Abstract
Activation of the sympathetic nervous system by stress increases breast cancer metastasis in vivo. Preclinical studies suggest that stress activates β-adrenoceptors (βARs) to enhance metastasis from primary tumors and that β-blockers may be protective in breast cancer. However, the subtype of βAR that mediates this effect, as well as the signaling mechanisms underlying increased tumor cell dissemination, remain unclear. We show that the β2AR is the only functionally relevant βAR subtype in the highly metastatic human breast cancer cell line MDA-MB-231HM. β2AR activation results in elevated cAMP (formoterol pEC50 9.86 ± 0.32), increased intracellular Ca2+ (formoterol pEC50 8.20 ± 0.33) and reduced phosphorylated ERK (pERK; formoterol pIC50 11.62 ± 0.31). We demonstrate that a highly amplified positive feedforward loop between the cAMP and Ca2+ pathways is responsible for efficient inhibition of basal pERK. Importantly, activation of the β2AR increased invasion (formoterol area under the curve [AUC] relative to vehicle: 1.82 ± 0.36), which was dependent on the cAMP/Ca2+ loop (formoterol AUC in the presence of 2′5′-dideoxyadenosine 0.64 ± 0.03, or BAPTA-AM 0.45 ± 0.23) but independent of inhibition of basal pERK1/2 (vehicle AUC with U0126 0.60 ± 0.30). Specifically targeting the positive feedforward cAMP/Ca2+ loop may be beneficial for the development of therapeutics to slow disease progression in patients with breast cancer.—Pon, C. K., Lane, J. R., Sloan, E. K., Halls, M. L. The β2-adrenoceptor activates a positive cAMP-calcium feedforward loop to drive breast cancer cell invasion.
Keywords: adrenergic receptor, GPCR, metastasis, stress
Stress causes activation of the sympathetic nervous system and the release of the catecholamines epinephrine and norepinephrine, the endogenous agonists of adrenoceptors. Preclinical studies have shown that stress or treatment with β-adrenoreceptor (βAR) agonists can accelerate cancer progression and the development of metastasis in distant organs (1–4). Furthermore, an association has been found between psychosocial factors such as chronic stress and depression and accelerated progression of cancer (5, 6). The effects of stress on breast cancer metastasis in vivo may be blocked by βAR antagonists (β-blockers), suggesting that βAR signaling is necessary for stress to enhance metastasis from primary tumors and that β-blockers may play a protective role in slowing breast cancer progression (2). Studies since have found an association between β-blocker use and improved breast cancer outcome (7–9). In particular, β-blocker use had a more favorable outcome in patients with triple-negative breast cancer (TNBC), which lack expression of estrogen, progesterone, and HER2 (human epidermal growth factor receptor 2) receptors and are a subset of more aggressive breast cancers (7, 9). TNBC patients prescribed β-blockers had improved relapse-free survival as well as reduced breast cancer–related recurrence, distant metastasis, and cancer-related death. Findings from these studies provide evidence that β-blockers may have potential as adjuvant therapy for patients with TNBC.
βARs are prototypical GPCRs that signal through G proteins to regulate various cellular events that are important for cancer progression, including proliferation, invasion, and activation of immune response (10). βAR expression has been reported in both tumor and stromal cells in the local tumor microenvironment (2, 11), suggesting that βARs in multiple cell types may be activated by stress. Consistent with this, βAR activation by stress drives recruitment of immune cells to primary mammary tumors (2). However, far less is known about whether stress can also directly activate βARs on tumor cells. Breast cancer cells express functional βARs, as seen by increased production of intracellular cAMP in response to βAR agonists (2, 12) and inhibition of basal phosphorylated ERK (pERK) in some breast cancer cells (13, 14). In these studies, activation of the βAR led to an inhibition of cell proliferation and decreased growth of primary tumors in vivo (13, 14). However, stress or βAR activation do not consistently increase primary tumor growth despite accelerating metastasis (2, 4). As there was a more favorable association reported between β-blocker use and outcome in TNBC, this may suggest that βARs play a unique role in the progression of this highly aggressive subset of breast cancer.
Given the increasing evidence that activation of βARs may promote tumor metastasis in TNBC, it is important to decipher which βAR subtype is activated in response to stress or βAR agonist treatment, to discover if there is a direct effect of βAR activation on the tumor cells, and to identify the signaling pathways involved in mediating these effects. In this present study, we examined the subtype of βAR activated in response to βAR agonist treatment in the highly metastatic variant human TNBC cell line MDA-MB-231HM. We identified the signaling pathways downstream of βAR activation and investigated the signaling mediators that control breast cancer cell invasion.
MATERIALS AND METHODS
Reagents
The following compounds were purchased from Sigma-Aldrich (St. Louis, MO, USA): (−)-propranolol, (−)-epinephrine, (−)-norepinephrine, 2′5′-dideoxyadenosine (ddA), KT5720, ESI-09, 8-bromoadenosine-3′,5′-cyclic monophosphate (8-Br-cAMP), N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (db-cAMP), forskolin, and ionomycin. Salbutamol hemisulfate, formoterol hemifumarate, salmeterol xinafoate, ICI-118551 hydrochloride, CGP-20712A dihydrochloride, and gallein were purchased from Tocris Bioscience (Bristol, United Kingdom). NF023 and NF449 were from Calbiochem (San Diego, CA, USA). [125I]-(−)-Cyanopindolol (ICYP) was purchased from PerkinElmer (Waltham, MA, USA). BAPTA-AM and U0126 were from Cayman Chemical (Ann Arbor, MI, USA). Compounds were dissolved in H2O or DMSO, and DMSO was used as the vehicle control in all experiments.
Cell culture and transfection
The highly metastatic variant of MDA-MB-231 human breast adenocarcinoma cells (MDA-MB-231HM) was the gift of Z. Ou (Fudan University Shanghai Cancer Center, Yangpu, Shanghai, China) (15), and cell identity was verified by CellBank Australia. The 66cl4 mammary adenocarcinoma cells were the gift of R. Anderson (Peter MacCallum Cancer Centre). Cells were cultured at 37°C in a 5% CO2 incubator. MDA-MB-231HM and parental MDA-MB-231 cells were cultured in DMEM supplemented with 5% v/v fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies, Carlsbad, CA, USA). 66cl4 cells were cultured in αMEM supplemented with 10% v/v FBS. For siRNA experiments, cells were electroporated with 50 nM On-Targetplus Smartpool siRNA (GE Dharmacon, Lafayette, CO, USA) using the Nucleofector Kit V and Amaxa Nucleofector (Lonza, Basel, Switzerland). Cells were seeded in 96-well plates at 4 × 104 cells per well for cAMP, Ca2+, and pERK1/2 assays or at 1 × 104 cells per well for proliferation assays, then serum starved overnight.
Gene expression
RNA was extracted using the RNeasy Kit (Qiagen, Limburg, The Netherlands), and quantitative real-time PCR (qRT-PCR) was performed in triplicate from 100 ng RNA using the iScript One-Step RT-PCR Kit (Bio-Rad, Hercules, CA, USA) and CFX96 Real Time System (Bio-Rad). TaqMan probes were from Applied Biosystems (Foster City, CA, USA): ADRB1 (Hs02330048_s1), ADRB2 (Hs00240532_s1), ADRB3 (Hs00609046_m1), and ACTB (Hs99999903_m1). Data were analyzed using the 2−ΔCt method and are expressed relative to ACTB (16).
Radioligand binding
Membranes were prepared from cells grown to 90% confluence. Cells were rinsed and scraped in homogenization buffer [5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 5 mM EDTA, pH 7.4], then homogenized with a Dounce homogenizer (10 strokes per pestle) and centrifuged (800 g, 10 min, 4°C). The supernatant was collected and the cell pellet subjected to another round of homogenization and centrifugation. Supernatants were pooled and centrifuged (40,000 g, 10 min, 4°C), and the pellet was resuspended in membrane buffer (50 mM Tris, 5 mM MgCl2, 1 mM EDTA, 200 mM sucrose, pH 7.2) and stored at −80°C.
