Abstract
Purpose
The risk of biochemical recurrence is inversely related to the relapse-free interval after radical prostatectomy. We examined predictors of late biochemical recurrence, and the relationship between timing of biochemical recurrence and long-term survival outcomes.
Materials and Methods
Of 10,609 men treated with radical prostatectomy 1,684 had biochemical recurrence. We examined predictors of late biochemical recurrence (more than 10 years after radical prostatectomy), and calculated metastasis- free and cancer specific survival rates from the time of biochemical recurrence. In the subset of 1,583 men with an undetectable prostate specific antigen at 10 years we calculated actuarial metastasis-free and cancer specific survival estimates at 20 years after radical prostatectomy.
Results
Of the biochemical recurrence studied 77.0%, 16.6%, 4.9% and 1.5% occurred at 5 or less, greater than 5 to 10, greater than 10 to 15 and more than 15 years postoperatively. Late recurrence was associated with more favorable pathological features, as well as higher metastasis-free and cancer specific survival rates. For men with an undetectable prostate specific antigen at 10 years the actuarial probability of biochemical recurrence and metastasis at 20 years varied by stage and grade, with no metastases in patients with a prostatectomy Gleason score 6 or less. A single patient with an undetectable prostate specific antigen at 10 years died of prostate cancer within 20 years after radical prostatectomy.
Conclusions
Men with an undetectable prostate specific antigen for more than 10 years have a low risk of subsequent biochemical recurrence, with correspondingly lower rates of metastasis and death. These patients should be counseled that their risk of subsequent cancer related morbidity and mortality is low. Furthermore, these results suggest that annual prostate specific antigen testing may be safely discontinued after 10 years for men with a prostatectomy Gleason score 6 or less and/or limited life expectancy.
Keywords: prostatic neoplasms, prostatectomy, recurrence, time factors
Despite controversy regarding its use in prostate cancer screening, PSA is a sensitive marker for recurrence after radical prostatectomy.1 Although BCR is biologically heterogeneous, it serves as a harbinger of possible local and/or systemic progression.2 Among men with an initially undetectable postoperative PSA, subsequent PSA increases typically precede the onset of metastatic disease and disease specific death by 8 and 13 years, respectively.
Risk factors for BCR after RP include preoperative PSA, biopsy features, clinical stage and adverse prostatectomy pathology.3 Many nomograms and prediction tools have been designed to help predict the risk of BCR.4 Prior studies have suggested that the risk of BCR is time varying, with the majority occurring in the first few years after RP. For example, with an average of 6.8 followup PSA measurements per patient, Amling et al reported that 94% of BCR occurred within 5 years of RP.5 In an earlier report which also had limited followup, Pound et al reported 45% of BCR within the first 2 years, 77% within 5 years and 96% by 9 years after radical prostatectomy.2
Previous studies have also demonstrated divergent risk profiles for early and late BCR. For example, Caire et al reported on 1,207 men with BCR after radical prostatectomy divided into early (less than 5 years) and late (more than 5 years) groups.6 There was a greater odds of Gleason score less than 7 and preoperative PSA less than 10 ng/ml in the delayed BCR group compared to the earlier failures. They also found a nonsignificant improvement in metastasis-free survival (p = 0.062) and significantly higher cancer specific survival (p = 0.025) in patients with BCR at more than 5 years.
We examined this issue during a longer followup interval. Specifically we calculated annual hazard rates of BCR for more than 25 years postoperatively. In addition, we compared the clinical implications of early, intermediate and late BCR with respect to metastasis and cancer specific survival. Finally, we examined the likelihood and risk factors for subsequent BCR for men with an undetectable PSA 10 years after RP. This information could be useful in determining whether and for whom PSA testing is necessary after 10 years without recurrence.
METHODS
From 1978 to 2009, 10,720 men with clinically localized prostate cancer underwent RP by multiple surgeons at the Johns Hopkins Hospital with followup data. After RP, PSA and digital rectal examination were performed quarterly for the first year, semiannually during year 2 and annually thereafter. A total of 111 men who received radiation therapy or hormonal therapy before BCR were excluded from analysis, resulting in a final study population of 10,609. Overall 1,684 (15.9%) men had BCR, defined as a postoperative PSA greater than 0.2 ng/ml. These men were classified into 4 groups based on the timing of BCR as early (5 years or less), intermediate (greater than 5 to 10 years or less), late (greater than 10 to 15 years or less) and very late (greater than 15 years).
