Abstract
Background
The United States has recently experienced increases in both its rate of obesity and its cesarean rate. Our objective was to use a new item measuring prepregnancy body mass index (BMI) on the U.S. Standard Certificate of Live Birth to examine at a population level the relationship between maternal obesity and primary cesarean delivery for women at otherwise low risk for cesarean delivery.
Methods
By 2012, 38 states with 86 percent of United States births had adopted the U.S. Standard Certificate. The sample was limited to the 2,233,144 women who had a singleton, vertex, term (37–41 weeks) birth in 2012 and no prior cesarean. We modeled the likelihood of a primary cesarean by BMI category, controlling for maternal socio-demographic and medical characteristics.
Results
Overall, 46.4 percent of otherwise low-risk mothers had a prepregnancy BMI in the overweight (25.1%) or obese (21.3%) categories, with the obese category distributed as follows: obese I (BMI 30.0–34.9, 12.4%); obese II (BMI 35.0–39.9, 5.5%); and obese III (BMI 40+, 3.5%). Obesity rates were highest among American Indian and Alaska Native (32.5%) and non-Hispanic black mothers (30.5%). After adjustment for demographic and medical risks, the adjusted risk ratios (95% confidence intervals) of cesarean for low-risk primiparas were: 1.61 (1.60–1.63) for obese I, 1.86 (1.83–1.88) for obese II, and 2.21 (2.18–2.25) for obese III mothers compared with mothers in the normal weight category.
Discussion
A relationship between prepregnancy obesity and primary cesarean delivery among relatively low-risk mothers remained even after controlling for social and medical risk factors.
Keywords: cesarean delivery, obesity, prepregnancy obesity
The long-term increase in obesity in the United States has begun to plateau (1). However, current high levels of obesity have resulted in a wide range of problematic maternal and infant health outcomes (2–7). Rates of prepregnancy obesity were found in a multi-state study to have increased slightly over the past decade with a reported 20.5 percent of mothers being obese as they began pregnancy in 2009, up from 17.6 percent in 2003 (8). A study focusing on low-income women found 28 percent of a 2008 cohort to be obese before pregnancy (9). One of the consequences of prepregnancy obesity has been a greater likelihood of medical interventions, most notably cesarean delivery (10–13).
Prior studies on the link between obesity and cesarean delivery have been limited by several factors, including relying on survey samples rather than population-based surveillance (11), drawing on data sets from individual medical centers (12), or examining earlier time periods when obesity and cesarean rates in the United States were lower (10–12). New items added to the U.S. Standard Certificate of Live Birth provide an opportunity for the examination of the relationship between prepregnancy body mass index (BMI) and cesarean births on a much larger scale than has been possible in the past. This paper uses 2012 birth certificate data from the 38 states (14) which included the new items to examine the hypothesis that prepregnancy BMI will be related to the likelihood of a primary cesarean for low-risk women even after controlling for other risk factors.
Methods
This retrospective cohort study took advantage of newly available data based on the 2003 revision of the U.S. Standard Certificate of Live Birth, which now includes information on the mother’s height and prepregnancy weight that are needed to compute BMI. These data are primarily obtained from the first prenatal visit, based on direct measurement of the mother’s height and weight (when the first visit occurred early in the pregnancy), or from the mother’s recall of her prepregnancy weight when the first visit occurred later in the pregnancy. The 2003 revision was slowly adopted by states until by 2012, 38 states and the District of Columbia included prepregnancy BMI (see Table 1 for a list of the states) (14,15). We used standard definitions for BMI categories: underweight (BMI < 18.5); normal weight (18.5–24.9); overweight (25.0–29.9); obese (30.0+); and the three subcategories of obesity: obese I (30–34.9); obese II (35.0–39.9), and obese III (40+) (16).
Table 1.
