Abstract
Vascular smooth muscle (VSM) expresses calcium/calmodulin-dependent protein kinase II (CaMKII)-δ and -γ isoforms. CaMKIIδ promotes VSM proliferation and vascular remodeling. We tested CaMKIIγ function in vascular remodeling after injury. CaMKIIγ protein decreased 90% 14 d after balloon injury in rat carotid artery. Intraluminal transduction of adenovirus encoding CaMKIIγC rescued expression to 35% of uninjured controls, inhibited neointima formation (>70%), inhibited VSM proliferation (>60%), and increased expression of the cell-cycle inhibitor p21 (>2-fold). Comparable doses of CaMKIIδ2 adenovirus had no effect. Similar dynamics in CaMKIIγ mRNA and protein expression were observed in ligated mouse carotid arteries, correlating closely with expression of VSM differentiation markers. Targeted deletion of CaMKIIγ in smooth muscle resulted in a 20-fold increase in neointimal area, with a 3-fold increase in the cell proliferation index, no change in apoptosis, and a 60% decrease in p21 expression. In cultured VSM, CaMKIIγ overexpression induced p53 mRNA (1.7 fold) and protein (1.8-fold) expression; induced the p53 target gene p21 (3-fold); decreased VSM cell proliferation (>50%); and had no effect on expression of apoptosis markers. We conclude that regulated CaMKII isoform composition is an important determinant of the injury-induced vasculoproliferative response and that CaMKIIγ and -δ isoforms have nonequivalent, opposing functions.—Saddouk, F. Z., Sun, L.-Y., Liu, Y. F., Jiang, M., Singer, D. V., Backs, J., Van Riper, D., Ginnan, R., Schwarz, J. J., Singer, H. A. Ca2+/calmodulin-dependent protein kinase II-γ (CaMKIIγ) negatively regulates vascular smooth muscle cell proliferation and vascular remodeling.
Keywords: Camk2g, Camk2d, restenosis
Differentiated vascular smooth muscle (VSM) undergoes a phenotypic switch after injury or primary cell culture to a synthetic phenotype characterized by proliferation, migration, extracellular matrix remodeling, and proinflammatory function (1). This process includes changes in expression of proteins involved in intracellular Ca2+ homeostasis (2, 3). A significant challenge is to understand how Ca2+ signals are transduced to regulate the VSM phenotype switch and synthetic phenotype function and whether specific Ca2+ signaling pathways can be targeted to inhibit the vascular remodeling associated with injury and disease. As a mediator of Ca2+ signals, the multifunctional serine/threonine Ca2+/calmodulin-dependent protein kinase II (CaMKII) has been shown to regulate contractile activity (4, 5) and vascular remodeling (2).
There are 4 highly conserved CaMKII genes in mammals (types α, β, δ, and γ), and each undergoes extensive alternative splicing to produce a large number of variants (6). Although the 4 CaMKII genes encode highly homologous proteins, CaMKII genes and splice variants are differentially expressed in cells and tissues. Major CaMKII isoforms in VSM have been identified as alternatively spliced products from δ (7) and γ (8) genes, which form large heteromultimeric holoenzymes. A generally accepted concept is that CaMKII isoform diversity is functionally important, because specific, variably expressed domains can affect holoenzyme localization and protein interactions. Well-documented examples include an 11 aa sequence, that results in nuclear targeting of holoenzymes (9) and variable domains in some β subunits that link the kinase to the actin cytoskeleton (10). Variable domains in γ (4), and δ subunits (11) have been implicated in binding interactions that could link Ca2+ signals to intracellular signaling pathways involving nonreceptor tyrosine kinases and ERK1/2 activation. These and yet-to-be-discovered structural variations of CaMKII isoforms could have important functional consequences related to CaMKII-dependent regulation of VSM cell function.
Transition of VSM from a differentiated to synthetic phenotype after injury (12) or cell culture (13) includes marked changes in CaMKII holoenzyme composition with increased expression of the CaMKIIδ2 [or CaMKIIδC, by alternative nomenclature (7)]. Preventing increases in CaMKIIδ expression using short hairpin small interfering (si)RNA (13), or global genetic deletion of CaMKIIδ (14) inhibits VSM cell proliferation and migration in vitro and injury-induced vascular remodeling in vivo, indicating a role for CaMKIIδ in promoting VSM synthetic phenotype function. However, a major limitation for interpreting previous CaMKIIδ loss-of-function studies is the background expression of endogenous CaMKIIγ, which is normally expressed in mature smooth muscle at a level comparable to that of CaMKIIδ (12). Possible redundant, or even opposing, functions of CaMKIIγ in vascular remodeling have not been tested or incorporated into the interpretation of existing studies.
We used conditional knockout and adenoviral transduction–mediated overexpression approaches to test for the first time the function of CaMKIIγ gene products in VSM synthetic phenotype function. We demonstrated nonredundant, opposing net functions for the CaMKII-γ and -δ isoforms in regulating injury-induced vascular remodeling in vivo and direct inhibitory effects of CaMKIIγ on VSM cell proliferation, mediated by increased expression of the cell cycle inhibitors p53 and p21. The results indicate an important function for regulated expression of CaMKIIγ isoforms during VSM phenotype switching and in synthetic VSM phenotype function. We propose that decreased expression of CaMKIIγ is permissive of CaMKIIδ-dependent regulation of VSM synthetic phenotype function and that re-expression of CaMKIIγ after injury limits progression of vascular remodeling.
MATERIALS AND METHODS
Adenoviral constructs
CaMKIIγc and CaMKIIδ2 adenovirus vectors were constructed by using the AdEasy system (15) (Agilent Technologies, Santa Clara, CA, USA). CaMKIIγc was cloned from the previously described pRC/CMV-γc plasmid (8) with the forward primer 5′-AGA TCT GCC GCC ATG GAG CAG AAA CTC ATC TCT GAA GAG GAT CTG ATG GCC ACC ACC GCC ACC TGC A-3′ and the reverse primer 5′-AAG CCT GAG CTC ACT GCA GCG GTG CGGCA-3′. CaMKIIδ2 was cloned from the pBluescript II-KS+ plasmid (Agilent Technologies) (7), with the forward primer 5′- GAA GAT CTG CCA CCA TGG ATT ACA AGG ATG ACG ACG AAT AGA TGG CTT CGA CCA CCT GCA CCC-3′ and the reverse primer 5′-GAG AGA GCG GCC GCA GAA GAC CCA AAT GTG AAT-3′. The empty vector served as the green fluorescent protein (GFP) control. Adenoviruses expressing myocardin (16) and a short hairpin (sh)RNA of serum response factor (SRF) (17) were a generous gift from Dr. Joseph Miano (University of Rochester, Rochester, NY, USA). The viruses were amplified and purified as previously described (18).
Balloon angioplasty and adenoviral vector–mediated gene delivery in vivo
The use of experimental animals for all the procedures in this study was reviewed and approved by the Albany Medical College Institutional Care and Use Committee and Institutional Biosafety Committee. Male Sprague-Dawley rats (Taconic Farms, Germantown, NY, USA) were anesthetized with xylazine and ketamine (4.6 g and 70 mg/kg, respectively, i.p.) and balloon angioplasty was performed (12). In brief, a 2-French Fogarty balloon embolectomy catheter was inserted through a small arteriotomy in the external carotid artery and passed into the common carotid artery. After balloon inflation at 2 atm of pressure (6×), the catheter was partially withdrawn and reinserted 3 times. Adenoviral solutions encoding GFP, CaMKIIγc, or Ad-CaMKIIδ were infused via the arteriotomy through a 20-gauge catheter and incubated for 30 min. A ligation placed around the internal carotid artery proximal to the arteriotomy maintained pressure. The animals were allowed to recover after receiving a postoperative dose of analgesic (buprenorphine, 0.2 mg/kg, i.m.).
Genetic mouse models
Camk2d and Camk2g smooth muscle knockout (SMKO) mice were generated by breeding mixed-background B6;129;FVB floxed Camk2d (19) and Camk2g founders (20) with the Transgelin-Cre mouse line [B6.Cg-Tg (Tagln-Cre) 1Her/J; Jackson Laboratory, Bar Harbor, ME, USA].
Carotid artery ligation model
Mice (10–12-wk-old) were anesthetized with ketamine and xylazine (0.1 and 0.01 mg/g, respectively, i.p.). The left carotid artery was completely ligated just proximal to the carotid bifurcation after a midline incision of the neck. The right carotid artery served as the uninjured control. The left and right carotid arteries were harvested at the time points indicated in the figures for real-time quantitative PCR (qPCR), Western blot, or histologic analysis.
