Abstract
We previously defined that the mitochondria-localized PKCδ signaling complex stimulates the conversion of pyruvate to acetyl-coenzyme A by the pyruvate dehydrogenase complex. We demonstrated in vitro and ex vivo that retinol supplementation enhances ATP synthesis in the presence of the PKCδ signalosome. Here, we tested in vivo if a persistent oversupply of retinol would further impair glucose metabolism in a mouse model of diet-induced insulin resistance. We crossed mice overexpressing human retinol-binding protein (hRBP) under the muscle creatine kinase (MCK) promoter (MCKhRBP) with the PKCδ−/− strain to generate mice with a different status of the PKCδ signalosome and retinoid levels. Mice with a functional PKCδ signalosome and elevated retinoid levels (PKCδ+/+hRBP) developed the most advanced stage of insulin resistance. In contrast, elevation of retinoid levels in mice with inactive PKCδ did not affect remarkably their metabolism, resulting in phenotypic similarity between PKCδ−/−hRBP and PKCδ−/− mice. Therefore, in addition to the well-defined role of PKCδ in the etiology of metabolic syndrome, we present a novel PKCδ signaling pathway that requires retinol as a metabolic cofactor and is involved in the regulation of fuel utilization in mitochondria. The distinct role in whole-body energy homeostasis establishes the PKCδ signalosome as a promising target for therapeutic intervention in metabolic disorders.—Shabrova, E., Hoyos, B., Vinogradov, V., Kim, Y.-K., Wassef, L., Leitges, M., Quadro, L., Hammerling, U. Retinol as a cofactor for PKCδ-mediated impairment of insulin sensitivity in a mouse model of diet-induced obesity.
Keywords: vitamin A, PKCδ signalosome, metabolic syndrome
Shift in choices toward high-caloric, industrially processed food has led to an unprecedented epidemic in obesity and related metabolic disorders associated with oxidative stress and mitochondrial dysfunction, including insulin resistance, type 2 diabetes, cardiovascular disease, and cancer (1–4). In eukaryotic cells, mitochondria play a central role in energy metabolism (5). To maintain an adequate energy supply, mitochondria constantly monitor the cellular metabolic state and accordingly translate signals from molecular sensors to adjust the flux of fuel to the energy demands. Reversible phosphorylation is a ubiquitous mechanism that enables metabolic adaptation of bioenergetics to nutrient availability (6, 7). The soluble adenylate cyclase/PKA signaling cascade is an example of a molecular pathway that adjusts oxidative phosphorylation in accordance with the metabolically generated CO2, thus linking ATP synthesis and reactive oxygen species (ROS) production with nutrient metabolism (8, 9).
We recently identified PKCδ as a key component of a mitochondrial feedback loop that regulates acetyl-coenzyme A (CoA) production from pyruvate. In this pathway, PKCδ acts simultaneously as the sensor of the reduction potential of cytochrome c, reflective of the electron transfer chain (ETC) workload, and as the signal transducer that controls the pyruvate dehydrogenase complex (PDHC) activity (10). In this manner, the PKCδ pathway adjusts the fuel flux from glycolytic sources to the intensity of mitochondrial respiration, thereby controlling oxidative phosphorylation (11). The adapter protein, p66Shc, has been postulated to assemble PKCδ and cytochrome c into a signaling complex, named the PKCδ signalosome (11). In its oxidized form, cytochrome c (cytochrome c3+) oxidizes the PKCδ C1b activation domain (11, 12), leading to kinase activation (13, 14). However, the use of cytochrome c3+ as oxidant limits the electron flow from the PKCδ activation domain to the heme group of cytochrome c to a single electron at a time, although at a minimum, 1 pair needs to be moved. To facilitate the passage of electrons, PKCδ employs retinol (vitamin A alcohol) as an electron bridge (15, 16). Therefore, retinol emerged as an indispensable cofactor that modulates the activity of PKCδ and, hence, the synthesis of acetyl-CoA from pyruvate. Genetic ablation of any protein component of the PKCδ signalosome, mutations that disrupt protein or retinol-binding sites on PKCδ, as well as retinol depletion attenuate ATP synthesis and decrease oxygen consumption. In contrast, retinol supplementation enhances ATP synthesis (10–12). Results from cell culture experiments indicate that cell viability is deeply compromised in the absence of vitamin A (17, 18). Furthermore, retinol supports cell survival in conditions of energy deprivation, potentially due to enhanced efficiency of fuel oxidation (19).
Specifically, we previously showed that the increase in ATP synthesis from pyruvate upon retinol supplementation was induced by the activation of the PKCδ signaling pathway that up-regulates the activity of PDHC in vitro and ex vivo (11). We therefore hypothesize that a persistent oversupply of retinol in vivo could alter utilization of glucose, predominantly in the major tissue sites of ATP synthesis and glucose disposal, ultimately affecting whole-body glucose metabolism. We speculate that elevated tissue levels of retinol would increase the proportion of fully assembled and functional PKCδ signalosome, thereby escalating PKCδ signaling. Chronic enforcement of pyruvate utilization would then persistently overload the ETC and consequently generate mild, but chronic, oxidative stress that would ultimately impair insulin signaling.
Retinol-binding protein (RBP or RBP4), the sole specific carrier of retinol in the circulation, is predominantly expressed in the liver, the major tissue storage of retinoids (vitamin A and its derivatives). Hepatic retinol bound to RBP is mobilized toward the periphery of the body. Peripheral tissues, including visceral adipose, also express RBP, although to a lesser extent (20). To date, the function of extrahepatic RBP has remained elusive. Mice overexpressing human retinol-binding protein (hRBP) under the muscle creatine kinase (MCK) promoter (MCKhRBP) represent a well-established model of elevated holo- and apo-RBP levels in the circulation and retinoid concentrations in the peripheral tissues (21). Moreover, these mice have been shown to be susceptible to the development of insulin resistance. This evidence and other findings from Yang et al. (22) and Moraes-Vieira et al. (23) suggested a potential link between RBP and obesity and insulin resistance. Although a controversy remains (24–26), numerous studies confirmed the correlation of increased levels of serum RBP with the magnitude of obesity and insulin resistance in humans (27–30) and rodent models (22, 31, 32). Two independent mechanisms have recently been proposed to account for the impairment of insulin signaling by RBP: 1) the activation of TLR4 and JNK signaling cascades by apo-RBP in resident macrophages of adipose tissue (23, 33); and 2) the activation, in muscle and adipose tissue, of the Janus kinase 2/signal transducer and activator of transcription (STAT)5 cascade upon binding of holo-RBP to its putative receptor, stimulated by retinoid acid 6. This pathway has been shown to lead to increased expression of STAT5 target genes, including suppressor of cytokine signaling 3 and peroxisome proliferator-activated receptor γ (PPARγ), which impair insulin signaling and promote lipid accumulation, respectively (34, 35).
PKCδ is involved in numerous signal transduction cascades and has also been implicated in the development of metabolic syndrome (MS) (36). Extensive studies from Bezy et al. (37) on the role of PKCδ in the susceptibility to insulin resistance in mice revealed a robust positive association, regardless of whether PKCδ expression was altered by genetic modifications in a tissue-specific or global manner or naturally occurred in different strains at different levels. We speculate that the proportion of the fully assembled and functional PKCδ signalosome, determined by the level of PKCδ, could be an additional factor affecting insulin sensitivity. Accordingly, inactivation of the PKCδ signaling pathway in the PKCδ knockout mouse model would contribute to the resistance to obesity-related disorders.
In this study, we crossed MCKhRBP mice (21) with PKCδ knockout mice (38) and analyzed the resulting progeny, representing 4 distinct genotypes, for their susceptibility to the development of obesity and insulin resistance when maintained on a high-fat diet. We show that, despite the similarity of the initial phenotypes between groups with the same PKCδ genotype but different retinoid status, overexpression of hRBP significantly escalates insulin resistance upon high-fat feeding only in the presence of a functional PKCδ signalosome.
MATERIALS AND METHODS
Animals, diet, and tissue collection
Mice expressing normal levels of murine RBP and an hRBP transgene under the control of the mouse MCK promoter (originally called MCKhRBP) were generated by Quadro et al. (21). PKCδ knockout mice were contributed by M.L. (38). Both founder mouse strains were heterozygous for the genetic traits in question and were on a mixed genetic background (C57Bl/6 × 129/sv). They were crossed to generate PKCδ+/−MCKhRBP+/− and PKCδ+/− mice. Their progenies were bred for >3 generations to even their genetic background. The 4 resulting genotypes representing our 4 experimental groups were 1) PKCδ+/+, referred to as wild-type (WT); 2) PKCδ+/+MCKhRBP+/−, referred to as PKCδ+/+hRBP; 3) PKCδ−/−MCKhRBP+/−, referred to as PKCδ−/−hRBP; and 4) PKCδ−/−.
PKCδ genotype was confirmed by PCR (37). Genotyping of hRBP-transgenic mice was performed as described below. The presence of hRBP in the mouse circulation was confirmed by Western blot (21). No developmental defects were associated with any of these genotypes. Of note, all 4 strains expressed endogenous murine RBP. Mice were maintained on a standard chow diet (Purina Mouse Chow 5015, 18 IU/g vitamin A; Purina Co., St. Louis, MO, USA) in a temperature-controlled facility (25°C) with a 12:12 h light-dark cycle and given both diet and water ad libitum, except when food withdrawal was required. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and were approved by the Memorial Sloan-Kettering Cancer Center Institutional Committees on Animal Care (Protocol #04-06-018).
At 5 wk of age, male mice of each genotype (2–4 mice per cage) were placed on a high-fat diet with 40% calories from fat and 6.5 IU/g vitamin A, referred to as Western high-fat diet (WD) (Western Diet for rodents; TestDiet, St. Louis, MO, USA) for 12 wk (i.e., until 17 wk of age). Food intake and body weights (BWs) were recorded weekly. After 5 wk on the WD (i.e., at 10 wk of age), body composition analysis was performed on subgroups of mice (3–6 mice per group) by using a PIXImus Densitometer (Lunar, Madison, WI, USA). On the next day, these mice were withheld food for 4 h and then sacrificed to collect plasma and tissues. Glucose tolerance tests (GTTs) were performed on subgroups of mice at 5 and 10 wk of age and on all mice by the end of WD feeding (at ∼16 wk of age); insulin tolerance tests (ITTs) were performed on all mice at 17 wk of age.
Fed blood samples were collected at 5 and 17 wk of age, and serum samples were frozen for further analysis. When mice were unfed before killing, food was given back for 1 h after 2 h food withdrawal (starting 1 h before the dark cycle) then withdrawn for 16–17 h overnight. Mice were killed by CO2 inhalation. Selected organs, including liver, muscle, spleen, and epididymal and mesenteric fat (MF), were dissected and weighed. All tissue samples and sera were frozen immediately and kept at −80°C until further analysis.
Animal genotyping
PCR genotyping was performed using tail biopsies. Crude DNA extracts were prepared by the HotSHOT method (39). To identify the hRBP allele, a specific 300 bp PCR product was amplified by using a forward primer on the MCK promoter region (5′-CCTGGCTAGTCACACCCTG-3′) and a reverse primer on the second exon of hRBP sequence (5′-GCGAGCCTTGTCGAAGTTC-3′). The following conditions were used for PCR amplification: 94°C for 30 s, followed by 40 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 60 s, and final incubation at 72°C for 10 min. The PKCδ genotype was established according to published procedures (37).
Blood chemistry
Serum triglyceride (TG), nonessential fatty acid (NEFA), and total cholesterol (TC) levels were measured using colorimetric assay kits (Wako Chemicals USA, Wako Pure Chemical Industries, Ltd., Tokyo, Japan). Blood glucose was measured via tail snips using the OneTouch Ultra Glucose Meter (LifeScan Inc., Milpitas, CA, USA). Leptin, resistin, and adiponectin were measured using the Ultra Sensitive Rat/Mouse Insulin Assay Kit (Crystal Chem Inc., Downers Grove, IL, USA) and leptin (mouse/rat) EIA Kit, resistin (murine) EIA Kit, and adiponectin (mouse) EIA Kit (Cayman Chemical Company, Ann Arbor, MI, USA), respectively.
Glucose tolerance and ITTs
Glucose (2 g/kg BW) was injected intraperitoneally after 6 h food withdrawal, starting at 10 am. Blood glucose was measured via tail snips using the OneTouch Ultra Glucose Meter at 0, 15, 30, 60, and 90 min after injection. Insulin (1 U/kg BW) was injected intraperitoneally after 4 h of food withdrawal, starting at 9 am. Blood glucose was measured at 0, 15, 30, 45, and 75 min after injection.
Tissue lipid and glycogen content
Total lipids from liver and muscle samples were extracted according to the Folch extraction protocol (40). The extracts, dissolved in chloroform with 2% Triton X-100, were evaporated under nitrogen and dispersed in water, followed by measurement of TG by a colorimetric assay kit. Tissue glycogen was extracted as previously described (41), repeating the ethanol-precipitation step 3 times. The glycogen content was estimated by measuring glucose released after overnight digestion at 37°C of ethanol-precipitated material by amyloglucosidase (Sigma-Aldrich, St. Louis, MO, USA) using the Glucose AutoKit (Wako Chemicals USA, Wako Pure Chemical Industries, Ltd.).