Percent Distribution of BMI Categories for Term, Singleton, Vertex Births to Mothers with No Prior Cesarean by Selected Characteristics: 38 States And DC, 2012
| Underweight | Normal weight | Overweight | Obese I | Obese II | Obese III | ||
|---|---|---|---|---|---|---|---|
| Characteristics of mother |
Total | (BMI < 18.5) (n = 88,638) |
(BMI 18.5–24.9) (n = 1,066,630) |
(BMI 25.0–29.9) (n = 542,296) |
(BMI 30.0–34.9) (n = 266,872) |
(BMI 35.0–39.9) (n = 118,111) |
(BMI ≥ 40.0) (n = 74,795) |
| All | 100.0 | 4.1 | 49.4 | 25.1 | 12.4 | 5.5 | 3.5 |
| Race and Hispanic origin | |||||||
| Hispanic | 100.0 | 3.1 | 44.4 | 29.1 | 14.7 | 5.6 | 3.0 |
| Non-Hispanic | |||||||
| White | 100.0 | 4.0 | 52.3 | 23.9 | 11.3 | 5.2 | 3.3 |
| Black | 100.0 | 3.7 | 38.7 | 27.1 | 16.1 | 8.0 | 6.3 |
| AIAN | 100.0 | 3.1 | 37.1 | 27.3 | 17.7 | 9.1 | 5.7 |
| Asian | 100.0 | 9.5 | 66.9 | 17.5 | 4.7 | 1.0 | 0.3 |
| NHOPI | 100.0 | 2.2 | 32.3 | 27.4 | 20.5 | 11.2 | 6.4 |
| Age of mother | |||||||
| Under 20 | 100.0 | 7.5 | 55.3 | 22.1 | 9.7 | 3.6 | 1.8 |
| 20–24 | 100.0 | 5.3 | 47.6 | 24.7 | 12.9 | 5.9 | 3.6 |
| 25–29 | 100.0 | 3.7 | 48.4 | 25.6 | 12.7 | 5.8 | 3.8 |
| 30–34 | 100.0 | 3.0 | 50.8 | 25.4 | 12.0 | 5.3 | 3.5 |
| 35–39 | 100.0 | 2.7 | 48.9 | 26.3 | 12.8 | 5.6 | 3.7 |
| 40 or more | 100.0 | 2.3 | 47.1 | 27.4 | 14.1 | 5.6 | 3.4 |
| Education of mother | |||||||
| Less than high school | 100.0 | 5.2 | 45.6 | 27.0 | 13.7 | 5.4 | 3.1 |
| High school diploma | 100.0 | 4.7 | 44.8 | 25.4 | 14.1 | 6.6 | 4.4 |
| Some college/Associate’s degree | 100.0 | 3.6 | 45.0 | 26.1 | 13.9 | 6.7 | 4.6 |
| Bachelor’s degree | 100.0 | 3.5 | 58.0 | 23.5 | 9.3 | 3.8 | 2.0 |
| Master’s or Doctorate | 100.0 | 3.6 | 62.6 | 21.9 | 7.6 | 2.8 | 1.5 |
| Birthplace of mother | |||||||
| Born in the 50 states and DC | 100.0 | 3.8 | 48.4 | 24.8 | 12.8 | 6.1 | 4.1 |
| Born outside the 50 states and DC | 100.0 | 5.1 | 53.0 | 26.5 | 10.8 | 3.2 | 1.3 |
| Live birth order | |||||||
| First | 100.0 | 4.9 | 52.7 | 23.4 | 10.8 | 4.9 | 3.2 |
| Second | 100.0 | 3.9 | 50.0 | 25.3 | 12.3 | 5.3 | 3.2 |
| Third | 100.0 | 3.2 | 45.3 | 27.4 | 14.2 | 6.1 | 3.7 |
| Fourth or higher | 100.0 | 2.6 | 39.2 | 29.2 | 16.8 | 7.5 | 4.8 |
| Month prenatal care began | |||||||
| First trimester | 100.0 | 3.9 | 49.9 | 25.1 | 12.2 | 5.4 | 3.4 |
| After 1st trimester or none | 100.0 | 4.7 | 47.9 | 25.4 | 12.8 | 5.6 | 3.5 |
| Source of payment | |||||||
| Medicaid | 100.0 | 3.4 | 53.7 | 24.3 | 10.9 | 4.8 | 2.9 |
| Private insurance | 100.0 | 4.8 | 44.3 | 25.8 | 14.1 | 6.5 | 4.4 |
| Self pay | 100.0 | 4.9 | 54.3 | 24.7 | 11.0 | 3.6 | 1.6 |
| Other | 100.0 | 4.0 | 48.8 | 27.0 | 12.4 | 5.0 | 2.9 |
| WIC1 | 100.0 | 4.5 | 43.5 | 26.2 | 14.5 | 6.7 | 4.5 |
| Not WIC1 | 100.0 | 3.7 | 54.5 | 24.2 | 10.6 | 4.4 | 2.6 |
| Smoker2 | 100.0 | 6.4 | 44.5 | 24.1 | 13.8 | 6.8 | 4.4 |
| Nonsmoker2 | 100.0 | 3.9 | 50.0 | 25.2 | 12.2 | 5.3 | 3.3 |
| Infant breastfed3 | 100.0 | 3.9 | 50.5 | 25.2 | 12.0 | 5.2 | 3.2 |
| Infant not breastfed3 | 100.0 | 4.9 | 44.2 | 24.6 | 14.1 | 7.1 | 5.1 |
| Diabetes | |||||||
| Prepregnancy | 100.0 | 1.2 | 23.6 | 24.5 | 21.3 | 14.7 | 14.7 |
| Gestational | 100.0 | 2.1 | 30.5 | 27.0 | 20.0 | 11.3 | 9.1 |
| Hypertension | |||||||
| Prepregnancy | 100.0 | 0.8 | 19.6 | 22.7 | 21.2 | 16.3 | 19.3 |
| Gestational | 100.0 | 1.9 | 31.1 | 27.3 | 19.3 | 11.1 | 9.4 |
Includes California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Mexico, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Washington, Wisconsin, and Wyoming.
Mother received WIC (Special Supplemental Nutrition Program for Women, Infants, and Children) food during this pregnancy.
Excludes Michigan, which does not report smoking during pregnancy in a form comparable to that of the national standard.
Infant was breastfed in the period between birth and hospital discharge. Excludes California, which does not report this item.
