Abstract
The transient receptor potential melastatin (TRPM)-3 channel is critical for various physiologic processes. In somatosensory neurons, TRPM3 has been implicated in temperature perception and inflammatory hyperalgesia, whereas in pancreatic β-cells the channel has been linked to glucose-induced insulin release. As a typical representative of the TRP family, TRPM3 is highly polymodal. In cells, it is activated by heat and chemical agonists, including pregnenolone sulfate (PS) and nifedipine (Nif). To define the nuances of TRPM3 channel activity and its modulators, we succeeded in incorporating the TRPM3 protein into planar lipid bilayers. We found that phosphatidylinositol-4,5-bisphosphate (PIP2) or clotrimazole is necessary for channel opening by PS. Unlike PS, the presence of Nif alone sufficed to induce TRPM3 activity and demonstrated distinct gating behavior. We also performed an extensive thermodynamic analysis of TRPM3 activation and found that TRPM3 exhibited slight temperature sensitivity in the bilayers. In the absence of other agonists TRPM3 channels remained closed upon heat-induced stimulation, but opened in the presence of PIP2, although with only a low open-probability profile. Together, our results elucidate the details peculiar to TRPM3 channel function in an isolated system. We confirmed its direct gating by PS and PIP2, but found a lack of the strong intrinsic temperature sensitivity common to other thermosensitive TRP channels.—Kunitoshi, U., Demirkhanyan, L., Asuthkar, S., Cohen, A., Tominaga, M., Zakharian, E. Stimulation-dependent gating of TRPM3 channel in planar lipid bilayers.
Keywords: reconstituted system, pregnenolone sulfate, nifedipine, clotrimazole, PIP2
The representative TRP channel of the melastatin subfamily TRPM3 is expressed in various tissue types, including the nervous system and epithelial cells of the kidney, pancreas, and testis. The prevalent expression pattern of TRPM3 has been detected in the kidney and at lesser levels in the brain, testis, and spinal cord (1, 2). Similar to other TRP channels, TRPM3 is a nonselective cation channel with a higher preference for divalent over monovalent cations (1). An interesting feature of TRPM3 is that its selectivity and permeation may vary widely among the different splice variants of the channel (3). Possessing a greater selectivity for Ca2+ and Mg2+, TRPM3 channels have been suggested to play an important role in homeostasis of these cations.
Among the identified TRPM3 agonists are a neurosteroid, pregnenolone sulfate (PS), and a pharmacological compound, 1,4- dihydropyridine nifedipine (Nif) (4). Alternatively, the channel can be activated by warm temperatures (∼37°C) (5).
To test the biophysical and pharmacological properties of the channel, we sought to incorporate the purified TRPM3 protein into planar lipid bilayers and examined single-channel activity in various conditions. Specifically, we investigated induction of TRPM3 channel activity by different agonists and characterized its temperature-dependent properties. Although we were able to confirm gating of TRPM3 with all its identified chemical agonists, the temperature sensitivity of the channel differed from that observed in the cellular system.
MATERIALS AND METHODS
Construction of Myc-tagged mouse TRPM3 cDNA
Oligonucleotide primers (forward, ATGGGCAAGAAGTGGAGGGATG, and reverse, TTAGTTGTGCTTGCTTTCAAAGC) were used to amplify TRPM3 transcripts after oligo dT12-18-primed RT of mouse dorsal root ganglion total RNA. We used the SuperScript First-Strand Synthesis System (Thermo Scientific, Waltham, MA, USA) for RT and Phusion High-Fidelity DNA Polymerase (Thermo Scientific) for amplification. Sequencing both strands of subcloned fragments identified independent clones encoding TRPM3α2 (GenBank accession number AJ544535). We introduced 2 Myc tags into the N terminus of TRPM3α2, which were subsequently cloned into pcDNA3.1.
In this work, we focused only on the splice variant TRPM3α2, which will be referred to hereafter as TRPM3.
Cell culture
Human embryonic kidney 293 (HEK-293) cells were maintained in minimum essential medium (Thermo Scientific–Invitrogen, San Diego, CA, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Thermo Scientific–Invitrogen), at 37°C in 5% CO2. For purification purposes, the TRPM3 expression vector was transfected into the HEK-293 cells with Effectene transfection reagent (Qiagen, Chatsworth, CA, USA). To increase the protein pool along with the transient expression of TRPM3, we developed an HEK-293 cell line that stably expressed the protein (Supplemental Figs. 1 and 2). The stable line was developed in a manner similar to the one described for TRPM8 (6).
For Ca2+-imaging experiments, TRPM3 expression vector and DsRed cDNA in Opti-MEM were transfected with Lipofectamine reagent (both from Thermo Scientific–Invitrogen).
Ca2+-imaging experiments
The Ca2+-imaging experiments were performed 1 d after transfection. HEK-293 cells grown on coverslips were placed into an open chamber and superfused with standard bath solution [140 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES), and 10 mM glucose (pH 7.4)]. Cytosolic Ca2+-free concentrations were measured by dual-wavelength Fura-2 (Thermo Scientific–Invitrogen) microfluorometry with excitation at 340/380 nm and emission at 510 nm. The ratio image was calculated and acquired with an IP Lab (Scanalytics Inc., Milwaukee, WI, USA). All experiments were performed at room temperature, with the exception of heat stimulation.
Two-electrode voltage-clamp recordings
The TRPM3 variants were heterologously expressed in oocytes of the African clawed frog Xenopus laevis, and the 2-electrode voltage-clamp method was used for recording the current. cRNA (150 ng) was injected into defolliculated oocytes, and currents were recorded 4–5 d after the injection. The membrane potential of the oocytes was clamped at −60 mV. ND96 solution contained 93.5 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 2 mM MgCl2, and 5 mM HEPES (pH 7.5; with NaOH) was perfused, and all chemicals were dissolved in bath solution. For temperature stimulation, heated or cold ND96 solutions were applied by perfusion. Data were sampled at 5 kHz and filtered at 1 kHz for analysis on an oocyte clamp (OC-725C; Warner Instruments, Hamden, CT, USA) with pCLAMP software (Molecular Devices–Axon, Sunnyvale, CA, USA).
Preparation of the TRPM3 protein from HEK cells
HEK-293 cells expressing TRPM3 were washed and collected in cold PBS. The cells were harvested and resuspended in NaCl buffer, containing 500 mM NaCl, 50 mM NaH2PO4, 20 mM HEPES, and 10% glycerol (pH 7.5), with the addition of 1 mM concentration of the protease inhibitor PMSF and 5 mM 2-ΜΕ. The cells were lysed by the freeze–thaw method and centrifuged at 40,000 g for 2.5 h. The pellet was resuspended in NaCl buffer with the addition of a protease inhibitor mixture (Roche Diagnostics, Indianapolis, IN, USA), 0.1% Nonidet P-40 (Roche Diagnostics), and 0.5% dodecylmaltoside (DDM) (EMD Biosciences–CalBiochem, San Diego, CA, USA). The suspension was incubated overnight on a shaker with gentle agitation and centrifuged for 1 h at 40,000 g. The solubilized TRPM3 protein was purified by immunoprecipitation with magnetic beads (Thermo Scientific–Pierce, Rockford, IL, USA) conjugated with the anti-Myc antibodies (Sigma-Aldrich, St. Louis, MO, USA). All steps of purification were performed at 4°C. The TRPM3 protein was electrophoretically separated on 10% SDS polyacrylamide gels (Bio-Rad, Hercules, CA, USA) in Tris-glycine-SDS buffer (Bio-Rad) at a constant voltage of 180 V. Protein bands were visualized with Silver Stain (Bio-Rad). For Western blot analysis, the protein was transferred onto PVDF membranes (Bio-Rad) by using transfer buffer containing 25 mM Tris-glycine, 20% methanol, and 0.07% SDS. The transfer was made at 24 V for 2.5 h with a Genie Electrophoretic blotter (Idea Scientific, Minneapolis, MN, USA). The TRPM3 protein was detected with the anti-Myc-IgG antibody (Sigma-Aldrich).