Histology and immunohistochemistry
For histology, the vessels were perfused with PBS, fixed in 4% paraformaldehyde overnight, dehydrated, and embedded in paraffin. Paraffin-embedded arteries were cut in 10 μm serial cross sections starting from the ligature, and predefined sections were mounted on slides and stained with hematoxylin and eosin (H&E). Photoshop CS4 software (Adobe Systems Inc., San Diego, CA, USA) was used to measure the circumferences of the internal elastic lamina, external elastic lamina, and lumen, as well as the medial and intimal cross-sectional areas. Assuming that a maximum potential lumen area is defined by the internal elastic circumference, luminal stenosis caused by neointimal hyperplasia was calculated as 1 − (actual lumen area/internal elastic lamina area).
For immunohistochemistry, paraffin-embedded sections were dewaxed and rehydrated and underwent antigen retrieval per standard protocol. After a 10 min incubation with 3% H2O2, the sections were blocked in PBS supplemented with 0.1% Tween and 2% bovine serum albumin for 30 min and incubated overnight 4°C with the following rabbit polyclonal antibodies: 1:1000 anti-Ki67 (ab15580) and 1:800 anti-GFP (ab290) (Abcam; Cambridge, MA, USA); 1:500 anti-p21 (sc-397; Santa Cruz Biotechnology, Santa Cruz, CA, USA); and 1:800 anti-FLAG (2368) (Cell Signaling Technology, Danvers, MA, USA). The primary antibodies were detected with biotinylated antibody to rabbit IgG followed by incubation with avidin-biotin complex (ABC) reagent (Vectastain Elite ABC Kit; Vector Laboratories, Burlingame, CA, USA), according to the manufacturer’s instructions. Staining was visualized by 3′3-diaminobenzidine (DAB) peroxidase substrate kit (Vector Laboratories), according to the manufacturer’s instructions, followed by counterstaining with hematoxylin. The percentage of positively stained area was determined with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Primary VSM cell isolation, culture, and adenoviral expression
Primary VSM cells were obtained from the medial layer of the thoracic aortas of 300–350 g Sprague-Dawley rats (Taconic Farms, Germantown, NY, USA) and 4-wk-old mice (13, 21). After the removal of the adventitial and endothelial layers, medial VSM cells were enzymatically dispersed and cultured in DMEM/F12 (Thermo Fisher, Grand Island, NY, USA), supplemented with 20% fetal bovine serum (FBS; HyClone, Logan, UT, USA), 100 U/ml penicillin, and 100 g/ml streptomycin at 37°C. Primary mouse VSM cell passages 3–7 were incubated with adenoviruses expressing GFP, CaMKIIγc, or CaMKIIδ2 at an MOI (multiplicity of infection) of 250. Subsequent experiments were conducted 48–72 h after infection. In experiments in freshly dispersed rat VSM cells, the cells were allowed to plate for 1 h and were infected with Ad-GFP, Ad-CaMKIIγ, and Ad-CaMKIIδ2 at an MOI of 100, in the presence of 10% FBS.
Real-time qPCR
RNA was isolated from the medial layer of aorta from 8- to 10-wk-old mice. Whole aortas were excised and cleaned. The adventitia was peeled off, and the vessel was denuded of its endothelial layer. RNA was also prepared from carotid artery samples, isolated 3 d and 3 wk after ligation, and their contralateral part. RNA was extracted with a combination of mechanical homogenization in Trizol (Thermo Fisher) and RNeasy columns (Qiagen, Valencia, CA, USA). The equivalent amounts of RNA from each sample were reverse transcribed, using QuantiTect reverse transcription reagents (Qiagen). Real-time qPCR was performed on an Mx3000P QPCR System (Agilent Technologies) with iQ SYBR Green supermix (Bio-Rad, Hercules, CA, USA). mRNA levels are expressed relative to Hprt. The oligonucleotide primers are listed in Supplemental Table S1.
Protein extraction and Western blot analysis
Proteins from whole tissue excised from rat or mouse thoracic aorta, carotid arteries, or primary VSM cells were extracted (12, 13). The proteins were separated by standard SDS-PAGE and transferred onto NitroPure nitrocellulose membranes (GE Healthcare, Pittsburgh, PA, USA), blocked in 5% nonfat milk and Tris-buffered saline with Tween 20. The following primary antibodies were used: rabbit polyclonal anti-peptide antibody specific for the C terminus in CaMKIIδ2 (PSCIPNGKENFSGSTSLWQNI) (7), anti-peptide corresponding to all CaMKIIγ subunits (567LNVHYHCSGAPAAPL581) (13), and pan-CaMKII antibody (39EYAAKIINTKKLSARDHQKLEREARICRLLK69) against a peptide in the catalytic domain. The latter sequence is conserved among the known CaMKII subunits (8). Goat anti-SM22α (1:1000; ab10135) was purchased from Abcam; mouse anti-calponin (1:500; 58707) and rabbit anti-p21 (1:100; sc-397) from Santa Cruz Biotechnology; rabbit anti-smooth muscle myosin heavy chain (1:1000; 5620313) from Biomedical Technologies (Stoughton, MA, USA); mouse anti-p53 (1:1000; 2524), rabbit anti-phosphorylated p53 (1:1000; Ser15;9284), and rabbit anti-phosphorylated mouse double minute (Mdm)-2 (1:1000; Ser166;3521) from Cell Signaling Technology. Mouse anti-GAPDH (Sigma-Aldrich, St. Louis, MO, USA) was used as the loading control. Purified recombinant protein standards for CaMKIIγb and -γc, were a gift from Dr. Roger Colbran (Vanderbilt University, Nashville, TN, USA). Secondary horseradish peroxidase (HRP)-conjugated antibodies (GE Healthcare) and chemiluminescent signals (SuperSignal West Pico; Thermo Fisher-Pierce Biotechnology, Rockford, IL, USA) were imaged and quantified with a Fuji LAS4000 Imaging Station (Fuji Film Corp., Tokyo, Japan).
Statistical analyses
Quantitative data are presented as means ± sem. Student’s unpaired t test was used in the comparisons between 2 groups. Prism6 (GraphPad, San Diego, CA, USA) was used for analysis. Two-way ANOVA with the Holm-Sidak post hoc test was used in Fig. 2 to compare differences between groups and across levels proximal to the ligature. Statistics were analyzed with SigmaPlot. Results were considered to be significantly different at P < 0.05.
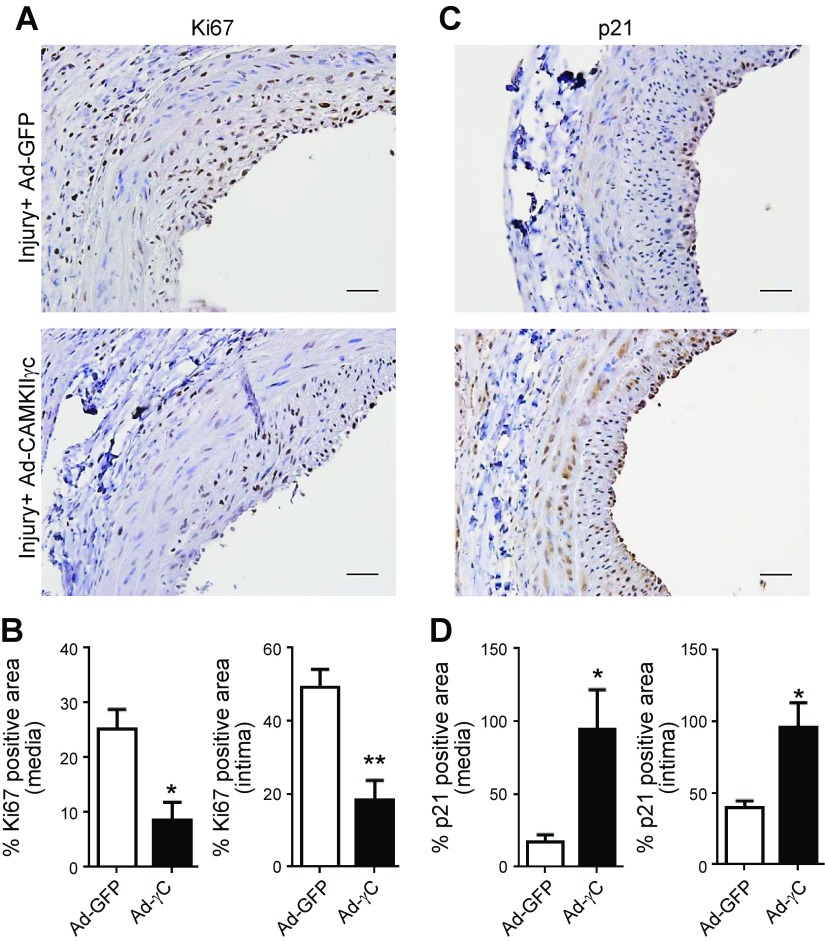
Figure 2.