Since more than 90 percent of mothers with a prior cesarean receive a repeat cesarean under any circumstances (17,18), we have chosen to focus our analysis on primary cesarean deliveries. To reduce variation based on perinatal characteristics, we limited our population to women at low risk for a cesarean delivery according to the Healthy People 2020 definition: singleton, vertex presentation, and 37+ weeks of gestational age (19,20), with one modification: we included only women delivering between 37 and 41 weeks of gestation, as postterm (42+ weeks) gestational age has been found to be related to cesarean delivery (21). Vertex presentation was defined as births with the cephalic presentation at birth checkbox marked on the birth certificate.
Although obesity itself is sometimes considered a risk factor for cesarean delivery, these women are low risk by the Healthy People 2020 definition; thus the designation “otherwise low risk” for this study population. Results are presented for the following non-Hispanic, single-race groups: white, black, American Indian or Alaska Native (AIAN), Asian, and Native Hawaiian or other Pacific Islander (NHOPI) women; and for Hispanic women of any race. The distribution of BMI categories by demographic and medical risk factors, and the likelihood of a primary cesarean by those same variables and by state was examined. Not stated responses were excluded before percentages were computed.
Because cesarean is a relatively common outcome, we did not use logistic regression but instead used general estimating equations with a Poisson distribution and log link to model the risk of cesarean delivery by obesity categories (underweight, normal weight, overweight, and obesity categories I–III). This approach allowed us to generate relative risks, as opposed to odds ratios. Normal prepregnancy weight was chosen as the reference group. In the multivariate model we included demographic and medical risk factors shown in the past to be related to the likelihood of a primary cesarean (21–23). The demographic factors included were: maternal age (< 20 years; 20–24 years [ref]; 25–29 years; 30–34 years; 35–39 years; 40+ years), race/ethnicity (groups shown above; non-Hispanic white [ref]), education (< 12 years completed [ref]; 12 years only completed; 13+ years completed), live birth order (first birth; 2+ births [ref]), maternal nativity (native born [ref]; foreign born), and source of payment for the birth (private insurance [ref]; Medicaid; self-pay). Maternal prenatal care (late/no prenatal care; prenatal care began in first 3 months [ref]) was also included as were chronic diabetes (no [ref]; yes), gestational diabetes (no [ref]; yes), and hypertension (no [ref]; yes).
We tested for interactions between BMI and maternal race/ethnicity, age, live birth order, and medical risk factors (diabetes and hypertension), as they had been related to cesareans in prior research (21,22). Of these, the only consistently significant interaction was with birth order and we choose to stratify our multivariate models by birth order. We also tested the possible influence of birthweight on the model by limiting the birthweight to infants between 2,500 and 3,999 g in a sensitivity analysis, and it did not change the results. Given that the population studied is more than 2.2 million, tests of statistical significance are not presented in Tables 1 and 2 as even the smallest differences were significant. Comparisons are across BMI categories unless otherwise noted. In the multivariate analysis, 95 percent confidence intervals are presented. The study was based on the publicly available Natality data set from the National Center for Health Statistics, and was exempt from Institutional Review Board review.
Table 2.
Percent of Births Delivered by Cesarean by BMI for Term, Singleton, Vertex Births to Mothers with No Prior Cesarean by Selected Characteristics: 38 States and DC, 2012
| Underweight | Normal weight | Overweight | Obese I | Obese II | Obese III | ||
|---|---|---|---|---|---|---|---|
| Characteristics of mother | Total* (n = 388,125)† |
(BMI < 18.5) (n = 10,512) |
(BMI 18.5–24.9) (n = 156,173) |
(BMI 25.0–29.9) (n = 101,844) |
(BMI 30.0–34.9) (n = 60,996) |
(BMI 35.0–39.9) (n = 32,369) |
(BMI ≥ 40.0) (n = 26,231) |