Mass spectrometry analysis
After immunoprecipitation, the TRPM3 preparation was separated by SDS-PAGE and stained with Coomassie blue. Apart from the heavy- and light-chain antibody bands derived from immunoprecipitation, the gel resulted in a prominent band at ∼212 kDa, corresponding to the molecular mass of the TRPM3 monomer. This band was excised from the gel and digested with trypsin for mass spectrometry (MS) analysis, according to the protocol of Mann et al. (7), with some modifications. In brief, the gel bands were reduced with 0.5 M DTT, alkylated with 0.7 M iodoacetamide, and digested with trypsin (Promega, Madison, WI, USA) for 12 h at 37°C. The peptides were extracted from the gel bands with 100 μl of a 50% acetonitrile, 5% formic acid solution. The extract was dried by vacuum centrifugation (SPD SpeedVac; Thermo Electron Corp.,Waltham, MA, USA), and the tryptic peptides were resuspended in 20 μl of a 3% acetonitrile, 0.5% formic acid solution.
Liquid chromatography–tandem MS (LC-MS/MS) was performed with a Nanoflow liquid chromatography system (Ultimate3000; Thermo Scientific) interfaced with a hybrid ion trap–orbitrap high-resolution tandem mass spectrometer (VelosPro; Thermo Scientific) operated in data-dependent acquisition (DDA) mode. In brief, 1 ml of each sample was injected into a capillary column (4 μm Jupiter C18 manually packed on a 30 cm × 75 μm ID PicoFrit Column; New Objective, Woburn, MA, USA) at a flow rate of 300 nl/min. Samples were electrosprayed at 1.2 kV with a dynamic nanospray ionization source. Chromatographic separation was performed with 90 min linear gradients (mobile phase A: 0.1% formic acid in MS-grade water; mobile phase B: 0.1% formic acid in MS-grade acetonitrile) from 3% B to 35% B over 60 min, then increasing to 95% B over 5 min. MS/MS spectra were acquired with both collision-induced dissociation (CID) and higher-energy collisional dissociation (HCD) for the top 15 peaks in the surveyed 30,000-resolution MS scan. The .raw files were acquired (Xcalibur) and exported to Proteome Discoverer 2.0 (both from Thermo Scientific) software for peptide and protein identification by using the SequestHT search algorithm (Thermo Scientific) (full trypsin digestion with 2 maximum missed cleavages, 10 ppm precursor mass tolerance, and 0.8 Da fragment mass tolerance). Database search was performed using Uniprot.
Planar lipid bilayer measurements
Planar lipid bilayers measurements were performed as has been described (6, 8, 9). Planar lipid bilayers were formed from a solution of synthetic 1-palmitoyl-2-oleoyl-glycero-3-phosphocoline (POPC) and 1-palmitoyl-2-oleoyl-glycero-3-phosphoethanolamine (POPE; Avanti Polar Lipids, Birmingham, AL, USA) at a 3:1 ratio in n-decane (Sigma-Aldrich). The solution was used to paint a bilayer in an aperture of ∼150 µm diameter in a Delrin cup (Warner Instruments) between symmetric aqueous bathing solutions of 150 mM KCl, 0.02 mM MgCl2, 1 μM CaCl2, and 20 mM HEPES (pH 7.2). Some experiments were performed in the presence of PIP2; specifically, 5 or 10 μM DiC8-PIP2 (Cayman Chemical, Ann Arbor, MI, USA) was dissolved in water and added to both compartments. All salts were ultrapure (>99%) (Sigma-Aldrich). Bilayer capacitances were in the range of 50–75 pF. After the bilayers had formed, 0.2 μl of the TRPM3 micellar solutions (0.001 μg/ml) was applied to the cis compartment with gentle stirring. Unitary currents were recorded with an integrating patch clamp amplifier (Axopatch 200B; Molecular Devices-Axon). The trans solution (voltage command side) was connected to the CV 201A head-stage input, and the cis solution was held at virtual ground via a pair of matched Ag-AgCl electrodes. Currents through the voltage-clamped bilayers (background conductance, <1 pS) were filtered at the amplifier output (low pass, −3 dB at 10 kHz, 8-pole Bessel response). Data were secondarily filtered at 100 Hz through an 8-pole Bessel filter (950 TAF; Frequency Devices, Ottawa, IL, USA) and digitized at 1 kHz with an analog-to-digital converter (Digidata 1322A), controlled by pClamp10.3 software (both from Molecular Devices-Axon). Single-channel conductance events, all-points histograms, open probability (Po), and other parameters were identified and analyzed with Clampfit10.3 software (Molecular Devices-Axon).
Experiments were performed at ∼23°C, except for the temperature studies.
Temperature studies
The bilayer recording chamber was fitted onto a conductive stage containing a pyroelectric heater/cooler that was driven by a temperature controller (CL-100; Warner Instruments). Deionized water was circulated through the stage and pumped into the system to remove the heat generated. The temperature of the bath was constantly monitored with a thermoelectric device in the cis chamber (the ground side) and was reliably controlled within ±0.5°C. The temperature coefficients (Q10) for Po or for the single-channel conductance were obtained by the calculation in Eq. 1:
 |
where T1 and T2 are temperatures in Kelvins, and X1 and X2 are Po obtained at relative temperatures.
Data analysis
Data are presented as means ± sem. The unpaired Student’s t test was used to test the significance of results in some experiments.
