanized. A, B) Hepatomegaly (A) and splenomegaly (B) are represented as the percentage of body weight. C, D) Parasite quantification is represented as LDUs for liver (C) and spleen (D). Data represent 1 of 2 independent experiments for 30 dpi and 1 of 3 experiments for 15 dpi (n = 3–5). **P < 0.05, ****P < 0.001.
Deficiency in IL-17A affects the Th1 and Th2 responses during VL
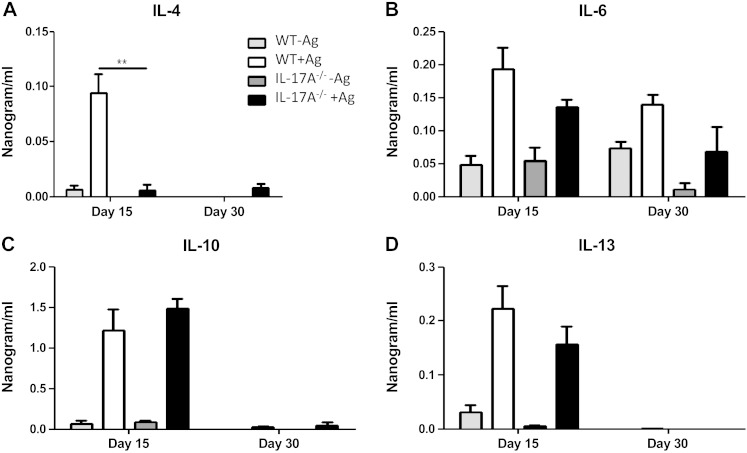
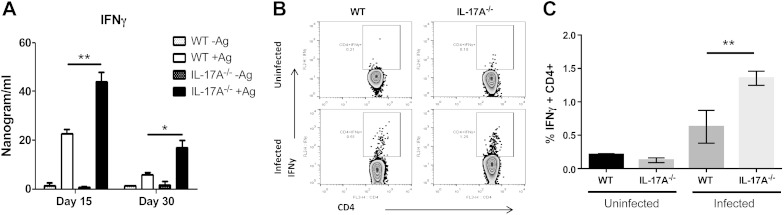
Next, we investigated the possible causes of resistance against L. donovani in the absence of IL-17A. We evaluated the cytokine production in splenocytes restimulated with L. donovani antigen from both WT and IL17A−/− mice. Interestingly, at 15 dpi IL-17A−/− mice did not produce IL-4 after restimulation in contrast with WT mice (Fig. 4A). IL-6 production was increased at 15 and 30 dpi in both WT and IL-17A−/− mice, and the levels of IL-6 were slightly decreased in IL-17A−/− mice; however, this difference was not statistically significant. IL-10 is an important cytokine causative of susceptibility during VL, but both experimental groups secreted similar amounts of IL-10 (Fig. 4C). We also evaluated IL-13 production, which helps to form the granuloma. IL-13 was slightly but not significantly reduced in IL-17A−/− mice at 15 and 30 dpi (Fig. 4D). Finally, we evaluated the secretion of IFN-γ, a main cytokine promoting parasite elimination. Importantly, we found statistically significant enhanced levels of IFN-γ at 15 and 30 dpi in splenocyte supernatants of IL-17A-deficient mice as compared with WT mice (Fig. 5A). To investigate in which cells IFN-γ production was enhanced, we investigated the source of IFN-γ by intracellular cytokine detection. The frequency of CD4+ T cells producing IFN-γ was augmented during VL, but was higher in absence of IL-17A (Fig. 5B, C).
Figure 4.
Altered cytokine response in the absence of IL-17A during VL. Cytokine response was evaluated in splenocyte supernatants of WT or IL-17A−/− mice after 15 or 30 dpi. Splenocytes were plated at 5 × 105 cells/well in 96 well plates and were stimulated or not with L. donovani soluble antigen (20 µg/ml). After 72 h the cell supernatants were recovered and analyzed by ELISA for IL-4 (A), IL-6 (B), IL-10 (C), or IL-13 (D) production. Data represent 1 of 2 independent experiments with similar results. **P < 0.01.
Figure 5.