Expression of CaMKIIγ in vivo inhibits balloon injury–induced VSM proliferation. A, C) Representative images of Ki67 (A) and p21 (CDKN1A) (C) immunohistochemistry on sections of rat carotid arteries transduced with Ad-GFP or CaMKIIγC and harvested 14 d after injury. Scale bars, 20 μm. B, D) Quantification of Ki67+ (B) and p21+ (D) medial and neointimal areas (n = 4). Data are means ± sem. *P < 0.05; **P < 0.01 vs. Ad-GFP-treated controls.
RESULTS
CaMKIIγ gene transfer prevents angioplasty-associated restenosis and VSM proliferation
Studies have established that expression of the CaMKIIδ isoform in VSM promotes vascular injury-induced remodeling (12, 14) and VSM cell proliferation (13, 14). Confirming our earlier observations (12), within 7 d after balloon angioplasty injury of the rat carotid artery, CaMKIIγ protein levels were suppressed 90%, coincident with a 70% down-regulation of the specific smooth muscle contractile protein marker, smooth muscle myosin heavy chain (Myh11) and Tagln (sm-22 sm-22α) compared with expression in the contralateral uninjured artery (Fig. 1A, B). To evaluate the functional consequences of CaMKIIγ down-regulation in this model, expression was rescued by luminal delivery of adenovirus encoding a GFP-tagged wild-type (WT) CaMKIIγC construct (Ad-CaMKIIγc) at the time of injury. Western blot analysis with an antibody specific to the CaMKIIγ C terminus indicated partial rescue of CaMKIIγc protein expression to ∼35% of uninjured control levels 7 d after injury, with distribution in both the medial and neointimal layers (Supplemental Fig. S1). CaMKIIγc rescue had no statistically significant effect on injury-induced CaMKIIδ levels or on expression of the smooth muscle differentiation markers Myh11 and Tagln (Fig. 1A, B).
Figure 1.
Expression of CaMKIIγ in vivo inhibits balloon angioplasty–associated restenosis. A) Adenovirus expressing GFP or GFP-tagged CaMKIIγc was administered immediately after balloon injury of rat carotid arteries. Seven days after injury, the arteries were harvested, and tissue lysates were immunoblotted (IB) with the indicated antibodies, followed by ECL detection. B) Quantification of ECL signals in (A), normalized to signals of the control uninjured contralateral artery (n = 4). C) Representative H&E-stained sections of balloon-injured rat carotid arteries transduced with the adenoviral constructs Ad-GFP, Ad-CaMKIIγC, or Ad-CaMKIIδ2 at the time of injury. Tissues were harvested 14 d after injury. A, adventitia; M, medial area, and N, neointimal area. Scale bars, 100 μm. Data are means ± sem (n = 4). *P < 0.05; ***P < 0.001 vs. Ad-GFP-treated controls.
Morphometric analysis of vascular wall medial and neointimal areas after 14 d indicated significant inhibition of vascular wall remodeling after CaMKIIγ expression compared to equivalent viral doses of Ad-GFP or Ad-CaMKIIδ2, which had no effect on remodeling (Fig. 1C). Cell proliferation (Ki67) and p21 expression were assessed by immunohistochemistry 2 wk after injury (Fig. 2A, C). Quantitative area analysis of Ki67 staining indicated a >60% reduction in medial wall and neointimal cell Ki67 staining in arteries treated with Ad-CaMKIIγc compared with that in Ad-GFP controls (Fig. 2B). Based on the same staining, the fraction of cells proliferating was decreased in the medial (0.69 ± 0.1 vs. 0.42 ± 0.04; n = 4; P < 0.05) and neointimal (0.90 ± 0.03 vs. 0.34 ± 0.14; n = 4; P < 0.05) areas. Quantitative area analysis of p21 staining was significantly enhanced in both the medial wall (∼5-fold) and neointima (∼2.5-fold) in arteries transduced with Ad-CaMKIIγc, compared with that in Ad-GFP controls (Fig. 2D). Based on the same staining, the fraction of cells expressing p21 was significantly higher in the medial wall (0.20 ± 0.05 vs. 0.78 ± 0.07; n = 3; P < 0.01) and in the neointima (0.50 ± 0.04 vs. 0.93 ± 0.01; n = 3; P < 0.001).
CaMKIIγ expression is down-regulated in response to carotid artery ligation in mice
A mouse vascular model of ligation-induced carotid artery injury (22) was implemented to take subsequent advantage of genetically engineered mouse lines for conditional knockout of CaMKIIγ (20). Western blot analysis of carotid artery extracts with a pan-CaMKII isoform antibody indicated expression of 2 major CaMKII subunits with apparent molecular masses of ∼52 and ∼54 kDa (Fig. 3A), similar to the expression pattern in rat VSM (12). Western blot analysis of extracts and recombinant CaMKIIγ variants with antibodies specifically recognizing the C terminus of CaMKIIγ or CaMKIIδ2 (Fig. 3C) identified the larger 54 kDa band as a CaMKIIγC subunit and the smaller 52 kDa band as CaMKIIδ2.
Figure 3.
CaMKIIγ expression is rapidly down-regulated after common carotid artery ligation in mice. A) Immunoblots (IB) with a pan-CaMKII antibody recognizing all isoforms and a CaMKIIγ isoform-specific antibody on uninjured carotid artery extracts from WT mice. Purified recombinant CaMKIIγC and -γB isoforms were used as standards. B) mRNA expression analysis by qPCR of Camk2 and smooth muscle contractile gene transcripts in carotid arteries 3 d after ligation, relative to contralateral nonligated vessels (n = 5). C) IBs with specific antibodies assessing protein expression 7 d after ligation. CaMKII was detected with isoform-specific antibodies, as indicated. D) Quantification of immunoblot ECL signals (n = 6). Data are means ± sem. *P < 0.05; **P < 0.01; ***P < 0.001, by unpaired Student's t test.
As early as 3 d after ligation, CaMKIIγ mRNA expression in the medial wall of carotid arteries decreased by 50%, coincident with decreases in mRNA expression of smooth muscle differentiated phenotype markers, including myocardin, Tagln (sm22α), smooth muscle α-actin, and calponin (Fig. 3B), indicative of a VSM phenotype switch. By 7 d, parallel decreases in protein expression were quantified, as determined by Western blot analysis with CaMKII isoform-specific and smooth muscle marker-specific antibodies (Fig. 3C, D). In contrast to the balloon injury model in rat (12), carotid ligation in mice did not result in induced expression of CaMKIIδ2 at these time points.
CaMKIIγ expression is increased in differentiated VSM cells
Coincident loss of CaMKIIγ and smooth muscle differentiation markers suggests some level of coregulated expression. This association was tested in cultured VSM cells by overexpressing myocardin, a transcriptional coregulator that interacts with SRF to promote smooth muscle differentiation and the contractile phenotype (23, 24). Ectopic expression of myocardin resulted in 3–4-fold increases in expression of Tagln, smooth muscle α-actin, calponin, and smooth muscle myosin heavy chain, with a parallel 2-fold increase in CaMKIIγ expression and no changes in CaMKIIδ expression (Fig. 4A–C). In contrast to the smooth muscle differentiation markers, basal (Fig. 4D) and myocardin-induced expression of CaMKIIγ (Fig. 4E) was found independent of SRF expression, when using a shRNA to suppress SRF expression. These results confirm the association of CaMKIIγ expression with the differentiated VSM phenotype.
Figure 4.
CaMKIIγ is up-regulated in differentiated VSM cells by a myocardin-dependent, SRF-independent mechanism. A) Mouse primary VSM cells were infected with adenovirus-expressing myocardin (Ad-MYOCD) or control (Ad-LacZ) at an MOI of 100 for 48 h. CaMKIIγ and CaMKIIδ mRNA expression and known myocardin-dependent smooth muscle differentiation markers were analyzed by real-time qPCR (n = 4). B) Protein expression after 72 h transduction with Ad-MYOCD or control Ad-LacZ. C) Quantification of (B) (n = 3). D) Mouse VSM cells were transduced with enhanced shGFP (shEGFP) or shSRF for 48 h, and gene expression was analyzed as indicated (n = 6). E) Mouse primary VSM cells were transduced with shEGFP or shSRF adenoviruses for 48 h, followed by infection with Ad-MYOCD or Ad-LacZ for 72 h, and gene expression was analyzed, as indicated (n = 3). Data are means ± sem. *P < 0.05; **P < 0.01; ***P < 0.001, vs. control-infected VSM cells.