RESULTS
Preparation of the TRPM3 protein
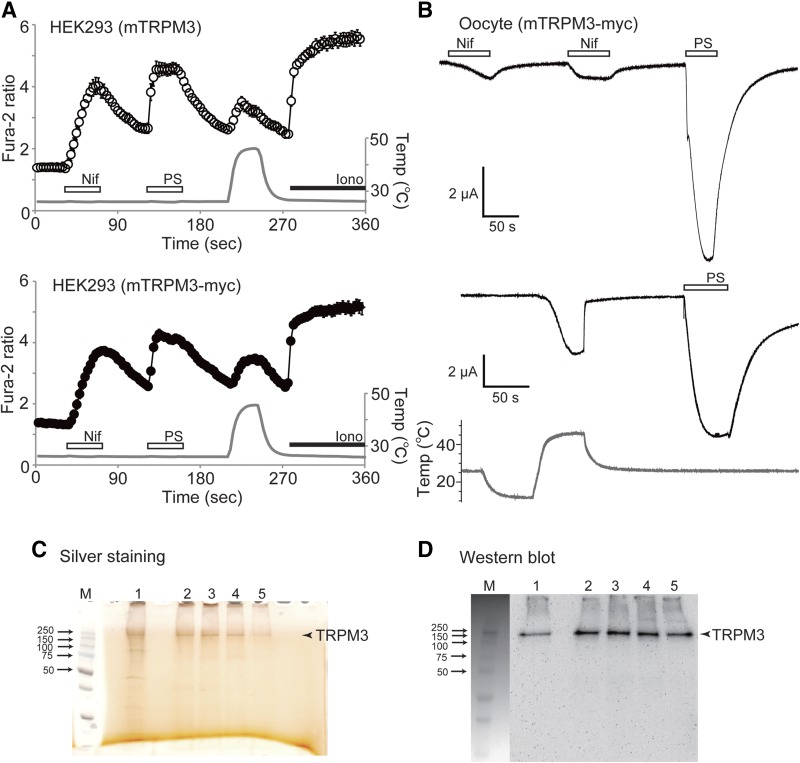
To characterize TRPM3 in the defined reconstituted system, we purified the Myc-tagged TRPM3 protein by immunoprecipitation. Initially, to check whether the Myc-tagged TRPM3 protein retained its functionality, we performed Ca2+-imaging experiments. Consistent with the Ca2+ responses obtained from the cells expressing nontagged TRPM3, Nif (100 μM), PS (50 μM), and temperature increase (∼45°C) elicited similar intracellular Ca2+ responses in HEK-293 cells transiently expressing Myc-tagged TRPM3 (Fig. 1A). In addition, we confirmed the function of Myc-tagged TRPM3 by the electrophysiological approach. Two-electrode voltage clamp recordings indicated the inward currents stimulated by 100 μM Nif (1.14 ± 0.41 μA; n = 8), 100 μM PS (12.41 ± 2.66 μA, n = 8), and temperature increase up to 48°C (5.47 ± 2.22, n = 4) in Xenopus oocyte expressing Myc-tagged TRPM3 (Fig. 1B). We detected that current potentiation elicited by Nif was smaller in comparison to PS, which could be caused by the interference of this lipophilic compound with oocyte membrane resulting in lesser accessibility to TRPM3. None of the agonists evoked currents in controls with solvent injected Xenopus oocyte (data not shown).
Figure 1.
Preparation of Myc-tagged mouse TRPM3 protein. A) A comparison of the responses to 100 μM Nif, 50 μM PS, and heat stimulation between native mouse TRPM3α2 (mTRPM3) and the Myc-tagged mouse TRPM3α2 (mTRPM3-Myc) by Ca2+-imaging. Ionomycin (5 μM) was applied at the end. Data are means ± sem (n = 38–46 cells). Gray trace: temperature of bath solution close to the cells. B) Representative traces of inward currents stimulated by application of 100 μM Nif (n = 8), 100 μM PS (n = 8), and heat stimulation (n = 4) in mTRPM3-Myc-expressing Xenopus oocytes. Membrane potential was at −60 mV. C) Silver staining of the purified mTRPM3-Myc protein. Lane 1, wash sample; lanes 2–5, eluates. Numbers on the left indicate the molecular mass of the markers in kilodaltons; M, markers;. D) Western blot of the purified mTRPM3-Myc protein immunoblotted with anti-Myc antibody.
Together, these results indicated that the Myc-tagged TRPM3 channel retains all its functional characteristics and may be further tested in the reconstituted system.
Using immunoprecipitation with anti-Myc antibody, we obtained the detergent-solubilized Myc-tagged TRPM3 protein. The protein purification was accomplished analogously to TRPM8, as we have described (8), except for using lauryl maltose neopentyl glycol (LMNG) instead of DDM in the elution buffer. To preserve the functionality of the channel, TRPM3 was eluted from the magnetic beads conjugated with anti-Myc antibody, using Myc peptide and LMNG containing buffer. The eluted protein was detected as a single band, as judged by silver staining and Western blot analysis with anti-Myc antibody, where the protein migrated at ∼212 kDa, corresponding to the molecular mass of the TRPM3 monomer (Fig. 1C, D).
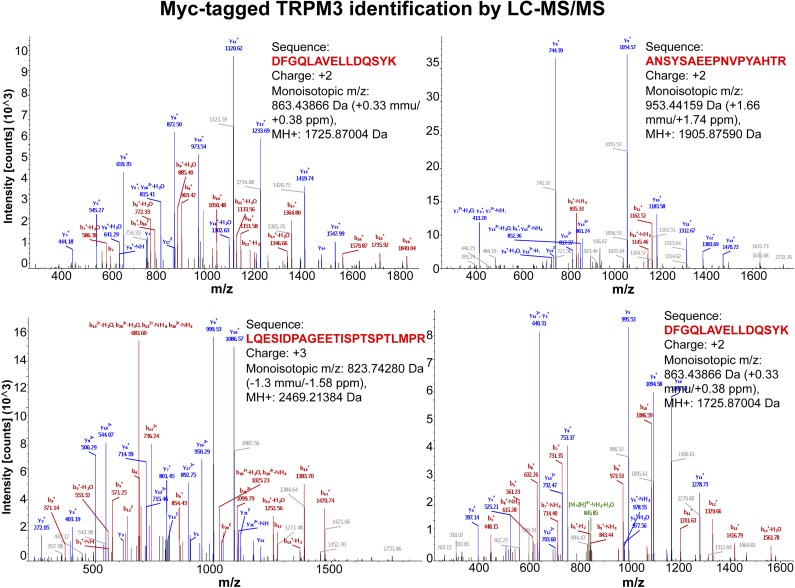
To further confirm the TRPM3 protein identity in the purified prep, we performed extensive MS analysis, using the LC-MS/MS approach. The results indicated that, in the purified samples, TRPM3 was present at the highest abundance, and its identity was confirmed by recognition of the multiple specific peptides totaling 64% of sequence coverage, which is considerably high. The representative spectra from several TRPM3 peptides are shown in Fig. 2. These results further confirmed the high homogeneity of the purified TRPM3 protein and absence of other ion channel contaminants present at the detectable level.
Figure 2.
The TRPM3 protein identification using LC-MS/MS analysis. For the analysis, the Myc-tagged TRPM3 protein immunoprecipitated with anti-Myc antibodies was removed from the magnetic beads in denaturing conditions, separated on an SDS-polyacrylamide gel, and stained with Coomassie blue. The bands were excised from the gel, trypsin-digested, and examined by LC-MS/MS. The TRPM3 protein was identified as the prevalent protein, with 64% of the protein sequence coverage retained. The representative spectra show the specific TRPM3 peptides that were identified.