Saturation binding experiments were performed at room temperature in binding buffer (50 mM Tris, 5 mM MgCl2, 1 mM EDTA, pH 7.2) in 96-well plates. Membranes (5 μg) were incubated with 1 pM to 100 pM ICYP for 1 h with and without antagonists: propranolol (700 nM, 100 × KD for β1AR), CGP-20712A (200 nM, 100 × KD for β1AR) or ICI-118551 (50 nM, 100 × KD for β2AR) (17). Reactions were terminated by filtration through presoaked GF/C filters (1% v/v polyethyleneimine, 30 min) using a Packard Cell Harvester (PerkinElmer). Filters were washed 4 times with 50 mM Tris (pH 7.4, 4°C), and radioactivity was measured using a Packard Top Count device (PerkinElmer). Results were corrected for nonspecific binding, determined by 700 nM propranolol.
cAMP accumulation
Cells were treated with antagonists or inhibitors in stimulation buffer (140 mM NaCl, 5 mM KCl, 800 nM MgSO4, 200 nM Na2HPO4, 440 nM KH2PO4, 5 mM HEPES, 1.3 mM CaCl2, 5.6 mM glucose, 0.1% w/v bovine serum albumin [BSA], 500 μM 3-isobutyl-1-methylxanthine, pH 7.4) for 30 min at 37°C. Agonists were diluted in stimulation buffer, and cells were stimulated for 10 min at 37°C. Cells were lysed in 50 μl ice-cold ethanol, evaporated, and reconstituted in 50 μl detection buffer (5 mM HEPES, 0.3% v/v Tween-20, 0.1% w/v BSA, pH 7.4). Cell lysates (5 μl) were transferred to a 384-well OptiPlate (PerkinElmer) and cAMP detected using the cAMP AlphaScreen Kit (PerkinElmer). Data are expressed as pmol/well or basal subtracted and expressed relative to the 100 μM forskolin response, as stated.
ERK1/2 phosphorylation
Changes in ERK1/2 phosphorylation (pERK) were detected using the AlphaScreen SureFire pERK1/2 Kit (PerkinElmer). Cells were treated with antagonists or inhibitors diluted in serum-free medium for 30 min at 37°C, followed by agonists for 15 min. Medium was aspirated, cells were lysed in 100 μl lysis buffer, and cell lysates (4 μl) were transferred to 384-well ProxiPlates (PerkinElmer) for detection. Data are expressed relative to basal.
Calcium mobilization
Cells were washed twice with assay buffer (150 mM NaCl, 2.6 mM KCl, 1.8 mM MgCl2, 10 mM d-glucose, 10 mM HEPES, 2.2 mM CaCl2, 0.5% w/v BSA, 4 mM probenecid, pH 7.4) and incubated with 1 μM Fluo4-AM (Life Technologies) for 1 h at 37°C. Cells were washed and treated with antagonists or inhibitors for 30 min at 37°C. Fluorescence was detected at 485 nm excitation and 525 nm emission for 30 s, agonists were added, and the fluorescence was measured every 1.5 s for 4 min using a FlexStation 3 (Molecular Devices, Sunnyvale, CA, USA). Data are expressed as the vehicle-subtracted area under the curve (AUC) or as the baseline-subtracted increase in fluorescence, as stated.
Cell proliferation
Cells were treated with vehicle or formoterol in 1% v/v FBS medium for 48 h, and proliferation was assessed using the CellTiter 96 Aqueous One Proliferation Assay (Promega, Madison, WI, USA). Data are expressed relative to vehicle-treated cells.
Cell invasion
Cell invasion assays were performed by xCELLigence Real-Time Cell Analysis (RTCA) using CIM-16 plates (Acea Biosciences, San Diego, CA, USA) (18) coated with 30 μl of 0.4 mg/ml Matrigel, and preincubated at 37°C for 4 h. A total of 180 μl of 10% v/v FBS medium containing ligand with or without inhibitors was added to the lower chambers, and 20 μl of serum-free medium with ligand and/or inhibitors was added to the upper chambers. The plate was incubated in the RTCA DP chamber (Acea Biosciences) at 37°C and 5% CO2 for 1 h, and a background measurement was taken. Cells were trypsinized and washed with serum-free medium; 3 × 104 cells were seeded in the upper chambers in serum-free medium containing ligands and/or inhibitors. After 30 min incubation at room temperature, measurements were taken every 15 min for the first 4 h, followed by 1 reading every hour for 120 h. The results are expressed relative to vehicle-treated cells at 120 h.
In vivo chronic stress metastasis model
All procedures involving mice were performed under protocols approved by the Institutional Animal Ethics Committee and in accordance with the animal ethics guidelines of the National Health and Medical Research Council of Australia. MDA-MB-231HM or 66cl4 cells were injected into the fourth left mammary fat pad of Balb/c nu/nu or Balb/c mice, respectively, and stress was induced as previously described (2, 4). Bioluminescence imaging (IVIS Lumina II; PerkinElmer) was used to track metastatic progression (2, 4). Data are expressed relative to nonstressed animals.
Statistical analysis
Data are expressed as means ± sem from at least 3 independent experiments performed in at least duplicate. GraphPad Prism 6 software (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis. Data were analyzed by 1-way ANOVA using Dunnett’s or Tukey’s multiple comparison test, or Fisher’s least significant difference test.
RESULTS
The β2AR is highly expressed in MDA-MB-231HM cells
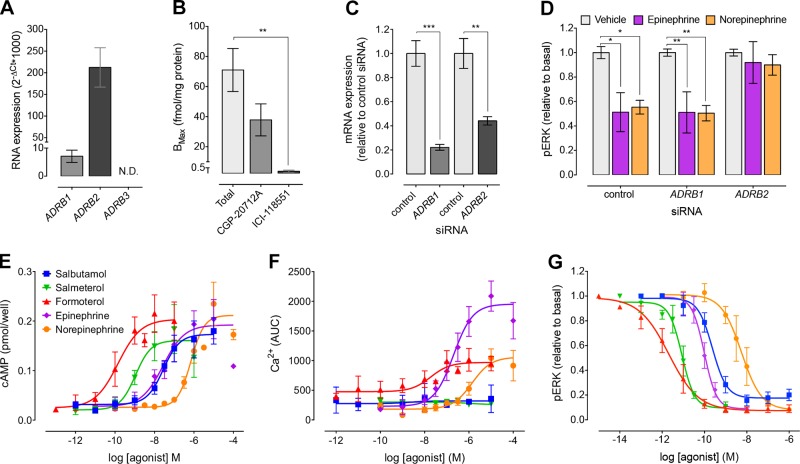
We used qRT-PCR to identify the βAR subtypes that are expressed in MDA-MB-231HM cells. These cells express both ADRB1 and ADRB2; however, ADRB3 mRNA levels were undetectable (Fig. 1A). Radioligand binding was used to quantitate the relative protein expression of β1AR and β2AR on cell membranes. The total number of βAR binding sites was 71 ± 14.31 fmol/mg protein (Fig. 1B). There was no significant effect on radioligand binding after the addition of a β1AR-selective antagonist, CGP-20712A (200 nM) (P = 0.086), which is 500-fold more selective for the β1AR than the β2AR (17). Conversely, addition of ICI-118551 (50 nM), a β2AR-selective antagonist that is 550-fold more selective for the β2AR than the β1AR (17), resulted in a 98% reduction in radioligand binding. Therefore, the β2AR is the predominant subtype expressed in MDA-MB-231HM cells. This is consistent with other studies showing higher expression of the β2AR relative to the β1AR in TNBC cell lines (12, 19).
Figure 1.
βAR subtype expression and signaling in MDA-MB-231HM cells. A) mRNA expression of βAR subtypes in MDA-MB-231HM cells (n = 5). B) Saturation binding in MDA-MB-231HM cell membranes using ICYP with/without CGP-20712A (200 nM) or ICI-118551 (50 nM) (n = 4). C) ADRB1 and ADRB2 mRNA was decreased in MDA-MB-231HM cells after expression of targeted siRNA (n = 4). D) Knockdown of β2AR, but not β1AR, abolished 10 nM epinephrine- and 100 nM norepinephrine-induced inhibition of pERK (n = 3). E) βAR agonists caused concentration-dependent increases in cAMP (n = 3–6). F) Only full βAR agonists caused concentration-dependent increases in intracellular Ca2+ (n = 4–5). G) βAR agonists caused concentration-dependent inhibition of basal pERK (n = 3–6). Symbols/bars represent means, error bars sem. ND, not detected. *P < 0.05, **P < 0.01, ***P < 0.001, 1-way ANOVA with Dunnett’s (A–C) or 2-way ANOVA with Tukey’s posttest (D).
