Abstract
Joint injury often leads to post-traumatic osteoarthritis (PTOA). Acute injury responses to trauma induce production of pro-inflammatory cytokines and catabolic enzymes, which promote chondrocyte apoptosis and degrade cartilage to potentiate PTOA development. Recent studies show that the rate-limiting step for transcriptional activation of injury response genes is controlled by cyclin-dependent kinase 9 (CDK9), and thus it is an attractive target for limiting the injury response. Here, we determined the effects of CDK9 inhibition in suppressing the injury response in mechanically-injured cartilage explants. Bovine cartilage explants were injured by a single compressive load of 30 % strain at 100 %/s, and then treated with the CDK9 inhibitor Flavopiridol. To assess acute injury responses, we measured the mRNA expression of pro-inflammatory cytokines, catabolic enzymes, and apoptotic genes by RT-PCR, and chondrocyte viability and apoptosis by TUNEL staining. For long-term outcome, cartilage matrix degradation was assessed by soluble glycosaminoglycan release, and by determining the mechanical properties with instantaneous and relaxation moduli. Our data showed CDK9 inhibitor markedly reduced injury-induced inflammatory cytokine and catabolic gene expression. CDK9 inhibitor also attenuated chondrocyte apoptosis and reduced cartilage matrix degradation. Lastly, the mechanical properties of the injured explants were preserved by CDK9 inhibitor. Our results provide a temporal profile connecting the chain of events from mechanical impact, acute injury responses, to the subsequent induction of chondrocyte apoptosis and cartilage matrix deterioration. Thus, CDK9 is a potential disease-modifying agent for injury response after knee trauma to prevent or delay PTOA development.
Keywords: CDK9, Flavopiridol, inflammatory cytokines, chondrocytes, cartilage
Introduction
Osteoarthritis (OA) is a disease characterised by progressive articular cartilage degradation and loss of mechanical properties, joint pain and dysfunction (Blumberg et al., 2008; Guilak et al., 2004). Although most OA cases are idiopathic, a major risk factor is traumatic joint injury such as an Anterior Cruciate Ligament (ACL) or meniscus tear. Roughly, half of the people with these types of knee injury will develop post-traumatic osteoarthritis (PTOA) in 5–20 years (Lohmander et al., 2007). Shortly after an injury, an acute injury and inflammatory response is triggered at the cellular level to prevent infection and to initiate healing. However, excessive inflammation can lead to adverse secondary effects, such as cartilage and subchondral bone erosion that is not detected at the time of injury, but becomes apparent a few days later in our mouse model of ACL rupture (Christiansen et al., 2012; Lockwood et al., 2014). Activation of the inflammatory cascade can disrupt joint tissue homeostasis through augmentation of the catabolic response, causing overexpression of extracellular enzymes (Lee et al., 2005), such as the various matrix metalloproteinases and aggrecanases that degrade the cartilage matrix (Imgenberg et al., 2013). In addition, the inflammatory response can also trigger chondrocytes apoptosis (D’Lima et al., 2001; Rosenzweig et al., 2012) that further accelerates cartilage erosion. Therefore, a strategy to prevent excessive inflammation-induced secondary damage after knee injury may prevent or delay the onset of PTOA.
Inflammation is initiated at the cellular level, by the activation of the primary response genes involved in the inflammatory process. Recent advances demonstrate that the rate-limiting step in primary response gene activation is controlled by the general transcription factor cyclin-dependent kinase 9 (CDK9). A unique feature of primary response genes is their instant activation upon stimulation without de novo protein synthesis. In order to achieve instant activation, the basal transcription of primary response genes is already pre-initiated by Ribonucleic Acid (RNA) Polymerase II (Pol II), even in the absence of inflammatory signals. However, only truncated mRNA transcripts are produced, because Pol II is paused shortly after the transcription start site (reviewed in (Zhou and Yik, 2006)). This promoter proximal pausing of Pol II at a basal resting state is currently recognised as a hallmark for all primary response genes (Fowler et al., 2011). Upon inflammatory signal activation, the transcription factor CDK9 is rapidly recruited to the transcription complex, where it phosphorylates Pol II to induce a conformational change that allows Pol II to overcome promoter pausing and continue to produce full-length mRNA transcripts (Zhou and Yik, 2006). Hence, the kinase activity of CDK9 is the rate-limiting step, and a common requirement for the activation of all primary response genes. Thus, CDK9 represents a novel attractive target efficiently and effectively to inhibit the acute inflammatory response, regardless of the source of inflammation.