Total RNA extraction and quantitative PCR analysis
RNA samples from liver and adipose tissues were extracted using RNA Bee reagent (Tel-Test, Inc., Friendswood, TX, USA) followed by DNase I treatment (Roche Diagnostics, Indianapolis, IN, USA) and a second round of RNA Bee extraction to remove DNase I. cDNA was prepared using qScript cDNA SuperMix and quantitative PCR (qPCR) performed using PerfeCTa SYBR Green FastMix (Quanta BioSciences, Gaithersburg, MD, USA) on an Eppendorf Mastercycler ep realplex System (Nijmegen, The Netherlands). Cyclophilin A was used as a reference gene. Primer sequences are presented in Supplemental Table S1.
Western blot analysis
Serum levels of IL-6 and hRBP were analyzed by Western blot. There were 0.5 µl aliquots of serum samples electrophoresed on 15% SDS-PAGE. Proteins were transferred to membranes and developed with a monoclonal rat anti-mouse IL-6 antibody (R&D Systems, Minneapolis, MN, USA) or a rabbit polyclonal anti-hRBP antiserum (21). Reference samples were loaded on each gel to allow for normalization between gels. Histidine-tagged human recombinant RBP reference standard was prepared as described (42), the histidine tag accounting for the higher apparent mass. Densitometry with Quantity One Software (Bio-Rad, Hercules, CA, USA) was used to quantify hRBP.
HPLC analysis of retinoids
Serum and tissue retinol and retinyl ester levels were measured by reverse-phase HPLC (21). Retinoids were separated on a 4.6 × 250 mm Denali C18 column preceded by a C18 guard column (both from Grace, Deerfield, IL, USA) using acetonitrile:methanol:methylene chloride (70:15:15, v:v) as the mobile phase at a flow rate of 1.8 ml/min. A Dionex UltiMate 3000 HPLC System and a computerized data analysis workstation with Chromeleon software were used (Thermo Scientific, Waltham, MA, USA). Retinol and retinyl esters were identified by comparing retention times and UV absorption spectra of experimental compounds with those of authentic standards. The concentrations of retinoids were determined by comparing respective peak integrated areas against those of known amounts of purified standards. Any losses during extraction were accounted for by adjusting for the recovery of retinyl acetate, added as an internal standard immediately after extraction of the tissues or sera.
Statistical analysis
Data are expressed as means ± se. WT group was used as a reference for quantitative analysis of mRNA. The 2-tailed unpaired Student’s t test with a threshold at 0.05 was used to calculate statistical significance. Slopes of regression lines were compared by the separate slope model with a threshold at 0.05. We performed principal component analysis (PCA) and varimax rotation (43) on the entire set of parameters obtained from mice at 17 wk of age, to reveal any latent structure in our experimental system. Variables with communalities <0.5 for 2-factor solution were excluded from further analysis. The remaining parameters carried forward for detailed analysis are listed in Table 1. We retained 2 principal components (PCs) with eigenvalue >1 (44) and loadings of individual variables >0.6. Finally, individual samples (i.e., mice) were placed into factor space according to their scores to reveal association of different genotypes with independent PCs representing combinations of original correlated parameters. Regression analysis and PCA were performed with SPSS software (IBM SPSS, Chicago, IL, USA).
TABLE 1.
Parameters used for PCA and correlation matrix generated by PCA
| PC1 |
PC2 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Label | BW | L | TG-fast | Glu-fast | Ins | ITT | GTT | EF | MF | Leptin | Adip |
| PC1 | ||||||||||||
| BW | BW | 1.0a | 0.7a | 0.7a | 0.8a | 0.6a | 0.6a | 0.5a | 0.4 | 0.4 | 0.3 | 0.1 |
| Liver | L | 1.0a | 0.5a | 0.4a | 0.4a | 0.4a | 0.3a | 0.1 | 0.4 | 0.0 | −0.2 | |
| Serum fasting TG | TG-fast | 1.0a | 0.6a | 0.7a | 0.4a | 0.5a | 0.2 | 0.1 | 0.2 | 0.0 | ||
| Fasting glucose | Glu-fast | 1.0a | 0.4a | 0.6a | 0.5a | 0.4 | 0.2 | 0.3 | 0.2 | |||
| Insulin | Ins | 1.0a | 0.6a | 0.5a | 0.5 | 0.5 | 0.5 | 0.1 | ||||
| ITT (AUC) | ITT | 1.0a | 0.6a | 0.4 | 0.7 | 0.4 | 0.3 | |||||
| GTT (AUC) | GTT | 1.0a | 0.3 | 0.2 | 0.2 | 0.2 | ||||||
| PC2 | ||||||||||||
| EF mass | EF | 1.0a | 0.7a | 0.9a | 0.8a | |||||||
| MF mass | MF | 1.0a | 0.7a | 0.5a | ||||||||
| Leptin | Leptin | 1.0a | 0.8a | |||||||||
| Adiponectin | Adip | 1.0a | ||||||||||
Correlation coefficients between parameters used for PCA are shown. Coefficients ≥0.5 are in bold. Coefficients for insulin ≥0.5 are underlined. aCorrelation coefficients between parameters assigned for the same cluster (with high loading for the same PC as indicated in the last column) are shown.
RESULTS
Susceptibility to obesity and obesity-related disorders of mice with different status of PKCδ signalosome and tissue retinoid levels
A total of 4 experimental groups generated through the cross of MCKhRBP (21) and PKCδ knockout mice (38) differed in their status of the PKCδ signalosome and tissue retinoid levels, as follows: 2 groups had a functional PKCδ signalosome (I WT, and II PKCδ+/+hRBP), and 2 groups had an inactive PKCδ (III PKCδ−/−hRBP, and IV PKCδ−/−). The latter will be referred to as PKCδ-null mice thereafter. Overexpression of hRBP in groups II (PKCδ+/+hRBP) and III (PKCδ−/−hRBP) (referred to as hRBP-transgenic) provided a supra-normal supply of retinol to the peripheral tissues, compared to groups I and IV, which solely expressed murine RBP. To determine the susceptibility of different genotypes to diet-induced obesity and obesity-related disorders, we placed male mice on the WD for 12 wk, starting at 5 wk of age, and tested subgroups of mice of each genotype after short time feeding (5 wk) with the WD. Physiologic parameters of mice prior to high-fat feeding and after 5 and 12 wk on the WD are shown in Tables 2, 3, and 4, respectively. All mice steadily accumulated fat and after 12 wk on the WD developed glucose intolerance, hyperinsulinemia, hyperleptinemia, hypercholesterolemia, and fasting hypertriglyceridemia, as expected (Table 3, and compare with Tables 2 and 4). The percentages of total body fat showed a significantly stronger correlation with epididymal fat (EF) mass compared to MF mass [R2 = 0.80 and P < 10−6 compared to R2 = 0.55 and P < 10−4, respectively; R(EF) vs. R(MF) P < 0.05, Supplemental Fig. S1A], suggesting that EF deposit was the primary site of fat accumulation upon development of obesity, regardless of mouse genotype.
TABLE 2.
Tissue weight, tissue lipid, glycogen, and retinoid content, and serum parameters of mice at 5 wk of age
| Parameter tested |
PKCδ+/+ (combined I and II) |
PKCδ−/− (combined III and IV) |
|||
|---|---|---|---|---|---|
| WT (I) | PKCδ+/+ hRBP (II) | PKCd−/− hRBP (III) | PKCd−/− (IV) | 5 vs. 17 wk | |
| Tissue weights and glycogen content | |||||
| BW (g)a | 21 ± 1 | 21 ± 1 | 17I, II ± 1 | 17I, II ± 1 | I–IV, (I + II), (III + IV) |
| 20.4 ± 0.4 | 17.4 ± 0.4 | ||||
| Liver (% BW)b | 3.1 ± 0.1 | 3.2 ± 0.1 | 3.5I ± 0.1 | 3.5 ± 0.2 | III, IV, (III + IV) |
| 3.2 ± 0.1 | 3.5 ± 0.1 | ||||
| EF (% BW) | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.0 | I–IV, (I + II), (III + IV) |
| MF (% BW) | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.1 | 0.6 ± 0.1 | I–IV, (I + II), (III + IV) |
| Spleen (% BW)b | 0.30 ± 0.04 | 0.27 ± 0.02 | 0.37II ± 0.01 | 0.36II ± 0.02 | I, II, IIIP = 0.05 |
| 0.28 ± 0.02 | 0.37 ± 0.01 | ||||
| Muscle glycogen (mg/g) | 10 ± 2 | 11 ± 1 | 15 ± 2 | 8III ± 1 | III |
| Liver glycogen (mg/g) |
4 ± 2 |
22 ± 7 |
19 ± 8 |
9 ± 4 |
II, III, (III + IV) |
| Retinoids: serum and liver | |||||
| Serum retinol (µg/dl) | 61 ± 8 | 77 ± 6 | 92I ± 2 | 45II, III ± 6 | No difference |
| Serum retinyl esters (µg/dl) | 4 ± 3 | 10I ± 1 | 2II ± 1 | 8 ± 5 | III |
| Total retinoids (µg/dl) | 62 ± 10 | 87I ± 6 | 94II ± 10 | 53III ± 6 | No difference |
| Liver retinol (µg/g) | 5 ± 1 | 5 ± 1 | 5 ± 1 | 5 ± 1 | No difference |
| Liver retinyl esters (µg/g) | 120 ± 14 | 107 ± 9 | 105 ± 11 | 121 ± 14 | II, III |
| Liver total retinoids (µg/g) |
125 ± 14 |
111 ± 9 |
110 ± 11 |
126 ± 14 |
II, III, (I + II), (III + IV) |
| Serum parameters of glucose metabolism | |||||
| Glucose (fed) (mM) | 8.3 ± 0.3 | 8.1 ± 0.2 | 8.0 ± 0.3 | 7.6I ± 0.3 | II, III, (I + II), (III + IV) |
| Glucose (6 h unfed) (mM)b | 7.1 ± 0.4 | 7.0I ± 0.4 | 5.9I, II ± 0.2 | 5.0I–III ± 0.2 | III and (III + IV) |
| 7.1 ± 0.3 | 5.4 ± 0.2 | ||||
| AUC (GTT) (M × 90 min)b | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 0.8I, II ± 0.0 | I–IV, (I + II), (III + IV) |
| 1.1 ± 0.1 | 0.9 ± 0.1 | ||||
| Resistin (unfed) (ng/ml)b | 10 ± 1 | 5I ± 1 | 11II ± 1 | 12II ± 1 | II, III, (I + II), (III + IV) |
| 8 ± 1 | 11 ± 1 | ||||
| Insulin (fed) (ng/ml)b |
0.7 ± 0.1 | 0.8 ± 0.1 | 0.3I, II ± 0.0 | 0.3I, II ± 0.0 | I, III, IV, (I + II), (III + IV) |
| 0.71 ± 0.07 |
0.31 ± 0.02 |
||||
| Serum parameters of lipid metabolism | |||||
| TG (fed) (mg/dl)b | 89 ± 10 | 94 ± 7 | 55I, II ± 4 | 52I, II ± 5 | (I + II) |
| 92 ± 6 | 54 ± 3 | ||||
| TG (fasting) (mg/dl)b | 72 ± 9 | 68 ± 7 | 55 ± 6 | 52 ± 5 | I, II, IV, (I + II), (III + IV) |
| 70 ± 6 | 53 ± 4 | ||||
| NEFA (unfed) (mmol/L) | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.1 | No difference |
| TC (fed) (mg/dl) | 117 ± 5 | 142I ± 9 | 116II ± 4 | 120 ± 8 | I–IV, (I + II), (III + IV) |
| TC (unfed) (mg/dl) |
141 ± 7 |
118I ± 4 |
126 ± 4 |
118I ± 3 |
I–IV, (I + II), (III + IV) |
| Serum adipokines | |||||
| Leptin (fed) (ng/ml)b | 4.1 ± 0.4 | 5.0 ± 0.8 | 3.3 ± 0.6 | 3.0I, II ± 0.3 | I–IV, (I + II), (III + IV) |
| 4.7 ± 0.5 | 3.1 ± 0.3 | ||||
| Adiponectin (unfed) (µg/ml) | 5.5 ± 0.3 | 5.6 ± 0.3 | 6.1 ± 0.3 | 6.0 ± 0.3 | I, (I + II) |
Data are expressed as means ± se. Fasting parameters were measured after overnight (16 h) food withdrawal, unless indicated otherwise. In the last column are indicated groups showing significant changes (P < 0.05; n = 5–12 animals) upon WD feeding for 12 wk (i.e., at 17 wk of age). Measurements at 17 wk are provided in Table 3. Roman numeric superscripts indicate statistically significant differences of measured parameters between groups. bSignificant difference (P < 0.05) between combined groups with intact PKCδ (I + II) and with inactive PKCδ (III + IV).
TABLE 3.