Smooth muscle–targeted CaMKIIγ deletion enhances vascular remodeling
Mice with floxed Camk2g alleles (20) were bred with a transgenic line expressing Cre recombinase under control of a Tagln (sm22α) promoter that has been shown to be active in smooth muscle (25) and in the developing heart (26). Analysis of CaMKIIγ mRNA and protein levels in the aortic medial layer from smooth muscle knockout mice (Camk2gSMKO) and littermate controls revealed essentially complete deletion of intact Camk2g mRNA and protein expression (Fig. 5A) with no significant compensation by increased expression of CaMKIIδ (Fig. 5B). CaMKIIγ deficiency had no observable effect on arterial wall structure and no effect on tail cuff resting blood pressure (data not shown).
Figure 5.
Genetic deletion of CaMKIIγ in smooth muscle enhances injury-induced neointimal hyperplasia. A) Validation of Tagln-Cre–driven smooth muscle Camk2gSMKO isoform knockout. Aortic medial smooth muscle Camk2g mRNA and CaMKIIγ protein expression in Camk2gSMKO mice compared to WT littermate controls. (n = 3–6). B) Aortic medial smooth muscle Camk2d mRNA and CaMKIIδ protein expression in Camk2gSMKO compared to WT littermate controls (n = 3–6). Immunoblots (IB) are of CaMKII isoform–specific antibodies and recombinant CaMKIIγ isoform controls. Note no compensation for decreased CaMKIIγ in Camk2gSMKO by CaMKIIδ. C) H&E-stained carotid artery sections at the indicated distances proximal to the ligature in Camk2gSMKO compared to WT controls 21 d after ligation. A, adventitia; M, media; N, neointima. Scale bar, 50 μm. D–F) Medial area (D), neointimal area (E), and lumen (F) stenosis in Camk2gSMKO compared to WT controls 21 d after ligation (n = 7–8). Data are means ± sem, analyzed across levels by 2-way ANOVA with significant between-group differences in the medial (P = 0.013) and neointimal (P = 0.002) areas and in lumen stenosis (P < 0.001). *P < 0.05; **P < 0.01; ***P < 0.001, by Holm-Sidak post hoc multiple-comparisons test.
Carotid artery ligation produced a prominent neointima in WT control mice by 3 wk after ligation (Fig. 5C). As has been documented (22, 27), this remodeling response diminishes with distance proximal to the ligature (Fig. 5C, E). Vascular wall remodeling in Camk2gSMKO mice was comparable to that in controls near the ligature (1100 μm), but was markedly enhanced proximally (1900 μm; Fig. 5C). Although there was substantial quantitative biologic variability, 2-way ANOVA indicated increased neointimal area (Fig. 5E; P = 0.002) and luminal stenosis (Fig. 5D; P < 0.001) in Camk2gSMKO vs. littermate control groups, which progressed proximal to the ligature. In addition to this constrictive (inward) remodeling, modest increases in medial area (Fig. 5D; P = 0.013) and external elastic lamina circumference (data not shown; P = 0.020) also indicated some degree of enhanced outward remodeling in Camk2gSMKO mice compared with that in littermate controls.
CaMKIIγ deletion increases VSM proliferation in vivo
Increased extent of vascular remodeling in ligated carotid arteries from Camk2gSMKO could be accounted for by increased VSM cell proliferation and decreased apoptosis. We observed a significant increase in Ki67 staining, first in the medial wall of arteries from Camk2gSMKO compared to control mice 14 d after ligation (Fig. 6B). By 21 d after ligation, a significant increase in both medial and neointimal Ki-67 staining was quantified in Camk2gSMKO arteries compared with that in littermate controls (Fig. 6A–C). Although CaMKII promotes apoptosis in cardiac myocytes (28, 29) and apoptosis may contribute to medial wall cell loss after carotid ligation (22), the apoptosis rate was not significantly different between Camk2gSMKO and littermate controls 21 d after ligation (Supplemental Fig. S2). Analysis of key cell cycle regulators indicated decreased expression of cyclin-dependent kinase inhibitors (CDKNs), with a statistically significant decrease in p21 (CDKN1A) in Camk2gSMKO compared to controls 21 d after ligation (Fig. 6D). These smooth muscle–specific CaMKIIγ loss-of-function results after carotid ligation are an exact opposite to those observed after carotid ligation in mice with global deletion of CaMKIIδ (14).
Figure 6.
Smooth muscle deletion of CaMKIIγ increases VSM cell proliferation. A) Representative Ki67-stained sections of carotid arteries from WT and Camk2gSMKO mice 3 wk after ligation. B. C) Quantification of Ki67+ area in the medial (B) and neointimal (C) areas at 14 (n = 4–5) and 21 (n = 5) d after ligation. D) Quantitative analysis of mRNA levels by real-time qPCR. Total RNA was extracted from carotids of Camk2gSMKO and littermate controls (WT) 21 d after ligation. Data are relative to contralateral nonligated vessels (n = 5–6). E) Immunoblot (IB) signals of CaMKIIδ expression in carotid artery extracts from Camk2gSMKO and control (WT) mice 21 d after ligation. F) Quantification of ECL signals. Ligated artery content vs. content of contralateral nonligated (control) vessels (n = 6). *P < 0.05; **P < 0.01.
As shown in WT mice (Fig. 3), decreased CaMKIIγ expression correlates with decreases in smooth muscle contractile gene expression after injury. We hypothesized that CaMKIIγ knockout may enhance the rate or extent of the VSM phenotype switch after injury, contributing to enhanced vascular remodeling. CaMKIIγ deficiency had no significant effects on baseline expression of smooth muscle differentiation markers (Supplemental Fig. S3A). Moreover, no differences in the levels of sm22α (Tagln) or smooth muscle Myh11 protein expression were observed between control and Camk2gSMKO carotid arteries harvested at 1 wk after ligation (Supplemental Fig. S3B, C). Analysis of CaMKIIδ protein expression at 1 and 3 wk (Fig. 6E, F) after carotid ligation indicated no significant differences in CaMKIIδ expression dynamics between control and CaMKIIγSMKO mice. Thus, enhanced VSM proliferation and vascular remodeling in Camk2gSMKO mice cannot be accounted for by enhanced VSM phenotype switching or enhanced absolute expression of CaMKIIδ after carotid ligation.
CaMKIIγ expression in synthetic VSM inhibits cell proliferation
To directly test the effects of CaMKIIγ on VSM synthetic phenotype function, VSM cell cultures were prepared from enzymatically dispersed mouse aortic medial smooth muscle and transduced with Ad-CaMKIIγ. Similar to cultured rat VSM (13), cultured murine VSM express mainly CaMKIIδ2 with little expression of CaMKIIγ isoforms (Fig. 7A). CaMKIIγC protein overexpression in WT cells (Fig. 6B; inset) significantly inhibited cell proliferation by ∼40%, as assessed by cell counts over 4 d (Fig. 7B) or BrdU incorporation 48 h after infection (Fig. 7C).
Figure 7.
Expression of CaMKIIγ attenuates VSM cell proliferation in vitro. A) Immunoblots (IB) of extracts from WT mouse aortic medial tissue and cultured mouse aortic VSM cells with a pan-CaMKII antibody recognizing all isoforms. B) Cultured mouse VSM cells (passages 3–6) were transduced with Ad-CaMKIIγC or control (Ad-GFP) 1 d after plating and were counted at the time points indicated (n = 4). Inset: representative immunoblot documenting CaMKIIγc overexpression. C) BrdU incorporation was assayed in cultured mouse VSM cells from WT mice infected with Ad-CaMKIIγc or Ad-GFP for 48 h (n = 3). D) Passaged VSM cells from Camk2dSMKO were infected with Ad-CaMKIIγC, Ad-CaMKIIδ2, or control Ad-GFP 1 d after plating and counted at the time points indicated. Inset: representative immunoblot documenting comparable CaMKIIγC and CaMKIIδ2 overexpression. E) Primary enzymatic isolates (passage 0) of rat aortic VSM cells were infected with Ad-CaMKIIγC, Ad-CaMKIIδ2, or Ad-GFP 1 hour after plating and were counted at the indicated time points (n = 3). Inset: representative Western blot documenting comparable CaMKIIγC and CaMKIIδ2 overexpression (n = 4). *P < 0.05; **P < 0.01; ***P < 0.001 vs. Ad-GFP-infected cells.