In a previous report, we highlighted the effectiveness of CDK9 inhibitors in protecting chondrocytes and cartilage explants from the catabolic effects of pro-inflammatory cytokines (Yik et al., 2014). In the presence of exogenously added inflammatory cytokines, we found that the small molecule CDK9 inhibitor Flavopiridol (Wang and Ren, 2010) significantly suppressed the transcriptional activation of inflammatory response genes as well as catabolic genes, resulting in reduced cartilage matrix degradation (Yik et al., 2014). While this study demonstrates the feasibility of targeting CDK9 as a viable strategy for protecting cartilage from exogenously added pro-inflammatory cytokines, the experimental conditions may not accurately represent the physical damage and inflammatory stimulation that the cartilaginous tissues may experience in the event of an actual traumatic knee injury. Various ex vivo impact injury models have been used for studying the effects of mechanical loading on cartilage explants (Borrelli et al., 2003; D’Lima et al., 2001; Imgenberg et al., 2013; Ko et al., 2013; Lee et al., 2005; Nishimuta and Levenston, 2012; Rosenzweig et al., 2012). Mechanical over-loading in cartilage explants can lead to chondrocyte cell death by both necrosis and apoptosis, and cause an inflammatory/catabolic response that damages the cartilage matrix and alters its physical properties (Hembree et al., 2007; Murray et al., 2004). These ex vivo impact injury models are invaluable tools to study the injury response in cartilage, since they recapitulate the physical injury and the subsequent biological response in the cartilage during traumatic knee injury.
In this study, we examined the therapeutic potential of the CDK9 inhibitor Flavopiridol in a single impact injury model with bovine cartilage explants. The ability of Flavopiridol to prevent the activation of the injury-induced inflammatory and catabolic responses, chondrocyte apoptosis, and cartilage matrix degradation was determined.
Materials and Methods
Cartilage explants
Bovine calf (~ 2 months old, n = 40 joints, sex unknown) stifle joints were obtained from a local slaughterhouse (Petaluma, CA) within 1 d of slaughtering. 6–8 cylindrical cartilage explants were harvested from each femoral condyle with a 6 mm biopsy punch inserted perpendicular to the weight bearing area of the articular surface. The explants were then trimmed into ~ 3 mm thickness (with the articular surface intact and the deep layer cut flat) using a custom jig. The explants were washed with phosphate buffered saline and cultured for 24 h in high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10 % foetal bovine serum (Invitrogen), penicillin (1 × 104 units/mL) and streptomycin (1 × 104 μg/mL) at 37 °C, 5 % CO2, and 95 % relative humidity.
Single impact ex vivo injury model
After a 24 h recovery and equilibration period, the cartilage explants were subjected to a single impact mechanical injury. The precise thickness of each individual explant was measured by a calliper before it was placed onto a custom-built unconfined loading chamber, with the articular surface facing upward. A 20 mm diameter stainless steel platen was lowered onto the explant surface to a pre-load of 0.5 N (~ 17.7 kPa) on a hydraulic material testing instrument (Instron 8511.20). All explants including the uninjured controls were subjected to this 0.5 N pre-loading step. To avoid potential variation due to the positional differences from where the explants were harvested on the condyles, two adjacent explants were purposefully matched as a control and injured pair for later comparison. After preloading, the Instron was programmed to deliver a single compression of 30 % strain at 100 %/s, followed by immediate release. After the single impact loading, the explant was sliced in half and weighed. One half of the explant was placed in 3 mL medium and the other half placed in medium containing 300 nM Flavopiridol (Sigma) and cultured for various times (see Fig. 1A). The medium was changed every other day, with fresh Flavopiridol added. The explants and the culturing medium were then subjected to further processing and analysis as described below.
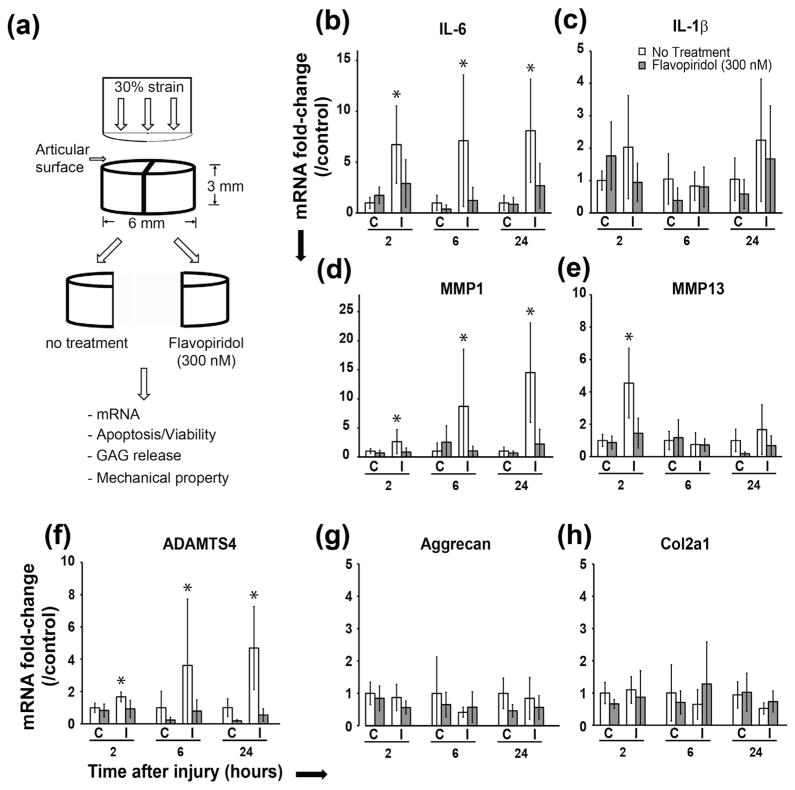
Fig. 1.