Tissue weight, tissue lipid and glycogen content, and serum parameters of mice at 10 wk of age
| Parameter tested |
PKCδ+/+ (combined I + II) |
PKCδ−/− (combined III + IV) |
10 vs. 5 wk | 10 vs. 17 wk | ||
|---|---|---|---|---|---|---|
| WT (I) | PKCδ+/+ hRBP (II) | PKCδ−/− hRBP (III) | PKCδ−/− (IV) | |||
| Tissue weights, lipid and glycogen content | ||||||
| BW (g)a | 29 ± 1 | 29 ± 1 | 25I, II ± 0 | 25I, II ± 1 | I–IV, (I + II), (III + IV) | I–IV, (I + II), (III + IV) |
| 29 ± 1 | 25 ± 0 | |||||
| Liver (% BW)a | 3.6 ± 0.2 | 3.6 ± 0.3 | 3.4 ± 0.1 | 3.3 ± 0.1 | I, (I + II) | III, (I + II), (III + IV) |
| EF (% BW) | 3.1 ± 0.5 | 3.2 ± 0.2 | 3.1 ± 0.2 | 3.1 ± 0.4 | I–IV, (I + II), (III + IV) | II, (I + II) |
| MF (% BW) | 1.2 ± 0.1 | 1.5 ± 0.1 | 1.5I ± 0.0 | 1.4 ± 0.1 | I–IV, (I + II), (III + IV) | II, III, (III + IV) |
| Spleen (% BW)a | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3II ± 0.0 | 0.3II ± 0.0 | II, (I + II) | II, IV |
| 0.19 ± 0.02 | 0.32 ± 0.04 | |||||
| Muscle TG (mg/g) | 28 ± 4 | 64I ± 7 | 36 ± 8 | 34 ± 13 | Not available |
|
| Muscle glycogen (mg/g) | 17 ± 1 | 18 ± 1 | 16 ± 1 | 16 ± 1 | ||
| Liver TG (mg/g) | 30 ± 10 | 69 ± 12 | 33II ± 6 | 34 ± 11 | ||
| Liver glycogen (mg/g) |
30 ± 5 |
19 ± 2 |
22 ± 2 |
26 ± 3 |
||
| Serum parameters of glucose and lipid metabolism | ||||||
| Glucose (6 h unfed) (mM)a | 7.6 ± 0.4 | 7.1 ± 0.2 | 4.8I, II ± 0.4 | 5.2I, II ± 0.7 | No differences | |
| 7.3 ± 0.2 | 5.0 ± 0.5 | |||||
| AUC (GTT) (M × 90 min)a | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.2I, II ± 0.1 | 1.2I, II ± 0.1 | I, II, IV, (I + II), (III + IV) | No differences |
| 1.60 ± 0.05 | 1.18 ± 0.08 | |||||
| Insulin (plasma) (ng/ml)a | 0.6 ± 0.1 | 0.9 ± 0.2 | 0.4II ± 0.1 | 0.6 ± 0.1 | Not available |
|
| 0.8 ± 0.2 | 0.5 ± 0.1 | |||||
| TG (mg/dl)a |
20 ± 2 | 16 ± 2 | 34 ± 5 | 24 ± 4 | ||
| 18 ± 2 |
30 ± 6 |
|||||
| Body composition (PIXImus) | ||||||
| Fat (g) | 8.1 ± 1.7 | 10.9 ± 1.3 | 7.7I, II ± 0.5 | 7.8 ± 1 | III, IV, (I + II), (III + IV)b | Not available |
| Fat (%) | 28 ± 4 | 34 ± 2 | 30 ± 1 | 29 ± 3 | ||
| Lean (g)a | 21 ± 1 | 21 ± 2 | 18I, II ± 0 | 18 ± 1 | ||
| 21 ± 1 | 18 ± 1 | III, IV, (III + IV) | ||||
| Lean (%) | 72 ± 4 | 66 ± 2 | 70 ± 1 | 71 ± 3 | III, IV, (I + II), (III + IV)b | |
| BMD (g/cm2)a | 0.054 ± 0.001 | 0.059 ± 0.003 | 0.048I, II ± 0.001 | 0.049I, II ± 0.001 | ||
| 0.056 ± 0.003 | 0.049 ± 0.001 | |||||
| BMC (g)a | 0.41 ± 0.02 | 0.42 ± 0.06 | 0.32I, II ± 0.01 | 0.33 ± 0.02 | ||
| 0.41 ± 0.04 | 0.32 ± 0.02 | |||||
Data are expressed as means ± se. Fasting parameters were measured after 4 h food withdrawal, unless indicated otherwise. In the penultimate column are indicated groups showing significant changes (P < 0.05; n = 5–12 animals) upon WD feeding for 5 wk. Measurements at 5 wk are provided in Table 2. In the last column are indicated groups showing significant changes (P < 0.05; n = 5–12 animals) upon WD feeding for 7 additional weeks (i.e., at 17 wk of age). Measurements at 17 wk are provided in Table 3. Roman numeric superscripts indicate statistically significant differences of measured parameters between groups. aSignificant difference (P < 0.05) between combined groups with intact PKCδ (I + II) and with inactive PKCδ (III + IV). bChanges were not evaluated for groups I and II due to an insufficient number of PIXImus measurements in the individual groups (I + II) at 5 wks.
TABLE 4.
Tissue weight, tissue lipid, glycogen, and retinoid content, and serum parameters of mice at 17 wk of age
| Parameter tested |
PKCδ+/+ (I + II) |
PKCδ−/− (III + IV) |
||
|---|---|---|---|---|
| WT (I) | PKCδ+/+hRBP (II) | PKCδ−/− hRBP (III) | PKCδ−/− (IV) | |
| Tissue weights, lipid and glycogen content | ||||
| BW (g) | 33 ± 1 | 34 ± 1 | 29I, II ± 1 | 29I, II ± 1 |
| Liver (% BW) | 3.1 ± 0.2 | 3.1 ± 0.2 | 2.9 ± 0.2 | 3.0 ± 0.1 |
| EF (mg/g)a | 3.8 ± 0.2 | 4.2 ± 0.2 | 3.8 ± 0.3 | 3.2II ± 0.3 |
| 4.0 ± 0.1 | 3.4 ± 0.2 | |||
| MF (mg/g) | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 |
| Spleen (% BW)a | 0.19 ± 0.01 | 0.22 ± 0.02 | 0.29I ± 0.04 | 0.42I–III ± 0.03 |
| Muscle TG (mg/g) | 26 ± 5 | 23 ± 2 | 21 ± 4 | 33 ± 5 |
| Muscle glycogen (mg/g) | 12 ± 2 | 10 ± 1 | 7 ± 1 | 9 ± 1 |
| Liver TG (mg/g) | 72 ± 9 | 74 ± 12 | 72 ± 4 | 71 ± 5 |
| Liver glycogen (mg/g) |
9 ± 2 |
3I ± 1 |
2.0I ± 0.4 |
4 ± 1 |
| Retinoids: serum, liver, muscle | ||||
| Serum retinol (µg/dl) | 49 ± 4 | 73 ± 10 | 73I ± 4 | 44II, III ± 4 |
| Serum retinyl esters (µg/dl) | 10 ± 6 | 18 ± 8 | 14 ± 4 | 12 ± 6 |
| Serum total retinoids (µg/dl) | 59 ± 6 | 90I ± 5 | 87I ± 6 | 56II, III ± 4 |
| Liver retinol (µg/g) | 4.4 ± 0.6 | 4.1 ± 0.5 | 3.7 ± 0.3 | 3.2 ± 0.6 |
| Liver retinyl esters (µg/g) | 151 ± 11 | 188I ± 5 | 154 ± 16 | 137 ± 26 |
| Liver total retinoids (µg/g) | 155 ± 11 | 192I ± 5 | 158 ± 16 | 140 ± 26 |
| Muscle retinol (ng/g) | 42 ± 4 | 194I ± 20 | 178I ± 14 | 43II, III ± 7 |
| Muscle retinyl esters (ng/g) | 22 ± 7 | 20 ± 8 | 15 ± 4 | 26 ± 11 |
| Muscle total retinoids (ng/g) |
63 ± 5 |
213I ± 28 |
193I ± 14 |
68II, III ± 13 |
| Serum parameters of glucose metabolism | ||||
| Glucose (fed) (mM)a | 7.7 ± 0.4 | 7.3 ± 0.3 | 6.7 ± 0.2 | 6.7 ± 0.4 |
| 7.5 ± 0.2 | 6.7 ± 0.2 | |||
| Glucose (6 h unfed) (mM)a | 6.4 ± 0.4 | 8.0I ± 0.4 | 4.4I, II ± 0.5 | 5.0I, II ± 0.2 |
| 7.1 ± 0.3 | 4.8 ± 0.2 | |||
| AUC (GTT) (M × 90 min)a | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.3 ± 0.1 | 1.2I, II ± 0.1 |
| 1.6 ± 0.1 | 1.3 ± 0.0 | |||
| AUC (ITT) (mM × 75 min)a | 194 ± 13 | 268I ± 23 | 197II ± 12 | 178II ± 8 |
| Resistin (ng/ml) | 13 ± 2 | 18 ± 4 | 18I ± 1 | 18 ± 2 |
| Insulin (fed) (ng/ml)a |
1.4 ± 0.2 | 1.3 ± 0.2 | 1.0 ± 0.1 | 0.7I–III ± 0.1 |
| 1.3 ± 0.1 |
0.8 ± 0.1 |
|||
| Serum parameters of lipid metabolism | ||||
| TG (fed) (mg/dl) | 60 ± 6 | 71 ± 10 | 46 ± 9 | 60 ± 9 |
| TG (mg/dl)a | 116 ± 7 | 106 ± 8 | 66I, II ± 7 | 82I ± 9 |
| 111 ± 5 | 76 ± 6 | |||
| NEFA (mmol/L) | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.05 ± 0.04 |
| TC (fed) (mg/dl) | 165 ± 13 | 197 ± 10 | 184 ± 7 | 172 ± 9 |
| TC (mg/dl) |
177 ± 7 |
176 ± 9 |
171 ± 8 |
165 ± 8 |
| Serum adipokines and cytokines | ||||
| Leptin (fed) (ng/ml) | 23 ± 2 | 30 ± 5 | 24 ± 5 | 20 ± 3 |
| TNF-α (fed) (pg/ml)a | 1.9 ± 0.4 | 4.5I ± 1 | 6.4I ± 1 | 5.2I ± 0.6 |
| Adiponectin (µg/ml) | 7.3 ± 0.4 | 7.2 ± 0.6 | 7.5 ± 0.5 | 6.2III ± 0.4 |
Data are expressed as means ± se. Fasting parameters were measured after 4 h food withdrawal, unless indicated otherwise. Roman numeric superscripts indicate statistically significant differences of measured parameters between groups. aSignificant difference (P < 0.05) between combined groups with intact PKCδ (I + II) and with inactive PKCδ (III + IV).
Impairment of PKCδ improved glucose tolerance, insulin sensitivity, and led to a lean phenotype, independently of the presence of hRBP
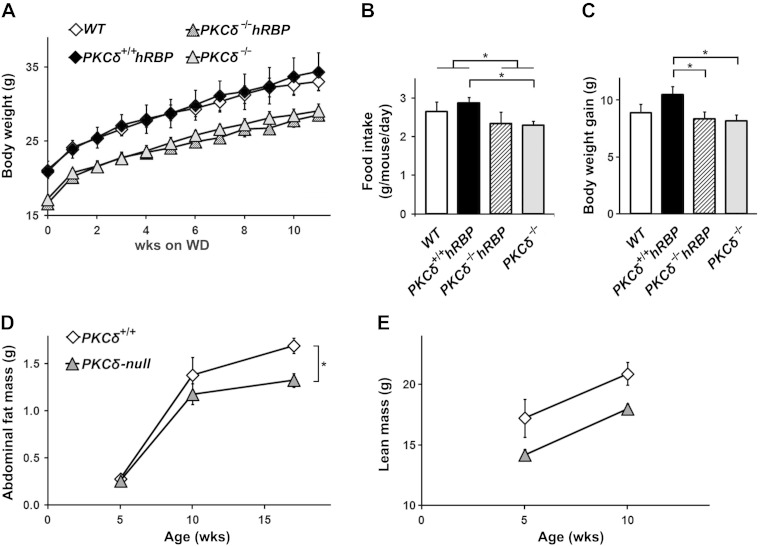
PKCδ-null mice (groups III and IV) remained hypoglycemic in the fed and unfed state, displayed milder glucose intolerance, lower BW, slightly reduced food intake, and lower fasting serum TG and insulin levels throughout the study (Fig. 1A, B and Tables 2–4). Their total weight gain was similar to WT mice (Fig. 1C). However, they appeared to be more resistant to developing central obesity upon high-fat feeding. Indeed, PKCδ-null mice (groups III and IV combined) gained less abdominal fat (AF; defined as the sum of EF and MF) after 12 wk on the WD (Fig. 1D), despite gaining similar lean mass (∼3 g in 5 wk, Fig. 1E) and lack of any differences in AF (and its components EF and MF) prior to the WD between groups (Fig. 1D and Table 2; data not shown).
Figure 1.
BW, adiposity, and food intake during WD feeding. A) BW changes. BW was measured weekly (n = 6–12 mice per group). B) Food intake. Average consumption from wk 2 till wk 10 on the WD was measured weekly (n = 3–4 cages per group). C) BW gain. Average BW gain from wk 2 until wk 11 on the WD was measured (n = 6–12 mice per group). D) Accumulation of AF during the 12 wk on the WD. Weight of AF was defined as the sum of epididymal and MF masses in mice at 5, 10, and 17 wk of age (n = 6–12 mice per group). E) Increase of lean mass during the initial 5 wk on the WD. Lean mass was determined by PIXImus at 5 and 10 wk of age (n = 3–8 mice per group). Symbols indicating the various groups are the same in (A, C) and (D, E). Data are expressed as means ± se. *P < 0.05.