| All | 18.0 | 11.9 | 14.6 | 18.8 | 22.9 | 27.4 | 35.1 |
| Race and Hispanic origin | |||||||
| Hispanic | 16.9 | 12.5 | 14.6 | 16.6 | 19.7 | 23.9 | 30.3 |
| Non-Hispanic | |||||||
| White | 17.5 | 10.8 | 13.8 | 18.9 | 23.9 | 28.4 | 36.4 |
| Black | 21.5 | 11.9 | 16.8 | 21.6 | 25.0 | 29.4 | 36.6 |
| AIAN | 15.7 | 11.2 | 11.6 | 15.2 | 18.1 | 21.8 | 29.8 |
| Asian | 19.2 | 15.1 | 18.3 | 22.4 | 25.3 | 29.5 | 35.6 |
| NHOPI | 17.4 | 15.4 | 13.4 | 16.9 | 19.0 | 21.9 | 28.1 |
| Age of mother | |||||||
| Under 20 | 16.9 | 10.4 | 14.0 | 19.1 | 24.2 | 30.5 | 39.6 |
| 20–24 | 16.9 | 11.0 | 13.4 | 17.5 | 22.0 | 27.0 | 34.9 |
| 25–29 | 17.1 | 11.6 | 13.8 | 17.7 | 21.5 | 25.4 | 33.3 |
| 30–34 | 18.1 | 13.0 | 14.9 | 18.9 | 22.7 | 27.1 | 34.6 |
| 35–39 | 21.2 | 15.5 | 17.8 | 21.6 | 25.8 | 30.8 | 37.9 |
| 40 or more | 27.6 | 20.0 | 24.0 | 28.2 | 31.6 | 36.7 | 45.0 |
| Education of mother | |||||||
| Less than high school | 14.5 | 10.8 | 12.3 | 14.4 | 17.3 | 21.8 | 28.4 |
| High school diploma | 17.5 | 11.4 | 13.9 | 17.5 | 21.7 | 25.9 | 33.5 |
| Some college/Associate’s degree | 19.0 | 11.9 | 14.9 | 19.3 | 23.7 | 28.1 | 36.1 |
| Bachelor’s degree | 19.1 | 12.8 | 15.5 | 21.8 | 27.2 | 32.8 | 41.3 |
| Master’s or doctorate | 19.8 | 14.1 | 16.6 | 23.1 | 29.6 | 34.8 | 43.6 |
| Birthplace of mother | |||||||
| Born in the 50 states and DC | 18.1 | 10.9 | 14.0 | 19.0 | 23.5 | 28.0 | 35.6 |
| Born outside the 50 states and DC | 17.6 | 14.4 | 16.6 | 18.0 | 20.2 | 23.6 | 29.5 |
| Live birth order | |||||||
| First | 26.9 | 16.3 | 21.6 | 29.6 | 36.5 | 43.0 | 52.8 |
| Second | 11.2 | 6.8 | 8.5 | 11.7 | 15.4 | 18.3 | 24.4 |
| Third | 9.8 | 5.8 | 7.3 | 10.0 | 12.7 | 15.6 | 21.1 |
| Fourth or higher | 8.7 | 5.6 | 6.3 | 8.5 | 10.7 | 13.3 | 17.0 |
| Month prenatal care began | |||||||
| First trimester | 18.5 | 12.1 | 15.0 | 19.4 | 23.7 | 28.3 | 36.0 |
| After first trimester or none | 16.4 | 11.1 | 13.5 | 16.8 | 20.5 | 24.9 | 32.6 |
| Source of payment | |||||||
| Medicaid | 17.3 | 11.6 | 14.0 | 17.2 | 20.9 | 25.5 | 32.7 |
| Private insurance | 19.4 | 12.5 | 15.6 | 21.1 | 26.1 | 30.8 | 39.3 |
| Self-pay | 11.6 | 9.9 | 9.8 | 12.4 | 15.4 | 18.2 | 23.9 |
| Other | 16.0 | 10.5 | 13.6 | 16.6 | 20.2 | 23.1 | 29.8 |
| WIC‡ | 17.7 | 11.7 | 14.2 | 17.5 | 21.4 | 26.1 | 33.7 |
| Not WIC‡ | 18.3 | 12.1 | 15.0 | 20.0 | 24.6 | 29.1 | 37.3 |
| Smoker§ | 17.6 | 11.4 | 13.9 | 17.9 | 22.1 | 26.4 | 33.8 |
| Nonsmoker§ | 18.0 | 12.0 | 14.7 | 18.8 | 22.9 | 27.5 | 35.2 |
| Infant breastfed¶ | 18.2 | 11.9 | 14.6 | 19.2 | 23.6 | 28.2 | 36.4 |
| Infant not breastfed¶ | 18.6 | 11.6 | 14.9 | 18.6 | 22.6 | 26.9 | 34.2 |
| Diabetes | |||||||
| Prepregnancy | 40.5 | 28.9 | 32.7 | 37.7 | 40.7 | 44.6 | 54.2 |
| Gestational | 26.4 | 15.6 | 20.1 | 23.9 | 28.6 | 34.6 | 41.9 |
| Hypertension | |||||||
| Prepregnancy | 31.9 | 17.6 | 21.9 | 27.4 | 30.8 | 37.7 | 44.3 |
| Gestational | 31.5 | 20.5 | 25.5 | 29.8 | 33.8 | 38.3 | 46.0 |
Includes California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Mexico, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Washington, Wisconsin, and Wyoming.
Excludes unknown BMI.
Number of cesareans.
Mother received WIC (Special Supplemental Nutrition Program for Women, Infants, and Children) food during this pregnancy.
Excludes Michigan, which does not report smoking during pregnancy in a form comparable to that of the national standard.
Infant was breastfed in the period between birth and hospital discharge. Excludes California, which does not report this item.
BMI = body mass index.
Results
From an initial 3,952,841 overall United States births in 2012, there were 3,412,436 births to residents of the 38-state and Washington, DC reporting area (86%). Limiting the study to mothers without a prior cesarean further reduces the study population to 2,905,024, and excluding cases of multiple births, malpresentation, and gestational ages < 37 weeks or more than 41 weeks results in a final study population of 2,233,144. Table 1 presents the distribution of prepregnancy BMI for mothers in the 38 states and Washington, DC in our study. Overall, 46.4 percent of mothers had a BMI in the overweight (25.1%) or obese (21.3%) categories, with the obese category distributed as follows: obese I (12.4%); obese II (5.5%); and obese III (3.5%).