The β2AR is the only functionally relevant subtype in MDA-MB-231HM cells: activation increases cAMP and Ca2+ mobilization but inhibits pERK
Because βARs preferentially couple to Gαs to activate adenylyl cyclase (AC) and increase cAMP, we first examined whether nonselective and βAR subtype-selective agonists could affect cAMP production in MDA-MB-231HM cells. Endogenous nonselective agonists (epinephrine and norepinephrine) and β2AR-selective agonists (salbutamol, salmeterol, and formoterol) all induced a concentration-dependent increase in cAMP (Fig. 1E). Although these cells expressed low levels of the β1AR at the RNA level, xamoterol, a β1AR-selective agonist, had no effect (data not shown). This suggests that the β2AR is the only functionally relevant subtype. Activation of the β2AR can also stimulate Ca2+ signaling (20), and Ca2+ signaling has been linked to tumor progression (21). Treatment of cells with formoterol, epinephrine, and norepinephrine increased Ca2+ mobilization; however, salbutamol and salmeterol (β2AR-selective partial agonists) and xamoterol (data not shown) had no effect (Fig. 1F). Previous studies in HEK293S cells overexpressing the β2AR also showed a very weak Ca2+ response to the partial agonists salbutamol and salmeterol compared to isoproterenol and epinephrine (20). The inability of the partial agonists to increase Ca2+ mobilization suggests that the β2AR may be less efficiently coupled to this pathway in MDA-MB-231HM cells. Finally, β2ARs can inhibit phosphorylation of ERK1/2 (pERK) in breast cancer cells (13, 14). Consistent with this, all βAR agonists except xamoterol (data not shown) caused a concentration-dependent inhibition of pERK in MDA-MB-231HM cells (Fig. 1G). Interestingly, all agonists were 100-fold more potent for pERK inhibition compared to activation of cAMP and Ca2+, suggesting that the β2AR is efficiently coupled to inhibition of pERK in these cells (Table 1). There was no effect of the βAR agonists on the activation of p38, JNK, Akt, and STAT3 measured using AlphaScreen assays (data not shown).
TABLE 1.
pEC50 and pIC50 values of βAR agonists for cAMP, Ca2+, and pERK
| Agonist | cAMP | Ca2+ | pERK |
|---|---|---|---|
| Formoterol | 9.86 ± 0.32 (4) | 8.20 ± 0.33 (5) | 11.62 ± 0.31 (6)*** |
| Salmeterol | 8.84 ± 0.14 (3) | ND | 11.11 ± 0.16 (3)*** |
| Salbutamol | 7.32 ± 0.21 (6) | ND | 9.69 ± 0.18 (4)*** |
| Epinephrine | 7.43 ± 0.33 (4) | 6.57 ± 0.12 (4) | 10.05 ± 0.11 (3)*** |
| Norepinephrine | 6.01 ± 0.01 (4) | 5.59 ± 0.15 (4) | 8.24 ± 0.28 (3)*** |
Agonist potencies expressed as mean ± sem pEC50 or pIC50 values for (n) experiments. ND, not detected. ***P < 0.001 compared to cAMP/Ca2+, 1-way ANOVA with Tukey’s posttest.
The epinephrine- and formoterol-induced changes in cAMP and pERK were further characterized by Schild analysis using propranolol, CGP-20712A, and ICI-118551 (nonselective, β1AR-selective, and β2AR-selective antagonists, respectively). Schild analysis is a pharmacologic method for receptor classification (22). It reports a pA2 value, which is a measure of the affinity of a competitive antagonist for its receptor, thereby allowing identification of the functionally relevant receptor subtype within a mixed receptor population. Both propranolol and ICI-118551 caused surmountable competitive antagonism of epinephrine and formoterol, as indicated by the rightward shift in the concentration–response curves in both cAMP and pERK signaling assays (Supplemental Figs. 1 and 2). In contrast, CGP-20712A had no effect. Propranolol, CGP-20712A, and ICI-118551 alone did not affect levels of cAMP, pERK1/2, or Ca2+ (data not shown). The pA2 values obtained (Table 2) were similar to the reported affinity (KD) of propranolol, CGP-20712A, and ICI-118551 for the β2AR (17). This indicates that βAR agonists modulate signaling via the β2AR, but not the β1AR, in MDA-MB-231HM cells. To confirm this, ADRB1 and ADRB2 expression was knocked down by at least 50% using targeted siRNA (Fig. 1C). In cells transfected with control siRNA, IC50 concentrations of epinephrine (10 nM) and norepinephrine (100 nM) inhibited pERK (Fig. 1D). There was no effect of ADRB1 siRNA; however, ADRB2 siRNA abolished this inhibition of pERK. Together, these results confirm that the β2AR is the only functionally relevant βAR subtype expressed in MDA-MB-231HM cells.
TABLE 2.
pA2 values for βAR antagonists from Schild analyses
| Signaling pathway | Agonist | Propranolol | CGP-20712A | ICI-118551 |
|---|---|---|---|---|
| cAMP | Formoterol | 10.31 ± 0.75 (3) | 6.14 ± 0.54 (3) | 9.45 ± 0.04 (3) |
| Epinephrine | 9.51 ± 0.38 (3) | 6.37 ± 0.35 (3) | 9.41 ± 0.13 (3) | |
| pERK | Formoterol | 10.25 ± 0.15 (5) | 5.91 ± 0.71 (5) | 10.11 ± 0.07 (5) |
| Epinephrine | 9.99 ± 0.11 (4) | 6.32 ± 0.26 (3) | 10.06 ± 0.08 (4) |
Data are expressed as means ± sem for (n) experiments.
A positive feedforward loop links cAMP and Ca2+ signaling in MDA-MB-231HM cells
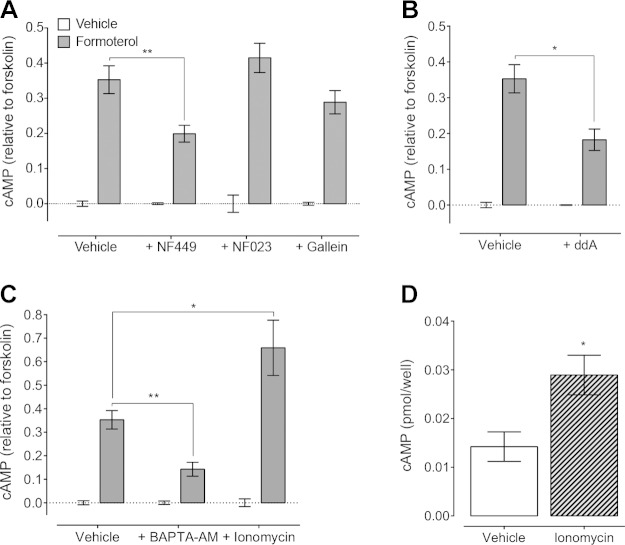
We next investigated the signaling hierarchy in MDA-MB-231HM cells after β2AR stimulation using a panel of inhibitors. To confirm that the β2AR stimulated cAMP production in a Gαs-dependent manner, cells were pretreated with a Gαs inhibitor, NF449 (23). Blockade of Gαs inhibited formoterol-induced cAMP, suggesting that the β2AR activates Gαs to increase cAMP (Fig. 2A). βARs can also couple to Gαi/o proteins (24, 25); however, NF023, a Gαi/o inhibitor (26), had no effect on formoterol-induced cAMP in these cells (P = 0.938). In addition to the Gα subunit, Gβγ subunits can also activate or inhibit different AC subtypes (27) to affect cAMP; however, treatment of cells with gallein (28) had no effect on cAMP after β2AR activation (P = 0.415). Finally, to determine if activation of AC was required for the increase in cAMP (rather than inhibition of the phosphodiesterases that degrade cAMP), cells were treated with ddA, an AC inhibitor (29). Preincubation of cells with ddA decreased formoterol-induced cAMP production (Fig. 2B). Therefore, the β2AR activates a Gαs-AC pathway to increase cAMP in MDA-MB-231HM cells.
Figure 2.
β2AR activation increases intracellular cAMP in MDA-MB-231HM cells through Gαs-AC and Ca2+ mobilization. A) Inhibition of Gαs (10 μM NF449), but not Gαi/o (10 μM NF023) or Gβγ subunits (50 μM gallein), decreased 1 nM formoterol-induced cAMP (n = 3–9). B, C) Formoterol (1 nM)-induced cAMP was also blocked after (B) inhibition of AC (100 μM ddA; n = 3–9) or (C) chelation of Ca2+ (20 μM BAPTA-AM; n = 4–9). Stimulation of Ca2+ influx by ionomycin (1 µM) further increased formoterol-induced cAMP (n = 5–9). D) Ionomycin alone increased basal cAMP production (n = 5–9). Bars represent means, error bars sem. *P < 0.05, **P < 0.01, ***P < 0.001, 2-way ANOVA with Sidak’s (B) or Dunnett’s (A, C) multiple comparison test or unpaired Student’s t test (D).
