Abstract
Potential drug–drug interactions mediated by the ATP-binding cassette (ABC) transporter and solute carrier (SLC) transporter families are of clinical and regulatory concern. However, the endogenous functions of these drug transporters are not well understood. Discussed here is evidence for the roles of ABC and SLC transporters in the handling of diverse substrates, including metabolites, antioxidants, signalling molecules, hormones, nutrients and neurotransmitters. It is suggested that these transporters may be part of a larger system of remote communication (‘remote sensing and signalling’) between cells, organs, body fluid compartments and perhaps even separate organisms. This broader view may help to clarify disease mechanisms, drug–metabolite interactions and drug effects relevant to diabetes, chronic kidney disease, metabolic syndrome, hypertension, gout, liver disease, neuropsychiatric disorders, inflammatory syndromes and organ injury, as well as prenatal and postnatal development.
What are usually considered to be ‘multispecific drug transporters’ come from two transporter superfamilies: the solute carrier (SLC) transporters and the ATP-binding cassette (ABC) transporters1. Because they have a crucial role in absorption, distribution, metabolism and elimination (ADME), these drug transporters are of considerable pharmacological significance (TABLE 1); indeed, owing to recent regulatory interest, the focus on these transporters has intensified1–6.
Table 1.
Examples of drugs that interact with multiple ABC and SLC drug transporters
| Drug (type of drug) |
Transporter name* |
Refs | |||
|---|---|---|---|---|---|
| ABC | SLC22 | SLCO | SLC47 | ||
| Methotrexate (anticancer, antirheumatic) |
• ABCC1 • ABCC2 • ABCC3 • ABCC4 • ABCC5 • ABCG2 |
• SLC22A6 • SLC22A8 • SLC22A11 |
• SLCO1A2 • SLCO1B3 |
– |
4,30–33, 36,76,77, 230,231 |
|
| |||||
| Acyclovir (antiviral) |
– | • SLC22A1 • SLC22A6 • SLC22A7 • SLC22A8 |
– | • SLC47A1 • SLC47A2 |
4,35,62, 232,233 |
|
| |||||
| Adefovir (antiviral) |
ABCC4 | SLC22A6 | – | – |
4,52, 234–236 |
|
| |||||
| Pravastatin (statin) |
ABCB11 | • SLC22A8 • SLC22A11 |
• SLCO1B1 • SLCO2B1 |
– |
4,55, 237–242 |
|
| |||||
| Rosuvastatin (statin) |
ABCG2 | SLC22A8 | • SLCO1A2 • SLCO1B1 • SLCO1B3 • SLCO2B1 |
– |
4,53, 243–245 |
|
| |||||
| Cimetidine (histamine H2 receptor antagonist) |
– | • SLC22A2 • SLC22A8 |
– | • SLC47A1 • SLC47A2 |
4,55,201, 246–249 |
|
| |||||
| Metformin (antidiabetic) |
– | • SLC22A1 • SLC22A2 • SLC22A3 |
– | • SLC47A1 • SLC47A2 |
4,41,42, 249–252 |
‘–’, not available, not applicable or insufficient information; ABC, ATP-binding cassette; SLC, solute carrier; SLCO, solute carrier organic anion.
The common names of the transporters in the table are shown in brackets in the following list: ABCC1 (MRP1); ABCC2 (MRP2); ABCC3 (MRP3); ABCC4 (MRP4); ABCC5 (MRP5); ABCB11 (BSEP); ABCG2 (BCRP); SLC22A1 (OCT1); SLC22A2 (OCT2); SLC22A3 (OCT3); SLC22A6 (OAT1); SLC22A8 (OAT3); SLC22A11 (OAT4); SLCO1A2 (OATP1A2); SLCO1B1 (OATP1B1); SLCO1B3 (OATP1B3); SLCO2B1 (OATP2B1); SLC47A1 (MATE1); SLC47A2 (MATE2).
Among the clinical issues driving this heightened focus on drug transporters is the concern about drug–drug interactions at the level of the transporter5,6. Drugs can potentially compete with each other for binding to the transporter, thereby leading to unexpected changes in serum and tissue drug levels and possible toxic side effects. The rationale for this concern is based on in vitro, ex vivo and in vivo laboratory studies, as well as clinical data.
The drug transporters that have so far received greatest attention are two ABC transporters (namely ABCB1 (also known as P-glycoprotein (PGP) or MDR1) and ABCG2 (also known as BCRP)) and five SLC transporters (namely SLC22A6 (also known as OAT1 or NKT), SLC22A8 (also known as OAT3 or ROCT), SLC22A2 (also known as OCT2), SLCO1B1 (also known as OATP1B1) and SLCO1B3 (also known as OATP1B3)). More recently, it has been suggested that the list should be expanded to include several other less-studied drug transporters, such as members of the SLC47 family (also known as multidrug and toxin extrusion proteins (MATEs))2,6 (FIGS 1,2). Thus, it is likely that academia and industry will continue to study SLC and ABC transporters in the context of their roles in ADME.
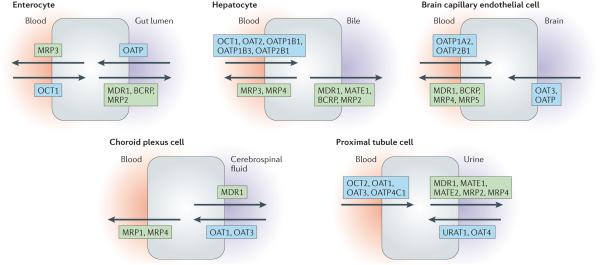
Figure 1. SLC and ABC drug transporters are expressed in most epithelial barriers.
Barrier epithelia play a major part in homeostasis by regulating the transcellular movement of solutes between body fluid compartments (for instance, between blood and urine, blood and cerebrospinal fluid, or blood and bile). Members of the solute carrier (SLC) and ATP-binding cassette (ABC) transporter families have been found in a variety of barrier epithelial cells (as well as in other cell types), where they regulate the movement of small-molecule xenobiotics (such as drugs and toxins) and endogenous metabolites into and out of the various tissues and fluid compartments. Examples of the arrangement of drug transporters within several different barrier epithelial cell types are depicted here. These transporters maintain homeostasis and mediate processes important for pharmacokinetics and toxicokinetics. Although the subsets of drug transporters that are expressed vary depending on the tissue, the movement of small-molecule substrates between body fluid compartments requires the activity of both SLC (generally influx) and ABC (efflux) transporters. Selected members of the SLC family (namely, organic anion transporters (OATs), organic anion-transporting polypeptides (OATPs), organic cation transporters (OCTs), organic cation/carnitine transporters (OCTNs) and multidrug and toxin extrusion proteins (MATEs)) are shown in blue boxes, and members of the ABC family (multidrug-resistance proteins (MDRs), breast cancer-resistance protein (BCRP) and multidrug-resistance-associated proteins (MRPs)) are shown in green boxes. In certain cases, specific tissue expression has only been clearly established in humans or mice, and localization to apical or basolateral surfaces remains tentative. Note that in this figure, the common names of the transporters are used; for formal designations, please refer to the main text, tables and/or FIG. 2. Many other SLC and ABC drug transporters and their relatives are also expressed in these tissues but are not shown here.
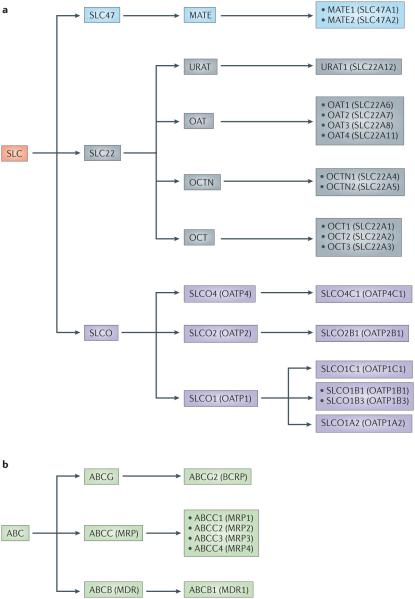
Figure 2. SLC and ABC drug transporters that have been implicated in the handling of xenobiotics and drugs.
Members of the solute carrier (SLC) and ATP-binding cassette (ABC) transporter families with roles in the absorption, disposition and elimination of xenobiotics and drugs have formal SLC and ABC designations as well as other designations that are commonly used in the field. The designations of many SLC and ABC drug transporters discussed in this article are clarified in this diagram. Within the SLC superfamily, members of the SLCO (also known as OATP and SLC21), SLC22 and SLC47 families have been shown to have key roles in drug transport. Transporters in the SLC22 family that are implicated in drug transport include members of the organic cation transporter (OCT), organic cation/carnitine transporters (OCTN) and organic anion transporter (OAT) families, as well as the uric acid transporter, URAT1 (SLC22A12). The SLC47 family has two members — SLC47A1 (also known as MATE1) and SLC47A2 (also known as MATE2, which has a kidney isoform MATE2K) — that have been implicated in drug transport. ABC transporters that have been implicated in drug handling can be divided into three subfamilies — namely, ABCB1 (also known as P-glycoprotein (PGP) or MDR1), the multidrug resistance-associated proteins (MRPs) of the ABCC family, and the breast cancer-resistance protein (BCRP; also known as ABCG2). Other transporters have also been implicated in drug transport but are not discussed in this article1.