Enhanced IFN-γ production in IL-17A-deficient mice. IFN-γ response was evaluated in splenocytes of infected WT or IL-17A−/−-deficient mice. A) IFN-γ detection by ELISA after 72 h of restimulation with L. donovani soluble antigen (20 µg/ml) at 15 or 30 dpi. B) Intracellular detection of IFN-γ by flow cytometry at 15 dpi. Splenocytes were stimulated with L. donovani soluble antigen (20 µg/ml) for 18 h and with ionomycin/PMA for the last 6 h in presence of Brefeldin A. CD3+ CD4+ T cells were gated and evaluated for IFN-γ production. C) Percentages of CD4+IFN-γ+ cells in uninfected and 15 d infected WT or IL-17A−/− mice. Data are representative of 1 of 2 independent experiments with groups of 3–5 mice. *P < 0.05, **P < 0.01.
IL-17A deficiency affects granuloma formation during VL
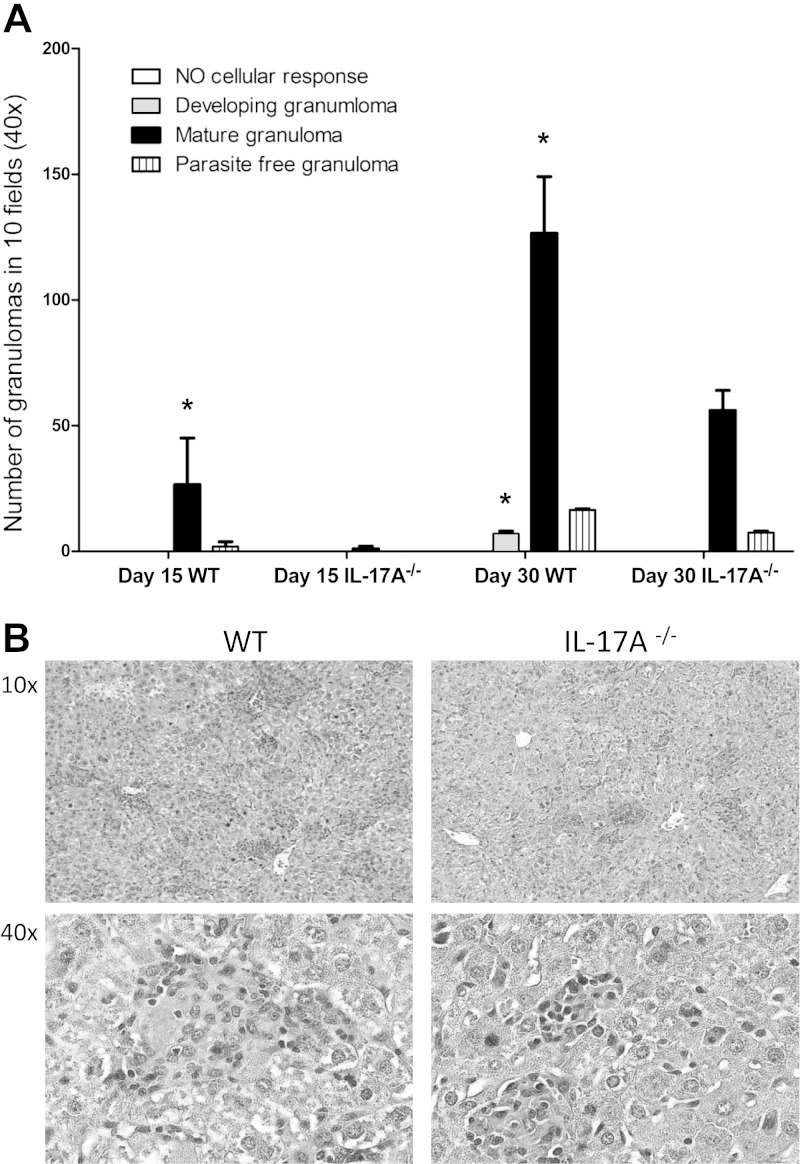
A well-known mechanism for L. donovani elimination from the liver is the assembly of the granuloma. This structure provides the necessary microenvironment to restrain and ultimately kill the parasite (26). IL-17A has been shown to play a role in granuloma formation during Mycobacterium bovis infection (27). Thus, we investigated the changes in granuloma formation in the liver of L. donovani-infected mice in the absence of IL-17A. Interestingly, IL17A deficiency resulted in delayed formation and reduced numbers of mature granulomas (Fig. 6A). Granulomas in WT mice were large and often fused together. In contrast, IL-17A−/− mice presented small and isolated granulomas (Fig. 6A, B).
Figure 6.