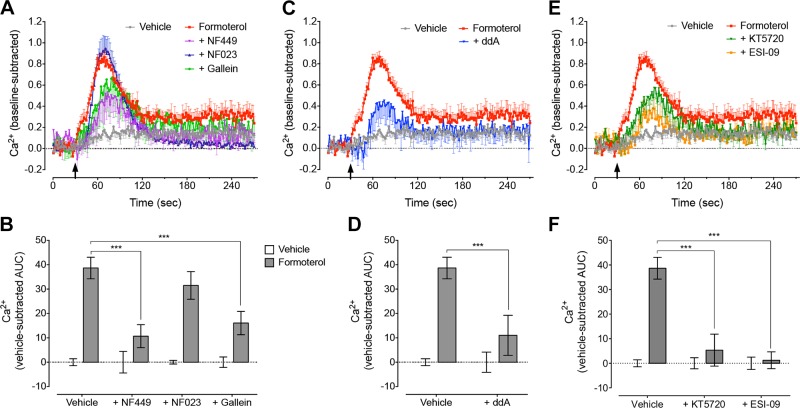
Next we investigated how activation of the β2AR induces Ca2+ mobilization. As observed for activation of cAMP signaling, increased Ca2+ mobilization was dependent on Gαs but independent of Gαi/o, as the Ca2+ response to formoterol was decreased after pretreatment of cells with NF449 but unaffected by NF023 (P = 0.543) (Fig. 3A, B). In contrast to the cAMP signaling pathway, increased Ca2+ mobilization was also dependent on Gβγ subunits, as preincubation of cells with gallein inhibited the formoterol-induced increase in intracellular Ca2+ (Fig. 3A, B). Stimulation of Ca2+ mobilization by formoterol was also dependent on cAMP signaling, as blockade of AC (ddA) or the cAMP effector proteins PKA or exchange protein directly activated by cAMP (Epac) with KT5720 (30) or ESI-09 (31), respectively, abolished formoterol activation of Ca2+ signaling (Fig. 3C–F). This suggests that 2 distinct pathways contribute to the increase in Ca2+ mobilization after activation of the β2AR: a direct activation by Gβγ subunits and an indirect activation that is dependent on cAMP and mediated by a Gαs-AC-cAMP-PKA/Epac pathway.
Figure 3.
Activation of β2AR induces Ca2+ mobilization by 2 pathways: directly by Gβγ subunits and indirectly by Gαs-AC-cAMP-PKA/Epac pathway. A, B) Formoterol (1 nM)-induced Ca2+ mobilization was lost after inhibition of Gαs (10 μM NF449) or Gβγ subunits (50 μM gallein), but not Gαi/o (10 μM NF023) (n = 3–6). C–F) Inhibition of AC (100 μM ddA) (C, D) and PKA or Epac (1 μM KT5720 or 50 μM ESI-09, respectively) (E, F) abolished Ca2+ responses to 1 nM formoterol (n = 3–9). Bars represent means, error bars sem. ***P < 0.001, 2-way ANOVA with Dunnett’s (B, F) or Sidak’s (D) multiple comparison test.
Cross-talk between Ca2+ and cAMP signaling pathways is well established (27). Given that β2AR-activated Ca2+ responses were partially dependent on cAMP (Fig. 3D, F), we next investigated whether Ca2+ signaling could also affect cAMP production. Interestingly, pretreatment of cells with BAPTA-AM (a Ca2+ chelator) (32) inhibited cAMP in response to formoterol (Fig. 2C). This suggests that the increase in intracellular Ca2+ induced by β2AR activation can lead to further cAMP production. This was confirmed by treatment of cells with ionomycin, a Ca2+ ionophore that causes Ca2+ influx (33). Ionomycin increased basal cAMP production and further increased formoterol-induced cAMP production to a greater degree than the effect of ionomycin on basal cAMP (Fig. 2C, D). These results suggest that a positive feedforward loop links cAMP and Ca2+ signaling in MDA-MB-231HM cells. Activation of the β2AR with formoterol stimulates a Gαs-AC pathway to increase cAMP; cAMP then activates PKA and Epac to increase Ca2+ mobilization, which is also influenced directly by Gβγ subunits; increases in intracellular Ca2+ then further increase cAMP.
The positive feedforward cAMP/Ca2+ loop leads to efficient inhibition of pERK
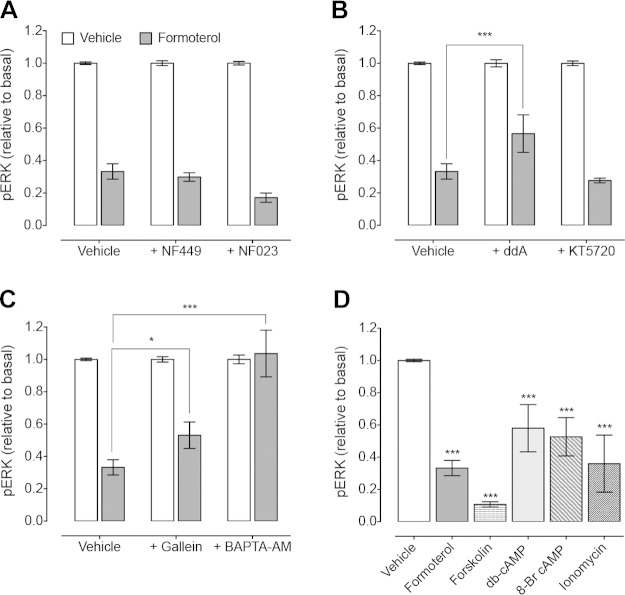
We then determined the signaling pathway that contributes to inhibition of pERK upon β2AR activation. There was no effect of inhibition of Gαs or Gαi/o using NF449 (P = 0.608) or NF023 (P = 0.069), respectively (Fig. 4A). However, inhibition of AC (ddA) partially reversed the formoterol-induced inhibition of pERK (Fig. 4B), suggesting that this response is dependent on cAMP. cAMP can inhibit ERK phosphorylation via a PKA-Src-Rap1 pathway, which blocks Ras activation, to prevent activation of the Ras-Raf1-ERK1/2 pathway (34–36). However, inhibition of PKA (KT5720) had no effect on the formoterol-induced inhibition of pERK (P = 0.668) (Fig. 4B). We could not determine any involvement of Epac, as preincubation of MDA-MB-231HM cells with ESI-09 significantly decreased basal levels of pERK such that no further inhibition was induced by formoterol (data not shown). However, inhibition of Ca2+ signaling (Gβγ subunits using gallein, or Ca2+ chelation with BAPTA-AM) reversed the formoterol-induced inhibition of pERK (Fig. 4C). These findings suggest that both cAMP and Ca2+ signaling contribute to the inhibition of pERK in response to formoterol.
Figure 4.
β2AR-mediated inhibition of pERK is dependent on the positive feedforward cAMP/Ca2+ loop. A) Formoterol (10 pM)-induced inhibition of pERK was unaffected after inhibition of Gαs (10 μM NF449) or Gαi/o (10 μM NF023) (n = 3–12). B) Blockade of AC (100 μM ddA) reversed inhibition of pERK by 10 pM formoterol, but inhibition of PKA (1 μM KT5720) had no effect (n = 5–11). C) Inhibition of Gβγ subunits (50 μM gallein) or Ca2+ chelation (20 μM BAPTA-AM) reversed 10 pM formoterol-induced inhibition of pERK (n = 3–11). D) Inhibition of pERK by 10 pM formoterol was mimicked by 10 μM forskolin, 100 μM db-cAMP, 100 μM 8-Br-cAMP, or 1 μM ionomycin (n = 5–11). Bars represent means, error bars sem. *P < 0.05, ***P < 0.001, 1-way ANOVA with Dunnett’s multiple comparison test (D) or 2-way ANOVA with Dunnett’s multiple comparison test (C, B).