Basal activity of TRPM3 in planar lipid bilayers
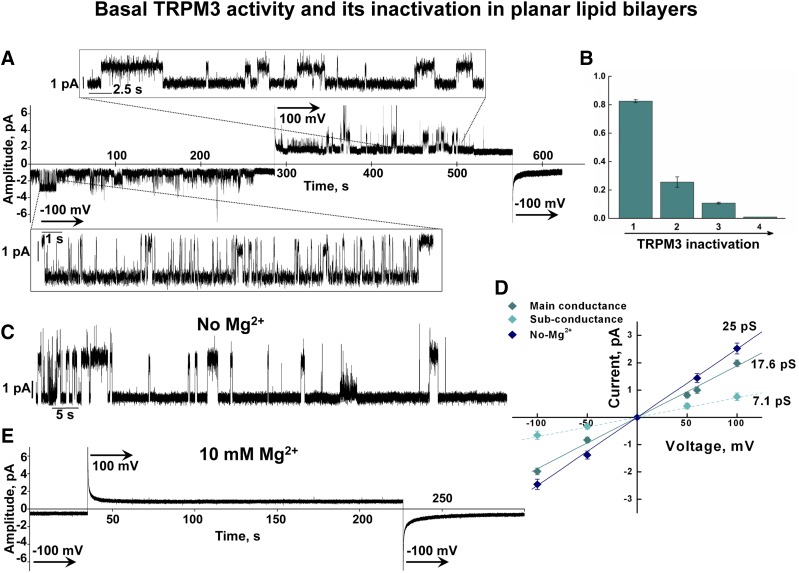
To assess the functionality of the purified TRPM3 protein, we reconstituted the channel into planar lipid bilayers formed from POPC/POPE (3:1) and performed electrophysiological recordings. We found that, soon after the incorporation into the bilayer system, TRPM3 exhibited basal channel activity in the absence of any added agonists. This feature may account for the constitutive TRPM3 activity evidenced in the cellular systems (1, 2, 10). In the bilayers, this constitutive activity lasted for only a few minutes and then gradually disappeared. Figure 3A demonstrates representative current traces from the TRPM3 channel that were obtained for several minutes of constitutive activity without additional ligands. This activation declined in a time-dependent manner and was completely abolished within ∼10 min. Distinctive TRPM3 openings demonstrate a gradual decrease in Po, where Po declined from ∼0.8 to ∼0.2 within 5 min (26,342 events analyzed; n = 7) (Fig. 3A, B). The basal activity was evidenced only after the incorporation and did not reappear, even when the channels were kept in the bilayers for several hours. The nature of this constitutive activity is not clear. It is possible that this TRPM3 behavior is caused by a presence of an agonist/compound copurified with the channel that could further dissociate from the protein and not reactivate it because of the negligible concentrations (in 1 ml of the bilayers buffer in each of the compartments, trans and cis). On the other hand, Oberwinkler et al. (3) earlier reported that TRPM3 activity is tightly regulated by both monovalent and divalent cations that exert a side-specific inhibition evidenced in whole-cell patch clamp recordings. Therefore, the inactivation of the constitutive activity in the bilayers could be causative of the inhibitory effect exerted by cations. However, unlike the reconstituted channels, in cells this inhibition was not complete (3); thus, it cannot explain its complete inactivation in the bilayers.
Figure 3.
Basal activity of the purified TRPM3 channel in planar lipid bilayers. A) Representative single-channel trace recordings of TRPM3 obtained in the absence of additional agonists. Traces demonstrate a slow decline of the basal activity and its inactivation within 10 min; voltages steps are indicated. TRPM3 basal activity was obtained in the presence of 0.02 mM Mg2+ to support the channel stability. B) Time-dependent changes in Po of TRPM3 without ligands obtained at −100 mV. C) Representative current recording shows TRPM3 activity obtained in the absence of Mg2+ (with addition of 2 mM EDTA to both bilayer compartments), which caused similar inactivation within ∼10 min. This basal activity was evidenced in both the absence and presence of free 10 μM Ca2+. D) Current–voltage relationship of TRPM3 basal activity obtained in the presence of 0.02 mM Mg2+ (main and subconductance states) and in the absence of Mg2+. Data are means ± sem of results in 22 independent experiments. E) Representative traces show complete inhibition of TRPM3 in the presence of 10 mM Mg2+, added to both compartments of the bilayers.
The constitutive activity of TRPM3 channels obtained in cells was shown to be inhibited by millimolar intracellular Mg2+ concentrations (3). In our experiments, we obtained constitutive activity in the presence of low Mg2+, at a concentration of 0.02 mM. The reason for maintaining low Mg2+ in the buffer was to support the protein’s stability. We noticed that the TRPM3 protein incorporated into the bilayers for a prolonged time in the absence of this cation demonstrated strong destabilization, evidenced by highly disorganized activity (data not shown). A similar requirement for the presence of Mg2+ to support the channel’s stability had been indicated earlier for the TRPM8 channel (6). Even though the appearance of the basal TRPM3 activity in the absence of Mg2+ was more frequent in comparison to that obtained in its presence, it showed a similar inactivation tendency (Fig. 3C). On the other hand, addition to the bilayers of 10 mM Mg2+ completely abolished TRPM3 activity (Fig. 3E), which is in line with previous work (3).
To this end, to prevent destabilization of the channel, the rest of the bilayer experiments were performed in the presence of low-Mg2+ concentrations.
PS activates TRPM3 in the presence of clotrimazole or PIP2
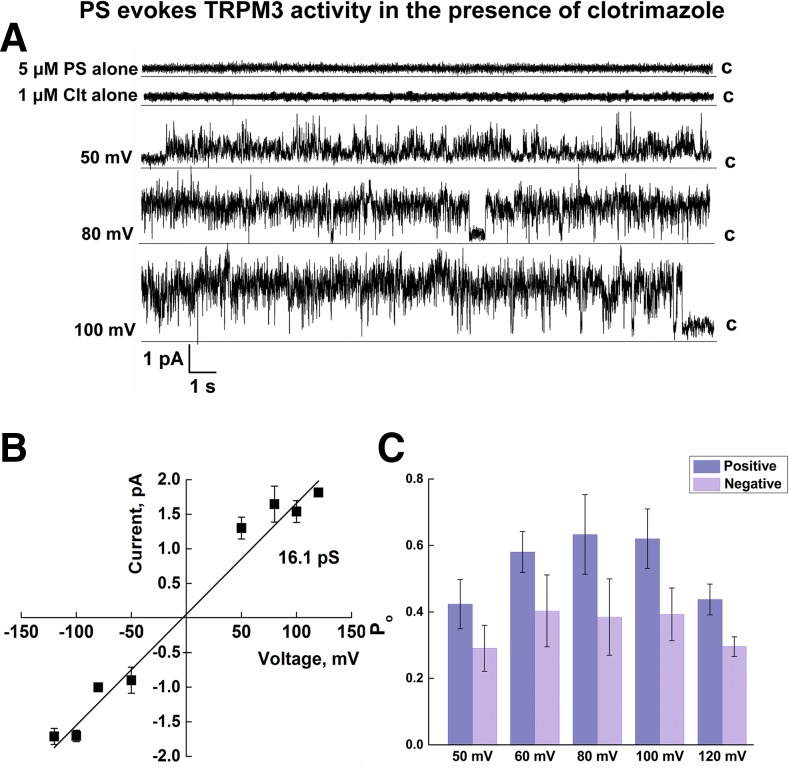
Next, we analyzed ligand-induced TRPM3 behavior. First, TRPM3 activity was tested in the presence of PS. We found that PS alone was insufficient to stimulate TRPM3 channel openings and required other additives. When, along with PS, we added to the bilayers a chemical compound, clotrimazole, the combined treatment stimulated TRPM3 channel openings. Clotrimazole is an antifungal compound that has been shown to enhance the PS-induced activity of TRPM3 in cells (11). As shown in Fig. 4A, 1 μM clotrimazole, together with 5 μM PS, resulted in activation of TRPM3, whereas clotrimazole alone was ineffective. The chord conductance of TRPM3 activated by PS and clotrimazole obtained from the regression slope was ∼16.1 pS and did not differ between positive and negative membrane potentials (Fig. 4B). Furthermore, TRPM3 openings exhibited greater Po at positive membrane potentials than that at negative membrane potentials (Fig. 4C), which may account for the outward rectification of the channel evidenced in cellular systems. The total number of events analyzed for PS/clotrimazole recordings was 324,408 (n = 15).