Decreased granuloma formation in IL-17A-deficient mice. Granuloma formation was evaluated in liver sections of mice infected for 30 d. A) Analysis of liver granuloma development in 10 ×40 power fields of WT or IL-17A−/− mice at 15 and 30 dpi. Data from 3 mice per group from 2 independent experiments. *P < 0.05. B) Representative hematoxylin and eosin–stained tissue sections of liver from WT or IL-17A−/− mice after 30 dpi.
Reduced accumulation of neutrophils and monocytes in absence of IL-17A
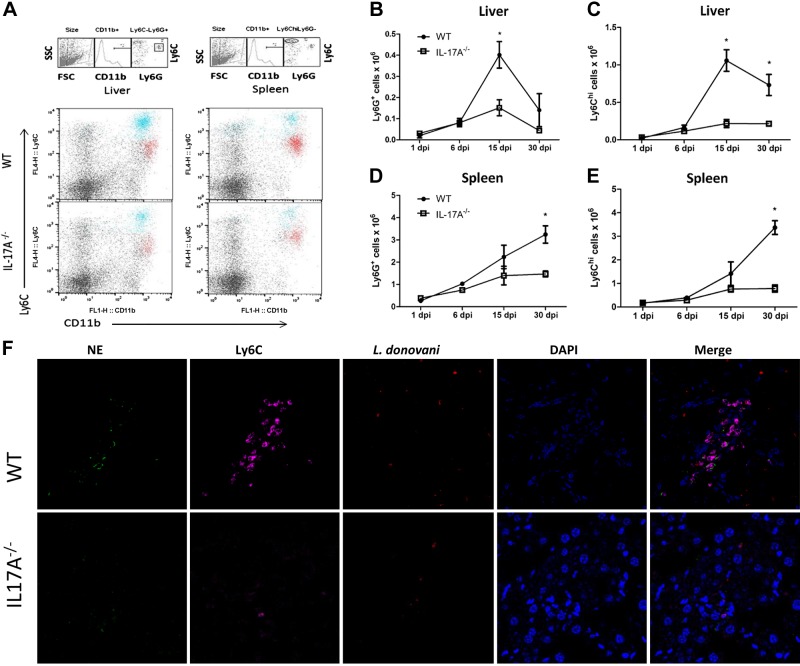
Granuloma formation is accompanied by recruitment of monocytes, neutrophils, and T cells (26). It has been reported that IL-17A is involved in the recruitment of neutrophils and monocytes (20, 28, 29). The impaired granuloma formation suggested reduced infiltration of these cells. To investigate whether immune populations were affected in the liver and spleen, we analyzed these tissues by flow cytometry. Neutrophils were identified as CD11b+Ly6CmedLy6G+ cells, and inflammatory monocytes were identified as CD11b+Ly6ChiLy6G− cells (Fig. 7A). The number of infiltrating neutrophils in the liver was similar at 1 and 6 dpi in both groups. In WT mice, neutrophil infiltration reached maximum numbers at 15 dpi and decreased at 30 dpi. However, in IL-17A−/− mice, the number of neutrophils remained low throughout the course of the infection (Fig. 7B). In the spleens of both groups, the number of neutrophils increased over time; however, IL-17A−/− mice showed decreased accumulation of neutrophils at 30 dpi compared with WT mice (Fig. 7D). The accumulation of Ly6Chi monocytes was also affected in IL-17A−/− mice only during chronic infection. The number of Ly6Chi monocytes in the liver was higher in WT mice at 15 dpi and 30 dpi compared with IL-17A−/− mice (Fig. 7C). Differences in Ly6C monocytes were also observed in the spleen where the number of inflammatory monocytes was reduced in the absence of IL-17A at 30 dpi (Fig. 7E). We next analyzed liver sections at 15 dpi, when neutrophil accumulation was higher in WT mice. To this end, monocytes were identified as Ly6C+ and neutrophils as Ly6C+ and neutrophil elastase-positive. In WT mice, neutrophils and monocytes often clustered together and were found in close distance or associated with parasites. However, in IL-17A−/− mice both neutrophils and monocytes were scattered among the tissue and did not form clusters as compared with WT mice (Fig. 7F).
Figure 7.