However, the endogenous roles of these transporters have received much less attention. After briefly illustrating the importance of selected transporters in drug transport, this article discusses a growing body of data pertaining to their endogenous functions, including evidence of their roles in regulating the transport of rate-limiting metabolites, antioxidants and signalling molecules; the evolutionary conservation of the transporters across a wide range of organisms (BOX 1); their role in morphogenesis and organ development (BOX 2); and their potential role in remote communication between organs and organisms (BOX 3). Understanding what drug transporters really do could be important for appropriately addressing the possibility of drug–metabolite interactions in drug discovery and drug development, as well as for clarifying disease mechanisms.
Box 1. Evolutionary conservation and clustering of drug transporters.
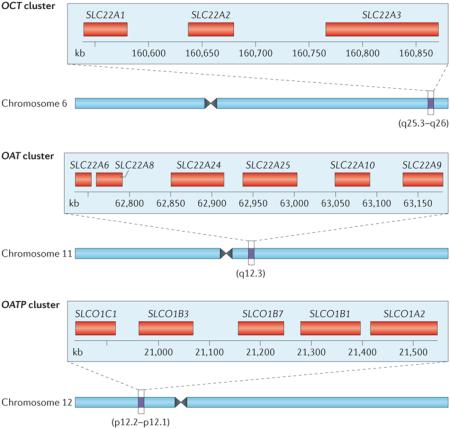
Many solute carrier (SLC) and ATP-binding cassette (ABC) transporters are conserved evolutionarily, although further back in phylogeny it is often difficult to assign the particular orthologue212. In bacteria, the main role of the closest, albeit still very distant, relatives of mammalian drug transporters seems to be the transport of nutrients, metabolites and toxic substances213. Among what are termed multidrug-resistance transporters, certain bacterial transporters, such as NorM, seem to be structurally and functionally homologous to their mammalian SLC counterparts, the SLC47 family of multidrug and toxin extrusion proteins (MATEs)214. In mammals, many genes for SLC and ABC subfamily members are found in pairs and clusters215,216, which presumably arose through gene duplication216. For example, among the genes of SLC drug transporters of current regulatory interest, those that encode organic cation transporters (OCTs), organic anion transporters (OATs) and organic anion transporting polypeptides (OATPs) exist in gene clusters12,215,217.
The figure shows a three-member OCT cluster (top panel), a six-member OAT cluster (middle panel) and a five-member OATP cluster (bottom panel) in humans. Beneath each cluster is a schematic representation of the location of the cluster on the corresponding human chromosome217. In the mouse genome, certain duplications in the OAT cluster — which consists of eight members — seem to be evolutionarily recent216, raising the possibility of active selection. The clustering has also been suggested to relate to the coordinated expression of transporter genes in close proximity within the same tissue (for example, within the kidney, choroid plexus or liver)215. Nevertheless, some genes in the OCT, OAT and OATP clusters are co-expressed in certain tissues, whereas in other tissues this is not so (FIG. 1; TABLE 3). Thus, if there is a relationship between the gene clustering and tissue expression patterns, it may not be straightforward.
Box 2. Developmental roles of SLC and ABC drug transporters.
Because of the focus on the pharmacological and toxicological roles of solute carrier (SLC) and ATP-binding cassette (ABC) transporters in mature mammalian organs, less attention has been given to their developmental expression patterns. These patterns were noted early on46, and there is high expression of a number of these transporters in developing neural and other tissues, which changes considerably during organ development21,56,218. Such observations have raised the possibility that drug transporters play a part in mammalian morphogenesis56. Given the ability of drug transporters to transport cyclic nucleotides, prostaglandins and other molecules of potential morphogenetic importance, their role in mammalian morphogenesis is worthy of further study. In Drosophila, the ABC transporter gene mdr49 is essential for early development219. Certain fly SLC22 homologues have been speculated to play a part in development as well220. In the sea urchin, ABC transporters have also been suggested to have a developmental role206, whereas in plants, ABC transporters with identified small-molecule substrates (that are similar in structure to those transported by mammalian ABC and SLC drug transporters) have an important role in development and physiology (for example, in auxin transport)189–191,221. ABC transporters also seem to be of developmental importance in Dictyostelium222. Thus, in non-mammalian model organisms, there is accumulating evidence for a developmental role for drug transporter-like genes, possibly resulting from the transport of small-molecule and/or peptide morphogens.
Moreover, as the placenta expresses many drug transporters223, it is probable that maternal small molecules, of physiological as well as toxicological significance, cross the maternal–fetal barrier in mammals via a set of SLC and ABC transporters. Furthermore, it is at least conceivable that a different set of drug transporters that is transiently expressed in developing tissues can take up and/or extrude these molecules. This could be important in maternal–fetal small-molecule-mediated communication and may represent a mechanism by which toxins (including certain drugs) to which the mother is exposed have developmental consequences for the fetus.29,56
During postnatal development, there is another phenomenon that is potentially relevant to xenobiotic handling in young children. Several drug transporters in the kidney, together with those in the hepatobiliary system, are responsible for the elimination of endogenous and exogenous toxins — including drugs that are given to newborns — that are modified by hepatic drug-metabolizing enzymes (for instance, by sulphation or glucuronidation)185,224. In developing animal organs such as the kidney and liver, the expression of drug transporters and drug-metabolizing enzymes is relatively low at birth and then increases considerably during the postnatal period and through juvenile stages21,29,225–227.
Low drug transporter expression may be a particularly important issue in premature infants29,224. It may be that there is some type of remote communication between the liver and kidney to ensure that the neonatal kidney can excrete potentially toxic metabolites produced by hepatic drug-metabolizing enzymes. Earlier physiological studies indicated that there may be a postnatal ‘developmental window’ of regulation for renal drug elimination207. There is evidence for substrate inducibility and hormone-mediated regulation of postnatal drug transporter function21,29,207,228. Delineation of these physiological mechanisms could be helpful for designing therapeutic approaches to enhance organ maturation in premature infants; for example, by using small molecules that can induce drug transporter expression in the kidney, liver and biliary tract.
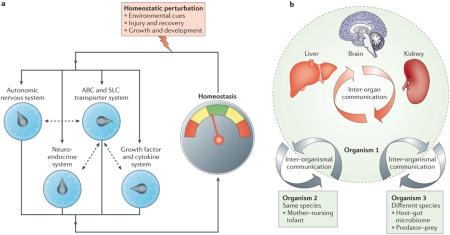
Box 3. Remote sensing and signalling by ABC and SLC drug transporters.
The ‘remote sensing and signalling’ hypothesis proposes that ATP-binding cassette (ABC) and solute carrier (SLC) drug transporters that are expressed in different cells and tissues — particularly those interfacing with body fluids — help coordinate the efflux and influx of small molecules involved in signalling, the regulation of cellular redox and nutritional states, and the rate-limiting or key steps in metabolism7,14,50. The hypothesis implies that a ‘sensing’ mechanism exists to enable the active regulation during normal physiology of ABC and SLC transporter expression and/or function in different cells and tissues, thereby fostering remote communication via small molecules between different cells and tissues. This remote sensing and signalling system has loose similarities with and works in parallel with other well-studied regulatory systems that are centred around hormones, cytokines and growth factors (see the figure, part a; dashed arrows indicate crosstalk between the SLC and ABC transporter system and the other systems; for simplicity, the crosstalk between the other systems is not shown).
Similar to these systems, the remote sensing and signalling system consisting of ABC and SLC drug transporters is proposed to have a complex organization and emergent properties, particularly after perturbation. In this latter scenario, the perturbed state is sensed as a result of metabolic, nutritional or redox alterations; this leads to changes in sets of ABC and SLC drug transporters in different tissues through altered activity and/or expression levels and thereby helps to restore tissue function and normal systemic physiology (including normal remote communication). Notably, the action of growth factors, cytokines and neuroendocrine influences on the regulation of drug transporters may be particularly important in acute and chronic disease states. Consequently, all of these regulatory systems are both parallel and interacting systems.
In this way, levels of small molecules involved in signalling and metabolism — such as prostaglandins, cyclic nucleotides, G protein-coupled receptor ligands, antioxidants, hormones, nutrients, rate-limiting small metabolites and nuclear receptor ligands — can be actively regulated in distinct cells, tissues and body fluids, thereby effecting both local and global physiological alterations. The multispecificity of ABC and SLC transporters, as well as their differential and modifiable tissue expression and/or trafficking profiles, may provide flexibility for restoring homeostasis after injury to a particular organ and in other dynamic settings, such as during organ development7,14,56,96,198 (BOX 2).