CDK9 inhibition prevents injury-induced upregulation of pro-inflammatory cytokine and catabolic genes. A) Schematic representation of the cartilage explant injury and drug treatment, and the subsequent tests. B–F) Suppression of pro-inflammatory cytokine and catabolic enzyme genes by Flavopiridol in cartilage explants (C = control uninjured, I = injured) within 24 h post-injury, G–H) Flavopiridol does not affect the expression of the anabolic genes aggrecan and collagen 2A. All values were the mean ± standard deviation obtained from n = 6 individual donors (* p < 0.05).
Quantitative real-time PCR
At 2, 6 and 24 h post-injury, the explants were frozen in liquid nitrogen and pulverised with a pestle and mortar while frozen. Total RNA was isolated with the miRNeasy Mini Kit (Qiagen) according to the manufacturer’s instruction, with the exception that the RNA was extracted twice with the Qiazol reagents to adequately remove the cartilage matrix constituents. The quantity and quality of the total RNA were determined by a Nanodrop-2000 spectrophometer. 2.5 μg of total RNA from each sample was used for reverse transcription with the SuperScript First-Strand RT kit (Invitrogen). Individual mRNA expression was determined with quantitative real-time PCR performed in triplicates in a 7900HT system (Applied Biosystems). Results were normalised to the 18s rRNA (catalogue no. 4319413E, Applied Biosystems) and calculated as fold-change in mRNA expression relative to control, using the 2−ΔΔCT method. Probes used for individual bovine genes were custom made by Integrated DNA Technologies.
Chondrocyte viability
The live and dead cells in the explants 5 d after mechanical injury were stained using a Live/Dead Viability/Cytotoxicity kit (catalogue no. L3224, Invitrogen), according to the manufacturer’s protocol. The percentages of live and dead cells were determined by counting the cell numbers in 3 random fields of the cross-sectional images of the explants (n = 6 for each sample group) captured using a Nikon TE2000 inverted fluorescence microscope and a 20 × objective.
Staining for apoptotic cells
After 1, 3 and 5 d after injury, the explants were fixed with 4 % paraformaldehyde for 24 h and transferred to 75 % ethanol, followed by sectioning for histological analysis. In situ detection of apoptosis was performed on 5 μm-thick whole explant cross-section using the DeadEnd Fluorometric TUNEL system kit (Promega). This kit measures the fragmented DNA of apoptotic cells by catalytically incorporating fluorescein-12-dUTP at 3′-OH DNA ends using the Terminal Deoxynucleotidyl Transferase recombinant enzyme (rTdT). Nuclei were counter-stained with DAPI. The sections were mounted and examined under a fluorescence microscope. The percentage of apoptotic cells (n = 3 different donors) was determined by counting the number of TUNEL positive cells (green) and calculated as a percentage of the total cells (DAPI). Sections incubated with DNase I were used as positive control while those incubated with buffer only were used as negative control.
GAG release
At 5 d post-injury, the culturing medium was collected and the amount of glycosylaminoglycan (GAG) was determined by the dimethyl-methylene blue (DMMB) colorimetric assay with chondroitin sulphate as the standard. Total GAG released into the medium was calculated and normalised to the wet weight of the explant (determined at the day of injury).
Cartilage mechanical properties
To test if CDK9 inhibition preserves the mechanical properties of cartilage explants after injury, injured and control explants were cultured for 4 weeks in media with or without Flavopiridol. Cartilage sample compressive properties were assessed by stress-relaxation testing in unconfined compression using a mechanical testing system (Instron 5565, Norwood, MA). Prior to testing, a 3 mm diameter, 2 mm thick compression sample was prepared using a dermal biopsy punch, then placed in phosphate buffered saline and centred beneath a 16 mm stainless steel platen. The platen was slowly lowered until a preload of 0.2 N was observed, indicating contact between the platen and the cartilage sample. The sample was then preconditioned by fifteen cycles of 5 % strain. All strains, including the preconditioning, were applied at a strain rate of 10 % per second. Immediately following preconditioning, the sample was subject to 10 % compressive strain; the 10 % strain was held constant and the load recorded for 380 s. At the end of the 10 % strain application, the compressive strain was increased to 20 % and held constant while the load was recorded for an additional 530 s. The compressive properties: instantaneous modulus, relaxation modulus, and coefficient of viscosity, were calculated from the individual stress-relaxation curves using data analysis software (MATLAB R2013a, Natick, MA), according to a standard linear solid model of viscoelasticity as previously described (Allen and Athanasiou, 2006). This mechanical test and model were chosen for their simplicity and accuracy in approximating the viscoelastic behaviour of cartilage. The compressive properties of freshly isolated (day 0) bovine cartilage explants from 6 donors were determined as baseline values for comparison to 4 week post-injury samples.
Statistical analysis
Values of all measurements were expressed as the mean ± standard deviation. Changes in gene expression were analysed by one-way Analysis of Variance (ANOVA) with SPSS 16.0 software. The fold-change in mRNA was used as variables to compare samples between different treatment groups. The least significant difference post-hoc analysis was conducted with a significance level of p < 0.05.
Changes in compressive properties were analysed by one-way ANOVA with Tukey’s post hoc test using JMP Pro software (version 11.2.0) with a significance level of p < 0.05.