Reduced accumulation of neutrophils and Ly6Chi monocytes in absence of IL-17A. L. donovani–infected WT or IL-17A−/− mice were euthanized at 15 or 30 dpi. Flow cytometry was performed on cell suspensions from liver and spleen. A) Gating strategy and representative dot plots of immune populations in the liver and spleen of WT or IL-17A−/− mice at 15 dpi. Neutrophils were identified as CD11b+Ly6GhiLy6Cmed (red region), whereas Ly6Chi monocytes were identified as CD11b+Ly6ChiLy6G− cells (blue region). Gated populations were overlaid on total lymphocytes from liver and spleen and analyzed by Ly6C and CD11b expression. B–E) Kinetic of total number of neutrophils (B, D) and monocytes (C, E) in the liver (B, C) or spleen (D, E) of WT or IL-17A mice during L. donovani infection. F) Aggregates of monocytes and neutrophils were found in WT mice at 15 dpi but not in IL-17A. Liver (15 dpi) sections were examined by confocal microscopy for the expression of Ly6C (magenta), neutrophil elastase (green), and DAPI (blue), DsRed expressing parasites are shown in red (×40 magnification). Data are representative of 2 independent experiments (n = 3–4). *P < 0.05.
DISCUSSION
In this investigation, we show evidence that absence of IL-17A resulted in reduced number of parasites in liver and spleen during experimental VL caused by L. donovani. Our data show that the IL-17A response was mediated by γδ T cells and Th17 cells. Additionally, the absence of IL-17A resulted in elevated levels of IFN-γ production by CD4+ T cells, suggesting a regulatory role for IL-17A in the protective immune response against L. donovani. Interestingly, absence of IL-17A resulted in reduced accumulation of neutrophils and inflammatory monocytes into the infected tissues. Together these data identify IL-17A as an unexpected contributor for VL progression.
The expression of IL-17A during VL was found mainly in γδ T cells followed by CD4+ T cells. It is known that γδ T cells are a prominent source of IL-17A at early stages of Listeria and Mycobacterium infection (27, 30). γδ T cells produce IL-17A after stimulation with dendritic cell–derived cytokines such as IL-1β or IL-23 or after direct contact with pathogens (31). On the other hand, stimulation with TGF-β and IL-6 or IL-23 triggers IL-17A production by CD4 T cells (13). How IL-17 production is activated during VL needs to be further investigated. However, macrophages produce TGF-β and IL-6 after Leishmania infection and represent a candidate to induce IL-17A production during VL (32). Interestingly, both TGF-β and IL-6 play a detrimental role during VL. Indeed, mice deficient in IL-6 showed accelerated clearance of L. donovani and an increased IFN-γ response (33). Additionally, TGF-β has been largely related with susceptibility during Leishmaniasis, mainly by regulating IFN-γ production within the granuloma (34). It is not known whether deficiency in TGF-β or IL-6 results in a diminished IL-17A response during VL. It is possible that both TGF-β and IL-6 skew the T-cell response favoring Th17 while down-regulating Th1 generation. In fact, TGF-β and IL-6 promote the development of CD4+IL-17+IL-10+ cells, which are anti-inflammatory rather than inflammatory during EAE (35).
The role of IL-17A in regulating the IFN-γ response has been reported previously. In some scenarios, IL-17A is important for optimal IFN-γ production, whereas other reports have shown increased IFN-γ production in the absence of IL-17. During fungal infection, an IL-23-dependent Th17 response impaired fungal clearance by down-regulating IFN-γ production (36). Furthermore, during T. cruzi and Toxoplasma gondii infection, absence of IL-17RA resulted in an enhanced IFN-γ response, which caused exaggerated inflammation in the infected organs and enhanced mortality (37, 38). Additionally, the number of CD8+ and CD4+ T cells producing IFN-γ during EAE was augmented significantly in IL-17A−/− mice (14). Despite the fact that these data support a role for IL-17A in the negative regulation of IFN-γ production, other reports have found a synergism of IL-17A and IFN-γ acting in conjunction to eliminate pathogens. Recent findings showed that IL-17RA deficiency resulted in reduced IFN-γ and iNOS expression and enhanced IL-10 levels, rendering mice susceptible against L. infantum/chagasi (39). Also during Listeria monocytogenes infection, IL-17A was necessary to induce an adequate CD8+ cytolytic response (30). Additionally, IL-17A was required for an efficient Th1 response during Francisella tularensis infection by inducing IL-12 production by dendritic cells (15). Thus, IL-17A plays a dual role during infections depending on the pathogen. So far, it is not clear why in some scenarios IL-17A is protective and in others detrimental; however, a recent finding identified that Th17 cells induced by IL-23 were detrimental during EAE and Th17 cells induced by IL-6 and TGF-β were not (35). It is possible that the differential role of IL-17A during infection is the result of the cytokines involved in the priming of the IL-17 response.