The distribution of prepregnancy weight varied substantially across certain demographic and behavioral characteristics and medical risk factors. For example, the proportion of American Indian and Alaska Native (AIAN) (32.5%) and non-Hispanic black mothers in the obese categories (30.5%) is more than five times higher than the comparable figure among Asian mothers (6.1%).
Likewise, native-born mothers (23.0%) were more likely to be obese than foreign-born mothers (15.4%) and mothers with private insurance (25.0%) as compared with those on Medicaid (18.5%). There were increases in the prevalence of obesity associated with each successive birth (birth 1—19.0% obese; 2—20.8%; 3—24.1%; 4+–29.0%). Prepregnancy obesity was also associated with a variety of medical conditions, most notably prepregnancy hypertension—19.6 percent of those with chronic hypertension were normal weight prepregnancy and 56.8 percent were obese.
Table 2 presents primary cesarean rates for singleton, vertex births at 37–41 weeks by demographic and medical risk factors. Compared with a mother in the normal weight range prepregnancy (primary cesarean rate 14.6%), the primary cesarean rate for mothers in each obese category increased from 22.9 percent in obese I to 27.4 percent in obese II and 35.1 percent in obese III. Primary cesarean rates were consistently higher among mothers in the obese III category and particularly among mothers with prepregnancy diabetes (54.2%) or gestational hypertension (46.0%). More than two in five first-time mothers (40.9%) with prepregnancy obesity had a cesarean with rates increasing by obese category (obese I—36.5%; obese II—43.0%; obese III—52.8%).
Given that the population was limited to mothers without a prior cesarean, high order births involved mothers who had given birth vaginally in the past and primary cesarean rates decreased with birth order. Although primary cesarean rates were higher in older age groups, the differences between obese and normal weight mothers declined with increasing age. The greatest difference was for younger mothers, with a primary cesarean rate for obese category III mothers that was 2.8 times that for normal weight mothers (39.6–14.0%). In comparison, for mothers age 40+, the difference was smaller in relative (1.7 times) and absolute terms (45.0–24.0%). Mothers with births that were self-paid had lower obesity rates, and also distinctly lower primary cesarean rates at each BMI category, including for obese category III (23.9%) (Tables 1 and 2). This rate was substantially lower than the primary cesarean rate for mothers in obese category III on Medicaid (32.7%) or private insurance (39.3%).
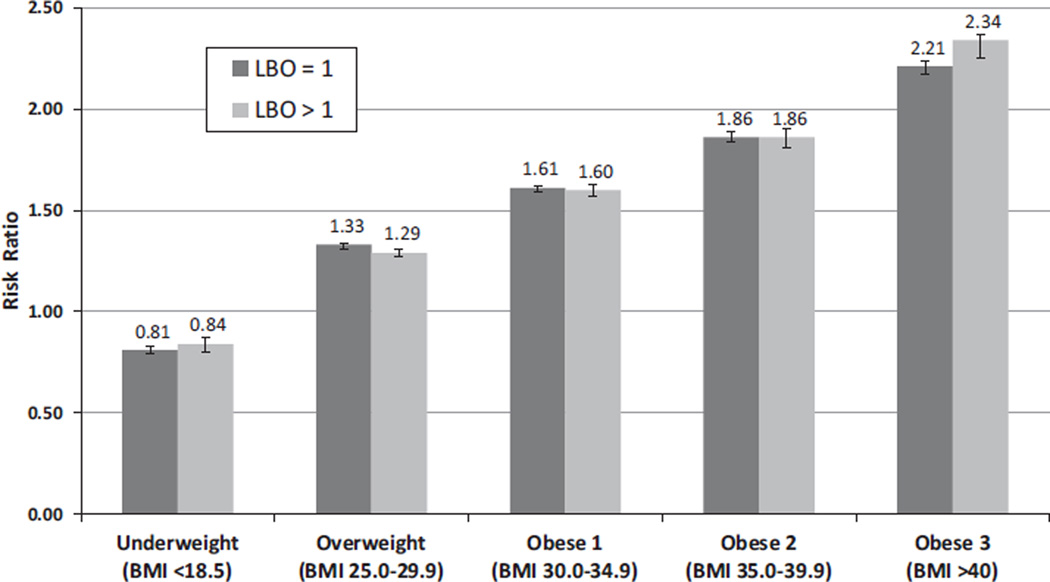
Figure 1 presents the results of the multivariate analysis stratified by parity. After controlling for demographic and medical risk factors, the relationship of prepregnancy obesity with the likelihood of a primary cesarean is pronounced. The adjusted risk ratio increases monotonically across the BMI categories for both primiparous and multiparous mothers. Because of the large sample size, the confidence intervals are very small with all differences by obesity category significant at p < 0.05.
Fig. 1.
Adjusted* risk ratios† for cesarean delivery by body mass index (BMI), low-risk‡ mothers with no prior cesarean, 38 states, 2012. *Adjusted for maternal age, race/ethnicity, education, trimester prenatal care began, nativity (US or foreign-born), method of payment for the delivery, and prepregnancy and gestational diabetes and hypertension. †“Normal weight” (BMI 18.5–24.9) was the reference group; error bars represent 95% confidence intervals. ‡Singleton, vertex, 37–41 weeks of gestational age. LBO = live birth order.