The lack of effect of Gαs and PKA blockade on the formoterol-induced inhibition of pERK may be due to the very efficient coupling of the β2AR to this pathway, as suggested by the increased potency of β2AR agonists for pERK compared to both cAMP and Ca2+ (Table 1). Therefore, an alternative approach was used to confirm that activation of the cAMP/Ca2+ feedforward loop by formoterol leads to inhibition of basal pERK. Cells were treated with either forskolin, which activates AC to increase cAMP, or the synthetic cAMP analogs 8-Br-cAMP or db-cAMP (Fig. 4D). All compounds mimicked formoterol inhibition of pERK, reducing basal pERK levels by 90%, 53%, and 46%, respectively. Treatment of cells with ionomycin, a Ca2+ ionophore, also reduced basal pERK levels by 72%. This suggests that activation of the cAMP/Ca2+ feedforward loop can inhibit basal pERK.
Only the feedforward cAMP/Ca2+ loop, and not pERK inhibition, contributes to increased invasion
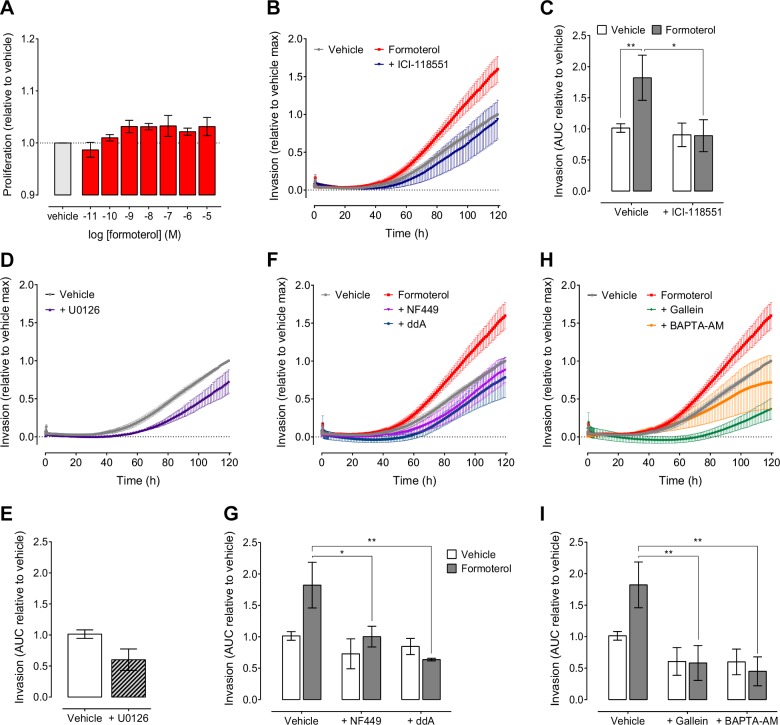
To determine the functional consequence of β2AR signaling in TNBC cells, we measured proliferation and invasion, 2 key steps in stress-induced metastasis. The MAPK signaling cascade has a major role in regulating cell growth and proliferation (37). Because activation of β2AR signaling in MDA-MB-231HM cells inhibited pERK, we examined whether proliferation was altered in these cancer cells. However, increasing concentrations of formoterol had no effect on cell proliferation (Fig. 5A). We then assessed tumor cell invasion in real time using xCELLigence (18). An EMax concentration of formoterol (0.5 μM) significantly increased the invasion of MDA-MB-231HM cells over 5 d compared to vehicle-treated controls (Fig. 5B, C), and this was abolished in the presence of ICI-118551, suggesting that increased invasion is due to activation of the β2AR. To determine which signaling pathways were responsible for the β2AR-dependent increase in MDA-MB-231HM cell invasion, we used a panel of inhibitors. Treatment of cells with U0126, a MEK inhibitor, had no effect on the basal level of cell invasion (Fig. 5D, E). This suggests that inhibition of pERK after β2AR activation is not directly associated with increased cell invasion. Next we assessed whether formoterol-induced breast cancer cell invasion required activation of the cAMP/Ca2+ feedforward loop. Inhibition of Gαs and AC with NF449 and ddA, respectively, completely reversed formoterol-induced invasion (Fig. 5F, G). Moreover, blockade of Gβγ subunits with gallein and Ca2+ chelation with BAPTA-AM also abrogated cell invasion induced by formoterol (Fig. 5H, I). The inhibitors alone had no significant effect on basal invasion. These results indicate that formoterol induces breast cancer cell invasion through the activation of the β2AR-dependent positive feedforward cAMP/Ca2+ loop.
Figure 5.
Activation of β2AR by formoterol increases breast cancer cell invasion. A) Increasing concentrations of formoterol had no effect on proliferation of MDA-MB-231HM cells (n = 3). B, C) Formoterol (0.5 μM) induced invasion of MDA-MB-231HM cells over 5 d, and this was blocked by 100 nM ICI-118551 (n = 3–10). D, E) Inhibition of MEK (10 μM U0126) had no effect on invasion (n = 3). F–I) The effect of formoterol on invasion was blocked after inhibition of Gαs (10 μM NF449) or AC (100 μM ddA) (F, G) or Gβγ subunits (50 μM gallein) or Ca2+ chelation (20 μM BAPTA-AM) (H, I) (n = 3–10). Symbols/bars represent means, error bars sem. *P < 0.05, **P < 0.01, ***P < 0.001, 1-way ANOVA with Fisher’s least significant difference test.
To determine whether the positive feedforward cAMP/Ca2+ loop was a common feature in other breast cancer cells, we used the less aggressive parental MDA-MB-231 cells and a 66cl4 mammary adenocarcinoma cell line that metastasizes in response to stress in vivo via βARs (2). The 66cl4 cells showed a significantly delayed onset of metastasis in response to stress compared to MDA-MB-231HM cells (Supplemental Fig. 3A). Parental MDA-MB-231 cells expressed both ADRB1 and ADRB2, whereas the 66cl4 cells only expressed adrb 2 at the mRNA level (Supplemental Fig. 3B, C). Interestingly, in both cell lines, β2AR agonists stimulated a much smaller increase in cAMP production compared to MDA-MB-231HM cells (Supplemental Fig. 3D–F). Moreover, there was no effect of the β2AR agonists on Ca2+ mobilization, and β2AR activation had varying effects on pERK (Supplemental Fig. 3G–L). It is possible that the lack of a Ca2+ signal, and therefore the absence of the feedforward cAMP/Ca2+ loop in these cells, may explain the low levels of cAMP production and the slower development of metastasis in response to stress.
DISCUSSION
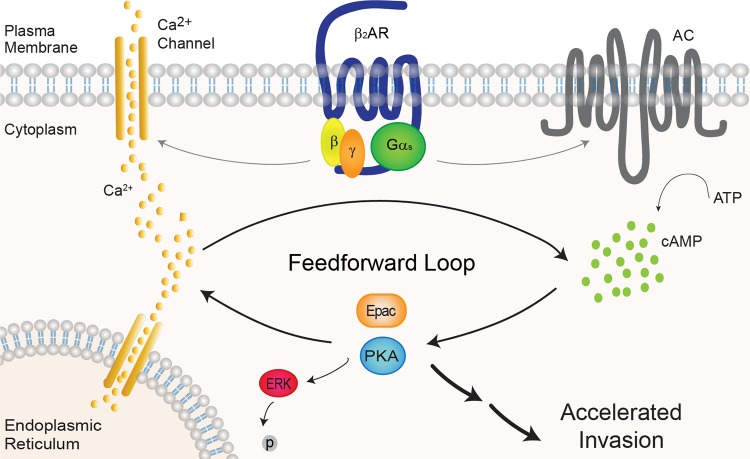
We have previously reported that activation of the βAR by chronic stress increases metastasis from primary tumors in mouse orthotopic models of cancer (2, 4). Retrospective studies have also demonstrated a clinical association between β-blocker usage and reduced distant metastasis, cancer recurrence, and mortality (7–9, 11). However, the subtype of βAR that mediates this effect, whether the increased sympathetic activity acts directly on the tumor and/or via the tumor microenvironment, and the cellular mechanisms underlying increased metastatic dissemination, remain unclear. The results presented here uncover molecular mechanisms that may underlie the capacity of stress to promote cancer metastasis at the level of the tumor cell. We show that the β2AR is highly expressed and is the only functionally relevant βAR subtype in MDA-MB-231HM breast cancer cells. β2AR activation results in elevated cAMP, increased intracellular Ca2+, and reduced pERK levels. We reveal a highly amplified positive feedforward loop between the cAMP and Ca2+ pathways, which is responsible for efficient inhibition of basal pERK (Fig. 6). Importantly, we link the positive cAMP/Ca2+ loop, but not inhibition of pERK, to increased invasion of MDA-MB-231HM cells.
Figure 6.