More detailed discussions of the remote sensing and signalling hypothesis and/or potential examples (mostly related to organic anion transporters (OATs)), have been provided elsewhere7,11,14,34,50,96,198,204,229. In addition to inter-organ communication, this hypothesis also emphasizes the role of ABC and SLC drug transporters and their close relatives in inter-organismal communication (see the figure, part b), such as the regulated, drug-transporter-mediated movement of small molecules across the intestine, enabling communication of the body with the gut microbiome61,159. These transporters and/or their close relatives also regulate the movement of volatile odorants into the urine, as well as the movement of key metabolites and/or signalling molecules into breast milk or across the placental barrier7,14,98,112,116,223. Information, in the form of small molecules that reflect (and affect) cell states and/or tissue physiology, can therefore be transmitted to other cells, organs and/or other organisms of the same or different species via transported substrates (see the figure, part b).
SLC and ABC drug transporters
SLC drug transporters do not rely directly on ATP hydrolysis and are largely, but not exclusively, uptake transporters; that is, they are generally involved in the uptake of small molecules into cells7–14 (FIG. 1). By contrast, ABC drug transporters utilize ATP hydrolysis and function as efflux transporters1,4,8,9,15–18 (FIG. 1). Various isoforms of SLC and ABC drug transporters are highly expressed in epithelial cells (on apical and basolateral surfaces) that separate nearly all body fluid compartments (including those containing urine, cerebrospinal fluid or bile), as well as in brain endothelial and other cells, such as circulating cells in the blood1,3,4,7–17,19–23 (FIG. 1).
Many recent articles have provided summaries, often in tabular form, of the substrate specificities of the principal drug transporter families4,7–10,12–14,16,20,24–29. A key concept emerging from examining these summaries is the overlapping substrate specificities of individual drug transporters within the SLC and ABC transporter families, and also among different members of these two superfamilies (TABLE 1). For instance, SLC22A6 (OAT1) and SLC22A8 (OAT3) share many common organic anion substrates, as do SLC22A2 (OCT2) and SLC47A2 (also known as MATE2) for organic cations (TABLE 1). In addition, there is overlap in substrate specificity among the SLC22, the SLCO (also known as OATP or SLC21) and the ABC subfamily C (ABCC; also known as MRP) transporters. Nevertheless, the academic fields of SLC drug transporters and ABC drug transporters have tended to be quite separate. This is often true even of families within the SLC superfamily (such as SLC22 and SLCO) and even within sub-families such as the organic anion transporters (OATs), the organic cation transporters (OCTs) and the organic cation/carnitine transporters (OCTNs), which all belong to the SLC22 family. Sometimes, the focus is on a single transporter. For instance, owing to its early discovery and relevance to resistance to cancer chemotherapy, the ABC drug transporter ABCB1 (P-glycoprotein; MDR1) is one of the best-studied mammalian transporters, from the atomic level to clinical populations18–20. Furthermore, drug transporter research has historically focused on a few epithelial tissues, such as the liver, kidney and intestine; however, with the discovery of drug transporters in the olfactory epithelia, choroid plexus, blood–brain barrier, placenta, retina, testes, mammary glands and many other tissues, this focus is changing.
The aforementioned factors can make it challenging to define the most physiologically relevant transport pathway or pathways for a particular drug. For example, methotrexate can be transported by multiple SLC and ABC transporters of different families with different tissue expression patterns, such as SLC22A6 and SLC22A8 from the SLC22 family, SLCO1B3 from the SLCO family, and ABCG2 and ABCC transporters from the ABC superfamily30–33 (TABLE 1). This picture is typical of many common drugs, including statins and antivirals4,12,13,17,34,35 (TABLE 1). In some cases, however, there are marked differences in the affinities of the substrate for different transporters, in addition to their relatively distinct tissue expression patterns. Knockout studies and certain human clinical phenotypes have helped to further clarify the relative importance of particular transporters for a given drug or toxin33,36–43. For instance, it seems that the principal diuretic transporters that work in parallel on the basolateral side (in contact with the blood) of kidney proximal tubule cells are SLC22A6 (OAT1) and SLC22A8 (OAT3)38–40, and the main transporters in kidney proximal tubule cells that work in series for net excretion of the antidiabetic drug metformin seem to be SLC22A2 (OCT2; on the basolateral surface) and a MATE transporter (on the apical surface)41–43.
In vitro, in vivo and clinical data indicate that drug transporters handle a diverse set of drugs and toxins
Before discussing recent data regarding the endogenous physiological roles of drug transporters, it is worth considering the large amount of data on their role in drug and toxin transport. In recent years, studies in knockout mice and clinical populations have supported this role, which previously had mostly been based on in vitro transport studies. Here, two well-studied SLC transporters, SLC22A6 (OAT1) and SLC22A8 (OAT3), are used to illustrate the types of pharmacological and toxicological data now available for clinically important drug transporters.
SLC22A6 (OAT1) is the prototypical member of the OAT subfamily of SLC22 transporters. Before the cloning of this transporter as NKT in 1996 (REFS 44–46), there was more than 50 years’ worth of rich physiological literature relating to processes mediated by this transporter, which was operationally defined as the para-aminohippurate (PAH) transporter and/or the probenecid-sensitive organic anion transporter47–50. Although SLC22A6 is also present in the choroid plexus and olfactory mucosa, most research has focused on its role in the kidney.
Over the past 20 years, considerable evidence from transfected cell lines or Xenopus oocytes that overexpress SLC22A6 (OAT1) indicate that this transporter can bind and/or transport various small anionic drugs (as well as toxins and metabolites), including many antibiotics, nonsteroidal anti-inflammatory drugs, antivirals, diuretics, statins, antihypertensives and chemotherapeutic agents13,35,40,51–62. Recent studies in Slc22a6 (Oat1)-knockout mice, or in tissues derived from such mice, have shown impaired handling of many of these compounds, as well as of environmental toxins35,38,40,61–63. For example, mercury, which can cause kidney and nervous system toxicity and remains a major environmental concern, is thought to be conjugated in vivo to cysteine (among other molecules); it is effectively ‘handled’ by SLC22A6 in a manner similar to an organic anion57,63,64. The Slc22a6 (Oat1)-knockout mouse kidney is notably protected from mercury toxicity because mercury conjugates cannot enter kidney proximal tubule cells from the blood side, owing to the lack of SLC22A6 in these animals63. Perfluorinated carboxylates are other examples of environmental toxins that are handled by SLC22A6 (REF. 65).
There is a similar concordance of in vitro and in vivo data with the other major transporter of organic anionic drugs and toxins, SLC22A8 (OAT3), which is closely related to SLC22A6 (OAT1)66. Apart from its role in drug elimination, SLC22A8 is important for the renal handling of aristolochic acid, a dietary toxin that is thought to cause a type of chronic kidney disease as a result of SLC22A8-mediated uptake by kidney proximal tubule cells67. Although the Slc22a8 (Oat3)-knockout mouse appears healthy, it has low blood pressure, a finding that has prompted a closer look at salt handling, neuroendocrine and metabolic changes, which might affect blood pressure68. SLC22A6 and SLC22A8 have overlapping substrate preferences. It is also worth noting that although SLC22A6 and SLC22A8 are categorized as OATs, they are closely related to SLC22A1 (also known as OCT1) and SLC22A2 (OCT2) — which are members of the OCT subfamily of SLC22 transporters — and can transport some organic cations51. This may be physiologically important for molecules such as creatinine, which is widely used in the clinic as a marker of renal function69 and is transported by OCTs, MATEs and OATs69–73.
The human relevance of many of these findings is becoming clear. For years, the drug probenecid, which competitively inhibits SLC22A6 (OAT1) and SLC22A8 (OAT3) and, to a lesser extent, other drug transporters, has been used to increase the half-life of antibiotics, such as penicillin48 and, more recently, that of antiviral drugs74. As many drugs can bind SLC22A6 (OAT1) and SLC22A8 (OAT3), there has been concern about drug–drug interactions owing to competition at the level of the transporter; this is thought to be one possible explanation for the rare but potentially severe toxicity of methotrexate (a drug used in chemotherapy and rheumatological disease) when nonsteroidal anti-inflammatory drugs are concomitantly administered5,31,75–77.
In addition, coding or non-coding single-nucleotide polymorphisms (SNPs) that result in clinical phenotypes have been reported for SLC22A6 and SLC22A8 (REFS 39,64,78) (TABLE 2). Although more extensive phenotypic analyses of the effects of OAT SNPs needs to be performed, these limited studies seem to be consistent with the in vitro and in vivo knockout data on their role in drug and toxin handling. For example, polymorphisms in SLC22A6 (OAT1) and/or SLC22A8 (OAT3) have been associated with mercury toxicity64, response to diuretics and antibiotic-handling capacity39,78. Nevertheless, given their obvious importance in drug handling, it is somewhat surprising that more evidence has not yet emerged for SNPs in SLC22A6 and SLC22A8 that affect drug disposition.