For primiparous mothers, the risk ratios shift from a significantly lower likelihood for women who were underweight prepregnancy (risk ratio 0.81 [95% CI 0.79–0.83]) to 1.61 (1.60–1.63) for obese I, 1.86 (1.83–1.88) for obese II, and 2.21 (2.18–2.25) for obese III. A comparable pattern is found among multiparous mothers. In a related analysis, we limited the sample to mothers who did not have evidence of chronic or gestational diabetes or hypertension and found a comparable pattern of adjusted risk ratios, ranging from a significantly lower likelihood for women who were underweight prepregnancy (AOR 0.84 [95% CI 0.80–0.88]) to 1.76 [1.72–1.79] for obese I, 2.14 [2.08–2.19] for obese II, and 2.94 [2.85–3.03] for obese III (data not shown).
Discussion
Utilizing what is the largest United States data set yet available, we have identified a substantial relationship between prepregnancy obesity and the likelihood of a primary cesarean. Analyzing more than 2.2 million births from 38 states and Washington, DC for 2012, we found more than one in five (21.3%) mothers began their pregnancy with a BMI in the obese range and that the likelihood of a primary cesarean increased consistently across categories of obesity in a population of low-risk women who would otherwise be likely candidates for a vaginal birth, even after controlling for several demographic and medical risk factors. For primiparous mothers in the obese III category (BMI 40+), the odds of a primary cesarean were 2.2 times that of a mother in a normal weight category and for a multiparous mother the difference was 2.3 times. Limiting our sample to an even lower risk group of mothers, those without evidence of diabetes or hypertension did not alter the pattern of the findings, nor did limiting the sample to infants with birthweights between 2,500 and 3,999 g.
Despite its large sample, this study has several limitations. The data account for 86 percent of all births in the United States, but they do not include 12 states and therefore cannot be regarded as a representative sample of all United States births. Also limiting this study is the lack of indications for cesarean, though births involving some of the more common reasons for a cesarean (prior cesarean, malpresentation, postdate, twins) were excluded from the analysis.
The measurement of prepregnancy BMI on the birth certificate is a new item and there have been limited studies of the accuracy of birth certificate reporting of this item. Park et al compared the BMI measure on the birth certificate to WIC records for the same mothers and found the measure to be generally valid and reliable for population-based research (24). Bodnar et al compared BMI data from Pennsylvania birth certificates to medical records from a single hospital over time and found BMI reporting most reliable for normal, overweight, obese II, and obese III, but less accurate for underweight and obese 1 categories (25).
Although not directly focused on the birth certificate measure of BMI, Holland et al found maternal self-reported prepregnancy weight to be generally concordant with weight measured at first visit with 87 percent of mothers categorized in the same BMI category (26). Oken et al found a strong correlation (r = 0.99) between self-reported prepregnancy weight and weight recorded in a medical visit near the beginning of pregnancy (27). A recent study of the quality of medical and health items from the 2003 birth certificate found reasonable data quality for variables such as cesarean delivery, gestational age, trimester prenatal care began, and source of payment; however, diabetes and hypertension appear to be underreported in birth certificate data (28). Demographic items such as maternal age, race/ethnicity, and education have previously been found to be accurately reported on birth certificates (29). Some other factors, such as placenta previa and fetal anomalies, that would have been relevant to the analysis were not included in the multivariate model because of concerns about the quality of the measurement of those items on the birth certificate.
Both the finding of the rate of prepregnancy obesity and the greater likelihood of a primary cesarean associated with increasing levels of BMI are generally consistent with the limited past United States-based research that has been done on this topic. With respect to the rate of prepregnancy obesity, Fisher et al, using PRAMS data from 20 states in 2009, found 20.5 percent of mothers were in the obese category before pregnancy (8). Among studies linking obesity to the likelihood of a cesarean delivery, Dietz et al using PRAMS data from 19 states between 1998 and 2000 found very obese (BMI 35+) first-time mothers without other complications more than three times more likely to have a primary cesarean than mothers with a prepregnancy weight in the normal range (11), and Roman et al using medical records from a single hospital between 1994 and 2004 found a 2.8 times greater likelihood of a cesarean for low-risk mothers who were very obese (12).
At a clinical level, it is unclear why the primary cesarean rate is consistently higher in the obese population. Because of the added morbidity associated with surgery in obese patients (5–7), physicians often try to avoid cesareans in obese patients. We controlled for maternal age, hypertension, and diabetes, all contributors to placental insufficiency, to decrease the potentially confounding effect of nonreassuring fetal testing. In light of the greater risk of stillbirth in obese patients (4), they might be subject to greater numbers of ultrasounds and other fetal testing. Closer monitoring is often associated with more interventions, potentially increasing cesarean risk (30). In addition, labor management may be influenced by maternal weight, as practitioners may initiate surgery earlier to avoid an emergency procedure on an obese mother.
From a public health perspective, campaigns emphasizing preconception care (31) and a life course approach (32,33) to maternal health, including prevention of obesity, may be able to reduce the risks from a factor that is strongly associated with primary cesareans. More importantly, recent systematic reviews and meta analyses (2,4) suggest that reducing prepregnancy BMI could have a positive change to population-based research. Campaigns to prevent maternal obesity can be extremely challenging (34,35) but are necessary steps in mitigating the negative effects of obesity on maternal and infant health.