β2AR activates a positive feedforward cAMP/Ca2+ loop in MDA-MB-231HM cells to increase invasion. Upon agonist binding to β2AR, Gαs activates AC, which stimulates cAMP production. In highly metastatic breast cancer cells, Gβγ subunits activate Ca2+ mobilization, which is further increased by cAMP effector proteins PKA and Epac. This increase in Ca2+ feeds back to further stimulate cAMP production. Activation of the positive feedforward cAMP/Ca2+ loop identified in MDA-MB-231HM cells results in inhibition of pERK1/2 and independently accelerates breast cancer cell invasion.
The β2AR, a predominantly Gαs-coupled receptor, increased cAMP production in response to endogenous and β2AR-selective agonists, consistent with other studies in breast cancer cells (2, 12, 19). We show that this increase in cAMP is due to Gαs activation of AC and was not affected by Gαi/o. Activation of the β2AR increased intracellular Ca2+ by 2 pathways: directly via Gβγ subunits and indirectly via a pathway involving the cAMP-dependent PKA and Epac. Interestingly, this increase in Ca2+ signaling also activated the cAMP pathway. This suggests a novel feedforward mechanism activated by β2AR signaling in MDA-MB-231HM cells in which initial G protein coupling increases both cAMP and Ca2+ mobilization. The cAMP effectors PKA and Epac further increase intracellular Ca2+ levels, and elevated Ca2+ leads to further stimulation of AC and additional increases in cAMP to amplify the signal (Fig. 6). A synergistic relationship between cAMP and Ca2+ signaling has long been observed (27, 38, 39). cAMP itself, in addition to its effectors PKA and Epac, can have direct effects on numerous aspects of Ca2+ signaling, including activation of hyperpolarization-activated cyclic nucleotide–gated channels, voltage-gated Ca2+ channels, and inositol trisphosphate (IP3) receptors (27). Indeed, after β2AR activation, cAMP-dependent PKA can either directly phosphorylate l-type voltage-gated Ca2+ channels to stimulate Ca2+ influx (40) or activate the ryanodine receptor (41) to mobilize Ca2+ from IP3-gated intracellular stores (20). Epac can also directly increase intracellular Ca2+ by activating a Rap GTPase–phospholipase C-IP3 receptor pathway in HEK293 cells (42, 43). Conversely, it is also well established that Ca2+ signaling can regulate ACs and thereby modulate intracellular cAMP levels, either directly by Ca2+ and/or calmodulin binding or via effectors including calmodulin kinase, PKC, or calcineurin (27). In MDA-MB-231HM cells, activation of this positive feedforward loop mediates 2 distinct and independent events: highly efficient inhibition of pERK, demonstrated by significantly increased potency of all ligands in pERK assays compared to cAMP and Ca2+ assays (Table 1), and increased cell invasion.
The 2 cAMP effectors, PKA and Epac, have been linked to increased tumor cell invasion. PKA can activate Src, a kinase involved in the regulation of cell survival, motility, and invasion (44). Indeed, hyperactivation of PKA signaling and therefore elevated Src phosphorylation (Ser17 and Tyr419) are associated with accelerated mammary tumorigenesis (45). Moreover, previous studies have linked βAR-dependent PKA activation to the stimulation of Src and increased cancer cell invasion. In ovarian cancer cells, stimulation of βAR leads to cAMP-dependent PKA phosphorylation of Src at Ser17 and Tyr419, which was required for tumor cell invasion and stress-induced tumor growth (46). Similarly, activation of βARs in MDA-MB-231 cells caused PKA activation of Src, which was essential for invasion (47). There is also evidence for an involvement of cAMP-Epac pathways in tumor cell invasion. Activation of Epac is important for pancreatic cancer cell migration and invasion (31), and in fibrosarcoma cells, the Gαs-cAMP-Epac pathway was linked to the formation of invadapodia (48), plasma membrane–localized complexes that are important for invasion. Interestingly, there is also evidence that the Gαs-cAMP-Epac pathway can regulate Src kinase activity (49). As such, we favor the hypothesis that Src links the feedforward cAMP/Ca2+ loop to enhanced MDA-MB-231HM cell invasion.
The link between increased cell invasion and the positive feedforward cAMP/Ca2+ loop has important ramifications for TNBC, as the main cause of mortality in breast cancer patients is metastatic dissemination of the primary tumor (50). Tumor cells are exposed to multiple growth factors and cytokines in the local tumor microenvironment, which critically influences cancer progression to metastasis (51). The transformation of tumor cells into a metastatic phenotype in response to the tumor microenvironment requires epithelial to mesenchymal transition. In invasive breast cancer, this involves significant reorganization of plasma membrane domains and the underlying cytoskeleton (52, 53). In fact, the remodeling of lipid-rich plasma membrane domains is highly associated with the transition to more aggressive breast carcinomas (52, 54). Interestingly, Ca2+/calmodulin activation of AC requires direct interactions with A kinase anchoring protein, AKAP79, which in turn scaffolds the Ca2+ channel components STIM1 and Orai1 in proximity to the AC in lipid-rich domains (27, 55, 56). In addition to interacting with AC/STIM1/Orai1, AKAP79 can also interact with the β2AR to form large signaling complexes, often dependent on lipid-rich plasma membrane domains (57–60). It may be that the plasma membrane organization within these highly invasive cells facilitates the positive feedforward loop between cAMP and Ca2+ signaling pathways, thus promoting increased invasion.
Retrospective clinical studies have reported a variable strength of association between β-blocker use and cancer outcomes. Although some studies have reported that breast cancer patients receiving β-blockers have a more favorable outcome, defined by reduced distant metastasis, reduced recurrence, and reduced mortality (7–9, 11, 61), others found no association between β-blocker use and outcome (62–65). Interestingly, β-blocker use in TNBC patients was reported to have a more favorable outcome compared to estrogen receptor–positive breast cancer patients (7, 9). It is plausible that in addition to β2AR signaling within the tumor microenvironment (2), the positive feedforward cAMP/Ca2+ loop identified here may occur in a tumor cell–type dependent manner that could impact β-blocker efficacy in vivo. Indeed, previous studies have reported that β2AR-dependent inhibition of pERK leads to reduced cell proliferation and/or tumor growth in the breast cancer cell lines MDA-MB-231, IBH-4, and IBH-6 and in the normal mammary epithelial cell line MCF10A (13, 14, 60). The authors suggested that β2AR agonists may be useful adjuvant treatments for breast cancer. This directly contrasts with our finding that β-blockers would be useful treatments for TNBC, as they prevented invasion in the highly metastatic variant of the MDA-MB-231 cell line used in this study (15). These important differences in the outcome of β2AR activation in distinct tumor cell types could be influenced by factors including the organization and expression levels of proteins required for efficient coordination of cAMP and Ca2+ signaling. In support of this, the feedforward cAMP/Ca2+ loop was absent in parental MDA-MB-231 and 66cl4 cells, and this correlated with lower levels of cAMP after β2AR activation and a delayed onset of metastasis in response to stress. Alternatively, the disparate effects of β-blockers in retrospective clinical studies could be due to the subtype selectivity of different β-blockers: β1AR-selective β-blockers are more commonly used clinically and have been shown to have no beneficial effect on cancer outcome (8). It will be essential for future prospective studies to assess the contribution of subtype selective β-blockers and breast cancer subtypes on cancer outcome.
In summary, we have identified the β2AR as the only functionally relevant βAR subtype in MDA-MB-231HM cells. Activation of the β2AR in these breast cancer cells results in a positive feedforward loop between cAMP and Ca2+ signaling that causes increased invasion. The identification of a tightly coupled signaling loop that is activated by β2ARs may be beneficial for future development of therapeutics to slow cancer progression in patients with aggressive TNBC.
Supplementary Material
Acknowledgments
The authors thank R. J. Summers (Drug Discovery Biology Theme, Monash Institute of Pharmaceutical Sciences, Monash University) and members of the laboratories of M.L.H. and E.K.S. for critical discussion. This work was supported by a National Health and Medical Research Council R. D. Wright Fellowship (1061687; Canberra, ACT, Australia) to M.L.H., a Drug Discovery Biology Theme Strategic grant to M.L.H., J.R.L., and E.K.S., and funding from the Australian National Health and Medical Research Council (1008865), the U.S. National Institutes of Health/National Cancer Institute (CA160890), and the National Breast Cancer Foundation (ECR-11-11).