Table 2.
Established or potential clinical associations with drug transporter genes*
| Gene name (alternative name) |
Association | Refs |
|---|---|---|
| SLC22A2 (OCT2) | Metformin concentration, platinum-based drug toxicity |
41,42, 253,254 |
|
| ||
| SLC22A4 (OCTN1) | Inflammatory disease | 149-151 |
|
| ||
| SLC22A5 (OCTN2) | Systemic carnitine deficiency, inflammatory disease |
146,147, 150,151 |
|
| ||
| SLC22A6 (OAT1) | Mercury concentration, diuretic response | 39,64 |
|
| ||
| SLC22A8 (OAT3) | Mercury concentration, diuretic response, antibiotic handling |
39,64,78 |
|
| ||
| SLC22A12 (URAT1) | Hypouricaemia, hyperuricaemia | 101,132 |
|
| ||
| SLC47A1 (MATE1) | Metformin concentration | 42,43 |
|
| ||
| SLCO1B1 (OATP1B1) | Hyperbilirubinaemia, statin-induced myopathy | 80 – 84 |
|
| ||
| ABCB1 (MDR1) | Chemotherapy resistance, inflammatory bowel disease | 86,255,256 |
|
| ||
| ABCC2 (MRP2) | Dubin–Johnson syndrome | 154 |
|
| ||
| ABCG2 (BCRP) | Chemotherapy resistance, hyperuricaemia | 86,87,132 |
Includes data from the Online Mendelian Inheritance in Man (OMIM) Database (2014).
More striking results have been obtained with respect to human SNPs in genes encoding other SLC transporters. Similar to OATs, SLCO (OATP) transporters are involved in the uptake of many organic anions in the liver, kidney and other tissues12,79. SNPs in SLCO1B1 (also known as OATP1B1) are thought to cause altered handling of cholesterol-lowering statins, leading to statin-induced myopathy79–82 as well as hyperbilirubinaemia83,84. SLC22A2 (OCT2), SLC47A1 (also known as MATE1) and SLC47A2 (also known MATE2, which has a kidney isoform MATE2K) are the transporters that seem to be responsible for much of the kidney transepithelial transport of organic cations — including the anti-diabetic agent metformin — from the blood to the urine; indeed, SNPs in genes encoding these transporters are associated with altered elimination of metformin41–43,85.
Decades of research on tumour resistance mechanisms have uncovered several mutations in the genes encoding the ABC efflux transporters ABCB1 (PGP; MDR1) and ABCG2 (BCRP); notably, mutations in these genes serve as some of the primary explanatory factors for the resistance of certain tumours to chemotherapeutic agents86,87. Polymorphisms in genes encoding other ABC efflux drug transporters, such as various members of the ABCC (MRP) family, have also been found to affect the handling of drugs88–90.
Many of the substrates of ABCC (MRP) and other ABC drug transporters overlap with those handled by SLC transporters such as OATs, OCTs, OATPs (SLCOs) and MATEs (TABLE 1). This may not be surprising when one considers what is generally believed regarding the physiological basis of net drug transport across polarized epithelial cells (FIG. 1). For instance, in a kidney proximal tubule cell, net movement of an organic anionic drug from the blood side (the basolateral surface) to the urinary side (the apical or luminal surface) is thought to involve the basolateral influx transporters SLC22A6 (OAT1) and/or SLC22A8 (OAT3), the apical efflux ABC transporters ABCC2 (also known as MRP2) and/or ABCC4 (also known as MRP4), and probably other apical transporters7,14,27,29,50,91. Although it will be important in the future to trace the fate of individual small molecules as they move from the blood via basolateral transporters on the plasma membrane, through the cytosol and subcellular compartments, and then into the urine via apical membrane transporters, this overall view is supported by a wide variety of biochemical and physiological data.
For other polarized epithelia (for instance, intestinal, hepatic, biliary, mammary, placental and retinal epithelia), a similar picture of SLC-mediated influx and ABC-mediated efflux is usually presented. Nevertheless, it is worth reiterating that different sets of SLC and ABC family members, each with distinct substrate preferences and distinct regulation, predominate in different tissues1; unravelling the impact of this complexity on the ability of tissues to handle various substrates in normal and pathological conditions is a major task for the field3 (FIG. 1; TABLE 3). Furthermore, given the frequent occurrence of overlapping substrate specificities — and the fact that functionally important coding-region SNPs in genes encoding members of some transporter families may not be common (and may perhaps only subtly affect function) — it might be most fruitful to focus on combinations of coding-region SNPs in genes encoding transporters that work in parallel (for example, SLC22A6 and SLC22A8, or SLCO1B1 and SLCO1B3) or in series (for example, OATs and/or SLCOs and ABCC transporters, or OCTs and MATEs)50,92. Furthermore, given the paucity of non-synonymous coding-region SNPs, as well as the clustering of genes for many drug transporters with overlapping specificity in the genome (BOX 1) — which may potentially lead to a degree of coordinated regulation — the study of non-coding regulatory-region SNPs may be particularly important to understand the differences in drug handling22,93–95.
Table 3.
The SLC22 transporter genes
| Gene name | Common name(s) | Tissue distribution | Examples of interacting compounds |
|---|---|---|---|
| SLC22A1 | OCT1 | Liver, small intestine | Cationic drugs, polyamines |
|
| |||
| SLC22A2 | OCT2 | Kidney, small intestine |
Cationic drugs, acetylcholine, dopamine |
|
| |||
| SLC22A3 | OCT3 | Heart, skeletal muscle, CNS |
Cationic drugs, epinephrine |
|
| |||
| SLC22A4 | OCTN1 | Kidney, intestine | Carnitine, acetylcholine |
|
| |||
| SLC22A5 | OCTN2 | Skeletal muscle, kidney |
Carnitine, choline |
|
| |||
| SLC22A6 | OAT1 | Kidney, choroid plexus |
Anionic drugs, α-ketoglutarate, indoxyl sulphate |
|
| |||
| SLC22A7 | OAT2 | Liver, kidney | Anionic drugs, cyclic GMP |
|
| |||
| SLC22A8 | OAT3 | Kidney, choroid plexus, testes |
Anionic drugs, oestrone sulphate, flavonoids |
|
| |||
| SLC22A9 | OAT7 | Liver | Oestrone sulphate |
|
| |||
| SLC22A10 | – | Liver | – |
|
| |||
| SLC22A11 | OAT4 | Placenta, kidney | Urate, oestrone sulphate |
|
| |||
| SLC22A12 | URAT1 and RST | Kidney | Urate |
|
| |||
| SLC22A13 | OAT10 | Kidney, brain, colon | Organic anions, urate |
|
| |||
| SLC22A14 | OCTL2 | Kidney, colon | – |
|
| |||
| SLC22A15 | FLIPT1 | Kidney, brain, liver | – |
|
| |||
| SLC22A16 | FLIPT2, CT2 and OCT6 | Testes | Carnitine |
|
| |||
| SLC22A17 | BOIT and BOCT1 | Brain, choroid plexus | – |
|
| |||
| SLC22A18 | BWR1A and IMPT1 | Kidney | – |
|
| |||
| Slc22a19 | Oat5 | – | Oestrone sulphate |
|
| |||
| SLC22A20 | OAT6 | Olfactory | Odorants |
|
| |||
| Slc22a21 | Octn3 | – | – |
|
| |||
| Slc22a22 | Oatpg | – | Prostaglandins |
|
| |||
| SLC22A23 | – | Brain, liver | – |
|
| |||
| SLC22A24 | – | – | – |
|
| |||
| SLC22A25 | UST6 | Liver | – |
|
| |||
| SLC22A31 | – | Larynx, prostate | – |
Endogenous roles of drug transporters
The data discussed in the previous section highlight the pharmacological and toxicological importance of the many SLC and ABC ‘drug’ transporters that are expressed differently in epithelial and non-epithelial tissues throughout the body (FIG. 1). However, there is a growing body of recent data indicating that an endogenous function of these transporters is to regulate the transport of metabolites, nutrients, anti-oxidants, microbiome products, bile salts, neurotransmitters, other neuro-active molecules, hormones and signalling molecules. These data suggest the need for another perspective on what SLC and ABC drug transporters do. According to this viewpoint, the pharmaceutical and toxicological importance of drug transporters, and the justifiable bias of publications towards substrates of greater clinical interest, has meant that their physiological roles have received less attention7,14,34,50,96. The physiological roles of SLC and ABC drug transporters are the focus of the remainder of this article.