We calculated the annual hazard rates for BCR in the overall population. Hazard rates were calculated as the number of events (BCR) in each 1-year interval divided by the number at risk for that interval. Among men with BCR the chi-square test and ANOVA were used to compare clinical and pathological features among the early, intermediate, late and very late groups.
For men with BCR the Kaplan-Meier method was used to estimate metastasis-free and cancer specific survival rates stratified by the timing of BCR. Due to the small sample with very late BCR, the late (greater than 10 to 15 years or less) and very late (greater than 15 years) recurrence groups were combined for this analysis. The difference between survival curves was compared by the log rank test.
Finally, subset analysis was performed in the 1,583 men with an undetectable PSA at 10 years after RP. For this analysis men with earlier biochemical progression or 10 years or less of recurrence-free followup were excluded from study. In the men with more than 10 years of confirmed recurrence-free followup we examined the rates of subsequent BCR. Kaplan-Meier survival analysis was used to examine BCR-free and metastasis-free survival at 20 years after RP. The probabilities of BCR and metastasis at 20 years were calculated after stratifying for pathological stage and Gleason score. Multivariable analyses were not performed because the goal of the study was to determine whether a long recurrence-free interval identified a group of men at low risk for subsequent failure, but not to quantify the independent prognostic impact of the recurrence-free interval. All statistical analysis was performed using SAS® v9.2.
RESULTS
In the overall study population the mean age was 58.1 years (range 33 to 81) and the majority of men were white (table 1). Median preoperative PSA was 5.7 ng/ml. Clinical stage was T1c or less in 6,618 (62.8%) and biopsy Gleason score was less than 7 in 8,021 (76.0%) men. At radical prostatectomy 6,696 (63.3%) men had organ confined disease. Extracapsular extension, positive surgical margins, seminal vesicle invasion and lymph node metastases were reported in 3,099 (29.3%), 1,512 (14.3%), 449 (4.2%) and 333 (3.2%) men, respectively.
Table 1.
Comparison of clinicopathological features and treatment outcomes
| Overall | Early BCR | Intermediate BCR | Late BCR | Very late BCR | All BCR | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. sample size (%)* | 10,609 | 1,297 | (12.2) | 280 | (2.6) | 82 | (0.8) | 25 | (0.2) | 1,684 | (15.9) | Not applicable | |
| Median age at RP (range) | 59.0 (33.0–81.0) | 59.0 (38.0–75.0) | 60.0 (44.0–73.0) | 60.0 (44.0–69.0) | 62.0 (51.0–70.0) | 60.0 (38.0–75.0) | 0.438 | ||||||
| No. African American (%) | 789 | (7.5) | 120 | (9.3) | 22 | (7.9) | 3 | (3.7) | 0 | (0.0) | 145 | (8.6) | 0.119 |
| No. yr of RP (%): | |||||||||||||
| Before 1989 | 683 | (6.4) | 170 | (13.1) | 62 | (22.1) | 29 | (35.4) | 19 | (76.0) | 280 | (16.6) | <0.0001 |