At this point, it is not clear how IL-17A regulates IFN-γ production during VL. IL-17A may act indirectly by affecting the recruitment of regulatory populations. Indeed, during T. cruzi infection, IL-17A mediates the accumulation of IL-10-producing neutrophils, which impairs the IFN-γ response (37). Importantly, IL-17A promotes neutrophil expansion by enhancing granulocyte-colony stimulating factor production (40). The role of neutrophils during VL has been explored by depleting this population. Depletion of neutrophils during VL in C57BL/6 and BALB/c mice with the antibody RB6-8C5 resulted in enhanced parasitic loads in the liver and spleen (41). Also, depletion of neutrophils using a more specific antibody NIMP-R14 in BALB/c mice resulted in enhanced parasitic loads in the spleen and bone marrow, but not the liver. Neutrophil depletion also resulted in impaired granuloma formation, IFN-γ production, and iNOS expression (21). Interestingly, a recent study found that neutrophil depletion during infection with L. amazonensis resulted in enhanced IL-17 and IL-10 production (42). During VL, it is not known whether neutrophil depletion results in higher IL-17 production and has to be further analyzed. In our studies, the accumulation of neutrophils in the liver and spleen was unaffected by the absence of IL-17A during the first week of infection. However, later we found a significant reduction in neutrophil accumulation in the spleen and liver of IL-17A−/− mice. Our data suggest that IL-17A is not necessary for the initial accumulation of neutrophils during VL but is necessary in the chronic phase of the infection to maintain neutrophilic inflammation. A possible explanation for these contradictory findings is that neutrophils can play a protective role early during infection but a detrimental role during the chronic phase. Alternatively, other mechanisms unrelated with neutrophil accumulation may mediate the increased resistance of IL-17A−/− mice during VL. In contrast to the increased resistance of C57BL/6 against L. donovani in absence of IL-17A, administration of recombinant IL-17A enhanced protection against VL caused by L. donovani in BALB/c mice (24). The differences could be the result of the genetic differences in mice strains. We tested this possibility, and in our hands the administration of rIL-17A in C57BL/6 mice did not result in a significant change of the parasitic loads in the liver and spleen (data not shown), opening the possibility that IL-17A could have different roles depending on the mouse genetic background or L. donovani strain.
Absence of IL-17A during VL also resulted in reduced number of inflammatory monocytes. It is known that IL-17A also participates in the recruitment of monocytes (28, 29, 43). Despite studies showing that monocytes and neutrophils are related with protection against L. donovani (21, 44), we have recently found that accumulation of Ly6Chi monocytes is detrimental for L. donovani infection (unpublished results). Interestingly, the expression of IL-17RA in steady-state conditions is higher in Ly6Chi monocytes compared with other immune cells (28), suggesting that IL-17A may act mainly on inflammatory monocytes. The potential effect of IL-17A on inflammatory monocytes is not clear but may facilitate parasite uptake because IL-17A augmented the phagocytic activity of macrophages in vitro (45, 46). Also, treatment of inflammatory monocytes with IL-17 resulted in enhanced arginase activity and enhanced L. amazonensis replication (42). Thus, the resistant phenotype of IL-17A-deficient mice against L. donovani could be the result of the lack of accumulation of regulatory myeloid cells that may also serve as host for the parasite. In this regard, IL-17A could enhance the type of inflammation required to sustain the infection.
The granuloma formation in the liver of L. donovani–infected mice is largely associated with protection. Among the cytokines that induce the granuloma formation are IL-13 and IL-4 (9, 10). Here we found that absence of IL-17A resulted in reduced IL-4 production in the spleen and affected the number of granulomas found in the liver. Despite the low number of mature granulomas, IL-17A-deficient mice still controlled the infection, indicating that mature granulomas were not necessaries to restrain parasite burdens in IL-17A-deficient mice. Interestingly, IL-17A-producing γδ T cells were found to be important for the maturation of the granuloma in the lungs of M. bovis–infected mice (47). Interestingly, clusters of Ly6C monocytes and neutrophils were commonly found in WT mice but not in IL-17A−/− mice, suggesting that IL-17A may modulate the granuloma formation by facilitating the infiltration of these cells into the liver. Further detailed analysis of neutrophil and monocyte activation is necessary to explain their role during experimental VL. However, in this study we found similar levels of reactive oxygen species production in neutrophils of mice at 1 and 6 dpi and comparable expression levels of myeloperoxidase and neutrophil elastase in the liver of WT and IL-17A−/− mice at 6 dpi (not shown), indicating early neutrophil activation in the liver even in absence of IL-17A.