It should also be noted that research on these two aspects of transporter function does not need to be considered as mutually exclusive. Understanding the movement of endogenous molecules between tissues and body fluid compartments via individual SLC and ABC drug transporters could lead to new therapeutic approaches. This understanding might, for instance, have ramifications for selectively altering drug half-lives in particular tissues or body fluids (as a result of designing inhibitors of drug transporters61) or for tissue-specific targeting of drugs (by mimicking certain endogenous substrates). Moreover, although there is growing interest in drug–drug interactions at the level of the transporter, one can make similar arguments regarding drug–metabolite interactions, drug–toxin interactions, drug–nutrient interactions, exogenous-metabolite–endogenous-metabolite interactions, and toxin–metabolite interactions. Indeed, there is growing clinical concern about the metabolic and developmental consequences of long-term drug therapy in children29. Some of these metabolic and developmental effects may be due to competition among drugs and physiologically important metabolites, nutrients, antioxidants, hormones and/or signalling molecules for binding sites at the level of the transporter. The same may be true for environmental toxins.
The SLC22 family as example SLC transporters
Currently, a major focus of pharmacological and toxicological research on SLC transporters is on three families: SLCO (OATP), SLC22 (including OATs, OCTs and OCTNs) and SLC47 (MATEs) (FIG. 2). Here, we examine one family, SLC22, with particular emphasis on their role in the transport of metabolite and signalling molecules rather than of drugs and toxins. This SLC family, which consists of over 20 members in humans and rodents10–13,50,97 (TABLE 3), is particularly relevant as it contains three of the SLC drug transporters that are currently of regulatory interest — namely, SLC22A6 (OAT1), SLC22A8 (OAT3) and SLC22A2 (OCT2)3,4,6. The SLC22 family includes not only several multispecific drug transporters, but also a number of closely related transporters that have a high specificity for metabolites, antioxidants and signalling molecules. Moreover, mutations or SNPs in some of these transporters are associated with human metabolic disease, which is discussed further below (TABLE 2).
SLC22 transporters are generally divided into anionic (OATs), cationic (OCTs) and zwitterionic (including carnitine transporters such as OCTNs) (TABLE 3). Despite substantial in vitro transport data, the endogenous function of many of these transporters in organ and systemic physiology is unclear. The literature heavily emphasizes the roles of particular OATs and OCTs as drug and/or toxin transporters. However, although OATs and OCTs are clearly drug and/or toxin transporters, they also seem to be important for regulating physiologically relevant metabolic and signalling pathways.
Although many of the other 15 or so members of the SLC22 family have the ability to transport a limited set of drugs, these members also seem to be specialized for the transport of metabolites and signalling molecules (TABLE 3). This seems to be reflected in the restricted tissue expression of particular family members. For example, certain SLC22 family members are highly expressed in the olfactory mucosa (which expresses SLC22A20 (also known as OAT6))98–100, the choroid plexus (which expresses SLC22A17)62, the liver (which expresses SLC22A7 (also known as OAT2)), the kidney (which expresses SLC22A22 (also known as OATPG) and SLC22A12 (also known as URAT1))101,102, the placenta (which expresses SLC22A11 (also known as OAT4))103 and the testes (which express several SLC22 members)99,104 (TABLE 3). Accordingly, it is interesting that olfactory mucosa-expressed SLC22A20 (OAT6) can bind odorants98, SLC22A7 (OAT2) has a preference for cyclic GMP105 and SLC22A22 (OATPG) is a high-affinity prostaglandin transporter102 (as are several other SLC22 transporters)14,106–108. SLC22A12 (URAT1), which is known as RST in mice, is a transporter of uric acid14,101, and placentally expressed SLC22A11 (OAT4) transports sulphated sex steroids (as do SLC22A8 and SLC22A20)103 (TABLE 3).
Similarly, OCTs have differential tissue expression patterns; SLC22A1 (OCT1) is highly expressed in the liver, whereas SLC22A2 (OCT) is highly expressed in the kidney (TABLE 3). As with the OATs, the genes encoding OCTs have been perturbed in mice and, generally, the knockout and in vitro transport data are concordant. For example, mice lacking both SLC22A1 and SLC22A2 exhibit defective secretion of organic cations by the kidney109. SLC22A3 (OCT3) seems to have a key role in neurotransmitter uptake in the nervous system10,110,111. OCTNs, which include SLC22A4 and SLC22A5 (also known as OCTN1 and OCTN2, respectively), are highly expressed in muscle and in many other tissues, playing a crucial part in metabolism involving carnitine10 (TABLE 3). OCTNs also have the ability to transport drugs, although this seems to be more limited than that of OATs and OCTs. In the mammary gland, OCTNs are believed to be important for the transport of carnitine into breast milk, providing the neonate with a carnitine source for the β-oxidation of fatty acids14,112–116.
ABC transporters
With ABC drug transporters, a similar set of data as for SLC transporters exists. Although the most famous member of the family, ABCB1 (PGP; MDR1)117, is important in multidrug resistance of tumours, it also transports natural products, metabolites and signalling molecules4,15,118,119. Several other ABC transporters of various families can transport leukotrienes, bile acids, peptides, folate, uric acid, cyclic nucleotides and glutathione1,15–17,120. Frequently, the substrate specificities of ABC transporters for drugs and metabolites overlap considerably with those of SLC family transporters — for example, in the case of certain ABCC (MRP), OAT and OATP substrates (TABLE 1). Although the structure of mammalian SLC drug transporters awaits solution, the structure of ABCB1 is known18, and there is a major effort in the field to understand the molecular basis of ABC transporter multispecificity121.
Single, double and triple ABC transporter gene-knockout animals have been generated. The complex studies of these knockout animals are difficult to summarize here, but animals with a single gene knocked out generally tend to have minimal to mild physiological changes, at least under basal conditions. Generally, the data from animals with single and combination knockouts support the case for the redundancy of certain ABC transporters in vivo from the viewpoint of systemic physiology, which is consistent with overlapping substrate preferences of the various transporters (TABLE 1). These knockout studies have, however, revealed pharmacokinetic alterations depending on the gene or genes deleted, the compound studied and the tissue of focus (for example, the brain122,123 or liver33,124–127). Sometimes, a gene knockout results in a change in the expression of another transporter or drug-metabolizing enzyme, raising the possibility that the loss of one component of a physiological network induces compensatory changes124,128. Some of these studies are discussed further below.
Similar to the OATs and SLCO (OATP) transporters, many ABCC (MRP) members handle organic anions129. However, unlike the (usually) influx role of SLC drug transporters in the kidney proximal tubule, biliary tract and elsewhere, the ABCC transporters are thought to have a major role in the efflux of organic anionic drugs, toxins and metabolites1,129 (FIG. 1). As in the case of the OATs and SLCO transporters, data from knockout mice support this view. For example, Abcc3 (Mrp3)-knockout mice have a defect in the ability to transport morphine glucuronide and plant-derived compounds into bile17,126,128. In the liver, ABCC2 (MRP2) seems to work in concert with ABCC3 (MRP3), and the deletion of one increases the expression of the other, supporting the notion of compensatory upregulation of one drug transporter as a result of the loss of another128. ABCC2 and ABCC3 also regulate the handling of drugs by the intestine, although acting in opposite directions3,129,130.
Clinical syndromes involving drug transporters
Mutations in the genes that encode SLC and ABC drug transporter homologues can result in metabolic disease (TABLE 2). As with certain drugs (for example, methotrexate, antivirals and statins), certain metabolites (including uric acid, indole derivatives, prostaglandins, bile acids, bilirubin conjugates and conjugated steroids) can be transported by multiple SLC and ABC drug transporters and/or their close relatives. For example, several studies in humans indicate that SLC22A12 (URAT1; originally discovered as RST in mice131) is involved in regulating uric acid levels; certain SLC22A12 mutations are associated with altered uric acid levels, gout and kidney stones101,132–135. Of the human SLCs, SLC22A12 is one of the most closely related to SLC22A6 (OAT1) and SLC22A8 (OAT3). All three transport uric acid in vitro, and Slc22a12 (Rst), Slc22a6 and Slc22a8 single gene-knockout mice have altered renal urate handling91. Nevertheless, although in vitro and in vivo data indicate that SLC22A6 and SLC22A8 transport a broad range of metabolites, drugs, signalling molecules and toxins, SLC22A12 seems to primarily be a uric acid transporter (although it seems to bind and/or transport a few other substrates)4,13,91,101. Although debated, it is often suggested that uric acid is one of the key antioxidants in the body14,136; thus, uric acid levels have the potential to influence, albeit perhaps in a subtle fashion, a wide range of processes, including metabolism and intracellular signalling14. Some of these effects might be responsible for the posited role of uric acid in hypertension133,137.