| 1989–1991 | 539 | (5.1) | 94 | (7.2) | 43 | (15.4) | 18 | (21.9) | 6 | (24.0) | 161 | (9.6) | |
| 1992–2009 | 9,387 | (88.5) | 1,033 | (79.7) | 175 | (62.5) | 35 | (42.7) | 0 | (0.0) | 1,243 | (73.8) | |
| Median PSA (range) | 5.7 (0.1–151.0) | 8.6 (0.1–151.0) | 8.0 (0.1–116.0) | 6.6 (0.9–38.0) | 8.2 (0.7–31.4) | 8.4 (0.1–151.0) | 0.003 | ||||||
| No. biopsy Gleason 7 or greater (%) | 2,536 | (24.0) | 726 | (56.7) | 102 | (36.8) | 20 | (24.4) | 6 | (25.0) | 854 | (51.3) | <0.0001 |
| No. clinical stage T2+ (%) | 3,914 | (37.2) | 774 | (60.3) | 186 | (66.9) | 64 | (79.0) | 22 | (88.0) | 1,046 | (62.8) | <0.0001 |
| No. pathological stage (%): | |||||||||||||
| Organ confined | 6,696 | (63.3) | 215 | (16.7) | 62 | (22.4) | 22 | (27.2) | 6 | (24.0) | 305 | (18.2) | |
| Extracapsular extension | 3,099 | (29.3) | 597 | (46.2) | 165 | (59.5) | 48 | (59.2) | 17 | (68.0) | 827 | (49.4) | <0.0001 |
| Seminal vesicle invasion | 449 | (4.2) | 229 | (17.7) | 26 | (9.4) | 5 | (6.2) | 2 | (8.0) | 262 | (15.7) | |
| Lymph node metastases | 333 | (3.2) | 250 | (19.4) | 24 | (8.7) | 6 | (7.4) | 0 | (0.0) | 280 | (16.7) | |
| No. pos surgical margins (%) | 1,512 | (14.3) | 518 | (40.2) | 79 | (28.2) | 22 | (26.8) | 7 | (28.0) | 626 | (37.3) | 0.0003 |
| No. salvage therapy after BCR (%) | 776 | (46.1) | 599 | (46.2) | 136 | (48.6) | 32 | (39.0) | 9 | (36.0) | 776 | (46.1) | 0.338 |
| No. local recurrence (%) | 249 | (3.3) | 201 | (20.3) | 35 | (15.4) | 5 | (6.8) | 6 | (24.0) | 247 | (18.8) | † |
| No. metastasis (%) | 464 | (4.7) | 416 | (33.7) | 42 | (15.6) | 4 | (5.2) | 2 | (8.0) | 464 | (28.9) | † |
| No. prostate Ca death (%) | 265 | (2.5) | 250 | (19.3) | 14 | (5.0) | 0 | (0.0) | 1 | (4.0) | 265 | (15.7) | † |
Due to missing data for some variables, the denominator in some cells may not equal the column total.
Comparison of crude proportions not performed due to differences in followup. See figure for Kaplan-Meier metastasis-free and cancer specific survival curves.
Overall 1,684 (15.9%) men had BCR. There were no significant differences in age or race between men with earlier and later BCR. However, preoperative PSA, biopsy Gleason score, clinical stage, pathological stage and surgical margin status were significantly different among the groups. Of the patients with BCR 247 (18.8%) had local recurrence, 464 (28.9%) had metastases and 265 (15.7%) died of prostate cancer. The rate of salvage therapy after BCR was similar among the groups.
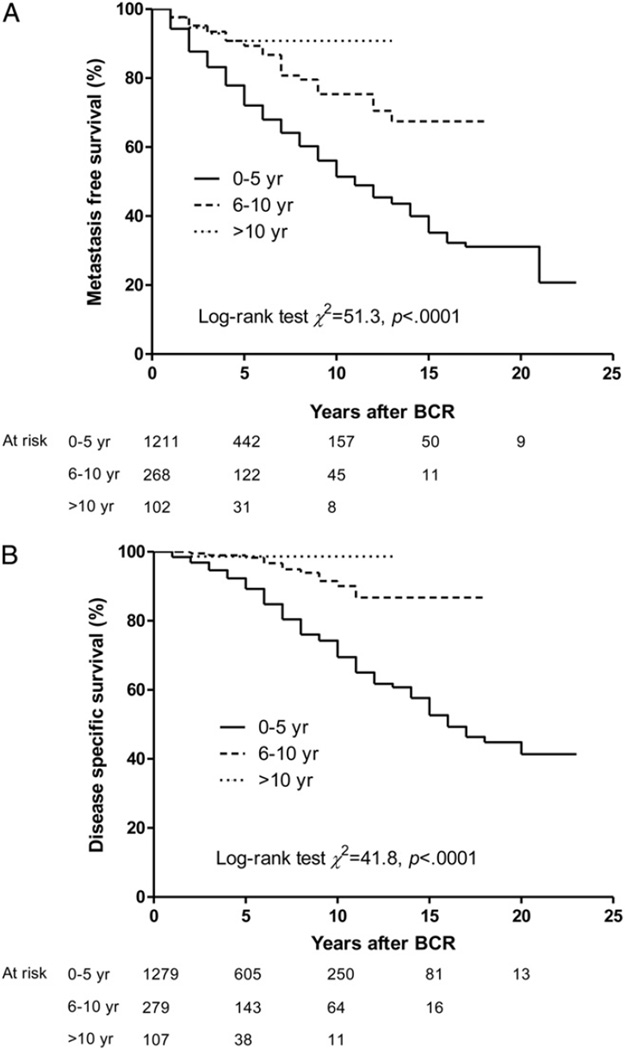
Part A of the figure shows the Kaplan-Meier estimates of metastasis-free survival after BCR, stratified by the timing of BCR. Overall early PSA failure was significantly associated with lower subsequent metastasis-free survival than intermediate and late BCR (p <0.0001). The median time from BCR to death or censoring was 4, 5 and 3 years in the early, intermediate and late BCR groups, respectively. Similarly, cancer specific survival following BCR was significantly lower for men with earlier BCR (p <0.0001, part B of figure).