Results
CDK9 inhibition suppresses injury-induced proinflammatory and catabolic genes
CDK9 controls the rate-limiting step of inflammatory gene activation (Hargreaves et al., 2009; Zippo et al., 2009) and we have previously shown that in vitro CDK9 inhibition protects chondrocytes and cartilage from the catabolic effects of exogenously added pro-inflammatory cytokines (Yik et al., 2014). However, the effects of CDK9 inhibition on cartilage that receives a direct impact injury, similar to what may happen in a knee injury have not been examined. We hypothesise that CDK9 inhibition in mechanically injured cartilage will prevent an inflammatory response, which in turn will reduce the subsequent deleterious effects on chondrocytes and the cartilage matrix. To test our hypothesis, bovine cartilage explants were mechanically injured by subjecting them to an impact loading at a 30 % strain rate (Fig. 1A). This magnitude of loading induces chondrocyte apoptosis and cartilage matrix degradation (Borrelli et al., 2003; D’Lima et al., 2001; Hembree et al., 2007; Loening et al., 2000; Morel and Quinn, 2004; Rosenzweig et al., 2012; Waters et al., 2014). The injured explants were cultured in the presence or absence of the CDK9 inhibitor Flavopiridol, for various times. The mRNA expression of inflammatory cytokines and catabolic genes induced in the injured explants were determined and compared to uninjured controls. The addition of Flavopiridol reduced the induction of cytokine mRNA by injury (Fig. 1B&C, grey bars). This effect was most pronounced in the expression of IL-6 mRNA, which was significantly induced by injury at all time points tested (Fig. 1B, open bars), and the IL-6 induction was markedly suppressed by Flavopiridol (Fig. 1B, grey bars). Similar trends were observed for IL-1β (Fig. 1C), although this did not reach statistical significance. These data indicate that, as expected, CDK9 inhibition in cartilage explants suppresses inflammatory cytokine induction in response to mechanical injury.
We next examined the injured-induced changes in mRNA expression of the catabolic genes MMP-1 and MMP-13, and ADAMTS4, which are induced by inflammatory cytokines and degrade the cartilage matrix. The results showed that injury induced MMP-1 and ADAMTS4 expression significantly at all time points and MMP-13 at the 2 h time-point (Fig. 1D–F, open bars). Inhibition of CDK9 effectively prevented the induction of these genes in the injured samples at all time points tested (Fig. 1D–F, grey bars). In contrast, the mRNA expression of the anabolic genes Aggrecan and Col2a1 were not affected by injury, whether or not Flavopiridol is present (Fig. 1G–H). Taken together, these results indicate that CDK9 inhibition suppressed injury-induced catabolic mediators of cartilage matrix degradation, while the basal levels of anabolic genes are not affected by injury or CDK9 inhibition at the time points tested.
CDK9 inhibition reduces injury-induced chondrocyte apoptosis
Besides inducing an inflammatory response, mechanical injury also causes chondrocyte apoptosis in cartilage explants (Borrelli et al., 2003; D’Lima et al., 2001; Hembree et al., 2007; Rosenzweig et al., 2012). We therefore investigated the effects of CDK9 inhibition on apoptosis, using our explant injury model. The mRNA expression of three selected genes (P53, Bcl-2 and PTEN) that are central to the apoptotic process were examined in the cartilage explants 24 h post-injury. P53 initiates apoptosis when DNA damage is irreparable (Amaral et al., 2010). Bcl-2 is the founding member of anti-apoptotic factors (Czabotar et al., 2014) and PTEN is a crucial regulator of apoptosis (Zheng et al., 2010). Although mechanical injury itself did not significantly change the mRNA expression of P53, Bcl-2, and PTEN (Fig. 2, open bars), Flavopiridol significantly decreased the expression of these apoptotic mediators in both the injured and uninjured groups (Fig. 2, grey bars).
Fig. 2.
CDK9 inhibition reduces mRNA expression of apoptotic mediators. Flavopiridol suppresses mRNA expression of the pro-apoptotic genes p53, Bcl-2, and PTEN. All values were the mean ± standard deviation obtained from n = 6 individual donors (* p < 0.05).
The reduced basal level of apoptotic mediators has prompted us to determine directly the number of apoptotic chondrocytes in the injured cartilage explants. Cartilage explants from three different donors collected at 1-, 3-, and 5-day post-injury were examined by TUNEL stain and the percentage of apoptotic cells relative to the total number of nuclei in each sample was determined. The results showed that injury increased the percentage of apoptotic chondrocytes to ~ 20 % at 1 d post-injury, and to ~ 60 % 3 d after injury (Fig. 3A). In contrast, CDK9 inhibition significantly reduced the percentage of apoptotic cells in the injured explants in all time points tested (Fig. 3A). These results were further corroborated by the data on live/dead staining of cartilage explants collected 5 d post-injury (Fig. 3B). The live/dead stain showed that injury significantly reduced the number of live chondrocytes from ~ 80 % to ~ 55 % (Fig. 3B, open bars). Flavopiridol treatment enhanced cell survival in the injured explants and did not significantly affect cell survival in uninjured explants (Fig. 3B, grey bars). Taken together, the above results indicate that CDK9 inhibition decreases injury-induced apoptosis in cartilage explants and enhance chondrocyte survival after impact injury.