Genome-wide association studies, as well as other studies, indicate that several additional transporters might have a role in regulating levels of uric acid in humans132–135. Of these, one of the most strongly implicated is the broadly expressed transporter ABCG2 (BCRP)132. This is one of the best-known ABC drug transporters and can transport, among other molecules, chemotherapeutic agents4,19,89 and, in addition to uric acid, other substrates of metabolic importance, such as riboflavin138. ABCG2 seems to be important in intestinal uric acid transport139,140 and, together with SLC22A12 (URAT1) and other kidney drug transporters, regulates uric acid homeostasis141–144. Intriguingly, intestinal ABCG2 expression is affected by loss of kidney mass144. Also involved in uric acid transport are the hexose transporter SLC2A9 and the phosphate transporters of the SLC17 family132,145. Thus, distinctions tend to blur as one considers how classic drug transporters cooperate with metabolite transporters to regulate uric acid homeostasis.
There are other examples of associations of ABC and SLC drug transporters with metabolic disorders. Mutations in SLC22A5 (also known as OCTN2), a gene that encodes a zwitterionic transporter of the same family as OATs and OCTs, cause systemic carnitine deficiency, leading to cardiomyopathy and other problems146–148. Moreover, variants of SLC22A4 (OCTN1) and SLC22A5 (also known as OCTN2) play a part in several inflammatory diseases148–151, although other SLC22 transporters probably also transport carnitine10,152,153. ABCC2 (MRP2) is a drug and metabolite transporter expressed in the liver, kidney and intestine that is involved in transport of bilirubin glucuronide124; mutations in ABCC2 result in Dubin–Johnson syndrome, which presents with conjugated hyperbilirubinaemia154 (TABLE 2). In addition, ABC and SLC transporters exhibit altered expression or function in several acute and chronic liver diseases or animal models of disease, and such changes may be of pathophysiological importance in certain instances155. Nevertheless, one must be careful in comparing animals and humans; a number of drug transporter isoforms are species specific, and expression patterns, as well as the physiological substrates and/or affected pathways, may be different. For example, there are differences in the pathways that regulate uric acid levels between animals and humans14,91,133,156.
Metabolomics analyses and metabolic reconstructions of knockouts
Although studies in knockout mice and knockout tissue have confirmed the importance of SLC and ABC drug transporters in the handling of many drugs and toxins as predicted by in vitro studies17,32,33,35–38,40,52,62,63,127,128,157,158, more extensive physiological analysis is needed. Evidence from targeted and untargeted metabolomics analyses in certain transporter-knockout animals, supplemented by in vitro transport studies, is beginning to support the view that drug transporters normally function in the systemic physiology of key metabolites, antioxidants, nutrients, microbiome-derived compounds, neuroactive molecules and signalling molecules38,61,91,128,159. This includes, in the case of the Slc22a6 (Oat1)-knockout and Slc22a8 (Oat3)-knockout animals, many important metabolites, such as citric acid cycle intermediates, uric acid, creatinine, gut microbiome products, flavonoids, signalling molecules and the so-called uraemic toxins of chronic kidney disease38,61,159,160 (TABLE 4). Metabolomics analyses also suggest that OATs have a central role in modulating oxidative metabolism, which is consistent with previous mechanistic studies that indicated the essential role of α-ketoglutarate in organic anion transport161. Similarly, Slco-knockouts and transgenic rodents that overexpress an OATP have alterations in metabolites that overlap with those of the Oat-knockout animals162–164. In vivo and in vitro data also indicate that multiple SLCO transporters and OATs play a key part in the hepatic and renal handling of bile acids165. Certain SLCO transporters can also transport thyroid hormones166; SLCO1C1 (also known as OATP1C1) is found in the brain capillary endothelium, and the Slco1c1 knockout seems to have alterations consistent with mild hypothyroidism in the central nervous system (CNS)167.
Table 4.
Selected metabolic alterations in knockout or transgenic rodents
| Gene name (alternative name) | Metabolites (alternative name) | Refs |
|---|---|---|
| Slc22a6 (Oat1) | 3-Hydroxybutyrate | 38 |
|
|
||
| Benzoate | 38 | |
|
|
||
| 2-Hydroxy-3-methylvalerate | 38 | |
|
|
||
| 2-Oxoglutarate (α-ketoglutarate) | 38 | |
|
|
||
| Indoxyl sulphate | 61 | |
|
|
||
| Indole lactic acid | 61 | |
|
|
||
| Kynurenine | 61 | |
|
|
||
| Pantothenic acid | 61 | |
|
|
||
| Urate | 61 | |
|
|
||
| Thymidine | 61 | |
|
|
||
| Orotic acid | 61 | |
|
|
||
| Amino-cresol sulphate | 61 | |
|
| ||
| Slc22a8 (Oat3) | Pongamoside A | 159 |
|
|
||
| 9-Amino-nonanoic acid | 159 | |
|
|
||
| Flavin mononucleotide | 68 | |
|
|
||
| Thymidine | 68 | |
|
|
||
| Urate | 91 | |
|
| ||
| Slco1a1 (Oatp1a1) | Trimethylamine | 162 |
|
|
||
| Gulonate | 162 | |
|
|
||
| Isethionic acid | 162 | |
|
| ||
| Slco1a4 (Oatp1a4) | Trimethylamine | 162 |
|
|
||
| Isethionic acid | 162 | |
|
| ||
| SLCO4C1 (OATP4C1)* | Asymmetric dimethylarginine | 163 |
|
|
||
| Guanidinosuccinic acid | 163 | |
|
|
||
| Trans-aconitate | 163 | |
|
| ||
| Abcc3 (Mrp3) | Enterodiol–glucuronide | 168 |
|
|
||
| Secoisolariciresinol–glucuronide | 168 | |
|
|
||
| Enterolactone–glucuronide | 168 | |
Human transporter gene transgenically overexpressed in the rat kidney.
Metabolomics studies in mice lacking ABCC3 (MRP3) suggest that this ABC transporter transports glucuronide conjugates of phytochemicals168. Along with defects in the handling of the anticancer drug topotecan, the Abcg2 (Bcrp)-knockout mouse is also defective in the handling of plant compounds and of at least one dietary carcinogen169,170. These mice also have defects in the secretion of xenobiotics, as well as riboflavin, into breast milk and bile138,170–172, whereas Abcc2 (Mrp2)-knockout mice have defects in the excretion of bilirubin and its conjugates124, as well as altered intestinal absorption130,173. To fully assess their roles in the handling of endogenous substrates and in physiological processes, additional metabolomics work needs to be carried out on the ABC and SLC drug transporter knockouts. So far, the accumulated data indicate that significant metabolic changes occur after the loss of certain members of both superfamilies.
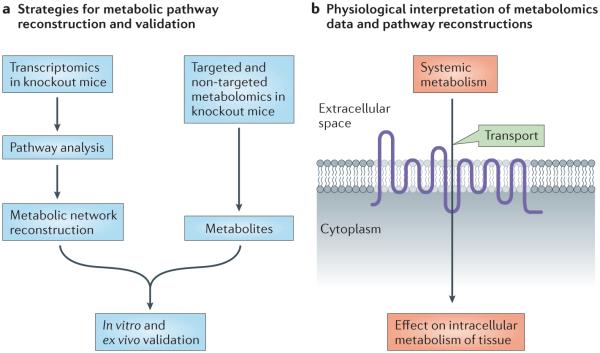
In a limited number of instances, there are metabolomics and microarray data from a drug transporter knockout mouse. Systems biology approaches to metabolic pathway reconstruction (for instance, genome-scale metabolic reconstruction) have advanced considerably in recent years174–176. Using such approaches, it has been possible to build and partly validate metabolic networks that are centred on a particular drug transporter159,177 (FIG. 3). In this way, it has been shown that SLC22A6 (OAT1) and SLC22A8 (OAT3) are directly or indirectly connected to many metabolic pathways, including those involved in the citric acid cycle, and polyamine, nucleotide and fatty-acid metabolism. Although these types of analyses are not without limitations, they suggest that there is a whole set of unexplored potential drug–metabolite interactions that may help to explain metabolic alterations and other side effects. Drug–metabolite interactions could be due to direct competition between a metabolite and a drug at the level of the transporter; however, drug-induced metabolic changes could also be the result of indirect (secondary) effects on metabolism, which are due to competition between a drug that is transported by a transporter and a key metabolic intermediate that is part of a larger metabolic pathway. These effects could influence the extracellular and/or intracellular concentration of one or more key metabolites in an unexpected way, thereby altering an entire metabolic pathway or set of pathways. Once comprehensive metabolomics and gene-expression data become available for all of the SLC and ABC drug transporter knockouts, it will be possible to perform similar reconstructions. On the basis of the types of data discussed in this article, it already seems reasonable to incorporate certain SLC and ABC ‘drug’ transporters into standard representations of metabolic pathways.