Figure 4.
Single-channel recordings of TRPM3 activated by PS with clotrimazole. A) Representative single-channel recordings of TRPM3 activated by 1 μM clotrimazole (Clt), with or without 5 μM PS. Membrane potential change from +50 to +120 mV. B) Current–voltage relationship of TRPM3 activated by 5 μM PS with 1 μM Clt. The slope conductance is ∼16.3 pS. C) Po of TRPM3 activated by 5 μM PS with 1 μM Clt obtained at different voltages. Data are means ± sem of results in 15 experiments.
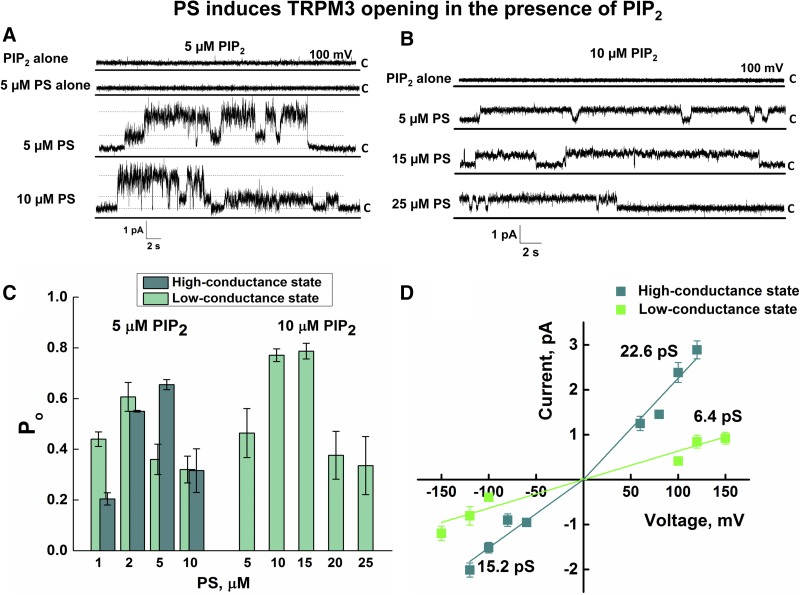
Phosphoinositides play important roles in the functional regulation of several TRP channels. Particularly, PIP2 has been reported to support activation of TRPM8 (12, 13), TRPV1 (14, 15), and TRPV6 (16), and its presence is also necessary to activate these channels in planar lipid bilayers (6, 9, 16, 17). Recent findings have indicated that PIP2 is also a critical factor for TRPM3 activity (18, 19). To clarify the involvement of PIP2 in TRPM3 activation, we tested the channel characteristics in the presence of PIP2, with or without other ligands. We found that addition of PIP2 along with PS readily stimulated TRPM3 channel openings (Fig. 5). Coapplication of 5 μM PIP2 with 5 μM PS was sufficient to activate the channel (Fig. 5A, C, D). The total number of events analyzed was 284,303.
Figure 5.
The effect of PIP2 on PS-stimulated TRPM3 activation. A, B) Representative single-channel recording of TRPM3 activated in the presence of various concentrations of PS and PIP2. Membrane potential was 100 mV. C) Dose-dependent changes in Po of TRPM3 activated by 1–10 μM PS with 5 μM PIP2 or 5–25 μM PS with 10 μM PIP2 at +100 mV. The TRPM3 channel activated with higher concentrations of PS and PIP2 preferably open to a low-conductance state. D) Current–voltage relationship of TRPM3 activated in the presence of PS/PIP2. Corresponding slope conductance values are shown. Data are means ± sem of results in 20 experiments.
Unlike the other PIP2-dependent TRP channels, PIP2 was not necessary for TRPM3 activation under all the conditions. For instance, TRPM3 basal channel activation or openings induced by combined PS/clotrimazole treatment were obtained in the absence of PIP2 (Figs. 3, 4).
TRPM3 channels activated with PS/PIP2 exhibited 2 distinct conductance states: a high-conductance state showed rectifying current with a mean slope conductance of ∼23 pS at positive voltages and ∼15 pS obtained at negative voltages. Unlike high-conductance, the low-conductance current–voltage relationship was linear and had a mean slope conductance of ∼6 pS (Fig. 5A, B, D). Among the observed conductance values, 15.2 pS at negative voltages was similar to the mean slope conductance of 16.1 pS obtained for the channel activated with PS/clotrimazole (Fig. 4).
The PS/PIP2-induced dose dependence of TRPM3 resulted in interesting observations. Increased concentrations of both PIP2 and PS resulted in the reduction of Po of the channel (1,058,142 events analyzed; n = 20) (Fig. 5C). Furthermore, with higher concentrations of PS/PIP2, TRPM3 acted only in the low-conductance mode, and high-conductance levels were no longer observed (Fig. 5B, C). These results suggest that PS/PIP2-induced activity of TRPM3 is concentration dependent and could represent a tuned activation/inactivation mechanism.
In the presence of 5 μM PIP2, PS induced TRPM3 with an EC50 of ∼1.3 at 100 mV (Supplemental Fig. 3), which was lower than that observed in cellular systems (EC50 = 23 μM) (4). This difference may account for a higher sensitivity of TRPM3 to its agonist in the pure reconstituted system.
Nifedipinef solely activates reconstituted TRPM3 channels
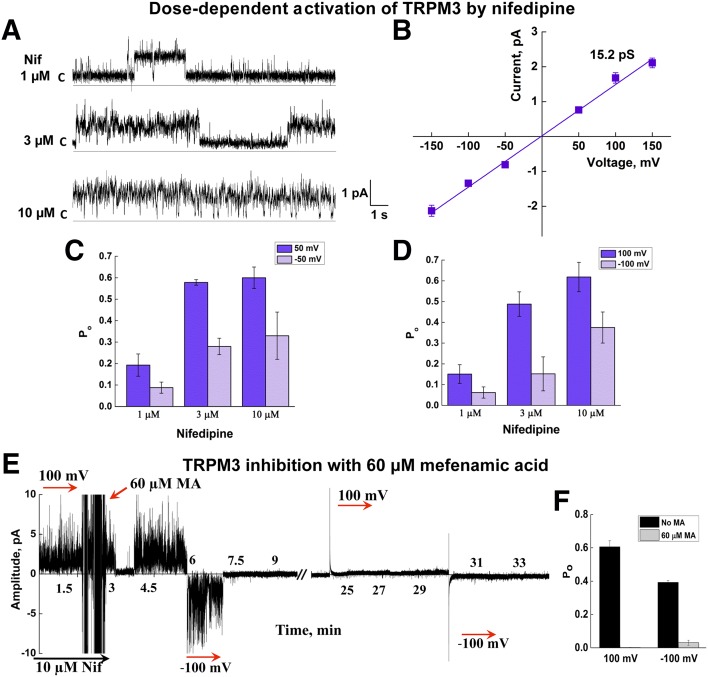
Next, we tested TRPM3 activity in the presence of Nif. Unlike PS, addition to the bilayers of Nif was sufficient to induce TRPM3 activity and did not require other additives. In the presence of Nif (3 μM) TRPM3 demonstrated the linear current–voltage relationship, but differential Po with the opposite voltages (Fig. 6). Nif-induced single channel conductance was ∼15.2 pS with higher Po at positive membrane potentials, similar to that obtained in the presence of PS/clotrimazole (Fig. 6A–D; 196,537 events analyzed; n = 15).