Fig. 3.
CDK9 inhibition rescues chondrocyte from injury-induced apoptosis. A) Flavopiridol treatment prevents chondrocyte apoptosis. At the indicated times, cartilage explants were processed for histological examination and TUNEL staining. Injury increased apoptosis in cartilage explants but Flavopiridol reduced the apoptotic cell population. Results were the mean ± standard deviation obtained from n = 3 individual donors (* p < 0.05). B) Flavopiridol enhances chondrocyte survival. At 5-days post-injury, cartilage explants were stained with LIVE/DEAD viability stain. Injury decreased cell viability but the cells were rescued by Flavopiridol. Results were the mean ± standard deviation obtained from n = 6 individual donors (* p < 0.05).
CDK9 inhibition prevents injury-induced cartilage matrix degradation
Since injury-induced catabolic response leads to upregulation of matrix degrading enzymes, we next investigated if CDK9 inhibition could prevent cartilage matrix degradation after injury. The cartilage explants were continuously cultured for 5 d in the presence or absence of 300 nM Flavopiridol after injury, the culturing media was collected and the GAG content released into the media was determined as a measure of matrix degradation. GAG release was significantly increased upon injury (Fig. 4, open bars); however, in samples treated with Flavopiridol, GAG release by injured cartilage was not increased above the uninjured control baseline. This result indicates that the CDK9 inhibition attenuates mechanical injury-induced cartilage matrix degradation and proteoglycan release.
Fig. 4.

Protection of cartilage from injury-induced matrix degradation. Mechanically injured cartilage explants were treated for 5 d with Flavopiridol. The amount of GAG released into the medium was measured by dimethylmethylene blue dye binding assay. Results were normalised to the wet weight of the explants. Injury caused cartilage degradation, as indicated by increased GAG release. In the presence of 300 nM Flavopiridol, levels of GAG release returned to baseline. Results were the mean ± standard deviation obtained from n = 6 individual donors (* p < 0.05).
CDK9 inhibition preserves the mechanical properties of cartilage explants after injury
The compressive properties of freshly isolated cartilage were determined from 6 individual donors and their mean values were indicated by the dotted lines in Fig. 5. The compressive properties of the cartilage explants following four weeks of culture demonstrate increased 10 % relaxation modulus, 10 % instantaneous modulus, and 10 % coefficient of viscosity in samples treated with Flavopiridol, when compared to injured, untreated samples (Fig. 5). Similar results were observed for the moduli and coefficients of viscosity calculated from the 20 % strain stress-relaxation curves (not shown). The positive effect of Flavopiridol on compressive properties was observed in both injured and uninjured cartilage samples; no significant difference was observed for any compressive property between injured and uninjured cartilage samples when treated with Flavopiridol. Although injured, untreated cartilage samples exhibited the lowest compressive properties among all groups, there was no significant difference between injured and uninjured cartilage samples in the absence of Flavopiridol. Interestingly, cartilage samples treated with Flavopiridol exhibited greater relaxation moduli than untreated, uninjured controls. Furthermore, injured cartilage samples treated with Flavopiridol also have greater instantaneous moduli and coefficients of viscosity than untreated, uninjured controls. These results indicate that Flavopiridol has a beneficial effect on the compressive properties of cartilage samples cultured in vitro.
Fig. 5.
CDK9 inhibition preserves the mechanical properties of injured cartilage. Cartilage explants were cultured for 4 weeks post-injury. The cartilage compressive properties were assessed by stress-relaxation testing in unconfined compression. The 10 % relaxation and instantaneous moduli, and coefficient of viscosity are shown. Flavopiridol treatment enhanced the compressive properties of both the injured and uninjured explants. Results were the mean ± standard deviation obtained from n = 6 individual donors. Within each chart, means that do not share a letter are significantly different from each other (p < 0.05).
Discussion
This study examined the effects of CDK9 inhibition on the biological and mechanical properties of cartilage explants after injury by compressive loading. This injury model caused a significant induction of pro-inflammatory cytokines and catabolic enzymes (Fig. 1) within the first 24 h. Although apoptotic genes were unchanged within this period (Fig. 2), apoptotic chondrocytes could be detected in the explants at 1 d following injury and peaked after 5 d (Fig. 3). Injury also accelerated cartilage matrix degradation, as measured by GAG release (Fig. 4). However, CDK9 inhibition by Flavopiridol suppressed all those changes and preserved the mechanical properties similar to those of native cartilage (Fig. 5), thus effectively protecting the chondrocytes and the cartilage from the harmful effects of physical injury and the inflammatory response that follows.