Figure 3. Reconstruction of metabolic networks from large-scale ‘omics’ data implicates drug transporters in metabolic pathways.
a | One type of computational approach based on ‘omics’ data for connecting transporters and metabolic pathways is schematically represented. In this strategy, transcriptomic data derived from transporter knockout mice are analysed using genome-scale metabolic reconstruction tools159,174–177. This approach can be used to support a potential linkage between a specific transporter and certain metabolic pathways that can be further validated by in vitro and other assays. Metabolomics data from knockout mice are also very valuable for supporting predictions. An approach related to this was used to link SLC22A6 (OAT1)-mediated transport to several metabolic pathways177. A number of other approaches have also been developed174 (not shown). b | The type of combined computational–wet-lab approach schematized in part a, once performed for multiple ATP-binding cassette (ABC) and solute carrier (SLC) drug transporters, may enable novel interpretations of the role of drug transporters in whole-body physiology, as well as in intracellular metabolism in specific tissues.
The implications of metabolomics studies and metabolic reconstructions from knockout mice may help us to more fully understand drug-induced metabolic syndromes such as those that occur with chronic treatment for HIV (which involves drugs that are transported by both SLC and ABC transporters)52,178–180 or long-term diuretic therapy (which involves SLC22A6- and SLC22A8-mediated transport)40,181. Without metabolic reconstructions, it may be difficult to determine the extent to which drug transporters are involved in normal metabolism and to systematically study potential drug–metabolite interactions and their broader metabolic consequences.
Drug transporters in the handling of dietary compounds and microbial metabolites
The metabolomics data from knockout mice also highlight the in vivo importance of SLC and ABC drug transporters in the elimination of dietary compounds, gut microbiome products and their phase I and phase II metabolites into bile and urine61,159,168. Future metabolomics studies of knockout animals will surely reveal many more examples of the parallels between the gut–liver–biliary tract axis and the gut–liver–kidney axis in the handling of metabolites in normal and disease contexts.
Chronic kidney disease is associated with the accumulation of small-molecule uraemic toxins, which are potentially toxic to cells and tissues via several mechanisms182–184. Many uraemic toxins are substrates of OATs, SLCOs (OATPs), ABCCs (MRPs) and other drug transporters, and some originate in the gut microbiome and are then modified by hepatic drug-metabolizing enzymes into more toxic compounds185. More than 100 putative uraemic toxins have been identified. These include indole derivatives, hippurate, kynurenine, polyamines and other molecules183,184. Although associated with toxicity, many of these molecules have important physiological roles: some are important metabolic intermediates; some have the potential to affect redox state, an ability demonstrated by indoxyl sulphate and uric acid; others can influence cell growth, as exemplified by polyamines; and some may act as ligands for signalling mediated by G protein-coupled receptors in remote tissues (kynurenine in the CNS and in other tissues186,187). Thus, in the uraemic syndrome of chronic kidney disease — a complex disorder of metabolism and other cellular processes — compounds that are derived from the gut microbiome that are absorbed, distributed, metabolized and eliminated by SLC and ABC drug transporters have the potential to remotely alter metabolic pathways, cellular redox state and signalling events. Indeed, indole derivatives may be causally important in the uraemic syndrome188. Intriguingly, structurally related molecules have an important role in normal plant physiology and morphogenesis, and are substrates for plant ABC transporters189–191 (BOX 2).
The symptoms of severe chronic kidney disease can vary considerably, and this may be partly due to different levels of uraemic toxins182,184,192; it would not be surprising if, ultimately, polymorphisms in multiple SLC and ABC drug transporters were shown to partly predict variation in the severity of the uraemic syndrome of chronic kidney disease, including the CNS symptoms. Thus far, metabolomics studies in knockout mice indicate the likely involvement of OATs and SLCOs (OATPs) in the handling of uraemic toxins61,159,163. Recent research also suggests a connection between metabolic alterations observed in diabetic kidney disease and the function of SLC22A6 (OAT1) and SLC22A8 (OAT3)193. Many metabolites associated with diabetes, metabolic syndrome and hepatic disease are also potential substrates for OATs, SLCO transporters, ABCC transporters, and other SLC and ABC drug transporters155,193–195. SNPs in the genes encoding these transporters could modulate the levels of metabolites of pathophysiological importance in diabetes, hepatobiliary syndromes and other diseases. Such interactions could help to explain the variability in clinical presentation and outcome of complex metabolic diseases.
Beyond the gut–liver–biliary axis and the gut–liver–kidney axis, there are many such ‘highways’ in the body through which the movement of small molecules involved in signalling is regulated by drug transporters. Given that drug transporters are highly expressed in the choroid plexus and the blood–brain barrier62,110,196,197 (FIG. 1), metabolomics analyses of drug-transporter-knockout brain tissue and cerebrospinal fluid, as well as plasma, will be important. Within various cells and structures of the CNS, there are also many SLC and ABC transporters, including those related to the drug transporters discussed here. SLC22A3 (OCT3), for instance, is expressed in the brain, where it is involved in neurotransmitter uptake, and it may be a therapeutic target for certain neuro-psychiatric disorders111. Thus, it will be very interesting to trace, in detail, the movement of substrates through the blood–brain barrier–CNS–choroid plexus axis. This should help to provide a firmer foundation for small-molecule drug therapy for CNS disorders and for understanding changes in the cerebrospinal fluid as a result of drug treatment, as well as in metabolic disease.
Do SLC and ABC drug transporters participate in a larger remote communication system?
There is a need to try to connect the disparate types of data described above to aim for a broader biological understanding of the roles of SLC and ABC transporters that could have clinical ramifications. After all, whether we are considering pharmacology, toxicology, physiology, metabolic disease or organ development and maturation (BOX 2), we are discussing roughly the same set of 20–40 drug transporters (or their close relatives) that handle structurally similar small molecules. Furthermore, whether for a metabolite such as uric acid or a drug such as methotrexate, it is now evident that the systemic picture often depends on multiple SLC and ABC drug transporters, expressed differentially in tissues and regulated in a complex fashion (such as through transcription, sorting and phosphorylation).
The ‘remote sensing and signalling’ hypothesis (the main points of which are summarized in BOX 3) aims to provide a systems-level understanding of drug transporters that goes beyond the mere shuttling of molecules and suggests that these transporters regulate metabolism and signalling within and between different cells (epithelial and non-epithelial cells), tissues and body fluid compartments — and possibly between organisms as well (for instance, between a mother and her nursing neonate, or between the gut microbiome and its host)7,14,50,98. This hypothesis aims to provide another perspective on drug transporters in normal biology and in acute or chronic disease states — and to reconcile non-pharmacological, non-toxicological data on drug transporters and their close relatives. However, this different perspective could have a major impact on understanding toxicity from drugs and environmental toxins. The details of the hypothesis, possible molecular mechanisms and potentially relevant examples of remote sensing and signalling have been considered elsewhere, mostly in the context of OAT biology7,11,14,50,96,198.
Some of the initial experimental results that led to the formulation of a broader hypothesis came from the discovery of SLC22A20 (OAT6) — an apparent odorant transporter in the olfactory epithelium of mice — which is closely related to SLC22A6 (OAT1) and SLC22A8 (OAT3)98,99. It was found that some of the highest affinities of tested SLC22A20 substrates were for volatile organic anion odorants that are apparently excreted into the urine via OATs in the kidney98. This observation led to the speculation, still unsubstantiated, that olfactory SLC22A20 might, in parallel with odorant receptors involved in olfaction, play a part in the ‘sensing’ by one organism (for instance, a dog) of volatile organic anion odorants excreted into the urine by another organism (such as another dog or a cat) — and thus the idea of inter-organismal and interspecific remote sensing and signalling arose7,14. Moreover, gut microbiome (also referred to as the enterobiome14,61) products that can affect metabolism and signalling in different tissues throughout the body are handled in the intestine by SLC and ABC transporters and accumulate in drug-transporter-knockout animals61,159. This observation seems to be an example of remote, interspecific communication that is mediated by drug transporters. Taking this one step further, there are potential examples of metabolites initially derived from the gut microbiome (for example, propionate)199 that may be excreted into the urine by drug transporters and that can activate odorant receptors14,50,98.
Moreover, SLC and ABC drug transporters may have a role in responding to pathological states96,155,200. After injury to the liver, SLC and ABC drug transporters in the kidney exhibit altered expression201, whereas loss of kidney tissue or function can affect the expression of liver transporters202,203. The mechanisms underlying this and other types of inter-organ communication remain unclear but have been considered in the context of remote sensing and signalling7,11,14,50,96,198,204. As mentioned, the hypothesis has so far been developed and interpreted almost entirely from the viewpoint of the expression patterns and function of various OAT isoforms; nevertheless, even with OATs, substantial amounts of additional data need to be gathered7,14,50. Insight into the potential relevance of this hypothesis to inter-organ communication via various drug transporters may be gained from the generation of different combinations of ABC and SLC drug-transporter-knockout animals that can be used to create well-described physiological models of disease (for example, partial hepatectomy, partial nephrectomy, bile-duct ligation, models of diabetes and acute injury), followed by detailed functional characterization, metabolomics, transcriptomics and other ‘omics’ analyses. More analyses of haematological, immune and other non-epithelial cells are warranted. Further exploration of the remote sensing and signalling hypothesis will probably also benefit from physiological and developmental studies in model organisms such as flies, worms, sea urchins and plants204–206 (BOX 2).