Figure 6.
TRPM3 is activated by Nif. A) Representative single-channel traces of TRPM3 activated by 3 μM Nif at +50 mV. B) Current–voltage relationship of TRPM3 activated by Nif. Mean slope conductance was ∼15.2 pS. C, D) Dose-dependent changes in Po of TRPM3 activated by Nif at ±50 and ±100 mV. Data are means ± sem of results in 15 experiments. E) Mefenamic acid induced inhibition of TRPM3 channel activated by 10 μM Nif (representative current trace at ±100 mV). F) Changes in Po upon TRPM3 inhibition by mefenamic acid. Data are means ± sem of results in 5 experiments.
Next, we tested the TRPM3 inhibitor’s effect on the channel activity obtained in the reconstituted system. Application of mefenamic acid to a Nif-induced TRPM3 channel resulted in prompt inhibition of its activity (Fig. 6E, F; n = 5). This result once again confirmed that the channel activity obtained in the bilayers was specific to TRPM3.
Similar to PS, the EC50 of Nif-evoked TRPM3 openings in the bilayers was much lower (∼1.7 μM) (Supplemental Fig. 3) compared with that obtained in cells (∼32 μM) (4), once again indicating a higher agonist efficiency in the isolated system.
The channel currents obtained with PS/clotrimazole or Nif exhibited noisy behavior (Figs. 4, 6). Initially, we reasoned that this activity might indicate that TRPM3 is not well folded. However, TRPM3 recordings during the basal activity demonstrated highly organized channel openings (Fig. 3), and even in 1 experiment, when we first obtained the basal activity, it was well organized, but then when TRPM3 was reactivated with PS/clotrimazole or Nif, the channel currents were noisy. Because this channel behavior was obtained repeatedly, we concluded that it is the specifics of the agonist itself. Most likely, upon binding to the channel, clotrimazole or Nif strongly affects TRPM3 conformation and that further exerts a disturbance along its permeation path. This observation perhaps is not surprising, as both ligands are pharmacological compounds and not physiologic TRPM3 agonists.
Reconstituted TRPM3 exhibits slight temperature sensitivity
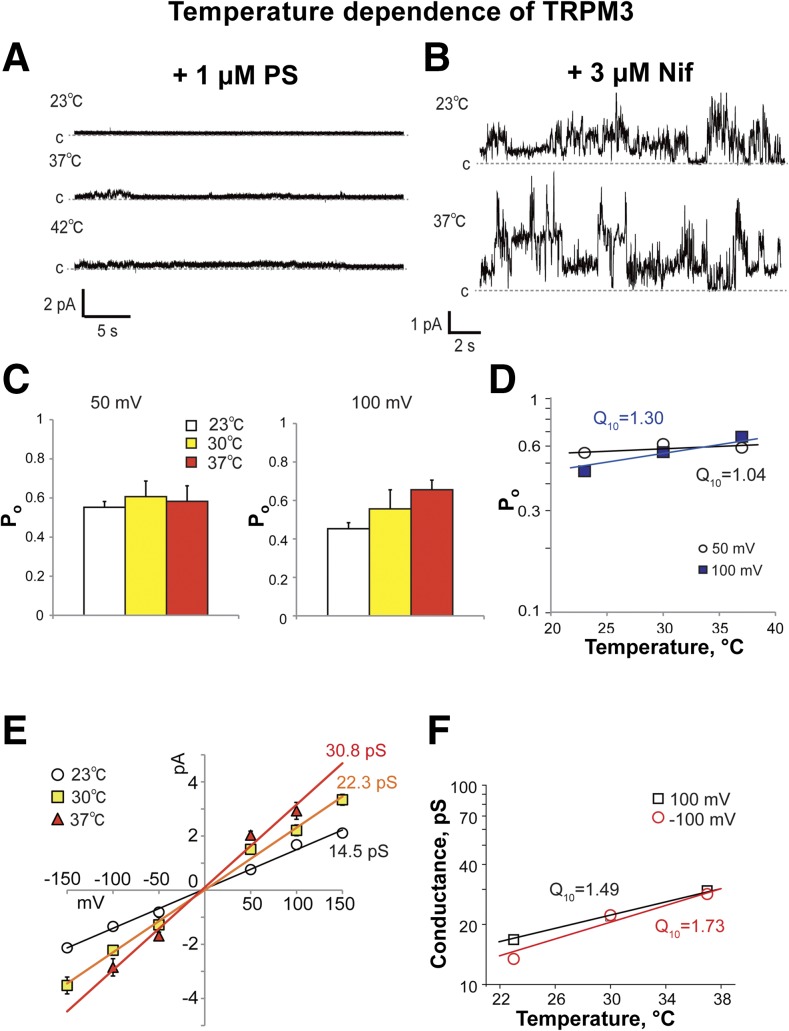
TRPM3 channels were reported to be involved in temperature sensation (5). In the current study, we tested TRPM3 temperature sensitivity in the reconstituted system. We found that a temperature increase (≤42°C) did not induce any noticeable channel activity of TRPM3. Furthermore, the addition of PS during the temperature test was also ineffective (Fig. 7A). On the other hand, TRPM3 challenged with temperature in the presence of Nif demonstrated the very low temperature dependence of both single-channel conductance and Po (Fig. 7). Q10 for the Po of Nif-induced TRPM3 were 1.0 and 1.3, at +50 and +100 mV, respectively (Fig. 7D). Although for the conductance change, Q10 values were 1.5 and 1.7 at +100 mV and −100 mV, respectively (Fig. 7F).
Figure 7.
Temperature sensitivity of TRPM3. A, B) Representative single-channel recordings of TRPM3 upon temperature increase from 23 to 42°C in the presence of 1 μM PS (A) or 3 μM Nif at 23 and 37°C (B). Membrane potential was +50 mV. C) Temperature-dependent changes in Po of TRPM3 activated by 3 μM Nif at +50 and +100 mV. D) Log of Po vs. temperature demonstrates Q10 averaged for currents at +50 and +100 mV, which was obtained from the regression slope. E) Current–voltage relationship of TRPM3 activated by Nif with temperature increase from 23 to 37°C. Data are means ± sem of results from 6 experiments. F) Log of conductance vs. temperature demonstrates Q10 averaged for currents at +100 and −100 mV, which were obtained from the regression slope.
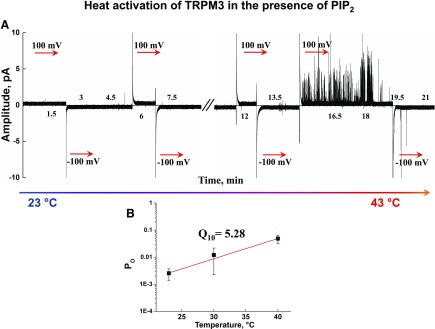
Previously, we demonstrated that both TRPM8 and TRPV1 require the presence of PIP2 for the temperature-induced activation in the planar lipid bilayers (9, 20). We were curious as to whether that would also be the case for TRPM3. This surmise motivated us to conduct the temperature experiments in the presence of PIP2.We found that heating the reconstituted TRPM3 in the presence of PIP2 can induce channel gating. However, it may only stimulate TRPM3 openings to a low Po mode (Po = 0.048 ± 0.016), and fully open channels were never obtained, even after extensive trials (n = 30) (Fig. 8).