Glossary
- 8-Br-cAMP
8-bromoadenosine-3′,5′-cyclic monophosphate
- AC
adenylyl cyclase
- AUC
area under the curve
- βAR
β-adrenoceptor
- BSA
bovine serum albumin
- db-cAMP
N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate
- ddA
2′5′-dideoxyadenosine
- Epac
exchange protein directly activated by cAMP
- FBS
fetal bovine serum
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- ICYP
[125I]-(−)cyanopindolol
- IP3
inositol trisphosphate
- pERK
phosphorylated ERK1/2
- qRT-PCR
quantitative real-time PCR
- TNBC
triple-negative breast cancer
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Thaker P. H., Han L. Y., Kamat A. A., Arevalo J. M., Takahashi R., Lu C., Jennings N. B., Armaiz-Pena G., Bankson J. A., Ravoori M., Merritt W. M., Lin Y. G., Mangala L. S., Kim T. J., Coleman R. L., Landen C. N., Li Y., Felix E., Sanguino A. M., Newman R. A., Lloyd M., Gershenson D. M., Kundra V., Lopez-Berestein G., Lutgendorf S. K., Cole S. W., Sood A. K. (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 12, 939–944 [DOI] [PubMed] [Google Scholar]
- 2.Sloan E. K., Priceman S. J., Cox B. F., Yu S., Pimentel M. A., Tangkanangnukul V., Arevalo J. M., Morizono K., Karanikolas B. D., Wu L., Sood A. K., Cole S. W. (2010) The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70, 7042–7052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell J. P., Karolak M. R., Ma Y., Perrien D. S., Masood-Campbell S. K., Penner N. L., Munoz S. A., Zijlstra A., Yang X., Sterling J. A., Elefteriou F. (2012) Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 10, e1001363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamkin D. M., Sloan E. K., Patel A. J., Chiang B. S., Pimentel M. A., Ma J. C. Y., Arevalo J. M., Morizono K., Cole S. W. (2012) Chronic stress enhances progression of acute lymphoblastic leukemia via β-adrenergic signaling. Brain Behav. Immun. 26, 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoni M. H., Lutgendorf S. K., Cole S. W., Dhabhar F. S., Sephton S. E., McDonald P. G., Stefanek M., Sood A. K. (2006) The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat. Rev. Cancer 6, 240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chida Y., Hamer M., Wardle J., Steptoe A. (2008) Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 5, 466–475 [DOI] [PubMed] [Google Scholar]
- 7.Melhem-Bertrandt A., Chavez-Macgregor M., Lei X., Brown E. N., Lee R. T., Meric-Bernstam F., Sood A. K., Conzen S. D., Hortobagyi G. N., Gonzalez-Angulo A. M. (2011) Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 29, 2645–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barron T. I., Connolly R. M., Sharp L., Bennett K., Visvanathan K. (2011) Beta blockers and breast cancer mortality: a population- based study. J. Clin. Oncol. 29, 2635–2644 [DOI] [PubMed] [Google Scholar]
- 9.Botteri E., Munzone E., Rotmensz N., Cipolla C., De Giorgi V., Santillo B., Zanelotti A., Adamoli L., Colleoni M., Viale G., Goldhirsch A., Gandini S. (2013) Therapeutic effect of β-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res. Treat. 140, 567–575 [DOI] [PubMed] [Google Scholar]
- 10.Barron T. I., Sharp L., Visvanathan K. (2012) Beta-adrenergic blocking drugs in breast cancer: a perspective review. Ther. Adv. Med. Oncol. 4, 113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powe D. G., Voss M. J., Habashy H. O., Zänker K. S., Green A. R., Ellis I. O., Entschladen F. (2011) Alpha- and beta-adrenergic receptor (AR) protein expression is associated with poor clinical outcome in breast cancer: an immunohistochemical study. Breast Cancer Res. Treat. 130, 457–463 [DOI] [PubMed] [Google Scholar]
- 12.Madden K. S., Szpunar M. J., Brown E. B. (2011) β-Adrenergic receptors (β-AR) regulate VEGF and IL-6 production by divergent pathways in high β-AR-expressing breast cancer cell lines. Breast Cancer Res. Treat. 130, 747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carie A. E., Sebti S. M. (2007) A chemical biology approach identifies a beta-2 adrenergic receptor agonist that causes human tumor regression by blocking the Raf-1/Mek-1/Erk1/2 pathway. Oncogene 26, 3777–3788 [DOI] [PubMed] [Google Scholar]
- 14.Pérez Piñero C., Bruzzone A., Sarappa M. G., Castillo L. F., Lüthy I. A. (2012) Involvement of α2- and β2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br. J. Pharmacol. 166, 721–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang X. Z., Li D. Q., Hou Y. F., Wu J., Lu J. S., Di G. H., Jin W., Ou Z. L., Shen Z. Z., Shao Z. M. (2008) Identification of the functional role of AF1Q in the progression of breast cancer. Breast Cancer Res. Treat. 111, 65–78 [DOI] [PubMed] [Google Scholar]
- 16.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 17.Baker J. G. (2005) The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br. J. Pharmacol. 144, 317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limame R., Wouters A., Pauwels B., Fransen E., Peeters M., Lardon F., De Wever O., Pauwels P. (2012) Comparative analysis of dynamic cell viability, migration and invasion assessments by novel real-time technology and classic endpoint assays. PLoS One 7, e46536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandewalle B., Revillion F., Lefebvre J. (1990) Functional beta-adrenergic receptors in breast cancer cells. J. Cancer Res. Clin. Oncol. 116, 303–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stallaert W., Dorn J. F., van der Westhuizen E., Audet M., Bouvier M. (2012) Impedance responses reveal β₂-adrenergic receptor signaling pluridimensionality and allow classification of ligands with distinct signaling profiles. PLoS One 7, e29420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteith G. R., Davis F. M., Roberts-Thomson S. J. (2012) Calcium channels and pumps in cancer: changes and consequences. J. Biol. Chem. 287, 31666–31673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arunlakshana O., Schild H. O. (1959) Some quantitative uses of drug antagonists. Br. Pharmacol. Chemother. 14, 48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hohenegger M., Waldhoer M., Beindl W., Böing B., Kreimeyer A., Nickel P., Nanoff C., Freissmuth M. (1998) Gsalpha-selective G protein antagonists. Proc. Natl. Acad. Sci. USA 95, 346–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abramson S. N., Martin M. W., Hughes A. R., Harden T. K., Neve K. A., Barrett D. A., Molinoff P. B. (1988) Interaction of beta-adrenergic receptors with the inhibitory guanine nucleotide-binding protein of adenylate cyclase in membranes prepared from cyc-S49 lymphoma cells. Biochem. Pharmacol. 37, 4289–4297 [DOI] [PubMed] [Google Scholar]
- 25.Xiao R. P., Ji X., Lakatta E. G. (1995) Functional coupling of the beta 2-adrenoceptor to a pertussis toxin–sensitive G protein in cardiac myocytes. Mol. Pharmacol. 47, 322–329 [PubMed] [Google Scholar]
- 26.Beindl W., Mitterauer T., Hohenegger M., Ijzerman A. P., Nanoff C., Freissmuth M. (1996) Inhibition of receptor/G protein coupling by suramin analogues. Mol. Pharmacol. 50, 415–423 [PubMed] [Google Scholar]
- 27.Halls M. L., Cooper D. M. (2011) Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harb. Perspect. Biol. 3, a004143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehmann D. M., Seneviratne A. M., Smrcka A. V. (2008) Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol. Pharmacol. 73, 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Londos C., Wolff J. (1977) Two distinct adenosine-sensitive sites on adenylate cyclase. Proc. Natl. Acad. Sci. USA 74, 5482–5486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kase H., Iwahashi K., Nakanishi S., Matsuda Y., Yamada K., Takahashi M., Murakata C., Sato A., Kaneko M. (1987) K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Commun. 142, 436–440 [DOI] [PubMed] [Google Scholar]
- 31.Almahariq M., Tsalkova T., Mei F. C., Chen H., Zhou J., Sastry S. K., Schwede F., Cheng X. (2013) A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol. Pharmacol. 83, 122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsien R. Y. (1981) A non-disruptive technique for loading calcium buffers and indicators into cells. Nature 290, 527–528 [DOI] [PubMed] [Google Scholar]