Because CaMKII isoforms are known to form and function as heteromultimeric holoenzymes, inhibitory effects of CaMKIIγ expression on VSM proliferation could be mediated indirectly through inhibition of CaMKIIδ-dependent proliferation. To investigate this possibility, we isolated and cultured VSM cells from thoracic aorta of mice with global deletion of CaMKIIδ and then transduced them with Ad-CaMKIIγc, Ad-CaMKIIδ2, or Ad-GFP control (Fig. 7D). In cells lacking CaMKIIδ expression, expression of CaMKIIγ nearly blocked cell proliferation compared with that of the control cells. Expression of comparable levels of CaMKIIδ2 expression in the cells had no effect on proliferation.
To test the function of CaMKIIγ expression in a system where VSM cells are actively undergoing phenotype switching, we observed rat aortic VSM cells in primary culture immediately after enzymatic dispersion and plating (Fig. 7E). Endogenous loss of CaMKIIγ expression in this system was rescued by adenoviral transduction of CaMKIIγc introduced 1 h after the cells were plated. Expression of CaMKIIγ strongly inhibited VSM cell proliferation in this system, whereas comparable induced expression of CaMKIIδ2 had no inhibitory effect, with a trend toward enhanced proliferation.
CaMKIIγ induces p53 and cell cycle inhibitory protein expression
CaMKIIγ gain-of-function (Fig. 1) and loss-of-function (Fig. 6) studies in vivo indicated increased and decreased expression, respectively, of the cell cycle inhibitory protein, CDKN1A (p21). Consistent with these data, CaMKIIγ overexpression in wild-type VSM cells significantly induced p21 mRNA expression and decreased expression of cell-division cycle-2 (Cdc2), Cdc20, and Cdc25b, cell cycle genes that are essential for progression of proliferating cells through the G2/M checkpoint (30) (Fig. 8A). No differences in smooth muscle phenotype marker genes, Tagln, or smooth muscle α-actin were observed, indicating no direct effects of CaMKIIγ expression on contractile phenotype. Expression of the apoptosis-related genes Bax and Puma was unaffected.
Figure 8.
Expression of CaMKIIγ in proliferating VSM cells induces p53 expression and cell cycle regulatory genes. A) Cultured mouse VSM cells were transduced with Ad-GFP or Ad-CaMKIIγc for 48 h, and total RNA was extracted for gene expression analysis by real-time qPCR (n = 4–6). Data are relative to Ad-GFP-infected cells. B, C) Cultured mouse VSM cells were transduced with Ad-GFP or Ad-CaMKIIγ for 72 h and immunoblotted for p53 (B) and p21 (C) expression, followed by quantification of ECL signals. D) Immunoblots of phosphorylated (p)p53 (Ser15), pMdm2 (Ser166), and GAPDH. E) Quantification of pp53 and pMdm2 expression (n = 5–6). Data are fold change relative to Ad-GFP-infected cells. F) p53 mRNA expression as indicated in (A) (n = 4). *P < 0.05; **P < 0.01. G) Model of the nonequivalent function of CaMKIIγ and -δ isoforms in the response to arterial injury.
A major upstream regulator of p21 is p53, and negative regulation of this pathway by CaMKIIδ explains CaMKIIδ-dependent VSM cell proliferation and injury-induced remodeling (14). In this study, CaMKIIγ expression in proliferative VSM cells increased p53 protein expression (∼2-fold) and its transcriptional target p21 (∼3-fold) (Fig. 8B, C). In normal, unstressed cells, p53 is inhibited by interaction with Mdm2, a p53-specific E3 ubiquitin ligase that also promotes p53 degradation. A well-characterized mechanism for regulating p53 protein expression is by disruption of the p53–Mdm2 interaction and p53 stabilization, mediated, at least in part, via both phosphorylation of Mdm2 and p53 (31–34). Analysis of p53 phosphorylation on Ser15 (Fig. 8D, E) indicated a 2-fold increase in p53 activity in VSM cells transduced with CaMKIIγ. However, Mdm2 phosphorylation on Ser166 was unaffected by CaMKIIγ overexpression, suggesting that p53 protein stability is not the major mechanism for increased p53 expression. Finally, analysis of p53 mRNA expression in CaMKIIγ-overexpressing VSM cells demonstrated a 1.5-fold increase compared to controls (Fig. 8F), pointing to a transcriptional or posttranscriptional mechanism of p53 regulation by CaMKIIγ.
DISCUSSION
CaMKII holoenzymes in VSM have been identified as mixtures of CaMKIIδ (Camk2d) and -γ (Camk2g) gene products (7, 8). In vitro, signaling through CaMKIIδ positively regulates VSM cell proliferation and migration (13), and in vivo molecular/genetic loss-of-function approaches indicate an important function of CaMKIIδ in promoting injury-induced vascular wall remodeling (12, 14), flow-dependent remodeling (35), and angiotensin II-induced vascular wall hypertrophy (36). We report for the first time an opposing function for signaling via CaMKIIγ-isoforms, which negatively regulate VSM proliferation in vitro and inhibit the vasculoproliferative response to balloon injury or flow cessation in vivo. These results contribute to growing recognition of the function of Ca2+-dependent signaling pathways to vascular remodeling responses (25, 37–41).
Based on the results in this study and previous observations (12), CaMKIIδ and CaMKIIγ expression are reciprocally up- and down-regulated, respectively, after balloon injury of rat carotid artery. This model results in endothelial barrier disruption and affords the approach of intraluminal delivery of viral constructs to transduce medial wall smooth muscle and manipulate gene or protein expression. Intraluminal adenoviral transduction of a flag-tagged CaMKIIγc construct at the time of injury partially rescued injury-induced decreases in VSM CaMKIIγ expression in both medial and neointimal smooth muscle and inhibited neointimal accumulation compared to Ad-GFP control or a comparable dose of Ad-CaMKIIδ2, which had no significant effects. The net effect of CaMKIIγ protein overexpression in this model is equivalent to CaMKIIδ mRNA suppression (12, 14). Because the expression of CaMKIIγ was not superphysiological and because a comparable dose of Ad- CaMKIIδ2 had no effect, we conclude that inhibition of remodeling was not related to increased CaMKII activity per se and was instead attributable to a CaMKIIγ isoform–specific effect.
Consistent with results in the rat balloon-injury model, CaMKIIγ expression was significantly down-regulated as early as 3 d at the mRNA level and 7 d at the protein level after carotid artery ligation in mice, correlating closely with loss of contractile phenotype markers (Fig. 3). In this model, medial wall CaMKIIδ expression did not increase at early time points and was only apparent 3 wk after ligation, when accumulation of neointima was near its maximum. These dynamics differ quantitatively from those measured after balloon injury in the rat, where CaMKIIδ expression rapidly increased preceding maximum cell proliferation and accumulation of neointima (12). At least some of this difference may be attributable to the nature of the 2 models. In the balloon-angioplasty model, vascular wall injury is abrupt, severe, and relatively uniform along the length of the artery. In the carotid-ligation model, vascular wall remodeling proximal to the ligation site is sensed and triggered by the endothelium in response to disrupted flow dynamics and appears to propagate progressively as a function of distance proximal to the ligature (22, 27). Thus, dynamics in medial wall smooth muscle CaMKII expression may be averaged over time and over the length of the ligated artery. Regardless, in either model there is an expected relative decrease in γ-isoform and an increase in δ-isoform content in CaMKII holoenzymes after vascular injury. Mechanisms controlling reciprocal CaMKIIγ and -δ isoform expression are not known in the present context and have not been investigated in other systems, including heart, where CaMKIIδ plays an important function in cardiac hypertrophy and progression of heart failure (42–44). The strong correlation observed in the present study between CaMKIIγ expression in dedifferentiating VSM after injury and differentiating VSM in vitro in response to myocardin overexpression provides some clues in the case of CaMKIIγ. More studies are needed to determine whether myocardin directly regulates CaMKIIγ through an SRF-independent mechanism, such as is the case for α8-integrin (45), or indirectly regulates expression through other mechanisms, such as mRNA stability, epigenetic modifications, micro-RNA, or long noncoding RNA.