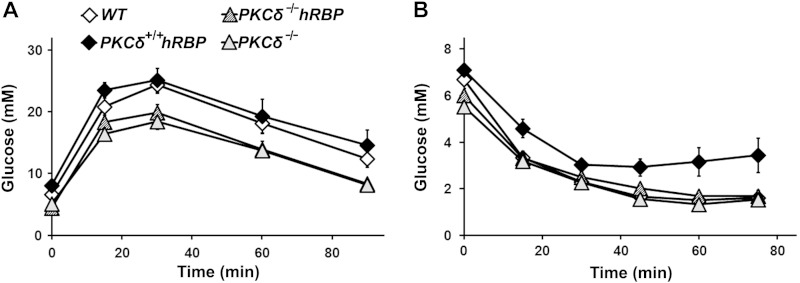
Although PKCδ-null mice (groups III and IV combined) display hypoinsulinemia, presumably due to impaired function of pancreatic β cells (45), overall, they managed to utilize glucose more efficiently during the insulin-sensitive stage (prior to high-fat feeding) (Table 2) and throughout the entire course of the WD regimen (Fig. 2A and Tables 3 and 4). Their areas under the curve (AUCs) for GTT and fasting glucose, as well as the fed glucose levels at 17 wk, were lower compared to mice with intact PKCδ (Fig. 2A and Table 4). Their response to insulin administration was identical to the response of WT mice (Fig. 2B). Fasting insulin levels (16 h food withdrawal) in PKCδ-null mice after 12 wk on the WD were significantly lower (0.45 ± 0.04 ng/ml) compared to mice with intact PKCδ (0.7 ± 0.1 ng/ml), despite the lack of differences between any groups at 5 wk of age (0.33 ± 0.02 ng/ml).
Figure 2.
Glucose and ITTs in mice at 17 wk of age. A) GTT. Glucose tolerance was performed after 11 wk on the WD. Glucose (2 g/kg) was injected intraperitoneally after 6 h food withdrawal, starting at 10 am. Blood glucose was measured at 0, 15, 30, 60, and 90 min after injection. B) ITT. ITT was performed after 12 wk on the WD. Insulin (1 U/kg) was injected intraperitoneally after 4 h food withdrawal, starting at 9 am. Blood glucose was measured at 0, 15, 30, 45, and 75 min after injection (n = 6–10 mice per group). Symbols indicating the various groups are the same in (A) and (B). Data are expressed as means ± se.
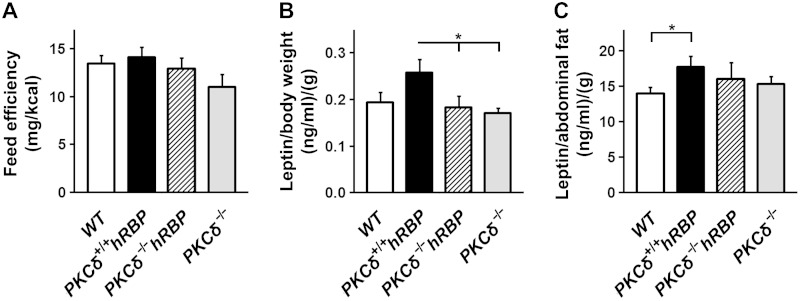
Feed efficiency calculated as a ratio of BW gain:amount of food consumed over 8 wk of the WD (from wk 2 till wk 10) (46) was similar among groups (Fig. 3A). However, PKCδ-null mice (groups III and IV combined) showed lower AF mass and markedly different levels of serum TG at 17 wk of age (Tables 2–4) compared to mice with intact PKCδ (groups I and II combined), which may reflect an involvement of PKCδ in whole-body lipid turnover and adipose tissue metabolism. Accordingly, the ablation of PKCδ led to decreased leptin levels before hyperleptinemia occurred (Fig. 3B, C and Table 4). Moreover, the strong correlation between AF mass and serum adiponectin levels of mice with an intact PKCδ (groups I and II) at 17 wk of age did not extend to PKCδ-null mice (groups III and IV; Supplemental Fig. S1B).
Figure 3.
Feed efficiency and serum leptin levels. A) Feed efficiency. BW gain normalized by food intake was measured from wk 2 till wk 10. B) Leptin prior to the WD. Serum leptin levels normalized by BW in mice at 5 wk of age were measured. C) Leptin at the stage of hyperleptinemia. Serum leptin levels normalized by AF mass in mice at 17 wk of age were measured (n = 5–12 mice per group). Data are expressed as means ± se. *P < 0.05.
PKCδ-null mice (groups III and IV combined) also displayed a unique cytokine profile due to the effects of PKCδ loss of function on different metabolic pathways. PKCδ-null mice showed elevated TNF-α levels in the circulation (Table 3), likely due to the hyperproliferation of B-lymphocytes reported for the PKCδ knockout mice (47, 48). In agreement with the literature, we detected a remarkable increase of IL-6 in the sera of PKCδ-null mice over the entire course of the experiment at 5, 10, and 17 wk of age (Supplemental Fig. S2A; data not shown). Finally, similar to humans with loss-of-function mutation in PKCδ (49), PKCδ-null mice displayed splenomegaly (Tables 2–4). Nevertheless, despite the elevated inflammatory background, the PKCδ-null mice (groups III and IV combined) preserved better insulin sensitivity on a high-fat diet compared to mice with intact PKCδ.
Overexpression of hRBP resulted in elevated RBP:retinol ratio in serum and increased retinoid levels in serum and skeletal muscle
As expected (21), serum levels of hRBP in hRBP-transgenic mice were ∼2-fold higher (5–6 µM, Supplemental Fig. S2B, C), comparable to the levels of murine RBP reported for obese mice (2–2.5 µM) (32). Serum retinol concentrations were likewise elevated in hRBP-transgenic mice (groups II and III) (Tables 2, 3, and 5) to levels similar to those reported for the parent MCKhRBP strain (21). Thus, the ratio of RBP:retinol was ∼1:1 in mice expressing only murine RBP (groups I and IV), whereas the ratio increased to ∼3:1 in hRBP-transgenic mice (groups II and III), suggesting that a proportion of RBP circulated in apo form in hRBP-transgenic mice.
TABLE 5.
Glycogen content, retinoid concentrations, and TC in mice with endogenous RBP (combined groups I + IV) and mice with transgenic hRBP (combined groups II + III)
| Parameter tested | 5 wk |
17 wk |
5 vs. 17 wk | ||
|---|---|---|---|---|---|
| Combined (I + IV) | Combined (II + III) | Combined (I + IV) | Combined (II + III) | ||
| Glycogen content | |||||
| Muscle glycogen (mg/g) | 9 ± 1 | 12I + IV ± 1 | 10 ± 1 | 9 ± 1 | (II + III) |
| Liver glycogen (mg/g) |
7 ± 2 |
21I + IV ± 5 |
9 ± 3 |
2.5 ± 0.4 |
(II + III) |
| Retinoids: serum, liver, muscle | |||||
| Serum retinol (µg/dl) | 52 ± 6 | 83I + IV ± 6 | 47 ± 3 | 73I + IV ± 5 | |
| Serum retinyl esters (µg/dl) | 6 ± 3 | 6 ± 2 | 11 ± 4 | 16 ± 4 | (II + III) |
| Total retinoids (µg/dl) | 57 ± 5 | 89I + IV ± 6 | 58 ± 4 | 89I + IV ± 4 | |
| Liver retinol (µg/g) | 5 ± 1 | 5 ± 1 | 3.8 ± 0.4 | 3.9 ± 0.3 | |
| Liver retinyl esters (µg/g) | 121 ± 9 | 106 ± 9 | 143 ± 14 | 171 ± 10 | (II + III) |
| Liver total retinoids (µg/g) | 125 ± 10 | 111 ± 7 | 147 ± 15 | 175 ± 10 | (II + III) |
| Muscle retinol (ng/g) | Not available |
42 ± 4 | 186I + IV ± 12 | ||
| Muscle retinyl esters (ng/g) | 24 ± 7 | 17 ± 5 | |||
| Muscle total retinoids (ng/g) |
66 ± 7 |
204I + IV ± 16 |
|||
| Serum TC levels | |||||
| TC (fed) (mg/dl) | 118 ± 5 | 127 ± 4 | 168 ± 8 | 191I + IV ± 6 | (I + IV), (II + III) |
| TC (unfed) (mg/dl) | 127 ± 5 | 121 ± 3 | 171 ± 5 | 171 ± 6 | (I + IV), (II + III) |
Data are expressed as means ± se. Parameters were obtained after overnight (16 h) food withdrawal. Roman numeric superscripts indicate statistically significant differences of measured parameters between groups.
In this study, skeletal muscle was the primary tissue of interest due to its central role in glucose utilization. As previously reported (21), overexpression of hRBP led to a remarkable elevation of retinol concentrations (4- to 5-fold) in skeletal muscle of hRBP-transgenic mice (groups II and III) compared to mice expressing only murine RBP (groups I and IV) (Tables 2, 3, and 5).
Total hepatic retinoid levels were similar among all groups. However, a moderate accumulation of hepatic retinyl esters was observed in hRBP-transgenic mice (groups II and III), but not in mice expressing only murine RBP (groups I and IV) (Tables 2, 3, and 5). In agreement, the 50% reduction in circulating RBP levels observed upon prolonged fenretinide treatment was accompanied by a decrease in hepatic retinyl ester content (31). The difference in hepatic retinyl esters reached statistical significance for PKCδ+/+hRBP (group II) vs. WT (group I) mice at 17 wk of age (Table 3). Furthermore, feeding with a high-fat diet (6.5 IU/g vitamin A) eliminated the initial difference in serum retinyl ester levels observed on chow diet (18 IU/g vitamin A) between PKCδ+/+hRBP (group II) mice and groups I and III (Tables 2 and 3).
Overexpression of hRBP accelerated insulin resistance in the presence, but not absence, of a functional PKCδ signalosome
An inferior response to insulin administration is a feature of advanced insulin resistance and diabetes. Compared to all other groups, PKCδ+/+hRBP mice (group II) showed the highest fasting glucose levels and an impaired ITT, as assessed by AUC values (Table 3), and glucose measurements at different time points during the ITT (Fig. 2B). Therefore, the PKCδ+/+hRBP mice displayed the most advanced stage of insulin resistance compared to the 3 other groups.
We did not observe considerable differences in serum cholesterol levels between groups, except a slight but significant elevation of serum TC in the hRBP-transgenic mice (groups II and III combined) vs. mice expressing only endogenous RBP (groups I and IV combined) at 17 wk of age (Table 5). Repression of lipoprotein lipase upon elevated retinoid levels in skeletal muscle can be one of the factors leading to elevated levels of TC in hRBP-transgenic mice (50).
PKCδ+/+hRBP mice (group II) showed higher lipid content in skeletal muscle and the liver measured in a basal state (after 4 h food withdrawal) at 10 wk of age (after 5 wk on the WD, Table 3). In the same group, differences in skeletal muscle lipid content reached statistical significance compared with WT mice (group I) and trended toward significance compared to mice in group III (P = 0.05). Hepatic lipids were similarly elevated in PKCδ+/+hRBP mice (group II), compared to the group III mice (P < 0.05) and groups I or IV (P = 0.06). The tendency to accumulate lipids in the lean tissues might be a result of preferred utilization of glycolytic fuel for ATP synthesis and consequent accumulation of unused lipids for storage in the muscle of PKCδ+/+hRBP mice (group II) (51, 52). Notably, hepatic and skeletal muscle lipid contents were similar after overnight food withdrawal in all mice after 12 wk of the WD (Table 3), being remarkably higher in the liver and lower in skeletal muscle, compared to those measured after 5 wk of the WD at the basal state (Table 4). Because basal and fasting tissue lipids were measured after a different duration of the WD, we cannot exclude that after prolonged high-fat feeding (12 wk), hepatic lipid levels might reach saturation levels that are similar in all groups. Moreover, in the insulin-resistant state, hepatic lipogenesis continues during food withdrawal (53, 54), thus likely increasing hepatic lipid content further. In contrast, lipid storage in muscle was markedly depleted to equal levels in all groups upon food withdrawal. PKCδ−/− mice (group IV) showed a trend toward lipid accumulation in skeletal muscle compared to PKCδ−/−hRBP mice (group III) (P < 0.08) (Table 3).
The transgenic hRBP expression in PKCδ−/− mice (group III) resulted in only a mild impairment of glucose metabolism. The 2 PKCδ-null groups (groups III and IV) differed in their fasting serum glucose prior to WD feeding (Table 2) and their insulin levels after 12 wk of the WD (Table 3). Because PKCδ has been shown to impair insulin signaling (55, 56), we expected that PKCδ-null mice would preserve insulin sensitivity longer than mice with intact PKCδ. Moreover, PKCδ-null mice displayed hypoinsulinemia, thus had lower insulin levels prior to high-fat feeding. Indeed, insulin levels were elevated in all the groups upon the WD, but PKCδ−/− mice (group IV) showed the lowest insulin rise (0.3 ± 0.1 ng/ml), whereas the average insulin rise varied from 0.7 to 1.0 ng/ml in other groups (Tables 2 and 3). These data support the notion of an involvement of RBP in the development of insulin resistance (23, 33, 35). However, GTT and ITT responses were not affected. Neither AUC values nor glucose measurements at any time point during the tolerance tests distinguished the PKCδ−/−hRBP from the PKCδ−/− group (Fig. 2 and Tables 2–4). Overall, PKCδ-null mice showed lower AUC GTT values, with the best GTT responses in the PKCδ−/− group (IV), significantly different from values measured in groups I or II throughout the study (Tables 2–4).