A high incidence of OA is associated with traumatic knee injury and hence the term post-traumatic OA (PTOA). Although the pathology of the development of PTOA from injury remains unclear, several lines of evidence point to the involvement of the inflammatory response in this process. Elevated levels of the inflammatory cytokines IL-1β and IL-6 are detected in the human joints within 24 h after an ACL injury (Irie et al., 2003). (Ko et al., 2013) demonstrate that inflammation and the accompanying dysregulated cytokines activities likely contribute to the disruption of the balance between anabolism and catabolism in OA (Goldring and Berenbaum, 2004). In vitro and in vivo studies have implicated pro-inflammatory cytokines, particularly IL-1β, in the destruction of articular cartilage in OA (Goldring and Berenbaum, 2004; Kobayashi et al., 2005). In cartilage, chondrocytes are the main target of pro-inflammatory cytokines, which dysregulate the expression of catabolic and anabolic genes. Cytokine-stimulated chondrocytes produce a variety of matrix-degrading enzymes, like MMP-1, −3, −13 and the aggrecanase ADAMTS-4, −5 (Lee et al., 2005; Nishimuta and Levenston, 2012). All these data implicate the involvement of the inflammatory response in cartilage matrix degradation. Our results in this study strongly indicate that CDK9 inhibition prevents induction of inflammatory and catabolic response genes and thus is a novel target for preventing injury-induced damage.
The influences of mechanical injury on chondrocyte anabolic gene expression are still controversial. It has been reported that Il-1β suppresses the expression of Col2a1 in chondrocytes in vitro (Okazaki et al., 2002). However, other OA studies show that in osteoarthritic cartilage, there is enhanced aggrecan and Col2a1 gene expression and biosynthesis, when compared to normal cartilage (Bau et al., 2002; Hermansson et al., 2004). Our results showed no significant change of anabolic gene expression in injured cartilage in the first 24 hours. This is supported by large-scale expression profiling studies use full-thickness cartilage, which demonstrated that many anabolic genes, including Col2a1, are only enhanced in late-stage OA (Aigner et al., 2006; Ijiri et al., 2008). More importantly, our result demonstrates that Flavopiridol has no adverse side effects on anabolic gene expression in the first 24 h after mechanical injury.
Mechanical injury to cartilage leads to chondrocyte apoptosis. D’Lima et al. (2001) applied a 30 % strain to injure cartilage explants and found 34 % apoptotic chondrocytes in 96 h, compared to only 4 % apoptosis in the control group. Similarly, our explant injury model also leads to chondrocyte apoptosis; we detected ~ 20 % apoptotic chondrocytes in 24 h and more than 60 % apoptotic cells after 72 h (Fig. 3A). Given that injury to cartilage and chondrocyte apoptosis lead to cartilage degradation, the development of drugs designed to block this could be beneficial in preventing PTOA development (Borrelli et al., 2003; D’Lima et al., 2001). For example, when mechanically injured cartilage explants were treated with caspase inhibitors, a 50 % reduction of apoptosis was seen (D’Lima et al., 2001). However, few studies have focused on the correlation between the acute injury response within hours, such as induction of inflammatory cytokine and catabolic genes, and the subsequent apoptosis that followed, usually at several days post-injury. Proinflammatory cytokines like IL-1β and IL-6 have the capacity to activate a diverse array of intracellular signalling pathways, such as c-Jun N-terminal Kinase (JNK), p38 Mitogen-activated Protein Kinase (MAPK) and NF-kB (Goldring et al., 2011), and further induce the expression of various pro-apoptotic genes like p53 (Amaral et al., 2010), BCL-2 (Czabotar et al., 2014) and PTEN (Zheng et al., 2010). In chondrocytes, JNK and p38 signalling pathways are thought to be pro-apoptotic in an injury response (Rosenzweig et al., 2012). Results from this study provide a temporal profile connecting the chain of events that happen after mechanical injury to cartilage, from the induction of inflammatory cytokines and catabolic genes, to the subsequent induction of chondrocyte apoptosis. Importantly, Flavopiridol treatment effectively blocks the initial phase of the injury response, and thus prevents subsequent damage to the cartilage.
In contrast to the anti-apoptotic property in this study, Flavopiridol has been reported to induce apoptosis in many cancer cells. Notably, Flavopiridol was originally known for its anti-proliferation properties by suppressing cell-cycle progression in rapidly dividing cells (e.g. cancers) (Wang and Ren, 2010). This is due to the off-target suppressive effect of Flavopiridol on other CDKs that directly regulate the cell cycle. We believe that mature chondrocytes, which do not normally divide (Kobayashi et al., 2005), are therefore less sensitive to the anti-proliferative effect of Flavopiridol.
Flavopiridol has a beneficial effect on the compressive, viscoelastic properties of injured cartilage explants after four weeks of culture. Specifically, both injured and uninjured cartilage samples treated with Flavopiridol have increased stiffness upon initial compression (instantaneous modulus) and upon reaching equilibrium during prolonged static compression (relaxation modulus) compared to injured-untreated samples. The increased instantaneous and relaxation moduli are likely associated with preservation of glycosaminoglycans (GAG) in the cartilage extracellular matrix (ECM) and of the elastic stiffness of the ECM, respectively. Furthermore, injured samples treated with Flavopiridol exhibit a slower rate of relaxation toward equilibrium following compression compared to injured-untreated samples as evidenced by a greater coefficient of viscosity in the treated samples. An increased coefficient of viscosity is likely associated with retention of GAG in the ECM, which resists movement of water out of cartilage during compression. Overall, treatment with Flavopiridol helps maintain the viscoelastic, compressive properties of cultured cartilage samples close to values observed for uncultured native cartilage samples (Fig. 5). Flavopiridol may preserve cartilage mechanical properties in vivo following injury as well as in vitro during culture.