The once rapid growth of prepregnancy obesity in the United States has apparently slowed, but the consequences of having hundreds of thousands of United States mothers begin their pregnancy obese remain. This study has documented a consistent relationship between prepregnancy obesity and primary cesarean delivery among mothers otherwise at relatively low risk. Given the low rate of vaginal birth after cesarean in the United States, subsequent cesareans for these mothers are likely as well. The response to the problem of the relationship of prepregnancy obesity with primary cesareans cannot, by definition, await the beginning of pregnancy and will necessitate efforts from medical practitioners and the public health community involved in women’s health in general to address this problem.
Conclusion
In this study of more than 2.2 million United States women, prepregnancy obesity was found to represent an independent risk factor for primary cesareans, even after controlling for maternal demographic characteristics and medical risk factors. For clinicians, further research is needed to clarify the factors associated with prepregnancy obesity that may be the basis for a decision to proceed with a primary cesarean. For the public health community, these findings suggest an additional justification for efforts to reduce rates of obesity among women of reproductive age.
Acknowledgments
The authors thank Thien Nguyen and Howard Cabral for their technical assistance on this project. ED’s work on the research partially supported by a grant from the U.S. Maternal and Child Health Bureau (R40MC 17172).
Footnotes
Disclosure of Interests
The authors have no interests to disclose.
Contributor Information
Eugene Declercq, Department of Community Health Sciences, Boston University School of Public Health, Boston, MA, USA.
Marian MacDorman, Maryland Population Research Center, University of Maryland, College Park, MD, USA.
Michelle Osterman, Division of Vital Statistics, Reproductive Statistics Branch, National Center for Health Statistics, CDC, Hyattsville, MD, USA.
Candice Belanoff, Department of Community Health Sciences, Boston University School of Public Health, Boston, MA, USA.
Ronald Iverson, Department of Obstetrics and Gynecology, Boston University School of Medicine, Boston, MA, USA.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meehan S, Beck CR, Mair-Jenkins J, et al. Maternal obesity and infant mortality: A meta-analysis. Pediatrics. 2014;133(5):863–871. doi: 10.1542/peds.2013-1480. [DOI] [PubMed] [Google Scholar]
- 3.Johansson S, Villamor E, Altman M, et al. Maternal overweight and obesity in early pregnancy and risk of infant mortality: A population based cohort study in Sweden. BMJ. 2014;349:g6572. doi: 10.1136/bmj.g6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aune D, Saugstad O, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: A systematic review and meta-analysis. JAMA. 2014;311:1536–1546. doi: 10.1001/jama.2014.2269. [DOI] [PubMed] [Google Scholar]
- 5.Crane J, Murphy P, Burrage L, Hutchens D. Maternal and perinatal outcomes of extreme obesity in pregnancy. J Obstet Gynaecol Can. 2013;35(7):606–611. doi: 10.1016/S1701-2163(15)30879-3. [DOI] [PubMed] [Google Scholar]
- 6.Papachatzi E, Dimitriou G, Dimitropoulos K, Vantarakis A. Prepregnancy obesity: Maternal, neonatal and childhood outcomes. J Neonatal Perinatal Med. 2013;6(3):203–216. doi: 10.3233/NPM-1370313. [DOI] [PubMed] [Google Scholar]
- 7.Garabedian MJ, Williams CM, Pearce CF, et al. Extreme morbid obesity and labor outcome in nulliparous women at term. Am J Perinatol. 2011;28(09):729–734. doi: 10.1055/s-0031-1280852. [DOI] [PubMed] [Google Scholar]
- 8.Fisher SC, Kim SY, Sharma AJ, et al. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med. 2013;56(6):372–378. doi: 10.1016/j.ypmed.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinkle S, Sharma A, Kim S, et al. Prepregnancy obesity trends among low-income women, United States, 1999–2008. Matern Child Health J. 2012;16(7):1339–1348. doi: 10.1007/s10995-011-0898-2. [DOI] [PubMed] [Google Scholar]
- 10.Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol. 2004;190(4):1091–1097. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 11.Dietz P, Callaghan W, Morrow B, Cogswell M. Population-based assessment of the risk of primary cesarean delivery due to excess prepregnancy weight among nulliparous women delivering term infants. Matern Child Health J. 2005;9(3):237–244. doi: 10.1007/s10995-005-0003-9. [DOI] [PubMed] [Google Scholar]
- 12.Roman H, Goffinet F, Hulsey TF, et al. Maternal body mass index at delivery and risk of caesarean due to dystocia in low risk pregnancies. Acta Obstet Gynecol Scand. 2008;87(2):163–170. doi: 10.1080/00016340701762975. [DOI] [PubMed] [Google Scholar]
- 13.Kyvernitakis I, Köhler C, Schmidt S, et al. Impact of maternal body mass index on the cesarean delivery rate in Germany from 1990 to 2012. J Perinat Med. 2014;43:449–454. doi: 10.1515/jpm-2014-0126. [DOI] [PubMed] [Google Scholar]