The hepatic and muscle glycogen contents measured after overnight food withdrawal were significantly higher in hRBP-transgenic mice (groups II and III) vs. groups I and IV in the insulin-sensitive state at 5 wk of age (Table 5). These levels drastically dropped after 12 wk of WD feeding only in groups II and III (Table 5), as expected in the insulin-resistant state (57, 58). Notably, in the basal state (after 4 h food withdrawal), glycogen levels were similar at 10 wk of age in all 4 groups (Table 4). Therefore, differences in tissue glycogen levels observed after overnight food withdrawal (Tables 2, 3, and 5) were likely determined by the rate of glycogen utilization rather than glycogen synthesis.
Overexpression of hRBP normalized substantially the production of adipokines in PKCδ-null mice prior to the WD at 5 wk of age. Adiponectin was elevated significantly in group I (32% raise; P < 0.05) and nearly significantly in groups II and III (29 and 22% raise, respectively; both P = 0.05) with developing adiposity (5 vs. 17 wk of age; compare Tables 2 and 3) (59, 60) but remained unchanged in the PKCδ−/− mice (group IV). Difference in adiponectin levels between PKCδ−/−hRBP and PKCδ−/− mice reached statistical significance at 17 wk of age (Table 3). Moreover, PKCδ−/− mice (group IV) showed the lowest leptin levels at 5 wk of age (Table 2).
Overexpression of hRBP also promoted an increase in TNF-α levels, supposedly through the mechanism proposed by Norseen et al. (33). Therefore, TNF-α levels were likely affected by the combined action of PKCδ and hRBP overexpression, leading to significantly higher values in all the groups compared to WT (Table 3). TNF-α has been shown to interfere with insulin signaling in a PKCδ-dependent manner (61). Therefore, this effect was likely to be restricted to the PKCδ+/+hRBP group.
Leptin levels normalized by BW were significantly higher in the PKCδ+/+hRBP group compared to both PKCδ−/− groups (III and IV) at 5 wk of age (Fig. 3B), despite the lower BW of PKCδ-null mice and the lack of significant difference in AF mass. PKCδ+/+hRBP mice showed relatively higher food intake (Fig. 1B) but similar feed efficiency (Fig. 3A). Therefore, the elevation of standardized leptin may signify an early predisposition to leptin resistance. The differences in food intake were marginal. Nevertheless, the slightly increased tendency toward higher food intake in PKCδ+/+hRBP mice suggests that chronic enforcement of glycolytic fuel utilization might increase food consumption. Without compensation in energy expenditure, this effect could lead to more extensive fat deposition.
In obese hyperleptinemic mice (after 12 wk on the WD), the differences in leptin levels (normalized for BW) disappeared (data not shown). However, when normalized by AF mass, leptin levels remained significantly higher in PKCδ+/+hRBP compared to WT (group I) (Fig. 3C). A strong correlation between insulin and leptin serum levels (R2 = 0.69; P = 10−6) was observed in mice at 5 wk of age (Supplemental Fig. S3A), consistent with the idea of adipogenic action of insulin, which was proposed to stimulate production and secretion of leptin (62). The correlation diminished significantly (R2 = 0.22; P = 0.02) after 12 wk of the WD (Supplemental Fig. S3B), likely reflecting dysregulation of hormone production upon development of obesity and insulin resistance.
Interestingly, the splenomegaly observed in PKCδ-null mice was remarkably ameliorated in the PKCδ−/−hRBP group by the end of the WD regimen (Table 3).
Effect of ablation of PKCδ and/or overexpression of hRBP on key genes of gluconeogenesis and lipogenesis
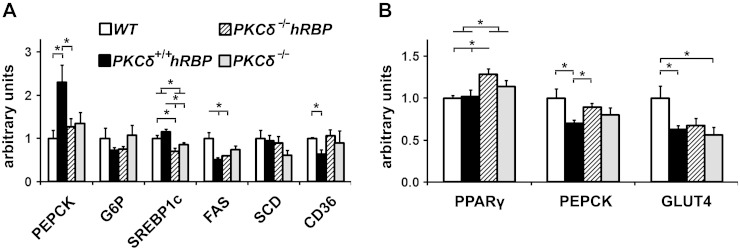
Phosphoenolpyruvate carboxykinase (PEPCK), one of the key enzymes of gluconeogenesis in the liver (63) and glyceroneogenesis in adipose tissue (64), was implicated in the development of accelerated insulin resistance in mice chronically injected with holo-RBP (22), in preservation of insulin sensitivity in PKCδ knockout mice (37) and obesity in mice overexpressing PEPCK in adipose tissue (64). Hepatic PEPCK levels, measured by qPCR in mice at 10 wk of age (5 wk of the WD, after 4 h food withdrawal), were significantly elevated in PKCδ+/+hRBP (group II) vs. PKCδ−/−hRBP (group III) or WT (group I) and tended toward significance vs. the PKCδ−/− (group IV) (Fig. 4A; P = 0.09). Notably, no differences in hepatic PEPCK expression were observed between PKCδ−/−hRBP and PKCδ−/− groups, suggesting a possible role of PKCδ in RBP-mediated activation of hepatic gluconeogenesis.
Figure 4.
qPCR analysis of genes involved in lipogenesis and gluconeogenesis. A) Gene expression in livers of mice unfed for 4 h after 5 wk on the WD. B) Gene expression in EF of mice unfed for 16 h after 12 wk on the WD (n = 3–6 mice per group). Symbols indicating the various groups are the same in (A) and (B). CD36, fatty acid translocase; FAS, fatty acid synthase; G6P, glucose 6-phosphatase; GLUT4, glucose transporter type 4; SCD, stearoyl-CoA desaturase; SREBP1c, sterol regulatory element-binding protein-1c. Data are expressed as means ± se. *P < 0.05.
Interestingly, hepatic mRNA levels of CD36, considered a major contributor to hepatic steatosis in obesity-prone C57Bl/6 mice (65), were down-regulated in PKCδ+/+hRBP (group II) compared to WT (group I) mice (Fig. 4A). PKCδ-null mice (groups III and IV) showed only a moderate down-regulation of hepatic sterol regulatory element-binding protein-1c expression, the transcription factor of hepatic lipogenesis (63), without further significant impact on its target genes, FAS and stearoyl-CoA desaturase (Fig. 4A). The latter finding did not conform to published results (37), but this discrepancy may be attributable to the mixed genetic background of our mice.
In adipose tissue of mice at 17 wk of age (after overnight food withdrawal), elevated TNF-α levels may account for the observed repression of glucose transporter type 4 in insulin-resistant PKCδ+/+hRBP and insulin-sensitive PKCδ−/− groups compared to WT (Fig. 4B), even though the difference for the PKCδ−/−hRBP group did not reach statistical significance. TNF-α has also been shown to down-regulate PEPCK expression in adipose tissue (66). However, in our study, this effect was limited to the PKCδ+/+hRBP group, possibly due to the overall higher insulin levels compared to PKCδ-null mice because insulin also down-regulates PEPCK expression (67).
Expression of PPARγ, a key transcription factor controlling adipogenesis (68), was significantly up-regulated in adipose of the PKCδ−/−hRBP mice (group III) compared to both groups with intact PKCδ (group I or II) (Fig. 4B). A strong inverse correlation of PPARγ expression in adipose with fasting insulin levels (R = −0.7; R2 = 0.53; P = 0.005) suggests that PPARγ may contribute to the maintenance of insulin sensitivity in PKCδ-null mice (69) (Supplemental Fig. S3C). On the other hand, PPARγ expression in adipose showed a moderate positive correlation with resistin (R2 = 0.37; P = 003) (Supplemental Fig. S3D), an adipokine implicated in impaired insulin sensitivity (70), corroborating the hypothesis of an indirect involvement of PPARγ in the regulation of resistin expression in mice (71).
PCA segregates hRBP-transgenic mice with intact PKCδ from those with inactive PKCδ
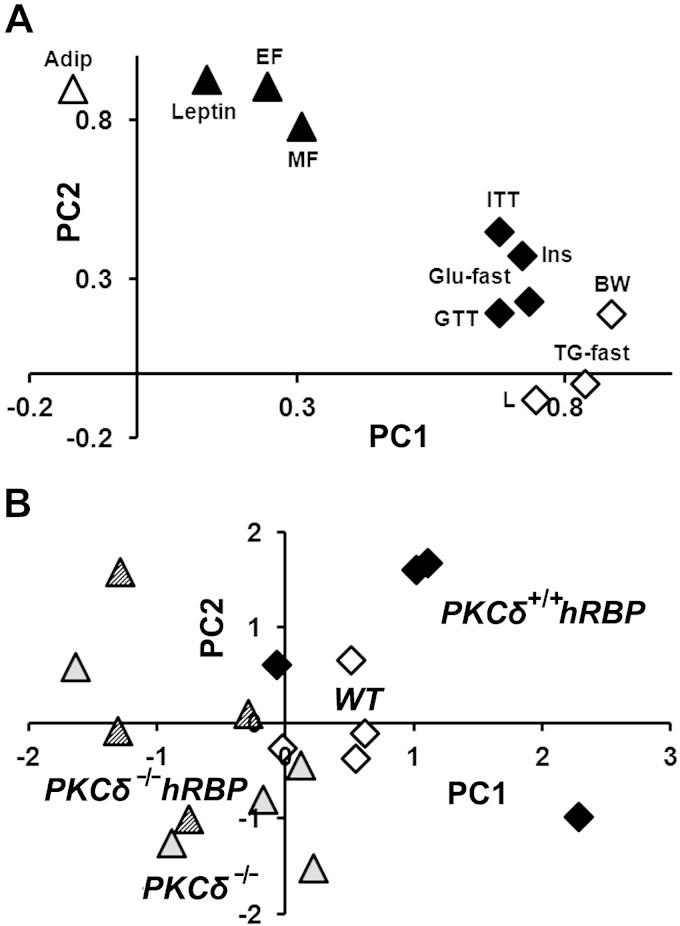
PCA was performed to extract independent factors with shared variance among parameters measured in mice at 17 wk of age. Parameters related to genetic background, but not relevant to obesity, were excluded from the analysis. Among these were spleen weight, a strong determinant for PKCδ-null mice that would separate them further from other groups, and retinoids as one of putative causes for the observed metabolic alterations. The analysis of variables with communalities >0.5 identified 2 independent PCs, which accounted for 70% of cumulative variance. PC1 explained 49% of total variance and was positively correlated with weight gain, serum fasting TG, and clustered parameters of glucose metabolism. Higher values of individual parameters in this cluster were associated with higher risk for insulin resistance and glucose intolerance (Fig. 5A). PC2 accounted for 22% of total variance and was loaded with a set of parameters of adipose metabolism, including weights of EF and MF pads and serum levels of adipokines (Fig. 5A). The matrix of parameter correlations showed high intracluster correlations and a lack of intercluster correlations, except for insulin (Table 1). In addition to correlations with parameters of glucose metabolism and BW, insulin was correlated with adiposity parameters defining MS loaded on PC2. These parameters included AF (both EF and MF) and leptin, but not adiponectin, which is considered a protective factor in obesity-related disorders (72). Accordingly, numerous studies reported insulin loaded on multiple factors [reviewed in Goodman et al. (73)]. Thus, higher positive coordinates for both PC1 and PC2 (upper-right quadrant in Fig. 5B) were associated with a higher cumulative risk of developing MS defined by a combination of MS biomarkers. Results of PCA for the individual samples are presented in Fig. 5B. PKCδ-null mice (groups III and IV) were indistinguishable, regardless of the presence of hRBP, and tended to localize in the lower-left and upper-left quadrants (Fig. 5B). In contrast, hRBP-transgenic mice with intact PKCδ (group II) were shifted toward a quadrant associated with the severity of MS, whereas WT mice (group I) settled in between (Fig. 5B).
Figure 5.
Graphic representation of PCA of physiologic parameters of mice at 17 wk of age. A) Plot of factor loadings. PC1 is marked by high loading on parameters of glucose metabolism including AUC values for ITT and GTT, fasting glucose (Glu-fast) levels, insulin (Ins), as well as body and liver (L) weight, fasting TGs (TG-fast; triangles), whereas parameters of adipose metabolism including adiponectin (Adip), leptin, epididymal, and MF mass (diamonds) are clustered on PC2. B) Plot of individual samples. Individual samples are plotted on PC space according to their scores.
DISCUSSION
A variety of signal transduction cascades driven by members of the PKC family of serine/threonine kinases has been shown to contribute to the etiology of MS [reviewed in Schmitz-Peiffer and Biden(36)]. PKCδ is a well-known pharmacologic target in obesity-related disorders. It modulates the activity of various proteins in the cytosol as well as in cellular membranes and organelles, including nucleus and mitochondria. It is activated by diacylglycerol accumulated upon lipid oversupply in obesity (36) as well as by hyperglycemia and oxidative stress, the hallmarks of diabetes (74). On the other hand, it contributes to the generation of oxidative stress (75, 76), one of the causative factors of insulin resistance and diabetic complications (74). PKCδ signaling pathways are directly involved in energy metabolism affecting glucose and lipid utilization, their storage, and de novo synthesis. Specifically, PKCδ is involved in regulation of glucose transport (77), gluconeogenesis (36), insulin secretion (45, 78), and signaling (55, 56). Thus, it is implicated in the development of insulin resistance as well as hepatic steatosis (37, 79).