The lack of difference in the mechanical properties between injured cartilage explants and uninjured control explants was an unexpected finding of this study. Although the injured explants cultured without Flavopiridol had the lowest instantaneous modulus and coefficient of viscosity among all treatment groups, the difference between injured and uninjured samples was not statistically significant (p > 0.05). Cartilage degradation and loss of GAG, known to occur during prolonged culture of immature cartilage (Bian et al., 2010), may have caused the loss of cartilage compressive properties observed in the untreated-uninjured cartilage explants. We suspect that testing samples after shorter culture duration may demonstrate a greater difference between the injured and uninjured cartilage.
In summary, out data demonstrated, for the first time, the effectiveness of CDK9 inhibition in the suppression of pro-inflammatory cytokine induced by mechanical injury and prevention of chondrocyte apoptosis in cartilage explants. In addition, our data strongly indicate that Flavopiridol is an effective agent to prevent cartilage matrix degradation and to preserve its mechanical properties after injury. Thus, CDK9 inhibition by Flavopiridol may provide a new strategy to prevent or delay PTOA after knee joint trauma.
Conclusion
Our data indicate that CDK9 inhibition by Flavopiridol prevents inflammation-induced apoptosis and protects cartilage from the deleterious effects of mechanical injuries. The immediate mechanical damage during an injury event can weaken cartilage and predispose the joint to future OA. Here, we show that CDK9-dependent cell-mediated secondary events initiated by the mechanical impact also contribute to OA-like degradative changes. This, perhaps, suggests that joint injuries should be treated during the acute injury response phase to suppress the secondary cell-mediated damage and alter the trajectory of OA progression. Our results suggest that CDK9 is a viable target to suppress the injury response effectively.
Acknowledgments
This study was supported by an Arthritis Foundation 2012 IRG award to DRH, a DOD PRMRP IIRA award #PR110507 to DRH, R21-AR063348 from NIAMS/NIH to DRH, Departmental Funds to DRH, and the National Natural Science Fund of China (81271971) to ZH and T32 OD 011147 to DDC.
No author received any financial support or other benefits from commercial sources for the work reported on in this manuscript, and no author has any other financial interest that could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
Footnotes
Competing interest
The authors declare that they have no competing interest.
References
- Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, Zien A, Obermayr F, Zimmer R, Bartnik E. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54:3533–3544. doi: 10.1002/art.22174. [DOI] [PubMed] [Google Scholar]
- Allen KD, Athanasiou KA. Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J Biomech. 2006;39:312–322. doi: 10.1016/j.jbiomech.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Amaral JD, Xavier JM, Steer CJ, Rodrigues CM. The role of p53 in apoptosis. Discov Med. 2010;9:145–152. [PubMed] [Google Scholar]
- Bau B, Haag J, Schmid E, Kaiser M, Gebhard PM, Aigner T. Bone morphogenetic protein-mediating receptor-associated Smads as well as common Smad are expressed in human articular chondrocytes but not up-regulated or down-regulated in osteoarthritic cartilage. J Bone Miner Res. 2002;17:2141–2150. doi: 10.1359/jbmr.2002.17.12.2141. [DOI] [PubMed] [Google Scholar]
- Bian L, Stoker AM, Marberry KM, Ateshian GA, Cook JL, Hung CT. Effects of dexamethasone on the functional properties of cartilage explants during long-term culture. Am J Sports Med. 2010;38:78–85. doi: 10.1177/0363546509354197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg TJ, Natoli RM, Athanasiou KA. Effects of doxycycline on articular cartilage GAG release and mechanical properties following impact. Biotechnol Bioeng. 2008;100:506–515. doi: 10.1002/bit.21778. [DOI] [PubMed] [Google Scholar]
- Borrelli J, Jr, Tinsley K, Ricci WM, Burns M, Karl IE, Hotchkiss R. Induction of chondrocyte apoptosis following impact load. J Orthop Trauma. 2003;17:635–641. doi: 10.1097/00005131-200310000-00006. [DOI] [PubMed] [Google Scholar]
- Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JH, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2012;20:773–782. doi: 10.1016/j.joca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- D’Lima DD, Hashimoto S, Chen PC, Colwell CW, Jr, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9:712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- Fowler T, Sen R, Roy AL. Regulation of primary response genes. Mol Cell. 2011;44:348–360. doi: 10.1016/j.molcel.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res. 2004:S37–46. doi: 10.1097/01.blo.0000144484.69656.e4. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzi RM, Marcu KB. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–220. doi: 10.22203/ecm.v021a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, Setton LA, Weinberg JB. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Horng T, Medzhitov R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell. 2009;138:129–145. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree WC, Ward BD, Furman BD, Zura RD, Nichols LA, Guilak F, Olson SA. Viability and apoptosis of human chondrocytes in osteochondral fragments following joint trauma. J Bone Joint Surg Br. 2007;89:1388–1395. doi: 10.1302/0301-620X.89B10.18907. [DOI] [PubMed] [Google Scholar]