Our in vivo studies have focused mainly on injury-induced changes in CaMKII isoform expression. CaMKII activation in vivo in response to carotid ligation has been demonstrated by measuring increases in levels of autonomous (Ca2+/calmodulin-independent) CaMKII activity, a response that is secondary to specific autophosphorylation and methionine oxidation events (14, 35, 46, 47). Potential initiating signals for injury-induced VSM CaMKII activation include growth factors, such as platelet-derived growth factor (PDGF) or angiotensin II, that have been shown to activate CaMKII in vitro (35, 48) and changes in blood flow dynamics that result in CaMKII oxidation in vivo (46). The relative importance of CaMKII oxidation has recently been tested in vivo with a mutant oxidation-deficient CaMKIIδ knock-in model (46, 47), with the noteworthy result that there was no net effect of this mutation on extent of neointimal remodeling after carotid ligation (47), although compensatory flow-induced remodeling in the contralateral artery was inhibited (46).
In the present study, we used mice with Camk2g floxed alleles and expressing Cre recombinase under the control of a Tagln (sm22α) promoter, an approach that allowed us to pinpoint the effects of CaMKIIγ loss-of-function, specifically in VSM cells (25) compared with endothelial cells (49), resident or circulating immune cells (50, 51), or mesenchymal stem cells (52), all of which may contribute to vascular remodeling. We found that lack of expression of CaMKIIγ in VSM or a closely related mesenchymal cell expressing Tagln, promoted VSM proliferation and progression of the injury-induced remodeling response, without compensatory increases in CaMKIIδ expression. This finding strongly suggests that endogenous CaMKIIγ serves as a functional brake on CaMKIIδ-mediated signaling, which otherwise promotes VSM proliferation and vascular wall remodeling.
Although there is accumulating evidence for CaMKII-dependent regulation of cell proliferation in other systems, the mechanisms coupling specific CaMKII isoforms to regulation of cell proliferation are not completely understood (30). CaMKIIδ silencing in vitro impairs VSM proliferation with increased numbers of multinucleated cells and cells accumulating at the G2/M checkpoint, as assessed by flow cytometry (13). A study in global CaMKIIδ knockout mice and in VSM cells from these mice indicated that decreased VSM cell proliferation and remodeling after carotid ligation in these animals is associated with increased expression of p21 and its transcriptional activator, p53 (14). These results were interpreted to indicate that CaMKIIδ-dependent proliferation is mediated by suppression of an endogenously active p53- and p21-dependent cell cycle inhibitory pathway. In the present study, conditional VSM knockout of CaMKIIγ in vivo had the opposite effect: increased VSM cell proliferation and decreased expression of p21 in ligated arteries. In vitro studies indicated that CaMKIIγ overexpression in passaged VSM strongly inhibited cell proliferation; increased expression of p53 and its target gene p21; and decreased expression of Cdc2 (Cdk1), Cdc20, and Cdc25, genes reported to be transcriptionally repressed by p53 (53–57). This pattern is consistent with cell cycle inhibition at both G1/S and G2/M checkpoints (54, 58). Based on results of other studies (12–14), we expected that overexpression of CaMKIIδ alone at comparable levels would enhance VSM proliferation, either in WT or CaMKIIδ-null cells. In fact, no effects were observed, suggesting that endogenous CaMKIIδ is sufficient for any effects on cell proliferation in WT cells. In passaged CaMKIIδ-knockout cells, cell cycle regulation may be adapted to lack of CaMKIIδ through cell selection pressure for rapidly proliferating CaMKIIδ-insensitive cells. Thus, reciprocal regulation of vascular remodeling by CaMKIIδ and -γ isoforms is replicated at the level of VSM cell proliferation and in the expression of specific cell cycle regulators. Regulation of expression and activity of p53 and one of its key regulators, the E3 ubiquitin ligase Mdm2, has proven to be complex, and a large number of signaling pathways have been reported to intersect with the pathways, including p38, AKT, and cAMP/PKZ (32–34, 59, 60). Most commonly Mdm2 levels or activity are reported to be a key point of p53 regulation, including that provided by CaMKIIδ in VSM (14). It is possible that expression of CaMKIIγ acts indirectly to suppress negative regulation of the p53/p21 pathway by CaMKIIδ. However, our in vitro studies in cells lacking CaMKIIδ and the regulation of p53 mRNA without apparent changes in Mdm2 activity suggest direct effects of CaMKIIγ in stimulating p53 expression with distinct targets, compared with CaMKIIδ.
The molecular mechanisms underlying differential function of highly homologous CaMKII isoforms in vascular remodeling are not yet known, but there are some precedents. Although CaMKII is multifunctional and all isoforms appear to have similar peptide substrate specificities and kinetics (61), it remains possible that specific isoforms may discriminate at the level of protein substrate specificity. Although we have demonstrated negative regulation of CAMP-responsive element–binding protein (CREB) activity in VSM by endogenous CaMKII isoforms (62), a recent study in neurons suggests a unique function for CaMKIIγ isoforms in regulating CREB activation through a mechanism involving calmodulin shuttling to the nucleus (63). Alternatively, isoform composition could affect intracellular targeting and function, as has been demonstrated in cardiomyocytes and heart for CaMKII splice variants containing nuclear localization domains (64, 65). In cultured VSM, the alternatively spliced 21 aa C terminus in the CaMKIIδ2 (or δC) isoform contains a proline-rich sequence that conforms to a consensus Src homology (SH)-3-binding domain. This domain appears to couple the kinase physically and functionally to regulation of the Src-family kinase, Fyn (11). In differentiated VSM, the association domain of a specific CaMKIIγ slice variant contains 2 overlapping SH3-binding motifs suggested to direct the kinase to a cytoskeletal domain and regulation of ERK1/2 signaling, contributing to contractile force maintenance in differentiated VSM (4). Taken together, the results of these and similar studies indicate that the isoform composition of CaMKII holoenzymes is likely an important determinant of kinase localization and function. Although our studies to date have focused on the most abundant CaMKIIδ and -γ isoforms in VSM, less abundantly expressed CaMKIIα or -β isoforms could play roles in the vascular response to injury by affecting function in other cell types such as endothelium (66), which is a key sensor and initiator of the vascular response to injury (67), or even in smooth muscle progenitor cells, that can contribute to vascular wall remodeling (68). A challenge going forward in this field is to recognize this potential complexity and to the extent possible use reagents and approaches that distinguish CaMKII isoform- and cell-specific functions.
In summary, the results of this study demonstrate independent regulation, and nonequivalent function of CaMKIIγ and -δ isoforms in the response to arterial injury (summarized in Fig. 8G). Based on coordinate regulation of CaMKIIγ- and VSM-differentiated phenotype marker gene expression (Fig. 1), we propose that decreased expression of endogenous CaMKIIγ in VSM after vascular injury is permissive of coupling CaMKIIδ-enriched holoenzymes to promotion of VSM synthetic phenotype functions. We have demonstrated a relative increase in CaMKIIγ/δ content after 7 d in medial smooth muscle and between 21 and 28 d in neointima after balloon injury, both responses correlating with postpeak proliferation rates in this model (12). Although it remains to be tested directly, re-expression of CaMKIIγ in synthetic phenotype VSM or acquisition of CaMKIIγ expression in differentiating VSM progenitor cells may be one factor that limits progression of vascular remodeling. This model also suggests a potentially useful therapeutic function for ectopically expressed CaMKIIγ in stabilizing neointimal lesions.
Supplementary Material
Acknowledgments
This research was supported by Grants R01HL049426 and R01HL092510 from the U.S. National Institutes of Health, National Heart, Lung, and Blood Institute (to H.A.S.) and by the Albany Medical College Weir Fund.