The present study furthers our understanding of the role of a novel PKCδ signaling pathway in mitochondrial bioenergetics. Upon translocation to the mitochondrial intermembrane space, PKCδ assembles into a signalosome comprising the p66Shc adapter protein, cytochrome c, and retinol. This tetrameric complex operates at a site upstream of the PDHC and enhances the conversion of pyruvate to acetyl-CoA [(10, 11); reviewed in Hoyos et al. (15)]. Genetic manipulations that impair any protein component, or disrupt the assembly of the PKCδ signalosome, attenuate this signaling. Thus, genetic ablation of PKCδ or p66Shc, mutations of the retinol-binding pocket on PKCδ, or of the sites required for binding of cytochrome c to p66Shc, or for binding of p66Shc to PKCδ, diminish the rate of ATP synthesis from glycolytic substrate and compromise responsiveness to retinol in vitro and ex vivo [reviewed in Hoyos et al.(15)].
Inactivation of PKCδ or p66Shc in mice results in complex metabolic phenotypes because both proteins are multifunctional. Nevertheless, PKCδ knockout and p66Shc knockout mice share a set of relevant characteristics. PKCδ-null and p66Shc-null mice display phenotypes resistant to diet-induced obesity and obesity-related disorders, even though these are linked to different alterations in lipid and glucose metabolism (37, 80, 81). Both PKCδ and p66Shc are indirect targets of insulin signaling, and both were linked to the generation of ROS in mitochondria (75, 82). Furthermore, impairment of p66Shc directly affects mitochondrial bioenergetics by increasing uncoupled respiration (80). Accordingly, the remarkable reduction of fat mass observed in p66Shc-null mice results from increased basal metabolic rate and defective adipogenesis (80). In contrast, based on the slightly reduced food intake with no alterations in feed efficiency, PKCδ-null mice likely have slower metabolic rate, even though they are also lean and display lower AF mass. In accord, increased lipid metabolism and diminished glycolysis were shown in cardiac muscle of PKCδ-null mice (83). It is noteworthy that hepatic expression levels of PKCδ were positively correlated with body mass index (37). Likewise, p66Shc levels were remarkably elevated in peripheral blood mononuclear cells of patients with type 2 diabetes (84).
Despite differences in function, both PKCδ and p66Shc seem to be examples of thrifty genes (79, 85), evolutionarily designed for survival during periods of food scarcity but detrimental when food is overabundant. The PKCδ signaling pathway might represent the thrifty pathway that is activated for lipid accumulation in the postprandial state but is inhibited upon chronic malnutrition or prolonged starvation. Nevertheless, enforcement of oxidative phosphorylation of glycolytic substrate due to the chronically activated PKCδ signaling pathway under conditions of excessive calorie and nutrient intake, when fuel flux for energy generation surpasses energy demand, would generate oxidative stress (86, 87), diminish the utilization of fatty acids as a fuel for ATP synthesis, and promote their esterification, ultimately leading to obesity (51, 52) and insulin resistance (86, 88, 89). In fact, constitutive activation of PDHC in mice lacking pyruvate dehydrogenase kinases 2 and 4 resulted in increased glucose oxidation and inhibition of lipid oxidation. These metabolic alterations were, however, accompanied by enhanced fatty acid esterification and consequent accumulation of diacyl- and triacylglycerol, as well as unexpected reduction of insulin-stimulated glucose uptake (88). Oxidation of glycolytic substrate over fatty acid achieved via constitutive activation of PDHC indeed led to profound muscle insulin resistance (88). Furthermore, apart from complex I and complex III of the ETC, which are considered the main sites of ROS production, the PDHC has been recently revealed as a potential source of H2O2 in skeletal muscle, under conditions of compromised redox status. PDHC-driven H2O2 emission is substrate (pyruvate) stimulated and extremely sensitive to mitochondrial glutathione levels. Therefore, a shift in redox status associated with aging and observed under a variety of common pathologic states, including nutrient overload and diet-induced insulin resistance, seems sufficient to trigger H2O2 emission (87).
Retinol, the nonprotein component of the PKCδ signalosome, functions as a cofactor required for the activation of PKCδ (10). We hypothesize that dietary retinol availability may be one of the signals that inhibits or stimulates the thrifty PKCδ pathway. Vitamin A is an essential nutrient that maintains the health of the body (90). Both vitamin A deficiency and excess compromise normal physiologic functions (91–94). The human body is designed to efficiently prevent vitamin A toxicity by storing retinoids in high amounts, predominantly in the liver, and by maintaining homeostatic levels of retinol-RBP in the circulation (94, 95). Therefore, instances of extreme vitamin A toxicity, due to nutrient overload, are not common. Moreover, toxicity of retinol metabolites would likely lead to acute symptoms rather than chronic pathologic states. Nevertheless, in developed countries, overnutrition and increased consumption of dietary supplements may enhance the risk of excessive but subtoxic uptake of vitamin A (94). Therefore, it is important to weigh the effects of potentially elevated, even though not toxic, retinoid levels in tissues of intensive ATP synthesis. Thus, even mild oxidative stress due to up-regulation of the mitochondrial PKCδ signaling pathway may exacerbate chronic or acute pathologic conditions.
Modifications of tissue retinoid levels via dietary vitamin A manipulation are impractical to control the PKCδ signaling pathway in experimental models. Therefore, to increase the proportion of functional PKCδ signalosome, we used the MCKhRBP mice, a unique mouse model with permanently elevated retinoid levels in the peripheral tissues (21). However, despite the marked elevation (4- to 5-fold) of muscle retinol concentrations above normal physiologic levels, we observed significant metabolic alterations in our experimental model only after 12 wk of the WD challenge.
How PKCδ is loaded with retinol is not known. Retinol is a lipophilic compound present in the extra- and intracellular environment bound with high affinity to RBPs [i.e., RBP in the circulation (Kd ∼ 20 nM) and tissue-specific intracellular RBPs, cellular retinol-binding protein(CRBP)I–CRBPIV, with Kd values ranging from ∼15 nM to ∼0.2 µM] (96–98). The Kd value for the retinol-binding site of PKCδ falls in the middle of this range (∼65 nM) (99), therefore justifying it as an equivalent member of the family of RBPs. Skeletal muscle (as well as the heart and adipose) expresses the CRBPIII isoform with affinity to retinol (∼0.1 µM) (98) comparable to PKCδ. Finally, the binding is unlikely limited by the concentration of PKCδ, which is a membrane-associated protein (100, 101), or by the concentrations of retinol in skeletal muscle (150 ± 10 nM in mice with only endogenous murine RBP and 650 ± 40 nM in hRBP-transgenic mice), which exceed the PKCδ Kd value. Thus, an increase in muscle vitamin A supply, as is the case of hRBP overexpression, would translate into a higher PKCδ occupancy and, therefore, a larger pool of fully assembled (functional) PKCδ signalosome.
Increase of the holo-RBP transport has been shown to impair insulin sensitivity in mouse models and humans (22, 102, 103). Conversely, impairment of such transport has been linked to improvement of insulin sensitivity in mice and humans (22, 31, 35, 104, 105). Despite the fact that RBP was identified as an adipokine (106), the contribution of the adipose tissue to elevated levels of RBP associated with insulin resistance in humans has never been directly assessed. Moreover, muscle retinol content was not measured in these human subjects. Nevertheless, these effects have been attributed to signaling cascades triggered by binding of RBP to TLR4 (33) or stimulated by retinoic acid 6 (34, 35, 107) in adipose and/or muscle tissues. However, the role of the cargo of RBP (i.e., retinol) has never been evaluated. We propose that the elevation of tissue retinol levels, as well as elevated levels of other components of PKCδ signalosome, may increase the proportion of the fully assembled, functional PKCδ complex that may contribute to insulin resistance. Accordingly, overexpression of hRBP in the MCKhRBP strain (21) or chronic injection of holo-RBP in mice (22) increases the availability of retinol to peripheral tissues, notably muscle, shifting the equilibrium of PKCδ toward the retinol-bound form. Genetic overexpression of PKCδ (37) would also increase the availability of the protein component, PKCδ. Likewise, difference in tissue PKCδ expression, including in skeletal muscle, could contribute to the remarkable difference in insulin sensitivity between 129S6/SvEvTac and C57Bl/6 mouse strains (79). Conversely, alterations of any protein component that attenuate the assembly of the PKCδ signalosome, and thus abrogate this insulin resistance-promoting pathway, would generate insulin-sensitive phenotypes, as is the case with the PKCδ knockout (37) and p66Shc knockout (81) mice.
Interestingly, the synthetic retinoid fenretinide increased insulin sensitivity in obese rodents (31) and enhanced the probability to improve the metabolic profile in overweight premenopausal women (104). We recently showed that fenretinide can attenuate PKCδ signaling in cell culture systems (108). These data suggest that an additional impact of PKCδ on the insulin-sensitizing effect of fenretinide, independent of its RBP-lowering effect, should be considered.
Our experimental model revealed PKCδ as a dominant factor in determining susceptibility to obesity-related disorders, outweighing the effect of apo- and holo-RBP excess. Despite the apparent slightly slower metabolism and higher inflammatory background, the PKCδ-null mice remained more glucose tolerant, as well as more resistant to the development of central obesity and hyperlipidemia throughout the duration of high-fat feeding. Overexpression of RBP in the absence of PKCδ resulted only in mild changes in the phenotype. Nevertheless, higher-fed insulin levels by the end of the WD in the PKCδ−/−hRBP group compared to the PKCδ−/− group confirmed the reported effect of RBP on the insulin signaling pathway (23, 33–35). Overexpression of RBP in the presence of PKCδ more profoundly exacerbated the phenotypic characteristics related to both glucose and lipid metabolism. Despite the lack of any significant difference with WT mice in regard to glucose metabolism prior to high-fat feeding, after 12 wk of the WD, PKCδ+/+hRBP mice showed the highest fasting glucose levels and a remarkable impaired response to insulin administration. Interestingly, the previously reported RBP-induced increase in hepatic PEPCK expression (22) was observed only in the presence of PKCδ. The PKCδ+/+hRBP group tended to accumulate more lipids in lean tissues, suggesting a higher susceptibility to lipid overload. Overall, a combination of mild changes in the complex set of parameters defining MS distinguished the PKCδ+/+hRBP mice from other strains by PCA.
Although additional research is required to unequivocally confirm our hypothesis, our data support the possibility that enhanced PDHC activity observed in our earlier in vitro and ex vivo experiments upon retinol supplementation (10, 11) can accelerate the development of insulin resistance in vivo. Enforcement of oxidative phosphorylation of glycolytic substrate by chronically enhanced PKCδ signaling diminishes the contribution of fatty acid β-oxidation. Thus, alteration of the proportion of glycolytic and lipid substrates for ATP synthesis impairs the mitochondrial redox potential, ultimately leading to various disorders related to oxidative stress. Particularly, it aggravates insulin resistance induced by high-fat feeding in mice and may have a similar effect in humans. Based on our findings, we propose that disruption of binding sites of any components that would prevent the assembling of the PKCδ complex and attenuate the PKCδ signaling pathway, including the retinol-binding site, may represent a novel pharmacologic approach to ameliorate skeletal muscle insulin resistance.
Supplementary Material
Acknowledgments
The authors acknowledge intellectual and material support by the members of the Weill-Cornell Pharmacology Department (New York, NY, USA), Drs. Lorraine Gudas, Jochen Buck, and Lonny Levine. The authors also thank Drs. Giovanni Manfredi and Anatoly Starkov (Cornell Medical College, New York, NY, USA) for manyfold advice and Dr. Malcolm Watford (Rutgers University, Piscataway, NY, USA) for critical reading of the manuscript. The authors are indebted to Dr. J. Friedman (Rockefeller University, New York, NY, USA) for help in PIXImus measurements. U.H. was supported by a grant from Memorial Sloan-Kettering Cancer Center, and L.Q. by grants from the U.S. National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD057493 and R01HD057493-02S1). E.S., L.Q., B.H., and U.H. were responsible for planning, oversight, and execution of the experiments, as well as of interpretation and validation of results. V.V., Y.-K.K., and L.W. carried out mouse breeding, maintenance and genotyping experiments, and assisted with various analytic procedures. M.L. provided breeding pairs of protein kinase Cδ knockout mice. The authors declare no conflicts of interest.