Immunity and susceptibility during VL have been difficult to fit in the classic Th1/Th2 paradigm (12). Contrary to most intracellular infections, the Th2 response has not been clearly related with susceptibility. In this regard, our work clearly demonstrates that the T-cell-derived cytokine IL-17A is involved in VL progression and highlights the complexity of cytokine regulation during this disease.
Acknowledgments
The authors thank Dr. Yoichiro Iwakura (Department of Biophysics and Biochemistry, The University of Tokyo, Tokyo, Japan) for providing the IL-17A−/− mice and Dr. Tracey L. Papenfuss (Department of Veterinary Biosciences, The Ohio State University) for evaluation of granuloma. P.B. was supported by U.S. National Institute of Health, National Institute of Allergy and Infectious Diseases Grant R01 AI043197.
Glossary
- CL
cutaneous leishmaniasis
- dpi
days postinfection
- EAE
experimental autoimmune encephalomyelitis
- GFP
green fluorescent protein
- LDU
Leishmania donovani units
- PMA
phorbol 12-myristate 13-acetate
- TCR
T-cell receptor
- Th
T helper
- VL
visceral leishmaniasis
- WT
wild-type
REFERENCES
- 1.McGwire B. S., Satoskar A. R. (2014) Leishmaniasis: clinical syndromes and treatment. QJM 107, 7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray H. W., Nathan C. F. (1999) Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 189, 741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oghumu S., Gupta G., Snider H. M., Varikuti S., Terrazas C. A., Papenfuss T. L., Kaplan M. H., Satoskar A. R. (2014) STAT4 is critical for immunity but not for antileishmanial activity of antimonials in experimental visceral leishmaniasis. Eur. J. Immunol. 44, 450–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W.-W., Mendez S., Ghosh A., Myler P., Ivens A., Clos J., Sacks D. L., Matlashewski G. (2003) Comparison of the A2 gene locus in Leishmania donovani and Leishmania major and its control over cutaneous infection. J. Biol. Chem. 278, 35508–35515 [DOI] [PubMed] [Google Scholar]
- 5.Chatelain R., Mauze S., Varkila K., Coffman R. L. (1999) Leishmania major infection in interleukin-4 and interferon-gamma depleted mice. Parasite Immunol. 21, 423–431 [DOI] [PubMed] [Google Scholar]
- 6.Rosas L. E., Snider H. M., Barbi J., Satoskar A. A., Lugo-Villarino G., Keiser T., Papenfuss T., Durbin J. E., Radzioch D., Glimcher L. H., Satoskar A. R. (2006) Cutting edge: STAT1 and T-bet play distinct roles in determining outcome of visceral leishmaniasis caused by Leishmania donovani. J. Immunol. 177, 22–25 [DOI] [PubMed] [Google Scholar]
- 7.Amprey J. L., Im J. S., Turco S. J., Murray H. W., Illarionov P. A., Besra G. S., Porcelli S. A., Späth G. F. (2004) A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J. Exp. Med. 200, 895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews D. J., Emson C. L., McKenzie G. J., Jolin H. E., Blackwell J. M., McKenzie A. N. J. (2000) IL-13 is a susceptibility factor for Leishmania major infection. J. Immunol. 164, 1458–1462 [DOI] [PubMed] [Google Scholar]
- 9.McFarlane E., Carter K. C., McKenzie A. N., Kaye P. M., Brombacher F., Alexander J. (2011) Endogenous IL-13 plays a crucial role in liver granuloma maturation during Leishmania donovani infection, independent of IL-4Rα-responsive macrophages and neutrophils. J. Infect. Dis. 204, 36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stäger S., Alexander J., Carter K. C., Brombacher F., Kaye P. M. (2003) Both interleukin-4 (IL-4) and IL-4 receptor alpha signaling contribute to the development of hepatic granulomas with optimal antileishmanial activity. Infect. Immun. 71, 4804–4807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stumhofer J. S., Hunter C. A. (2008) Advances in understanding the anti-inflammatory properties of IL-27. Immunol. Lett. 117, 123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander J., Bryson K. (2005) T helper (h)1/Th2 and Leishmania: paradox rather than paradigm. Immunol. Lett. 99, 17–23 [DOI] [PubMed] [Google Scholar]
- 13.Gu C., Wu L., Li X. (2013) IL-17 family: cytokines, receptors and signaling. Cytokine 64, 477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komiyama Y., Nakae S., Matsuki T., Nambu A., Ishigame H., Kakuta S., Sudo K., Iwakura Y. (2006) IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177, 566–573 [DOI] [PubMed] [Google Scholar]