Glossary
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- Cdc
cell-division cycle
- CDKN
cyclin-dependent kinase inhibitor
- CREB
CAMP-responsive element–binding protein
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- H&E
hematoxylin and eosin
- Mdm2
mouse double minute 2
- MOI
multiplicity of infection
- Myh11
myosin heavy chain 11
- qPCR
quantitative PCR
- SH
Src homology
- shRNA
short hairpin RNA
- SMKO
smooth muscle knockout (mouse)
- SRF
serum response factor
- Tagln
transgelin
- VSM
vascular smooth muscle
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Yoshida T., Owens G. K. (2005) Molecular determinants of vascular smooth muscle cell diversity. Circ. Res. 96, 280–291 [DOI] [PubMed] [Google Scholar]
- 2.Singer H. A. (2012) Ca2+/calmodulin-dependent protein kinase II function in vascular remodelling. J. Physiol. 590, 1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wamhoff B. R., Bowles D. K., Owens G. K. (2006) Excitation-transcription coupling in arterial smooth muscle. Circ. Res. 98, 868–878 [DOI] [PubMed] [Google Scholar]
- 4.Marganski W. A., Gangopadhyay S. S., Je H. D., Gallant C., Morgan K. G. (2005) Targeting of a novel Ca+2/calmodulin-dependent protein kinase II is essential for extracellular signal-regulated kinase-mediated signaling in differentiated smooth muscle cells. Circ. Res. 97, 541–549 [DOI] [PubMed] [Google Scholar]
- 5.Rokolya A., Singer H. A. (2000) Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am. J. Physiol. Cell Physiol. 278, C537–C545 [DOI] [PubMed] [Google Scholar]
- 6.Tombes R. M., Faison M. O., Turbeville J. M. (2003) Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene 322, 17–31 [DOI] [PubMed] [Google Scholar]
- 7.Schworer C. M., Rothblum L. I., Thekkumkara T. J., Singer H. A. (1993) Identification of novel isoforms of the delta subunit of Ca2+/calmodulin-dependent protein kinase II: differential expression in rat brain and aorta. J. Biol. Chem. 268, 14443–14449 [PubMed] [Google Scholar]
- 8.Singer H. A., Benscoter H. A., Schworer C. M. (1997) Novel Ca2+/calmodulin-dependent protein kinase II gamma-subunit variants expressed in vascular smooth muscle, brain, and cardiomyocytes. J. Biol. Chem. 272, 9393–9400 [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan M., Edman C. F., Schulman H. (1994) Alternative splicing introduces a nuclear localization signal that targets multifunctional CaM kinase to the nucleus. J. Cell Biol. 126, 839–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Leary H., Lasda E., Bayer K. U. (2006) CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Mol. Biol. Cell 17, 4656–4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginnan R., Zou X., Pfleiderer P. J., Mercure M. Z., Barroso M., Singer H. A. (2013) Vascular smooth muscle cell motility is mediated by a physical and functional interaction of Ca2+/calmodulin-dependent protein kinase IIδ2 and Fyn. J. Biol. Chem. 288, 29703–29712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.House S. J., Singer H. A. (2008) CaMKII-delta isoform regulation of neointima formation after vascular injury. Arterioscler. Thromb. Vasc. Biol. 28, 441–447 [DOI] [PubMed] [Google Scholar]
- 13.House S. J., Ginnan R. G., Armstrong S. E., Singer H. A. (2007) Calcium/calmodulin-dependent protein kinase II-delta isoform regulation of vascular smooth muscle cell proliferation. Am. J. Physiol. Cell Physiol. 292, C2276–C2287 [DOI] [PubMed] [Google Scholar]
- 14.Li W., Li H., Sanders P. N., Mohler P. J., Backs J., Olson E. N., Anderson M. E., Grumbach I. M. (2011) The multifunctional Ca2+/calmodulin-dependent kinase II delta (CaMKIIdelta) controls neointima formation after carotid ligation and vascular smooth muscle cell proliferation through cell cycle regulation by p21. J. Biol. Chem. 286, 7990–7999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He T. C., Zhou S., da Costa L. T., Yu J., Kinzler K. W., Vogelstein B. (1998) A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95, 2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long X., Bell R. D., Gerthoffer W. T., Zlokovic B. V., Miano J. M. (2008) Myocardin is sufficient for a smooth muscle-like contractile phenotype. Arterioscler. Thromb. Vasc. Biol. 28, 1505–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Streb J. W., Miano J. M. (2005) AKAP12alpha, an atypical serum response factor-dependent target gene. J. Biol. Chem. 280, 4125–4134 [DOI] [PubMed] [Google Scholar]
- 18.O’Carroll S. J., Hall A. R., Myers C. J., Braithwaite A. W., Dix B. R. (2000) Quantifying adenoviral titers by spectrophotometry. Biotechniques 28, 408–410, 412 [DOI] [PubMed] [Google Scholar]
- 19.Backs J., Backs T., Neef S., Kreusser M. M., Lehmann L. H., Patrick D. M., Grueter C. E., Qi X., Richardson J. A., Hill J. A., Katus H. A., Bassel-Duby R., Maier L. S., Olson E. N. (2009) The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc. Natl. Acad. Sci. USA 106, 2342–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backs J., Stein P., Backs T., Duncan F. E., Grueter C. E., McAnally J., Qi X., Schultz R. M., Olson E. N. (2010) The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc. Natl. Acad. Sci. USA 107, 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geisterfer A. A., Peach M. J., Owens G. K. (1988) Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ. Res. 62, 749–756 [DOI] [PubMed] [Google Scholar]
- 22.Kumar A., Lindner V. (1997) Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler. Thromb. Vasc. Biol. 17, 2238–2244 [DOI] [PubMed] [Google Scholar]
- 23.Huang J., Cheng L., Li J., Chen M., Zhou D., Lu M. M., Proweller A., Epstein J. A., Parmacek M. S. (2008) Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J. Clin. Invest. 118, 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D., Chang P. S., Wang Z., Sutherland L., Richardson J. A., Small E., Krieg P. A., Olson E. N. (2001) Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105, 851–862 [DOI] [PubMed] [Google Scholar]
- 25.Mancarella S., Potireddy S., Wang Y., Gao H., Gandhirajan R. K., Autieri M., Scalia R., Cheng Z., Wang H., Madesh M., Houser S. R., Gill D. L. (2013) Targeted STIM deletion impairs calcium homeostasis, NFAT activation, and growth of smooth muscle. FASEB J. 27, 893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leeper N. J., Raiesdana A., Kojima Y., Chun H. J., Azuma J., Maegdefessel L., Kundu R. K., Quertermous T., Tsao P. S., Spin J. M. (2011) MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J. Cell. Physiol. 226, 1035–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Althoff T. F., Albarrán Juárez J., Troidl K., Tang C., Wang S., Wirth A., Takefuji M., Wettschureck N., Offermanns S. (2012) Procontractile G protein-mediated signaling pathways antagonistically regulate smooth muscle differentiation in vascular remodeling. J. Exp. Med. 209, 2277–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y., Zhu W. Z., Joiner M. L., Zhang R., Oddis C. V., Hou Y., Yang J., Price E. E., Gleaves L., Eren M., Ni G., Vaughan D. E., Xiao R. P., Anderson M. E. (2006) Calmodulin kinase II inhibition protects against myocardial cell apoptosis in vivo. Am. J. Physiol. Heart Circ. Physiol. 291, H3065–H3075 [DOI] [PubMed] [Google Scholar]
- 29.Zhu W., Woo A. Y., Yang D., Cheng H., Crow M. T., Xiao R. P. (2007) Activation of CaMKIIdeltaC is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. J. Biol. Chem. 282, 10833–10839 [DOI] [PubMed] [Google Scholar]
- 30.Skelding K. A., Rostas J. A., Verrills N. M. (2011) Controlling the cell cycle: the role of calcium/calmodulin-stimulated protein kinases I and II. Cell Cycle 10, 631–639 [DOI] [PubMed] [Google Scholar]
- 31.Haupt Y., Maya R., Kazaz A., Oren M. (1997) Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 [DOI] [PubMed] [Google Scholar]
- 32.Lu X. (2010) Tied up in loops: positive and negative autoregulation of p53. Cold Spring Harb. Perspect. Biol. 2, a000984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayo L. D., Donner D. B. (2001) A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. USA 98, 11598–11603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou B. P., Liao Y., Xia W., Zou Y., Spohn B., Hung M. C. (2001) HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 3, 973–982 [DOI] [PubMed] [Google Scholar]