Glossary
- AF
abdominal fat
- AUC
area under the curve
- BW
body weight
- CoA
coenzyme A
- CRBP
cellular retinol-binding protein
- EF
epididymal fat
- ETC
electron transfer chain
- GTT
glucose tolerance test
- hRBP
human retinol-binding protein
- ITT
insulin tolerance test
- MCK
muscle creatine kinase
- MF
mesenteric fat
- MS
metabolic syndrome
- NEFA
nonessential fatty acid
- PC
principal component
- PCA
principal component analysis
- PDHC
pyruvate dehydrogenase complex
- PEPCK
phosphoenolpyruvate carboxykinase
- PPARγ
peroxisome proliferator-activated receptor γ
- qPCR
quantitative PCR
- RBP
retinol-binding protein
- ROS
reactive oxygen species
- STAT
signal transducer and activator transcription
- TC
total cholesterol
- TG
triglyceride
- WD
Western high-fat diet
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Lowell B. B., Shulman G. I. (2005) Mitochondrial dysfunction and type 2 diabetes. Science 307, 384–387 [DOI] [PubMed] [Google Scholar]
- 2.Wallace D. C. (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 39, 359–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishikawa T., Edelstein D., Du X. L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M. A., Beebe D., Oates P. J., Hammes H. P., Giardino I., Brownlee M. (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404, 787–790 [DOI] [PubMed] [Google Scholar]
- 4.Sorriento D., Pascale A. V., Finelli R., Carillo A. L., Annunziata R., Trimarco B., Iaccarino G. (2014) Targeting mitochondria as therapeutic strategy for metabolic disorders. ScientificWorldJournal 2014, 604685 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.McBride H. M., Neuspiel M., Wasiak S. (2006) Mitochondria: more than just a powerhouse. Curr. Biol. 16, R551–R560 [DOI] [PubMed] [Google Scholar]
- 6.Opalinska M., Meisinger C. (2014) Mitochondrial protein import under kinase surveillance. Microb. Cell 1, 51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagliarini D. J., Dixon J. E. (2006) Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem. Sci. 31, 26–34 [DOI] [PubMed] [Google Scholar]
- 8.Valsecchi F., Ramos-Espiritu L. S., Buck J., Levin L. R., Manfredi G. (2013) cAMP and mitochondria. Physiology (Bethesda) 28, 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acin-Perez R., Salazar E., Kamenetsky M., Buck J., Levin L. R., Manfredi G. (2009) Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 9, 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acin-Perez R., Hoyos B., Zhao F., Vinogradov V., Fischman D. A., Harris R. A., Leitges M., Wongsiriroy N., Blaner W. S., Manfredi G., Hammerling U. (2010) Control of oxidative phosphorylation by vitamin A illuminates a fundamental role in mitochondrial energy homoeostasis. FASEB J. 24, 627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acin-Perez R., Hoyos B., Gong J., Vinogradov V., Fischman D. A., Leitges M., Borhan B., Starkov A., Manfredi G., Hammerling U. (2010) Regulation of intermediary metabolism by the PKCdelta signalosome in mitochondria. FASEB J. 24, 5033–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao F., Ilbert M., Varadan R., Cremers C. M., Hoyos B., Acin-Perez R., Vinogradov V., Cowburn D., Jakob U., Hammerling U. (2011) Are zinc-finger domains of protein kinase C dynamic structures that unfold by lipid or redox activation? Antioxid. Redox Signal. 14, 757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konishi H., Tanaka M., Takemura Y., Matsuzaki H., Ono Y., Kikkawa U., Nishizuka Y. (1997) Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc. Natl. Acad. Sci. USA 94, 11233–11237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korichneva I., Hoyos B., Chua R., Levi E., Hammerling U. (2002) Zinc release from protein kinase C as the common event during activation by lipid second messenger or reactive oxygen. J. Biol. Chem. 277, 44327–44331 [DOI] [PubMed] [Google Scholar]
- 15.Hoyos B., Acin-Perez R., Fischman D. A., Manfredi G., Hammerling U. (2012) Hiding in plain sight: uncovering a new function of vitamin A in redox signaling. Biochim. Biophys. Acta 1821, 241–247 [DOI] [PubMed] [Google Scholar]
- 16.Hammerling U. (2013) The centennial of vitamin A: a century of research in retinoids and carotenoids. FASEB J. 27, 3887–3890 [DOI] [PubMed] [Google Scholar]
- 17.Buck J., Ritter G., Dannecker L., Katta V., Cohen S. L., Chait B. T., Hämmerling U. (1990) Retinol is essential for growth of activated human B cells. J. Exp. Med. 171, 1613–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buck J., Derguini F., Levi E., Nakanishi K., Hämmerling U. (1991) Intracellular signaling by 14-hydroxy-4,14-retro-retinol. Science 254, 1654–1656 [DOI] [PubMed] [Google Scholar]
- 19.Chiu H.-J., Fischman D. A., Hammerling U. (2008) Vitamin A depletion causes oxidative stress, mitochondrial dysfunction, and PARP-1-dependent energy deprivation. FASEB J. 22, 3878–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Ambrosio D. N., Clugston R. D., Blaner W. S. (2011) Vitamin A metabolism: an update. Nutrients 3, 63–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quadro L., Blaner W. S., Hamberger L., Van Gelder R. N., Vogel S., Piantedosi R., Gouras P., Colantuoni V., Gottesman M. E. (2002) Muscle expression of human retinol-binding protein (RBP). Suppression of the visual defect of RBP knockout mice. J. Biol. Chem. 277, 30191–30197 [DOI] [PubMed] [Google Scholar]
- 22.Yang Q., Graham T. E., Mody N., Preitner F., Peroni O. D., Zabolotny J. M., Kotani K., Quadro L., Kahn B. B. (2005) Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 [DOI] [PubMed] [Google Scholar]
- 23.Moraes-Vieira P. M., Yore M. M., Dwyer P. M., Syed I., Aryal P., Kahn B. B. (2014) RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 19, 512–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janke J., Engeli S., Boschmann M., Adams F., Böhnke J., Luft F. C., Sharma A. M., Jordan J. (2006) Retinol-binding protein 4 in human obesity. Diabetes 55, 2805–2810 [DOI] [PubMed] [Google Scholar]
- 25.McTernan P. G., Kumar S. (2007) Editorial: Retinol binding protein 4 and pathogenesis of diabetes. J. Clin. Endocrinol. Metab. 92, 2430–2432 [DOI] [PubMed] [Google Scholar]
- 26.Motani A., Wang Z., Conn M., Siegler K., Zhang Y., Liu Q., Johnstone S., Xu H., Thibault S., Wang Y., Fan P., Connors R., Le H., Xu G., Walker N., Shan B., Coward P. (2009) Identification and characterization of a non-retinoid ligand for retinol-binding protein 4 which lowers serum retinol-binding protein 4 levels in vivo. J. Biol. Chem. 284, 7673–7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho Y. M., Youn B. S., Lee H., Lee N., Min S. S., Kwak S. H., Lee H. K., Park K. S. (2006) Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care 29, 2457–2461 [DOI] [PubMed] [Google Scholar]
- 28.Graham T. E., Yang Q., Blüher M., Hammarstedt A., Ciaraldi T. P., Henry R. R., Wason C. J., Oberbach A., Jansson P. A., Smith U., Kahn B. B. (2006) Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 354, 2552–2563 [DOI] [PubMed] [Google Scholar]
- 29.Gavi S., Stuart L. M., Kelly P., Melendez M. M., Mynarcik D. C., Gelato M. C., McNurlan M. A. (2007) Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. J. Clin. Endocrinol. Metab. 92, 1886–1890 [DOI] [PubMed] [Google Scholar]
- 30.Jia W., Wu H., Bao Y., Wang C., Lu J., Zhu J., Xiang K. (2007) Association of serum retinol-binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. J. Clin. Endocrinol. Metab. 92, 3224–3229 [DOI] [PubMed] [Google Scholar]
- 31.Preitner F., Mody N., Graham T. E., Peroni O. D., Kahn B. B. (2009) Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 297, E1420–E1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manolescu D. C., Sima A., Bhat P. V. (2010) All-trans retinoic acid lowers serum retinol-binding protein 4 concentrations and increases insulin sensitivity in diabetic mice. J. Nutr. 140, 311–316 [DOI] [PubMed] [Google Scholar]
- 33.Norseen J., Hosooka T., Hammarstedt A., Yore M. M., Kant S., Aryal P., Kiernan U. A., Phillips D. A., Maruyama H., Kraus B. J., Usheva A., Davis R. J., Smith U., Kahn B. B. (2012) Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol. Cell. Biol. 32, 2010–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry D. C., Jin H., Majumdar A., Noy N. (2011) Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc. Natl. Acad. Sci. USA 108, 4340–4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry D. C., Jacobs H., Marwarha G., Gely-Pernot A., O’Byrne S. M., DeSantis D., Klopfenstein M., Feret B., Dennefeld C., Blaner W. S., Croniger C. M., Mark M., Noy N., Ghyselinck N. B. (2013) The STRA6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. J. Biol. Chem. 288, 24528–24539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz-Peiffer C., Biden T. J. (2008) Protein kinase C function in muscle, liver, and beta-cells and its therapeutic implications for type 2 diabetes. Diabetes 57, 1774–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bezy O., Tran T. T., Pihlajamäki J., Suzuki R., Emanuelli B., Winnay J., Mori M. A., Haas J., Biddinger S. B., Leitges M., Goldfine A. B., Patti M. E., King G. L., Kahn C. R. (2011) PKCδ regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. J. Clin. Invest. 121, 2504–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leitges M., Mayr M., Braun U., Mayr U., Li C., Pfister G., Ghaffari-Tabrizi N., Baier G., Hu Y., Xu Q. (2001) Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J. Clin. Invest. 108, 1505–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truett G. E., Heeger P., Mynatt R. L., Truett A. A., Walker J. A., Warman M. L. (2000) Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29, 52. [DOI] [PubMed] [Google Scholar]
- 40.Folch J., Lees M., Sloane Stanley G. H. (1957) A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 [PubMed] [Google Scholar]
- 41.Pederson B. A., Chen H., Schroeder J. M., Shou W., DePaoli-Roach A. A., Roach P. J. (2004) Abnormal cardiac development in the absence of heart glycogen. Mol. Cell. Biol. 24, 7179–7187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Y., Lashuel H. A., Miroy G. J., Dikler S., Kelly J. W. (1998) Recombinant human retinol-binding protein refolding, native disulfide formation, and characterization. Protein Expr. Purif. 14, 31–37 [DOI] [PubMed] [Google Scholar]
- 43.Jolliffe I., ed (2002) Principal Component Analysis, Vol. 29, Springer Verlag, New York [Google Scholar]
- 44.Kaiser H. F. (1960) The application of electronic computers to factor analysis. Educ. Psychol. Meas. 20, 141–151 [Google Scholar]
- 45.Uchida T., Iwashita N., Ohara-Imaizumi M., Ogihara T., Nagai S., Choi J. B., Tamura Y., Tada N., Kawamori R., Nakayama K. I., Nagamatsu S., Watada H. (2007) Protein kinase Cdelta plays a non-redundant role in insulin secretion in pancreatic beta cells. J. Biol. Chem. 282, 2707–2716 [DOI] [PubMed] [Google Scholar]
- 46.Sutherland T. M., Biondini P. E., Ward G. M. (1974) Selection for growth rate, feed efficiency and body composition in mice. Genetics 78, 525–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boussiotis V. A., Nadler L. M., Strominger J. L., Goldfeld A. E. (1994) Tumor necrosis factor alpha is an autocrine growth factor for normal human B cells. Proc. Natl. Acad. Sci. USA 91, 7007–7011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyamoto A., Nakayama K., Imaki H., Hirose S., Jiang Y., Abe M., Tsukiyama T., Nagahama H., Ohno S., Hatakeyama S., Nakayama K. I. (2002) Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature 416, 865–869 [DOI] [PubMed] [Google Scholar]
- 49.Kuehn H. S., Niemela J. E., Rangel-Santos A., Zhang M., Pittaluga S., Stoddard J. L., Hussey A. A., Evbuomwan M. O., Priel D. A., Kuhns D. B., Park C. L., Fleisher T. A., Uzel G., Oliveira J. B. (2013) Loss-of-function of the protein kinase C δ (PKCδ) causes a B-cell lymphoproliferative syndrome in humans. Blood 121, 3117–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haugen B. R., Jensen D. R., Sharma V., Pulawa L. K., Hays W. R., Krezel W., Chambon P., Eckel R. H. (2004) Retinoid X receptor gamma-deficient mice have increased skeletal muscle lipoprotein lipase activity and less weight gain when fed a high-fat diet. Endocrinology 145, 3679–3685 [DOI] [PubMed] [Google Scholar]
- 51.Houmard J. A. (2008) Intramuscular lipid oxidation and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1111–R1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mobbs C. V., Mastaitis J. W., Zhang M., Isoda F., Cheng H., Yen K. (2007) Secrets of the lac operon. Glucose hysteresis as a mechanism in dietary restriction, aging and disease. Interdiscip. Top. Gerontol. 35, 39–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry R. J., Camporez J. P., Kursawe R., Titchenell P. M., Zhang D., Perry C. J., Jurczak M. J., Abudukadier A., Han M. S., Zhang X. M., Ruan H. B., Yang X., Caprio S., Kaech S. M., Sul H. S., Birnbaum M. J., Davis R. J., Cline G. W., Petersen K. F., Shulman G. I. (2015) Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown M. S., Goldstein J. L. (2008) Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 [DOI] [PubMed] [Google Scholar]