Figure 8.
Heat-induced activation of the TRPM3 channel in the presence of PIP2. A) Representative current traces of the heat-induced activation of TRPM3 channels in the presence of 5 μM PIP2. The recordings were taken at temperatures ranging between 23 and 43°C. B) Logarithmic relationship of Po and temperature. Q10 values were calculated from the regression slope at +100 mV. Data are means ± sem of results in 30 experiments.
Together, these results indicate that the TRPM3 channel is intrinsically slightly temperature dependent.
DISCUSSION
Exploration of the physiologic designation of TRPM3 channels is still in its developing stage. The ambiguity about its function derives primarily from the diverse expression of the channel and its system-defined roles in a range of important physiologic processes. Hence, in the somatosensory neurons, TRPM3 is implicated in the development of heat hyperalgesia during inflammation (5). On the other hand, a distinctive role of TRPM3 in controlling insulin release has been indicated for the channel in pancreatic β-cells (4). In addition, TRPM3 is expressed in various other tissues, including the brain, pituitary gland, eye, kidney, and adipose tissue, where its role is not well understood (21). Another important physiologic implication of TRPM3 has recently emerged, where the channel was shown to evoke the release of calcitonin gene-related peptide (CGRP) from sensory nerve terminals (22). The diverse implication of TRPM3’s part in specific system-defined responses could derive from its regulatory role in affecting Ca2+ and Mg2+ homeostasis (21). However, how this activity is dictated by TRPM3 agonists is yet to be determined.
In general, the TRP channel family stands out among the other channels because of their diverse physiologic functions and implication in sensing a broad range of physical and chemical stimuli. TRPM3 channels are not an exception. Like other TRP channels, TRPM3 activity is driven by a plethora of exogenous and endogenous factors. To distinghish among the specific channel’s agonists, in this work we wanted to characterize the purified TRPM3 channel in the reconstituted system. An extensive analysis of TRPM3 activity in the planar lipid bilayers enabled us to define the channel’s characteristics and its direct regulators.
Earlier, electrophysiological studies indicated that TRPM3 function is involved in neurosteroid-induced signaling, with the neurosteroid PS being an agonist for the channel (4). Appreciating the physiologic significance of this interaction, we tested the effect of PS on TRPM3 in the bilayer system. In line with the previous finding, we detected that PS can directly induce TRPM3 channel activity in the presence of PIP2 or clotrimazole. Although the regulation of TRPM3 activity by clotrimazole in combination with PS is difficult to interpret physiologically, the channel dependence on PIP2 seems more relevant. Indeed, 2 recent publications documented TRPM3 dependence on the presence of this phosphoinositide (18, 19).
The PS/clotrimazole-induced TRPM3 activity observed in cell-attached patches indicated 2 distinct conductance states of ∼50 and ∼13 pS of magnitude (11). In the presence of PS/clotrimazole in the bilayer system, we observed only 1 state of single-channel conductance, which was of 16 pS magnitude. However, TRPM3 exhibited 2 distinct conductance states when the channel was activated with PS and PIP2. The large-conductance channel showed rectification and opened with ∼23 pS conductance at positive voltages and ∼15 pS at negative voltages. The small-conductance channels showed a linear current–voltage relationship and a slope conductance of ∼6 pS. These results indicate that clotrimazole and PIP2 in combination with PS differentially affect the TRPM3 pore conformation. Together, our results for PS-dependent TRPM3 activation confirm direct gating of the channel by the neurosteroid.
Hormonal regulation of the function of TRP may have significant physiologic implications. In light of direct channel gating by steroids, we recently demonstrated that the prevalent male hormone testosterone directly gates the TRPM8 channel (23, 24). These findings indicate that the dependence of both TRPM3 and TRPM8 on hormonal action present novel phenomena in TRP channel designation. At the same time, there are some particular differences between TRPM3 and TRPM8 sensitivity to their steroid agonists: whereas PS activates TRPM3 at micromolar concentrations (EC50 = 23 μM) (4), testosterone induces TRPM8 in a picomolar concentration range (23, 24).
Another interesting TRPM3 agonist that has been detected is Nif (4). It is a pharmacological compound and an inhibitor of L-type voltage-gated Ca2+ channels. At low micromolar concentrations, Nif readily activated TRPM3 in the planar lipid bilayers, but exhibited a different gating mode from that obtained with PS. Furthermore, Nif activated TRPM3 alone and, unlike PS, did not need additives. These results indicate that PS and Nif act on the channel differently, which is in agreement with the allosteric activation of TRPM3 with these compounds observed in cells (25).
One of the important TRPM3 activities was linked to the temperature sensation, where the channel plays the role of a heat receptor (5). To establish the role of the channel in temperature-induced activity, we performed an extensive study in the temperature-controlled system. Exposure of TRPM3 to heat alone or in the presence of PS was insufficient to stimulate channel openings. In a prior study, we demonstrated that temperature-induced activation in the bilayers of the cold receptor TRPM8 or heat receptor TRPV1 is effectively achieved in the presence of their permanent gating factor PIP2 (9, 20). We wondered whether PIP2 could also be involved in TRPM3 temperature-induced gating. Testing the addition of PIP2 to the bilayers upon heat-induced activation of TRPM3 indeed showed more efficiency than its absence, yet it could not stimulate sufficient conformational changes in the protein to extend it to the fully open channel and showed only a low Po mode (Fig. 8). Evaluating the thermodynamics of TRPM3 further posed the question of how strong the channel’s temperature sensitivity is in relation to the other thermo-TRPs. From one perspective, in the presence of PIP2, TRPM3 exhibited a Q10 of 5.3, which was fairly close to the one observed in cells (Q10 = 7.2) (5). However, these temperature-dependent properties of TRPM3 essentially differed from the other thermo-TRPs, in which the temperature change induced channel transition into the fully open state. Furthermore, the temperature dependence of TRPM3 in the bilayers with a Q10 of 5.3 was much lower than that obtained for TRPM8 (Q10 = 40) and TRPV1 (Q10 = 18) (9, 20). This contrast suggests that TRPM3 is intrinsically slightly temperature dependent.
An example of indirect temperature sensitivity was reported earlier for another TRP channel, TRPV4. Although heat-evoked TRPV4 activity was indicated in the whole-cell recordings, it was not observed in an inside–out configuration, indicating an additional heat-induced cellular mechanism upon TRPV4 activation (26).
In conclusion, we have provided insights into the mechanism of TRPM3 channel gating with its physiologic agonists. In parallel with earlier reports obtained from cell recordings, in the context of the planar lipid bilayers, TRPM3 was directly activated by PS and depended on the presence of PIP2. On the other hand, we detected dissimilarity related to the temperature sensitivity of TRPM3. Unlike its strong heat-induced activity and current potentiation in cells, in the artificial system, TRPM3 was only slightly temperature sensitive and could not transition into the fully open conformation when exposed to heat. These TRPM3 channel characteristics point toward an existence of other endogenous factors that regulate channel activity at the cellular level. However, taking into account the constitutive TRPM3 activity seen in cells and also in the bilayers, it could be the component that contributes to higher temperature sensitivity of the channel in cells.