Figure.
Kaplan-Meier curves for actuarial metastasis-free survival (A) and cancer specific survival (B) stratified by BCR timing.
In the subset of 1,583 men with more than 10 years of recurrence-free followup, the mean age at surgery was 58 years (range 34 to 73) and the median preoperative PSA was 5.6 ng/ml (81% had a PSA of 10 ng/ml or less). Although 55% had clinical stage T2 or greater disease, 85.9% had a biopsy Gleason score less than 7. At radical prostatectomy 58.4% had organ confined disease with negative surgical margins and 28.4% had a prostatectomy Gleason score of 7 or greater. Extraprostatic extension, seminal vesicle invasion and lymph node metastases were present in 38.5%, 2.2% and 0.95%, respectively. Men in this subset were followed for a median of 4.0 years after the 10-year mark (range 1 to 18) and 251 (16%) had complete followup out to 20 years after RP.
Overall 99 men (6.3%) in the subset with an undetectable PSA at 10 years had subsequent BCR. Table 2 shows the actuarial probability of BCR at 20 years for men with an undetectable PSA at 10 years, stratified by pathological stage and Gleason score. As expected, men with organ confined disease had a lower probability of BCR by 20 years, while the subgroups with positive surgical margins and extraprostatic disease had intermediate probabilities. There also appeared to be a greater probability of BCR with increasing Gleason score within each pathological stratum.
Table 2.
Actuarial probability of BCR and metastasis in men with greater than 10 years of recurrence-free followup after RP
| Organ Confined | Pos Surgical Margins |
Extraprostatic Extension |
Seminal Vesicle Invasion |
Lymph Node Metastases |
|
|---|---|---|---|---|---|
| Biochemical recurrence | |||||
| Gleason 6: | |||||
| No. pts | 754 | 95 | 373 | 9 | 3 |
| Actuarial probability (95% CI) | 3.9 (2.1–7.3) | 30.1 (18.5–46.7) | 17.9 (13.0–24.4) | 20.0 (3.1–79.6) | 66.7 (22.6–99.1) |
| Gleason 3+4: | |||||
| No. pts | 123 | 40 | 166 | 15 | 10 |
| Actuarial probability (95% CI) | 12.2 (3.6–36.7) | 16.7 (7.1–36.5) | 7.6 (3.6–15.3) | — | — |
| Gleason 4+3 or greater: | |||||
| No. pts | 44 | 14 | 66 | 8 | 1 |
| Actuarial probability (95% CI) | 14.1 (3.6–46.8) | 37.3 (11.7–82.8) | 25.7 (13.1–46.5) | 46.7 (13.7–93.2) | — |
| Metastasis | |||||
| Gleason 6: | |||||
| No. pts | 754 | 95 | 373 | 9 | 3 |
| Actuarial probability (95% CI) | 0 | 0 | 0 | 0 | 0 |
| Gleason 3+4: | |||||
| No. pts | 123 | 40 | 166 | 15 | 10 |
| Actuarial probability (95% CI) | 2.2 (0.3–14.7) | 0 | 0 | 0 | — |
| Gleason 4+3 or greater: | |||||
| No. pts | 44 | 14 | 66 | 8 | 1 |
| Actuarial probability (95% CI) | 0 | 14.3 (2.1–66.6) | 2.0 (0.3–13.1) | 20.0 (3.1–79.6) | — |
Of the men with an undetectable PSA at 10 years, 11 had local recurrence and 6 had metastatic disease by 20 years after RP. Table 2 also shows the actuarial probability of metastasis at 20 years for men with an undetectable PSA at 10 years. Although this analysis was limited by the low overall rates of metastasis, no patient with Gleason 6 disease and an undetectable PSA at 10 years after RP had metastasis by 20 years. Finally, 1 patient with Gleason 7 disease and seminal vesicle invasion experienced BCR at year 15, had metastasis at year 16 and died of disease at year 18. Another patient with Gleason 7 disease had an increased PSA at year 20, metastasis at year 21 and died of disease at year 23.