- 15.Lin Y., Ritchea S., Logar A., Slight S., Messmer M., Rangel-Moreno J., Guglani L., Alcorn J. F., Strawbridge H., Park S. M., Onishi R., Nyugen N., Walter M. J., Pociask D., Randall T. D., Gaffen S. L., Iwakura Y., Kolls J. K., Khader S. A. (2009) Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31, 799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopal R., Monin L., Slight S., Uche U., Blanchard E., Fallert Junecko B. A., Ramos-Payan R., Stallings C. L., Reinhart T. A., Kolls J. K., Kaushal D., Nagarajan U., Rangel-Moreno J., Khader S. A. (2014) Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 10, e1004099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho J. S., Pietras E. M., Garcia N. C., Ramos R. I., Farzam D. M., Monroe H. R., Magorien J. E., Blauvelt A., Kolls J. K., Cheung A. L., Cheng G., Modlin R. L., Miller L. S. (2010) IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120, 1762–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang W., Na L., Fidel P. L., Schwarzenberger P. (2004) Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 190, 624–631 [DOI] [PubMed] [Google Scholar]
- 19.Lopez Kostka S., Dinges S., Griewank K., Iwakura Y., Udey M. C., von Stebut E. (2009) IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J. Immunol. 182, 3039–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Lombana C., Gimblet C., Bacellar O., Oliveira W. W., Passos S., Carvalho L. P., Goldschmidt M., Carvalho E. M., Scott P. (2013) IL-17 mediates immunopathology in the absence of IL-10 following Leishmania major infection. PLoS Pathog. 9, e1003243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFarlane E., Perez C., Charmoy M., Allenbach C., Carter K. C., Alexander J., Tacchini-Cottier F. (2008) Neutrophils contribute to development of a protective immune response during onset of infection with Leishmania donovani. Infect. Immun. 76, 532–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitta M. G. R., Romano A., Cabantous S., Henri S., Hammad A., Kouriba B., Argiro L., el Kheir M., Bucheton B., Mary C., El-Safi S. H., Dessein A. (2009) IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. J. Clin. Invest. 119, 2379–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terrazas C., Oghumu S., Varikuti S., Martinez-Saucedo D., Beverley S. M., Satoskar A. R. (2015) Uncovering Leishmania-macrophage interplay using imaging flow cytometry. J. Immunol. Methods 423, 93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh K., Sharma G., Saha A., Kar S., Das P. K., Ukil A. (2013) Successful therapy of visceral leishmaniasis with curdlan involves T-helper 17 cytokines. J. Infect. Dis. 207, 1016–1025 [DOI] [PubMed] [Google Scholar]
- 25.Bermejo D. A., Jackson S. W., Gorosito-Serran M., Acosta-Rodriguez E. V., Amezcua-Vesely M. C., Sather B. D., Singh A. K., Khim S., Mucci J., Liggitt D., Campetella O., Oukka M., Gruppi A., Rawlings D. J. (2013) Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors RORγt and Ahr that leads to IL-17 production by activated B cells. Nat. Immunol. 14, 514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray H. W. (2001) Tissue granuloma structure-function in experimental visceral leishmaniasis. Int. J. Exp. Pathol. 82, 249–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umemura M., Yahagi A., Hamada S., Begum M. D., Watanabe H., Kawakami K., Suda T., Sudo K., Nakae S., Iwakura Y., Matsuzaki G. (2007) IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 178, 3786–3796 [DOI] [PubMed] [Google Scholar]
- 28.Barin J. G., Baldeviano G. C., Talor M. V., Wu L., Ong S., Quader F., Chen P., Zheng D., Caturegli P., Rose N. R., Ciháková D. (2012) Macrophages participate in IL-17-mediated inflammation. Eur. J. Immunol. 42, 726–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahrara S., Pickens S. R., Dorfleutner A., Pope R. M. (2009) IL-17 induces monocyte migration in rheumatoid arthritis. J. Immunol. 182, 3884–3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu S., Han Y., Xu X., Bao Y., Zhang M., Cao X. (2010) IL-17A-producing gammadeltaT cells promote CTL responses against Listeria monocytogenes infection by enhancing dendritic cell cross-presentation. J. Immunol. 185, 5879–5887 [DOI] [PubMed] [Google Scholar]