- 55.Braiman L., Alt A., Kuroki T., Ohba M., Bak A., Tennenbaum T., Sampson S. R. (2001) Insulin induces specific interaction between insulin receptor and protein kinase C delta in primary cultured skeletal muscle. Mol. Endocrinol. 15, 565–574 [DOI] [PubMed] [Google Scholar]
- 56.Greene M. W., Ruhoff M. S., Roth R. A., Kim J. A., Quon M. J., Krause J. A. (2006) PKCdelta-mediated IRS-1 Ser24 phosphorylation negatively regulates IRS-1 function. Biochem. Biophys. Res. Commun. 349, 976–986 [DOI] [PubMed] [Google Scholar]
- 57.Halse R., Bonavaud S. M., Armstrong J. L., McCormack J. G., Yeaman S. J. (2001) Control of glycogen synthesis by glucose, glycogen, and insulin in cultured human muscle cells. Diabetes 50, 720–726 [DOI] [PubMed] [Google Scholar]
- 58.Krssak M., Brehm A., Bernroider E., Anderwald C., Nowotny P., Dalla Man C., Cobelli C., Cline G. W., Shulman G. I., Waldhäusl W., Roden M. (2004) Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 53, 3048–3056 [DOI] [PubMed] [Google Scholar]
- 59.Li J., Yu X., Pan W., Unger R. H. (2002) Gene expression profile of rat adipose tissue at the onset of high-fat-diet obesity. Am. J. Physiol. Endocrinol. Metab. 282, E1334–E1341 [DOI] [PubMed] [Google Scholar]
- 60.Bullen J. W. Jr, Bluher S., Kelesidis T., Mantzoros C. S. (2007) Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am. J. Physiol. Endocrinol. Metab. 292, E1079–E1086 [DOI] [PubMed] [Google Scholar]
- 61.Rosenzweig T., Braiman L., Bak A., Alt A., Kuroki T., Sampson S. R. (2002) Differential effects of tumor necrosis factor-alpha on protein kinase C isoforms alpha and delta mediate inhibition of insulin receptor signaling. Diabetes 51, 1921–1930 [DOI] [PubMed] [Google Scholar]
- 62.Kieffer T. J., Habener J. F. (2000) The adipoinsular axis: effects of leptin on pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 278, E1–E14 [DOI] [PubMed] [Google Scholar]
- 63.Desvergne B., Michalik L., Wahli W. (2006) Transcriptional regulation of metabolism. Physiol. Rev. 86, 465–514 [DOI] [PubMed] [Google Scholar]
- 64.Franckhauser S., Muñoz S., Pujol A., Casellas A., Riu E., Otaegui P., Su B., Bosch F. (2002) Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes 51, 624–630 [DOI] [PubMed] [Google Scholar]
- 65.Colombo C., Haluzik M., Cutson J. J., Dietz K. R., Marcus-Samuels B., Vinson C., Gavrilova O., Reitman M. L. (2003) Opposite effects of background genotype on muscle and liver insulin sensitivity of lipoatrophic mice. Role of triglyceride clearance. J. Biol. Chem. 278, 3992–3999 [DOI] [PubMed] [Google Scholar]
- 66.Cawthorn W. P., Sethi J. K. (2008) TNF-alpha and adipocyte biology. FEBS Lett. 582, 117–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quinn P. G., Yeagley D. (2005) Insulin regulation of PEPCK gene expression: a model for rapid and reversible modulation. Curr. Drug Targets Immune Endocr. Metabol. Disord. 5, 423–437 [DOI] [PubMed] [Google Scholar]
- 68.Tontonoz P., Spiegelman B. M. (2008) Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 77, 289–312 [DOI] [PubMed] [Google Scholar]
- 69.Sugii S., Olson P., Sears D. D., Saberi M., Atkins A. R., Barish G. D., Hong S. H., Castro G. L., Yin Y. Q., Nelson M. C., Hsiao G., Greaves D. R., Downes M., Yu R. T., Olefsky J. M., Evans R. M. (2009) PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc. Natl. Acad. Sci. USA 106, 22504–22509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y., Jiang C., Xu G., Wang N., Zhu Y., Tang C., Wang X. (2008) Homocysteine upregulates resistin production from adipocytes in vivo and in vitro. Diabetes 57, 817–827 [DOI] [PubMed] [Google Scholar]
- 71.Hartman H. B., Hu X., Tyler K. X., Dalal C. K., Lazar M. A. (2002) Mechanisms regulating adipocyte expression of resistin. J. Biol. Chem. 277, 19754–19761 [DOI] [PubMed] [Google Scholar]
- 72.Spranger J., Kroke A., Möhlig M., Bergmann M. M., Ristow M., Boeing H., Pfeiffer A. F. (2003) Adiponectin and protection against type 2 diabetes mellitus. Lancet 361, 226–228 [DOI] [PubMed] [Google Scholar]
- 73.Goodman E., Dolan L. M., Morrison J. A., Daniels S. R. (2005) Factor analysis of clustered cardiovascular risks in adolescence: obesity is the predominant correlate of risk among youth. Circulation 111, 1970–1977 [DOI] [PubMed] [Google Scholar]
- 74.Brownlee M. (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 [DOI] [PubMed] [Google Scholar]
- 75.Majumder P. K., Mishra N. C., Sun X., Bharti A., Kharbanda S., Saxena S., Kufe D. (2001) Targeting of protein kinase C delta to mitochondria in the oxidative stress response. Cell Growth Differ. 12, 465–470 [PubMed] [Google Scholar]
- 76.Talior I., Tennenbaum T., Kuroki T., Eldar-Finkelman H. (2005) PKC-delta-dependent activation of oxidative stress in adipocytes of obese and insulin-resistant mice: role for NADPH oxidase. Am. J. Physiol. Endocrinol. Metab. 288, E405–E411 [DOI] [PubMed] [Google Scholar]
- 77.Braiman L., Alt A., Kuroki T., Ohba M., Bak A., Tennenbaum T., Sampson S. R. (1999) Protein kinase Cdelta mediates insulin-induced glucose transport in primary cultures of rat skeletal muscle. Mol. Endocrinol. 13, 2002–2012 [DOI] [PubMed] [Google Scholar]
- 78.Hennige A. M., Ranta F., Heinzelmann I., Düfer M., Michael D., Braumüller H., Lutz S. Z., Lammers R., Drews G., Bosch F., Häring H. U., Ullrich S. (2010) Overexpression of kinase-negative protein kinase Cdelta in pancreatic beta-cells protects mice from diet-induced glucose intolerance and beta-cell dysfunction. Diabetes 59, 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Almind K., Kahn C. R. (2004) Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes 53, 3274–3285 [DOI] [PubMed] [Google Scholar]
- 80.Berniakovich I., Trinei M., Stendardo M., Migliaccio E., Minucci S., Bernardi P., Pelicci P. G., Giorgio M. (2008) p66Shc-generated oxidative signal promotes fat accumulation. J. Biol. Chem. 283, 34283–34293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ranieri S. C., Fusco S., Panieri E., Labate V., Mele M., Tesori V., Ferrara A. M., Maulucci G., De Spirito M., Martorana G. E., Galeotti T., Pani G. (2010) Mammalian life-span determinant p66shcA mediates obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA 107, 13420–13425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C., Pelliccia G., Luzi L., Minucci S., Marcaccio M., Pinton P., Rizzuto R., Bernardi P., Paolucci F., Pelicci P. G. (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122, 221–233 [DOI] [PubMed] [Google Scholar]
- 83.Mayr M., Chung Y. L., Mayr U., McGregor E., Troy H., Baier G., Leitges M., Dunn M. J., Griffiths J. R., Xu Q. (2004) Loss of PKC-delta alters cardiac metabolism. Am. J. Physiol. Heart Circ. Physiol. 287, H937–H945 [DOI] [PubMed] [Google Scholar]
- 84.Pagnin E., Fadini G., de Toni R., Tiengo A., Calò L., Avogaro A. (2005) Diabetes induces p66shc gene expression in human peripheral blood mononuclear cells: relationship to oxidative stress. J. Clin. Endocrinol. Metab. 90, 1130–1136 [DOI] [PubMed] [Google Scholar]
- 85.Avogaro A., de Kreutzenberg S. V., Federici M., Fadini G. P. (2013) The endothelium abridges insulin resistance to premature aging. J. Am. Heart Assoc. 2, e000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fisher-Wellman K. H., Neufer P. D. (2012) Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol. Metab. 23, 142–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fisher-Wellman K. H., Gilliam L. A., Lin C. T., Cathey B. L., Lark D. S., Neufer P. D. (2013) Mitochondrial glutathione depletion reveals a novel role for the pyruvate dehydrogenase complex as a key H2O2-emitting source under conditions of nutrient overload. Free Radic. Biol. Med. 65, 1201–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rahimi Y., Camporez J. P., Petersen M. C., Pesta D., Perry R. J., Jurczak M. J., Cline G. W., Shulman G. I. (2014) Genetic activation of pyruvate dehydrogenase alters oxidative substrate selection to induce skeletal muscle insulin resistance. Proc. Natl. Acad. Sci. USA 111, 16508–16513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goodpaster B. H. (2013) Mitochondrial deficiency is associated with insulin resistance. Diabetes 62, 1032–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brun P. J., Yang K. J., Lee S. A., Yuen J. J., Blaner W. S. (2013) Retinoids: potent regulators of metabolism. Biofactors 39, 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Oliveira M. R. (2015) Vitamin A and retinoids as mitochondrial toxicants. Oxid. Med. Cell. Longev. 2015, 140267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sommer A., West K. P. (1996) Infectious morbidity. In Vitamin A Deficiency: Health, Survival and Vision, pp. 62–83, Oxford University Press, New York [Google Scholar]
- 93.West K. P., Jr (2002) Extent of vitamin A deficiency among preschool children and women of reproductive age. J. Nutr. 132 (9, Suppl), 2857S–2866S [DOI] [PubMed] [Google Scholar]
- 94.Penniston K. L., Tanumihardjo S. A. (2006) The acute and chronic toxic effects of vitamin A. Am. J. Clin. Nutr. 83, 191–201 [DOI] [PubMed] [Google Scholar]
- 95.Obrochta K. M., Kane M. A., Napoli J. L. (2014) Effects of diet and strain on mouse serum and tissue retinoid concentrations. PLoS One 9, e99435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Noy N., Blaner W. S. (1991) Interactions of retinol with binding proteins: studies with rat cellular retinol-binding protein and with rat retinol-binding protein. Biochemistry 30, 6380–6386 [DOI] [PubMed] [Google Scholar]
- 97.Noy N. (2000) Retinoid-binding proteins: mediators of retinoid action. Biochem. J. 348, 481–495 [PMC free article] [PubMed] [Google Scholar]
- 98.Vogel S., Mendelsohn C. L., Mertz J. R., Piantedosi R., Waldburger C., Gottesman M. E., Blaner W. S. (2001) Characterization of a new member of the fatty acid-binding protein family that binds all-trans-retinol. J. Biol. Chem. 276, 1353–1360 [DOI] [PubMed] [Google Scholar]
- 99.Imam A., Hoyos B., Swenson C., Levi E., Chua R., Viriya E., Hammerling U. (2001) Retinoids as ligands and coactivators of protein kinase C alpha. FASEB J. 15, 28–30 [DOI] [PubMed] [Google Scholar]
- 100.Kholodenko B. N., Hoek J. B., Westerhoff H. V. (2000) Why cytoplasmic signalling proteins should be recruited to cell membranes. Trends Cell Biol. 10, 173–178 [DOI] [PubMed] [Google Scholar]
- 101.Farese R. V., Standaert M. L., Francois A. J., Ways K., Arnold T. P., Hernandez H., Cooper D. R. (1992) Effects of insulin and phorbol esters on subcellular distribution of protein kinase C isoforms in rat adipocytes. Biochem. J. 288, 319–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu C., Jia W., Zhang R., Wang C., Lu J., Wu H., Fang Q., Ma X., Xiang K. (2008) Effect of RBP4 gene variants on circulating RBP4 concentration and type 2 diabetes in a Chinese population. Diabet. Med. 25, 11–18 [DOI] [PubMed] [Google Scholar]
- 103.Munkhtulga L., Nakayama K., Utsumi N., Yanagisawa Y., Gotoh T., Omi T., Kumada M., Erdenebulgan B., Zolzaya K., Lkhagvasuren T., Iwamoto S. (2007) Identification of a regulatory SNP in the retinol binding protein 4 gene associated with type 2 diabetes in Mongolia. Hum. Genet. 120, 879–888 [DOI] [PubMed] [Google Scholar]
- 104.Johansson H., Gandini S., Guerrieri-Gonzaga A., Iodice S., Ruscica M., Bonanni B., Gulisano M., Magni P., Formelli F., Decensi A. (2008) Effect of fenretinide and low-dose tamoxifen on insulin sensitivity in premenopausal women at high risk for breast cancer. Cancer Res. 68, 9512–9518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zemany L., Bhanot S., Peroni O. D., Murray S. F., Moraes-Vieira P. M., Castoldi A., Manchem P., Guo S., Monia B. P., Kahn B. B. (2015) Transthyretin antisense oligonucleotides lower circulating RBP4 levels and improve insulin sensitivity in obese mice. Diabetes 64, 1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tamori Y., Sakaue H., Kasuga M. (2006) RBP4, an unexpected adipokine. Nat. Med. 12, 30–31, discussion 31 [DOI] [PubMed] [Google Scholar]
- 107.Berry D. C., Noy N. (2012) Signaling by vitamin A and retinol-binding protein in regulation of insulin responses and lipid homeostasis. Biochim. Biophys. Acta 1821, 168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gong J., Hoyos B., Acin-Perez R., Vinogradov V., Shabrova E., Zhao F., Leitges M., Fischman D., Manfredi G., Hammerling U. (2012) Two protein kinase C isoforms, δ and ε, regulate energy homeostasis in mitochondria by transmitting opposing signals to the pyruvate dehydrogenase complex. FASEB J. 26, 3537–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.