DISCUSSION
For patients with prostate cancer treated with initial definitive therapy, the National Comprehensive Cancer Network Guidelines recommend PSA testing every 6 to 12 months for 5 years, then annual testing.7 No guidelines are provided regarding the requisite duration of followup, and more specifically whether PSA testing may be safely discontinued after a lengthy disease-free interval.
Nevertheless, the majority of BCR occurs within the first few years after RP.2,5 Indeed, numerous studies have calculated annual hazard rates for BCR after radical prostatectomy as a way to examine temporal patterns of failure. For example, Amling et al modeled the annual hazard rates for recurrence up to 10 years after radical prostatectomy in 2,784 men who underwent radical prostatectomy from 1987 to 1993, including 819 with disease recurrence.5 The highest hazard rates (8.8 progressions per 100 person-years) were found in the first year, which decreased to 4.0 at 9 to 10 years postoperatively. Similarly in our study the hazard rate for BCR was highest in the first year after radical prostatectomy, with a general decrease thereafter.
Clearly the likelihood of BCR is inversely related to the length of BCR-free followup after radical prostatectomy. Stephenson et al created a postoperative nomogram to predict the 10-year progression-free probability after radical prostatectomy in which the prediction adjusts for the number of months the patient has already remained progression-free.8 In addition to confirming these findings during a longer study period, we also show that late BCR is associated with a decreased risk of metastasis and prostate cancer mortality once BCR occurs. Moreover due to considerable stage migration, many contemporary patients with low risk disease have a low risk of BCR at any time after RP. These combined findings suggest there may be specific patient populations in which lifelong PSA followup after RP is unnecessary.
Tollefson et al recently reported on 2,219 patients with low risk prostate cancer (PSA less than 10 ng/ml, pT2N0R0, Gleason 6 or less) treated with radical prostatectomy at the Mayo Clinic from 1994 to 2004.9 BCR risk was inversely proportional to the BCR-free interval after RP. Among men with an undetectable PSA 5 years after radical prostatectomy, the subsequent 5-year BCR rate was only 1.3%. The authors concluded that annual PSA measurements are unnecessary for men with an undetectable PSA during the first 3 postoperative years, and that PSA testing every 2 years should capture the majority of low risk patients who will experience BCR.
More recently Ahove et al reported on a smaller cohort of 505 men with no evidence of BCR 5 years after radical prostatectomy.10 In this study the median overall followup was 10.7 years and the 10-year BCR-free probability was 88%. Pathological tumor features associated with BCR beyond 5 years included Gleason score 7 or greater, extracapsular extension and seminal vesicle invasion. Thus, the authors concluded that patients with these features warrant continued PSA followup at least annually beyond the 5-year mark, whereas a less intensive followup regimen may be reasonable for those with less aggressive pathology who remain progression-free at 5 years.
Our study expands on these findings in a large subset analysis of men with more than 10 years of BCR-free followup. Although this group was not limited to patients with low risk disease, by 20 years BCR occurred in only 6%, with 6 cases of metastatic disease. Furthermore, subsequent metastasis and prostate cancer death was a rare event in this population, occurring in 2 patients at 18 and 23 years after radical prostatectomy. Overall these results suggest that an undetectable PSA at 10 years is a reasonable marker for surgical cure. Particularly for men with a short life expectancy, PSA testing may be discontinued after 10 years given the low rate of subsequent events by 20 years.