- Hermansson M, Sawaji Y, Bolton M, Alexander S, Wallace A, Begum S, Wait R, Saklatvala J. Proteomic analysis of articular cartilage shows increased type II collagen synthesis in osteoarthritis and expression of inhibin betaA (activin A), a regulatory molecule for chondrocytes. J Biol Chem. 2004;279:43514–43521. doi: 10.1074/jbc.M407041200. [DOI] [PubMed] [Google Scholar]
- Ijiri K, Zerbini LF, Peng H, Otu HH, Tsuchimochi K, Otero M, Dragomir C, Walsh N, Bierbaum BE, Mattingly D, van Flandern G, Komiya S, Aigner T, Libermann TA, Goldring MB. Differential expression of GADD45beta in normal and osteoarthritic cartilage: potential role in homeostasis of articular chondrocytes. Arthritis Rheum. 2008;58:2075–2087. doi: 10.1002/art.23504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imgenberg J, Rolauffs B, Grodzinsky AJ, Schunke M, Kurz B. Estrogen reduces mechanical injury-related cell death and proteoglycan degradation in mature articular cartilage independent of the presence of the superficial zone tissue. Osteoarthritis Cartilage. 2013;21:1738–1745. doi: 10.1016/j.joca.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003;10:93–96. doi: 10.1016/s0968-0160(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Ko FC, Dragomir C, Plumb DA, Goldring SR, Wright TM, Goldring MB, van der Meulen MC. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 2013;65:1569–1578. doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52:2386–2395. doi: 10.1002/art.21215. [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Chu BT, Anderson MJ, Haudenschild DR, Christiansen BA. Comparison of loading rate-dependent injury modes in a murine model of post-traumatic osteoarthritis. J Orthop Res. 2014;32:79–88. doi: 10.1002/jor.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening AM, James IE, Levenston ME, Badger AM, Frank EH, Kurz B, Nuttall ME, Hung HH, Blake SM, Grodzinsky AJ, Lark MW. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381:205–212. doi: 10.1006/abbi.2000.1988. [DOI] [PubMed] [Google Scholar]
- Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- Morel V, Quinn TM. Cartilage injury by ramp compression near the gel diffusion rate. J Orthop Res. 2004;22:145–151. doi: 10.1016/S0736-0266(03)00164-5. [DOI] [PubMed] [Google Scholar]
- Murray MM, Zurakowski D, Vrahas MS. The death of articular chondrocytes after intra-articular fracture in humans. J Trauma. 2004;56:128–131. doi: 10.1097/01.TA.0000051934.96670.37. [DOI] [PubMed] [Google Scholar]
- Nishimuta JF, Levenston ME. Response of cartilage and meniscus tissue explants to in vitro compressive overload. Osteoarthritis Cartilage. 2012;20:422–429. doi: 10.1016/j.joca.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K, Li J, Yu H, Fukui N, Sandell LJ. CCAAT/enhancer-binding proteins beta and delta mediate the repression of gene transcription of cartilage-derived retinoic acid-sensitive protein induced by interleukin-1 beta. J Biol Chem. 2002;277:31526–31533. doi: 10.1074/jbc.M202815200. [DOI] [PubMed] [Google Scholar]
- Rosenzweig DH, Djap MJ, Ou SJ, Quinn TM. Mechanical injury of bovine cartilage explants induces depth-dependent, transient changes in MAP kinase activity associated with apoptosis. Osteoarthritis Cartilage. 2012;20:1591–1602. doi: 10.1016/j.joca.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Wang LM, Ren DM. Flavopiridol, the first cyclin-dependent kinase inhibitor: recent advances in combination chemotherapy. Mini Rev Med Chem. 2010;10:1058–1070. doi: 10.2174/1389557511009011058. [DOI] [PubMed] [Google Scholar]
- Waters NP, Stoker AM, Carson WL, Pfeiffer FM, Cook JL. Biomarkers affected by impact velocity and maximum strain of cartilage during injury. J Biomech. 2014;47:3185–3195. doi: 10.1016/j.jbiomech.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Yik JH, Hu Z, Kumari R, Christiansen BA, Haudenschild DR. Cyclin-dependent kinase 9 inhibition protects cartilage from the catabolic effects of proinflammatory cytokines. Arthritis Rheumatol. 2014;66:1537–1546. doi: 10.1002/art.38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Meng X, Wang J, Chen X, Yin D, Liang Y, Song X, Pan S, Jiang H, Liu L. PTEN- and p53-mediated apoptosis and cell cycle arrest by FTY720 in gastric cancer cells and nude mice. J Cell Biochem. 2010;111:218–228. doi: 10.1002/jcb.22691. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Yik JH. The Yin and Yang of P-TEFb regulation: implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol Mol Biol Rev. 2006;70:646–659. doi: 10.1128/MMBR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippo A, Serafini R, Rocchigiani M, Pennacchini S, Krepelova A, Oliviero S. Histone crosstalk between H3S10ph and H4K16ac generates a histone code that mediates transcription elongation. Cell. 2009;138:1122–1136. doi: 10.1016/j.cell.2009.07.031. [DOI] [PubMed] [Google Scholar]