- 33.Morgan A. J., Jacob R. (1994) Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem. J. 300, 665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu J., Dent P., Jelinek T., Wolfman A., Weber M. J., Sturgill T. W. (1993) Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science 262, 1065–1069 [DOI] [PubMed] [Google Scholar]
- 35.Vossler M. R., Yao H., York R. D., Pan M. G., Rim C. S., Stork P. J. (1997) cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 89, 73–82 [DOI] [PubMed] [Google Scholar]
- 36.Schmitt J. M., Stork P. J. (2002) PKA phosphorylation of Src mediates cAMP’s inhibition of cell growth via Rap1. Mol. Cell 9, 85–94 [DOI] [PubMed] [Google Scholar]
- 37.McCubrey J. A., Steelman L. S., Chappell W. H., Abrams S. L., Wong E. W., Chang F., Lehmann B., Terrian D. M., Milella M., Tafuri A., Stivala F., Libra M., Basecke J., Evangelisti C., Martelli A. M., Franklin R. A. (2007) Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 1773, 1263–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasmussen H. (1981) Calcium and cAMP as Synarchic Messengers, John Wiley & Sons, Hoboken, NJ [Google Scholar]
- 39.Berridge M. J. (1975) The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv. Cyclic Nucleotide Res. 6, 1–98 [PubMed] [Google Scholar]
- 40.Benitah J. P., Alvarez J. L., Gómez A. M. (2010) l-Type Ca(2+) current in ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 48, 26–36 [DOI] [PubMed] [Google Scholar]
- 41.Kushnir A., Marks A. R. (2010) The ryanodine receptor in cardiac physiology and disease. Adv. Pharmacol. 59, 1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt M., Evellin S., Weernink P. A., von Dorp F., Rehmann H., Lomasney J. W., Jakobs K. H. (2001) A new phospholipase–C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat. Cell Biol. 3, 1020–1024 [DOI] [PubMed] [Google Scholar]
- 43.Ivonnet P., Salathe M., Conner G. E. (2015) Hydrogen peroxide stimulation of CFTR reveals an Epac-mediated, soluble AC-dependent cAMP amplification pathway common to GPCR signalling. Br. J. Pharmacol. 172, 173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W., Kovacevic Z., Peng Z., Jin R., Wang P., Yue F., Zheng M., Huang M. L., Jansson P. J., Richardson V., Kalinowski D. S., Lane D. J., Merlot A. M., Sahni S., Richardson D. R. (2015) The molecular effect of metastasis suppressors on Src signaling and tumorigenesis: new therapeutic targets. [E-pub ahead of print] Oncotarget doi: 10.18632/oncotarget.5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beristain A. G., Molyneux S. D., Joshi P. A., Pomroy N. C., Di Grappa M. A., Chang M. C., Kirschner L. S., Privé G. G., Pujana M. A., Khokha R. (2015) PKA signaling drives mammary tumorigenesis through Src. Oncogene 34, 1160–1173 [DOI] [PubMed] [Google Scholar]
- 46.Armaiz-Pena G. N., Allen J. K., Cruz A., Stone R. L., Nick A. M., Lin Y. G., Han L. Y., Mangala L. S., Villares G. J., Vivas-Mejia P., Rodriguez-Aguayo C., Nagaraja A. S., Gharpure K. M., Wu Z., English R. D., Soman K. V., Shahzad M. M., Zigler M., Deavers M. T., Zien A., Soldatos T. G., Jackson D. B., Wiktorowicz J. E., Torres-Lugo M., Young T., De Geest K., Gallick G. E., Bar-Eli M., Lopez-Berestein G., Cole S. W., Lopez G. E., Lutgendorf S. K., Sood A. K. (2013) Src activation by β-adrenoreceptors is a key switch for tumour metastasis. Nat. Commun. 4, 1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dezong G., Zhongbing M., Qinye F., Zhigang Y. (2014) Carvedilol suppresses migration and invasion of malignant breast cells by inactivating Src involving cAMP/PKA and PKCδ signaling pathway. J. Cancer Res. Ther. 10, 998–1003 [DOI] [PubMed] [Google Scholar]
- 48.Harper K., Arsenault D., Boulay-Jean S., Lauzier A., Lucien F., Dubois C. M. (2010) Autotaxin promotes cancer invasion via the lysophosphatidic acid receptor 4: participation of the cyclic AMP/EPAC/Rac1 signaling pathway in invadopodia formation. Cancer Res. 70, 4634–4643 [DOI] [PubMed] [Google Scholar]
- 49.Shi G. X., Jin L., Andres D. A. (2010) Src-dependent TrkA transactivation is required for pituitary adenylate cyclase–activating polypeptide 38–mediated Rit activation and neuronal differentiation. Mol. Biol. Cell 21, 1597–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weigelt B., Peterse J. L., van ’t Veer L. J. (2005) Breast cancer metastasis: markers and models. Nat. Rev. Cancer 5, 591–602 [DOI] [PubMed] [Google Scholar]
- 51.Hanahan D., Coussens L. M. (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 [DOI] [PubMed] [Google Scholar]
- 52.Ostapkowicz A., Inai K., Smith L., Kreda S., Spychala J. (2006) Lipid rafts remodeling in estrogen receptor–negative breast cancer is reversed by histone deacetylase inhibitor. Mol. Cancer Ther. 5, 238–245 [DOI] [PubMed] [Google Scholar]
- 53.Finger E. C., Giaccia A. J. (2010) Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 29, 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spychala J., Lazarowski E., Ostapkowicz A., Ayscue L. H., Jin A., Mitchell B. S. (2004) Role of estrogen receptor in the regulation of ecto-5′-nucleotidase and adenosine in breast cancer. Clin. Cancer Res. 10, 708–717 [DOI] [PubMed] [Google Scholar]
- 55.Willoughby D., Everett K. L., Halls M. L., Pacheco J., Skroblin P., Vaca L., Klussmann E., Cooper D. M. (2012) Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca2+ and cAMP signaling. Sci. Signal. 5, ra29 [DOI] [PubMed] [Google Scholar]
- 56.Willoughby D., Halls M. L., Everett K. L., Ciruela A., Skroblin P., Klussmann E., Cooper D. M. (2012) A key phosphorylation site in AC8 mediates regulation of Ca(2+)-dependent cAMP dynamics by an AC8-AKAP79-PKA signalling complex. J. Cell Sci. 125, 5850–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fraser I. D., Cong M., Kim J., Rollins E. N., Daaka Y., Lefkowitz R. J., Scott J. D. (2000) Assembly of an A kinase-anchoring protein-beta(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr. Biol. 10, 409–412 [DOI] [PubMed] [Google Scholar]
- 58.Head B. P., Patel H. H., Roth D. M., Lai N. C., Niesman I. R., Farquhar M. G., Insel P. A. (2005) G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J. Biol. Chem. 280, 31036–31044 [DOI] [PubMed] [Google Scholar]
- 59.Wright P. T., Nikolaev V. O., O’Hara T., Diakonov I., Bhargava A., Tokar S., Schobesberger S., Shevchuk A. I., Sikkel M. B., Wilkinson R., Trayanova N. A., Lyon A. R., Harding S. E., Gorelik J. (2014) Caveolin-3 regulates compartmentation of cardiomyocyte beta2-adrenergic receptor-mediated cAMP signaling. J. Mol. Cell. Cardiol. 67, 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruzzone A., Saulière A., Finana F., Sénard J. M., Lüthy I., Galés C. (2014) Dosage-dependent regulation of cell proliferation and adhesion through dual β2-adrenergic receptor/cAMP signals. FASEB J. 28, 1342–1354 [DOI] [PubMed] [Google Scholar]
- 61.Powe D. G., Voss M. J., Zänker K. S., Habashy H. O., Green A. R., Ellis I. O., Entschladen F. (2010) Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 1, 628–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sørensen G. V., Ganz P. A., Cole S. W., Pedersen L. A., Sørensen H. T., Cronin-Fenton D. P., Garne J. P., Christiansen P. M., Lash T. L., Ahern T. P. (2013) Use of β-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and risk of breast cancer recurrence: a Danish nationwide prospective cohort study. J. Clin. Oncol. 31, 2265–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li C. I., Daling J. R., Tang M. T., Haugen K. L., Porter P. L., Malone K. E. (2013) Use of antihypertensive medications and breast cancer risk among women aged 55 to 74 years. JAMA Intern. Med. 173, 1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holmes S., Griffith E. J., Musto G., Minuk G. Y. (2013) Antihypertensive medications and survival in patients with cancer: a population-based retrospective cohort study. Cancer Epidemiol. 37, 881–885 [DOI] [PubMed] [Google Scholar]
- 65.Ganz P. A., Habel L. A., Weltzien E. K., Caan B. J., Cole S. W. (2011) Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast Cancer Res. Treat. 129, 549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.