Another interesting finding is that no metastatic disease occurred in men with Gleason 6 or less disease, in agreement with the study by Tollefson et al in low risk patients,9 and with Ahove et al who reported no distant metastases in the subset with Gleason 6 disease.10 These combined findings suggest that additional PSA testing may be unnecessary after 10 years for patients with Gleason 6 or less disease due to the low risk of subsequent adverse clinical outcomes.
An alternative approach is to perform PSA testing at different followup intervals depending on surgical pathology and risk classification. For example, the frequency of postoperative PSA testing might be individually tailored based on the risk of adverse outcomes with an individual’s specific clinical and pathological characteristics.
A limitation of our study is that all RPs were performed at a high volume academic center and, thus, the results may not be generalizable to other populations. In addition, our statistical analysis was limited by the low rate of metastases, particularly in men with late BCR, and the more limited followup after BCR in this group. Although Kaplan-Meier estimates account for differences in followup, nevertheless the late and very late BCR groups had relatively less opportunity for metastases to develop or to die of prostate cancer. If confirmed with longer followup these findings are encouraging with regard to their implications for patients with a lengthy disease-free interval after RP. Furthermore, our study included patients treated during a lengthy period (1978 to 2009) and the results could change in the future due to stage migration.
Strengths of our study include the long duration of followup, allowing the calculation of annual hazard rates beyond 10 years (the limit in prior studies) and the examination of a large subset with at least 10 years of BCR-free followup. The clinical importance of our study was not to quantify the independent prognostic contribution of late BCR, but rather to highlight that patients with a lengthy recurrence-free interval constitute a group at low risk for subsequent disease recurrence. If confirmed, these findings may be useful in establishing future guidelines regarding the duration of PSA testing necessary after RP.
CONCLUSIONS
The highest hazard rates for BCR are found in the first few years after radical prostatectomy, with a general decrease over time. More favorable pathology features at prostatectomy were more likely among later compared to earlier BCR. Following BCR, actuarial metastasis- free and cancer specific survival rates were significantly lower for patients with early (less than 5 years) compared to intermediate (more than 5 to 10 or less years) or late (more than 10 years) BCR. Patients with an undetectable PSA for more than 10 years have a low risk of subsequent disease related morbidity and mortality, suggesting the possibility of less frequent PSA followup. In particular, annual PSA testing after 10 years may be unnecessary for men with a prostatectomy Gleason score 6 or less and/or limited life expectancy.
Supplementary Material
Abbreviations and Acronyms
- BCR
biochemical recurrence
- PSA
prostate specific antigen
- RP
radical prostatectomy
Footnotes
Supplementary material for this article can be obtained at http://urology.jhu.edu/SupplementaryResources/Loeb_JUrol.php.
Nothing to disclose.
REFERENCES
- 1.Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 2.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 3.Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 4.Kattan MW, Eastham JA, Stapleton AM, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 5.Amling CL, Blute ML, Bergstralh EJ, et al. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol. 2000;164:101. [PubMed] [Google Scholar]
- 6.Caire AA, Sun L, Ode O, et al. Delayed prostate-specific antigen recurrence after radical prostatectomy: how to identify and what are their clinical outcomes? Urology. 2009;74:643. doi: 10.1016/j.urology.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. [Accessed January 21, 2009]; Available at http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf. [Google Scholar]
- 8.Stephenson AJ, Scardino PT, Eastham JA, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23:7005. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tollefson MK, Blute ML, Rangel LJ, et al. Lifelong yearly prostate specific antigen surveillance is not necessary for low risk prostate cancer treated with radical prostatectomy. J Urol. 2010;184:925. doi: 10.1016/j.juro.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 10.Ahove DA, Hoffman KE, Hu JC, et al. Which patients with undetectable PSA levels 5 years after radical prostatectomy are still at risk of recurrence?–implications for a risk-adapted follow- up strategy. Urology. 2010;76:1201. doi: 10.1016/j.urology.2010.03.092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.