- 31.Sutton C. E., Lalor S. J., Sweeney C. M., Brereton C. F., Lavelle E. C., Mills K. H. G. (2009) Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 [DOI] [PubMed] [Google Scholar]
- 32.Moore K. J., Matlashewski G. (1994) Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J. Immunol. 152, 2930–2937 [PubMed] [Google Scholar]
- 33.Murray H. W. (2008) Accelerated control of visceral Leishmania donovani infection in interleukin-6-deficient mice. Infect. Immun. 76, 4088–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson M. E., Young B. M., Davidson B. L., Mente K. A., McGowan S. E. (1998) The importance of TGF-beta in murine visceral leishmaniasis. J. Immunol. 161, 6148–6155 [PubMed] [Google Scholar]
- 35.McGeachy M. J., Bak-Jensen K. S., Chen Y., Tato C. M., Blumenschein W., McClanahan T., Cua D. J. (2007) TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 8, 1390–1397 [DOI] [PubMed] [Google Scholar]
- 36.Zelante T., De Luca A., Bonifazi P., Montagnoli C., Bozza S., Moretti S., Belladonna M. L., Vacca C., Conte C., Mosci P., Bistoni F., Puccetti P., Kastelein R. A., Kopf M., Romani L. (2007) IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 37, 2695–2706 [DOI] [PubMed] [Google Scholar]
- 37.Tosello Boari J., Amezcua Vesely M. C., Bermejo D. A., Ramello M. C., Montes C. L., Cejas H., Gruppi A., Acosta Rodríguez E. V. (2012) IL-17RA signaling reduces inflammation and mortality during Trypanosoma cruzi infection by recruiting suppressive IL-10-producing neutrophils. PLoS Pathog. 8, e1002658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guiton R., Vasseur V., Charron S., Arias M. T., Van Langendonck N., Buzoni-Gatel D., Ryffel B., Dimier-Poisson I. (2010) Interleukin 17 receptor signaling is deleterious during Toxoplasma gondii infection in susceptible BL6 mice. J. Infect. Dis. 202, 427–435 [DOI] [PubMed] [Google Scholar]
- 39.Nascimento M. S. L., Carregaro V., Lima-Júnior D. S., Costa D. L., Ryffel B., Duthie M., de Jesus A., de Almeida R. P., da Silva J. S. (2014) IL-17A acts synergistically with IFN-γ to promote protection against Leishmania infantum infection. J. Infect. Dis. 211, jiu531. [DOI] [PubMed] [Google Scholar]
- 40.Schwarzenberger P., Huang W., Ye P., Oliver P., Manuel M., Zhang Z., Bagby G., Nelson S., Kolls J. K. (2000) Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J. Immunol. 164, 4783–4789 [DOI] [PubMed] [Google Scholar]
- 41.Smelt S. C., Cotterell S. E., Engwerda C. R., Kaye P. M. (2000) B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J. Immunol. 164, 3681–3688 [DOI] [PubMed] [Google Scholar]
- 42.Sousa L. M. A., Carneiro M. B. H., Resende M. E., Martins L. S., Dos Santos L. M., Vaz L. G., Mello P. S., Mosser D. M., Oliveira M. A. P., Vieira L. Q. (2014) Neutrophils have a protective role during early stages of Leishmania amazonensis infection in BALB/c mice. Parasite Immunol. 36, 13–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shahrara S., Pickens S. R., Mandelin A. M. II, Karpus W. J., Huang Q., Kolls J. K., Pope R. M. (2010) IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J. Immunol. 184, 4479–4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cervia J. S., Rosen H., Murray H. W. (1993) Effector role of blood monocytes in experimental visceral leishmaniasis. Infect. Immun. 61, 1330–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erdmann H., Roßnagel C., Böhme J., Iwakura Y., Jacobs T., Schaible U. E., Hölscher C. (2013) IL-17A promotes macrophage effector mechanisms against Trypanosoma cruzi by trapping parasites in the endolysosomal compartment. Immunobiology 218, 910–923 [DOI] [PubMed] [Google Scholar]
- 46.Silverpil E., Glader P., Hansson M., Lindén A. (2011) Impact of interleukin-17 on macrophage phagocytosis of apoptotic neutrophils and particles. Inflammation 34, 1–9 [DOI] [PubMed] [Google Scholar]
- 47.Okamoto Yoshida Y., Umemura M., Yahagi A., O’Brien R. L., Ikuta K., Kishihara K., Hara H., Nakae S., Iwakura Y., Matsuzaki G. (2010) Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J. Immunol. 184, 4414–4422 [DOI] [PubMed] [Google Scholar]