- 35.Scott J. A., Xie L., Li H., Li W., He J. B., Sanders P. N., Carter A. B., Backs J., Anderson M. E., Grumbach I. M. (2012) The multifunctional Ca2+/calmodulin-dependent kinase II regulates vascular smooth muscle migration through matrix metalloproteinase 9. Am. J. Physiol. Heart Circ. Physiol. 302, H1953–H1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H., Li W., Gupta A. K., Mohler P. J., Anderson M. E., Grumbach I. M. (2010) Calmodulin kinase II is required for angiotensin II-mediated vascular smooth muscle hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 298, H688–H698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aubart F. C., Sassi Y., Coulombe A., Mougenot N., Vrignaud C., Leprince P., Lechat P., Lompré A. M., Hulot J. S. (2009) RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol. Ther. 17, 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipskaia L., del Monte F., Capiod T., Yacoubi S., Hadri L., Hours M., Hajjar R. J., Lompré A. M. (2005) Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ. Res. 97, 488–495 [DOI] [PubMed] [Google Scholar]
- 39.Liu Z., Zhang C., Dronadula N., Li Q., Rao G. N. (2005) Blockade of nuclear factor of activated T cells activation signaling suppresses balloon injury-induced neointima formation in a rat carotid artery model. J. Biol. Chem. 280, 14700–14708 [DOI] [PubMed] [Google Scholar]
- 40.Potier M., Gonzalez J. C., Motiani R. K., Abdullaev I. F., Bisaillon J. M., Singer H. A., Trebak M. (2009) Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 23, 2425–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W., Halligan K. E., Zhang X., Bisaillon J. M., Gonzalez-Cobos J. C., Motiani R. K., Hu G., Vincent P. A., Zhou J., Barroso M., Singer H. A., Matrougui K., Trebak M. (2011) Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circ. Res. 109, 534–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreusser M. M., Lehmann L. H., Keranov S., Hoting M. O., Oehl U., Kohlhaas M., Reil J. C., Neumann K., Schneider M. D., Hill J. A., Dobrev D., Maack C., Maier L. S., Gröne H. J., Katus H. A., Olson E. N., Backs J. (2014) Cardiac CaM Kinase II genes δ and γ contribute to adverse remodeling but redundantly inhibit calcineurin-induced myocardial hypertrophy. Circulation 130, 1262–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinreuter M., Kreusser M. M., Beckendorf J., Schreiter F. C., Leuschner F., Lehmann L. H., Hofmann K. P., Rostosky J. S., Diemert N., Xu C., Volz H. C., Jungmann A., Nickel A., Sticht C., Gretz N., Maack C., Schneider M. D., Gröne H. J., Müller O. J., Katus H. A., Backs J. (2014) CaM Kinase II mediates maladaptive post-infarct remodeling and pro-inflammatory chemoattractant signaling but not acute myocardial ischemia/reperfusion injury. EMBO Mol. Med. 6, 1231–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M., Hagenmueller M., Riffel J. H., Kreusser M. M., Bernhold E., Fan J., Katus H. A., Backs J., Hardt S. E. (2015) Calcium/calmodulin-dependent protein kinase II couples Wnt signaling with histone deacetylase 4 and mediates dishevelled-induced cardiomyopathy. Hypertension 65, 335–344 [DOI] [PubMed] [Google Scholar]
- 45.Kitchen C. M., Cowan S. L., Long X., Miano J. M. (2013) Expression and promoter analysis of a highly restricted integrin alpha gene in vascular smooth muscle. Gene 513, 82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott J. A., Klutho P. J., El Accaoui R., Nguyen E., Venema A. N., Xie L., Jiang S., Dibbern M., Scroggins S., Prasad A. M., Luczak E. D., Davis M. K., Li W., Guan X., Backs J., Schlueter A. J., Weiss R. M., Miller F. J., Anderson M. E., Grumbach I. M. (2013) The multifunctional Ca²⁺/calmodulin-dependent kinase IIδ (CaMKIIδ) regulates arteriogenesis in a mouse model of flow-mediated remodeling. PLoS One 8, e71550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu L. J., Klutho P. J., Scott J. A., Xie L., Luczak E. D., Dibbern M. E., Prasad A. M., Jaffer O. A., Venema A. N., Nguyen E. K., Guan X., Anderson M. E., Grumbach I. M. (2014) Oxidative activation of the Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) regulates vascular smooth muscle migration and apoptosis. Vascul. Pharmacol. 60, 75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abraham S. T., Benscoter H., Schworer C. M., Singer H. A. (1996) In situ Ca2+ dependence for activation of Ca2+/calmodulin-dependent protein kinase II in vascular smooth muscle cells. J. Biol. Chem. 271, 2506–2513 [DOI] [PubMed] [Google Scholar]
- 49.Wu X., Zou Y., Zhou Q., Huang L., Gong H., Sun A., Tateno K., Katsube K., Radtke F., Ge J., Minamino T., Komuro I. (2011) Role of Jagged1 in arterial lesions after vascular injury. Arterioscler. Thromb. Vasc. Biol. 31, 2000–2006 [DOI] [PubMed] [Google Scholar]
- 50.Furgeson S. B., Simpson P. A., Park I., Vanputten V., Horita H., Kontos C. D., Nemenoff R. A., Weiser-Evans M. C. (2010) Inactivation of the tumour suppressor, PTEN, in smooth muscle promotes a pro-inflammatory phenotype and enhances neointima formation. Cardiovasc. Res. 86, 274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roque M., Fallon J. T., Badimon J. J., Zhang W. X., Taubman M. B., Reis E. D. (2000) Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler. Thromb. Vasc. Biol. 20, 335–342 [DOI] [PubMed] [Google Scholar]
- 52.Majesky M. W., Dong X. R., Regan J. N., Hoglund V. J. (2011) Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ. Res. 108, 365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yun J., Chae H. D., Choy H. E., Chung J., Yoo H. S., Han M. H., Shin D. Y. (1999) p53 negatively regulates cdc2 transcription via the CCAAT-binding NF-Y transcription factor. J. Biol. Chem. 274, 29677–29682 [DOI] [PubMed] [Google Scholar]
- 54.Taylor W. R., Stark G. R. (2001) Regulation of the G2/M transition by p53. Oncogene 20, 1803–1815 [DOI] [PubMed] [Google Scholar]
- 55.Kidokoro T., Tanikawa C., Furukawa Y., Katagiri T., Nakamura Y., Matsuda K. (2008) CDC20, a potential cancer therapeutic target, is negatively regulated by p53. Oncogene 27, 1562–1571 [DOI] [PubMed] [Google Scholar]
- 56.Taylor W. R., Schonthal A. H., Galante J., Stark G. R. (2001) p130/E2F4 binds to and represses the cdc2 promoter in response to p53. J. Biol. Chem. 276, 1998–2006 [DOI] [PubMed] [Google Scholar]
- 57.Krause K., Haugwitz U., Wasner M., Wiedmann M., Mössner J., Engeland K. (2001) Expression of the cell cycle phosphatase cdc25C is down-regulated by the tumor suppressor protein p53 but not by p73. Biochem. Biophys. Res. Commun. 284, 743–750 [DOI] [PubMed] [Google Scholar]
- 58.Agarwal M. L., Agarwal A., Taylor W. R., Stark G. R. (1995) p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc. Natl. Acad. Sci. USA 92, 8493–8497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saha K., Adhikary G., Kanade S. R., Rorke E. A., Eckert R. L. (2014) p38δ regulates p53 to control p21Cip1 expression in human epidermal keratinocytes. J. Biol. Chem. 289, 11443–11453 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Hayashi S., Morishita R., Matsushita H., Nakagami H., Taniyama Y., Nakamura T., Aoki M., Yamamoto K., Higaki J., Ogihara T. (2000) Cyclic AMP inhibited proliferation of human aortic vascular smooth muscle cells, accompanied by induction of p53 and p21. Hypertension 35, 237–243 [DOI] [PubMed] [Google Scholar]
- 61.Gaertner T. R., Kolodziej S. J., Wang D., Kobayashi R., Koomen J. M., Stoops J. K., Waxham M. N. (2004) Comparative analyses of the three-dimensional structures and enzymatic properties of alpha, beta, gamma and delta isoforms of Ca2+-calmodulin-dependent protein kinase II. J. Biol. Chem. 279, 12484–12494 [DOI] [PubMed] [Google Scholar]
- 62.Liu Y., Sun L. Y., Singer D. V., Ginnan R., Singer H. A. (2013) CaMKIIδ-dependent inhibition of cAMP-response element-binding protein activity in vascular smooth muscle. J. Biol. Chem. 288, 33519–33529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma H., Groth R. D., Cohen S. M., Emery J. F., Li B., Hoedt E., Zhang G., Neubert T. A., Tsien R. W. (2014) γCaMKII shuttles Ca²⁺/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 159, 281–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gray C. B., Heller Brown J. (2014) CaMKIIdelta subtypes: localization and function. Front. Pharmacol. 5, 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang T., Kohlhaas M., Backs J., Mishra S., Phillips W., Dybkova N., Chang S., Ling H., Bers D. M., Maier L. S., Olson E. N., Brown J. H. (2007) CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J. Biol. Chem. 282, 35078–35087 [DOI] [PubMed] [Google Scholar]
- 66.Saura M., Marquez S., Reventun P., Olea-Herrero N., Arenas M. I., Moreno-Gómez-Toledano R., Gómez-Parrizas M., Muñóz-Moreno C., González-Santander M., Zaragoza C., Bosch R. J. (2014) Oral administration of bisphenol A induces high blood pressure through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB J. 28, 4719–4728 [DOI] [PubMed] [Google Scholar]
- 67.Deanfield J. E., Halcox J. P., Rabelink T. J. (2007) Endothelial function and dysfunction: testing and clinical relevance. Circulation 115, 1285–1295 [DOI] [PubMed] [Google Scholar]
- 68.Majesky M. W. (2007) Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 27, 1248–1258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.