- 14.Martin J, Hamilton B, Osterman M, et al. National Vital Statistics Reports. 9. Vol. 62. Hyattsville, Maryland: National Center for Health Statistics; 2013. Births: Final Data for 2012. [PubMed] [Google Scholar]
- 15.Osterman M, Martin J, Curtin S, et al. National Vital Statistics Reports. 4. Vol. 62. Hyattsville, Maryland: National Center for Health Statistics; 2013. Newly Released Data from the Revised U.S. Birth Certificate, 2011. [PubMed] [Google Scholar]
- 16.National Center for Health Statistics. User Guide to the 2012 Natality Public Use File. Hyattsville, Maryland: National Center for Health Statistics; 2013. [Google Scholar]
- 17.American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Safe Prevention of the Primary Cesarean Delivery. Am J Obstet Gynecol. 2014;210(3):179–193. doi: 10.1016/j.ajog.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Menacker F, MacDorman M, Declercq E. Neonatal mortality risk for repeat cesarean compared to vaginal birth after cesarean (VBAC) deliveries in the United States, 1998–2002 birth cohorts. Matern Child Health J. 2010;14(2):147–154. doi: 10.1007/s10995-009-0551-5. [DOI] [PubMed] [Google Scholar]
- 19.ACOG Task Force on Cesarean Delivery. Evaluation of Cesarean Delivery. Washington, DC: American College of Obstetricians and Gynecologists; 2000. [Google Scholar]
- 20.Cáceres IA, Arcaya M, Declercq E, et al. Hospital differences in cesarean deliveries in Massachusetts (US) 2004–2006: The case against case-mix artifact. PLoS ONE. 2013;8(3):e57817. doi: 10.1371/journal.pone.0057817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ford J, Grewal J, Mikolajczyk R, et al. Primary cesarean delivery among parous women in the United States, 1990–2003. Obstet Gynecol. 2008;112(6):1235–1241. doi: 10.1097/AOG.0b013e31818ce092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Getahun D, Strickland D, Lawrence JM, et al. Racial and ethnic disparities in the trends in primary cesarean delivery based on indications. Am J Obstet Gynecol. 2009;201(4):422.e1–422.e7. doi: 10.1016/j.ajog.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 23.Declercq E, Menacker F, MacDorman M. Maternal risk profiles and the primary cesarean rate in the United States, 1991–2002. Am J Public Health. 2006;96(5):867–872. doi: 10.2105/AJPH.2004.052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S, Sappenfield W, Bish C, et al. Reliability and validity of birth certificate prepregnancy weight and height among women enrolled in prenatal WIC Program: Florida, 2005. Matern Child Health J. 2011;15(7):851–859. doi: 10.1007/s10995-009-0544-4. [DOI] [PubMed] [Google Scholar]
- 25.Bodnar LM, Abrams B, Bertolet M, et al. Validity of birth certificate- derived maternal weight data. Paediatr Perinat Epidemiol. 2014;28(3):203–212. doi: 10.1111/ppe.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland E, Moore Simas T, Doyle Curiale D, et al. Self-reported pre-pregnancy weight versus weight measured at first prenatal visit: Effects on categorization of pre-pregnancy body mass index. Matern Child Health J. 2013;17(10):1872–1878. doi: 10.1007/s10995-012-1210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oken E, Taveras EM, Kleinman KP, et al. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196(4):322.e1–322.e8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin J, Wilson E, Osterman M, et al. Assessing the Quality of Medical and Health Data From the 2003 Birth Certificate Revision: Results From Two States. Hyattsville, Maryland: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 29.Zollinger TW, Przybylski MJ, Gamache RE. Reliability of Indiana birth certificate data compared to medical records. Ann Epidemiol. 2006;16(1):1–10. doi: 10.1016/j.annepidem.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Rossignol M, Chaillet N, Boughrassa F, Moutquin J-M. Interrelations between four antepartum obstetric interventions and cesarean delivery in women at low risk: A systematic review and modeling of the cascade of interventions. Birth. 2014;41(1):70–78. doi: 10.1111/birt.12088. [DOI] [PubMed] [Google Scholar]
- 31.Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recommendations & Reports. 2006;55(RR-6):1–23. [PubMed] [Google Scholar]
- 32.Witt W, Wisk L, Cheng E, et al. Determinants of cesarean delivery in the US: A lifecourse approach. Matern Child Health J. 2015;19(1):84–93. doi: 10.1007/s10995-014-1498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu M. Improving maternal and child health across the life course: Where do we go from here? Matern Child Health J. 2014;18(2):339–343. doi: 10.1007/s10995-013-1400-0. [DOI] [PubMed] [Google Scholar]
- 34.Morton SM, Grant CC, Wall CR, et al. Adherence to nutritional guidelines in pregnancy: Evidence from the Growing Up in New Zealand birth cohort study. Public Health Nutr. 2014;17(9):1919–1929. doi: 10.1017/S1368980014000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frey C, Farrell P, Cotton Q, et al. Wisconsin’s Lifecourse Initiative for Healthy Families: Application of the maternal and child health life course perspective through a regional funding initiative. Matern Child Health J. 2014;18(2):413–422. doi: 10.1007/s10995-013-1271-4. [DOI] [PubMed] [Google Scholar]