Supplementary Material
Acknowledgments
The authors thank N. Fukuta and C. Saito (National Institute of Physiological Sciences) for technical assistance, the laboratory members for helpful discussions, and H. Sahakyan (Bradley University) for proofreading the manuscript. This work was supported by Young Researcher Overseas Visit Program Expenses from The Graduate University for Advanced Studies (to K.U.); Grant-in-Aid 25871061 for Young Scientists (B) (to K.U.); and U.S. National Institutes of Health/National Institute of General Medicine Sciences Grant R01GM098052 (to E. Z).
Glossary
- DDM
dodecylmaltoside
- HEK
human embryonic kidney
- HEPES
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid
- LMNG
lauryl maltose neopentyl glycol
- Nif
nifedipine
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MS
mass spectrometry
- PIP2
phosphatidylinositol-4,5-bisphosphate
- Po
open probability
- POPC
1-palmitoyl-2-oleoyl-glycero-3-phosphocoline
- POPE
1-palmitoyl-2-oleoyl-glycero-3-phosphoethanolamine
- PS
pregnenolone sulfate
- TRPM
transient receptor potential melastatin
- TRPV
transient receptor potential vanilloid
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Grimm C., Kraft R., Sauerbruch S., Schultz G., Harteneck C. (2003) Molecular and functional characterization of the melastatin-related cation channel TRPM3. J. Biol. Chem. 278, 21493–21501 [DOI] [PubMed] [Google Scholar]
- 2.Lee N., Chen J., Sun L., Wu S., Gray K. R., Rich A., Huang M., Lin J.-H., Feder J. N., Janovitz E. B., Levesque P. C., Blanar M. A. (2003) Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3). J. Biol. Chem. 278, 20890–20897 [DOI] [PubMed] [Google Scholar]
- 3.Oberwinkler J., Lis A., Giehl K. M., Flockerzi V., Philipp S. E. (2005) Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J. Biol. Chem. 280, 22540–22548 [DOI] [PubMed] [Google Scholar]
- 4.Wagner T. F., Loch S., Lambert S., Straub I., Mannebach S., Mathar I., Düfer M., Lis A., Flockerzi V., Philipp S. E., Oberwinkler J. (2008) Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat. Cell Biol. 10, 1421–1430 [DOI] [PubMed] [Google Scholar]
- 5.Vriens J., Owsianik G., Hofmann T., Philipp S. E., Stab J., Chen X., Benoit M., Xue F., Janssens A., Kerselaers S., Oberwinkler J., Vennekens R., Gudermann T., Nilius B., Voets T. (2011) TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70, 482–494 [DOI] [PubMed] [Google Scholar]
- 6.Zakharian E., Thyagarajan B., French R. J., Pavlov E., Rohacs T. (2009) Inorganic polyphosphate modulates TRPM8 channels. PLoS One 4, e5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shevchenko A., Tomas H., Havlis J., Olsen J. V., Mann M. (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 [DOI] [PubMed] [Google Scholar]
- 8.Zakharian E. (2013) Recording of ion channel activity in planar lipid bilayer experiments. Methods Mol. Biol. 998, 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakharian E., Cao C., Rohacs T. (2010) Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J. Neurosci. 30, 12526–12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naylor J., Li J., Milligan C. J., Zeng F., Sukumar P., Hou B., Sedo A., Yuldasheva N., Majeed Y., Beri D., Jiang S., Seymour V. A., McKeown L., Kumar B., Harteneck C., O’Regan D., Wheatcroft S. B., Kearney M. T., Jones C., Porter K. E., Beech D. J. (2010) Pregnenolone sulphate- and cholesterol-regulated TRPM3 channels coupled to vascular smooth muscle secretion and contraction. Circ. Res. 106, 1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vriens J., Held K., Janssens A., Tóth B. I., Kerselaers S., Nilius B., Vennekens R., Voets T. (2014) Opening of an alternative ion permeation pathway in a nociceptor TRP channel. Nat. Chem. Biol. 10, 188–195 [DOI] [PubMed] [Google Scholar]
- 12.Liu B., Qin F. (2005) Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 25, 1674–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohács T., Lopes C. M., Michailidis I., Logothetis D. E. (2005) PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 8, 626–634 [DOI] [PubMed] [Google Scholar]
- 14.Lukacs V., Thyagarajan B., Varnai P., Balla A., Balla T., Rohacs T. (2007) Dual regulation of TRPV1 by phosphoinositides. J. Neurosci. 27, 7070–7080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ufret-Vincenty C. A., Klein R. M., Hua L., Angueyra J., Gordon S. E. (2011) Localization of the PIP2 sensor of TRPV1 ion channels. J. Biol. Chem. 286, 9688–9698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zakharian E., Cao C., Rohacs T. (2011) Intracellular ATP supports TRPV6 activity via lipid kinases and the generation of PtdIns(4,5) P₂. FASEB J. 25, 3915–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukacs V., Rives J. M., Sun X., Zakharian E., Rohacs T. (2013) Promiscuous activation of transient receptor potential vanilloid 1 (TRPV1) channels by negatively charged intracellular lipids: the key role of endogenous phosphoinositides in maintaining channel activity. J. Biol. Chem. 288, 35003–35013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badheka D., Borbiro I., Rohacs T. (2015) Transient receptor potential melastatin 3 is a phosphoinositide-dependent ion channel. J. Gen. Physiol. 146, 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tóth B. I., Konrad M., Ghosh D., Mohr F., Halaszovich C. R., Leitner M. G., Vriens J., Oberwinkler J., Voets T. (2015) Regulation of the transient receptor potential channel TRPM3 by phosphoinositides. J. Gen. Physiol. 146, 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun X., Zakharian E. (2015) Regulation of the temperature-dependent activation of transient receptor potential vanilloid 1 (TRPV1) by phospholipids in planar lipid bilayers. J. Biol. Chem. 290, 4741–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberwinkler J., Philipp S. E. (2014) Trpm3. Handbook Exp. Pharmacol. 222, 427–459 [DOI] [PubMed] [Google Scholar]
- 22.Held K., Kichko T., De Clercq K., Klaassen H., Van Bree R., Vanherck J. C., Marchand A., Reeh P. W., Chaltin P., Voets T., Vriens J. (2015) Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release. Proc. Natl. Acad. Sci. USA 112, E1363–E1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asuthkar S., Demirkhanyan L., Sun X., Elustondo P. A., Krishnan V., Baskaran P., Velpula K. K., Thyagarajan B., Pavlov E. V., Zakharian E. (2015) The TRPM8 protein is a testosterone receptor: II. Functional evidence for an ionotropic effect of testosterone on TRPM8. J. Biol. Chem. 290, 2670–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asuthkar S., Elustondo P. A., Demirkhanyan L., Sun X., Baskaran P., Velpula K. K., Thyagarajan B., Pavlov E. V., Zakharian E. (2015) The TRPM8 protein is a testosterone receptor: I. Biochemical evidence for direct TRPM8-testosterone interactions. J. Biol. Chem. 290, 2659–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drews A., Mohr F., Rizun O., Wagner T. F., Dembla S., Rudolph S., Lambert S., Konrad M., Philipp S. E., Behrendt M., Marchais-Oberwinkler S., Covey D. F., Oberwinkler J. (2014) Structural requirements of steroidal agonists of transient receptor potential melastatin 3 (TRPM3) cation channels. Br. J. Pharmacol. 171, 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe H., Vriens J., Suh S. H., Benham C. D., Droogmans G., Nilius B. (2002) Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J. Biol. Chem. 277, 47044–47051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.