Although there is evidence for the substrate induction of drug transporters in cells and periods of developmental responsiveness to substrates and hormones207, the actual sensors in the communication system are not understood. There is a growing body of data on the basic mechanisms of transcriptional and post-transcriptional regulation of drug transporters that may prove relevant22,95,208,209. These mechanisms conceivably involve hormones, growth factors and small molecules (some of which are sometimes substrates of the transporters) that are known to be important in tissue recovery and regeneration. Indeed, recent in vitro and older in vivo physiological studies suggest that the neuroendocrine, cytokine and growth factor regulatory systems operate alongside the broader system of SLC and ABC transporters, which are involved in the transport of drugs, toxins, metabolites, nutrients, hormones, antioxidants, vitamins and signalling molecules, and, in many respects, the various systems seem to interact7,14,50,159,166. Cyclic nucleotides, prostaglandins and hormones — good substrates for several drug transporters — have well-documented roles in physiology. Transcriptional programmes are frequently activated by nuclear receptors that are known to bind certain endogenous substrates (for example, bile acids, fatty acids, eicosanoids and gut microbial products). All of these substrates are transported by SLC and/or ABC drug transporters and their close relatives. For example, in the liver and kidney, nuclear receptors seem to regulate the expression of drug transporters in addition to that of numerous phase I and phase II drug-metabolizing enzymes1,22,95,210.
Thus, remote sensing and signalling by SLC and ABC drug transporters in different tissues might represent an essential regulatory system that works along with the neuroendocrine, cytokine and growth factor systems to maintain and restore homeostasis. Importantly, the understanding of the roles of drug transporters in restoring homeostasis might be a place at which the toxicological and the physiological views could be reconciled. For instance, similar (or overlapping) sets of transporters might be required to both eliminate toxins that cause injury and, after injury, to restore the normal physiology involved in remote sensing and signalling, by regulating the movement of metabolites, signalling molecules, antioxidants and nutrients. Beyond inter-organ and inter-organismal communication, an ecological perspective with SLC and ABC drug transporters playing a central role is implied, but remains to be explored14.
Concluding perspectives
There is a growing concern about drug toxicities related to interactions with SLC and ABC drug transporters. With the current focus by industry, regulatory agencies and academic institutions on mechanisms of drug elimination and distribution, as well as drug–drug interactions, it seems that the already substantial literature on the roles of SLC and ABC transporters in drug transport will continue to increase rapidly. This article is partly motivated by the concern that this may further skew the focus on SLC and ABC drug transporters towards the transport of drugs and away from what might prove to be very interesting endogenous biological roles. That said, in light of the types of data discussed here, one might argue that a deeper understanding of these transporters in metabolic pathways, systems physiology and morphogenesis will ultimately shed light on unexplained side effects of drugs and complex disease states. Considered in the context of recent genetic information on human metabolic diseases (such as those affecting uric acid), the metabolite transport data and metabolomics analyses of knockout animals suggest that genetic variants of one or more SLC and ABC drug transporters could exert a modifying influence in common diseases such as obesity, diabetes, metabolic syndrome, chronic kidney disease, chronic liver disease and inflammatory states, as well as in recovery from acute tissue injury.
Consequently, it is crucial to remain cognizant of the endogenous functions of these transporters and their participation in systemic physiology. It is also evident from a few of the instances presented here that some of these physiological functions of SLC and ABC drug transporters are closely tied to metabolic pathways involving phase I and phase II drug-metabolizing enzymes. It may be that what we often call ‘ADME’ is largely the co-opting by drugs of SLC and ABC transporter-mediated pathways that are part of a larger remote sensing and signalling system — or something akin to this — that works in parallel with the neuroendocrine, cytokine and growth factor systems. If so, perhaps a significant proportion of the vast number of the adverse reactions to drugs reported in the Physician’s Desk Reference211 represent manifestations of drug–metabolite interactions at the level of SLC and ABC drug transporters — and the upstream and downstream consequences that result from the dysregulation of metabolism and signalling pathways as well as of remote communication. Exploration of the functional relationships between drug transporters, growth factors and the hormonal system may further help to explain unexpected side effects that go beyond simple drug–drug interactions and drug–metabolite interactions.
Evolutionary analyses might make it easier to reconcile the physiological roles of drug transporters with their roles in xenobiotic handling. It is clear that drug transporters play an essential part in the elimination of many environmental toxins, such as heavy metals63,64 and aristolochic acid67, as well as many of the man-made toxins of concern to governmental and non-governmental agencies65. More studies also seem necessary to analyse the function of drug transporters (BOX 1) in the comparative physiologies of flies, worms, fish, mice, humans and possibly even plants.
Equally important, and intriguing, is the production of endogenous toxins by the gut microbiome that are, as shown in knockout mice and in vitro studies, eliminated by drug transporters such as OATs and ABCC transporters. As mentioned above, some of these are uraemic toxins that are associated with chronic kidney disease. Whether the driving force in higher organisms for the evolution of SLC and ABC drug transporters (and perhaps even of a remote sensing and signalling system) was the need to deal with natural environmental toxins, gut microbial metabolites and toxins, or metabolic requirements may remain a ‘chicken or egg’ story, but with the availability of vast numbers of genomes and the ability to mine big data in novel ways, a plausible scenario may soon emerge.
Acknowledgements
The author gratefully acknowledges the considerable help of W. Wu and K. T. Bush in providing comments, as well as in the preparation of figures, tables and references. Also, the author is indebted to students, postdoctoral fellows and many colleagues within and outside the general field of drug transporters for stimulating discussions. Given the broad scope of this subject, it was not possible to discuss many important papers, and there is a risk of oversimplification; the reader can therefore refer to more specialized authoritative reviews such as those cited in this article. This work was partly supported by the US National Institutes of Health Grants (GM098449, GM104098 and HD07160 (U54)).
Footnotes
Competing interests statement
The author declares competing interests: see Web version for details.
References
- 1.You G, Morris ME, editors. Drug Transporters: Molecular Characterization and Role in Drug Disposition. John Wiley & Sons; 2014. [Google Scholar]
- 2.Giacomini KM, et al. International Transporter Consortium commentary on clinically important transporter polymorphisms. Clin. Pharmacol. Ther. 2013;94:23–26. doi: 10.1038/clpt.2013.12. [DOI] [PubMed] [Google Scholar]
- 3.Giacomini KM, et al. Membrane transporters in drug development. Nature Rev. Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrissey KM, et al. The UCSF-FDA TransPortal: a public drug transporter database. Clin. Pharmacol. Ther. 2012;92:545–546. doi: 10.1038/clpt.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamek-Gliszczynski MJ, et al. ITC recommendations for transporter kinetic parameter estimation and translational modeling of transport-mediated PK and DDIs in humans. Clin. Pharmacol. Ther. 2013;94:64–79. doi: 10.1038/clpt.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SC, Zhang L, Huang SM. In: Drug Transporters: Molecular Characterization and Role in Drug Disposition. You G, Morris ME, editors. Vol. 495. John Wiley & Sons; 2014. [Google Scholar]
- 7.Ahn SY, Nigam SK. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol. Pharmacol. 2009;76:481–490. doi: 10.1124/mol.109.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeGorter MK, Xia CQ, Yang JJ, Kim RB. Drug transporters in drug efficacy and toxicity. Annu. Rev. Pharmacol. Toxicol. 2012;52:249–273. doi: 10.1146/annurev-pharmtox-010611-134529. [DOI] [PubMed] [Google Scholar]
- 9.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol. Rev. 2010;62:1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koepsell H. The SLC22 family with transporters of organic cations, anions and zwitterions. Mol. Aspects Med. 2013;34:413–435. doi: 10.1016/j.mam.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Emami Riedmaier A, Nies AT, Schaeffeler E, Schwab M. Organic anion transporters and their implications in pharmacotherapy. Pharmacol. Rev. 2012;64:421–449. doi: 10.1124/pr.111.004614. [DOI] [PubMed] [Google Scholar]
- 12.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm. Drug Dispos. 2010;31:1–71. doi: 10.1002/bdd.693. [DOI] [PubMed] [Google Scholar]
- 14.Wu W, Dnyanmote AV, Nigam SK. Remote communication through solute carriers and ATP binding cassette drug transporter pathways: an update on the remote sensing and signaling hypothesis. Mol. Pharmacol. 2011;79:795–805. doi: 10.1124/mol.110.070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J. Bioenerg. Biomembr. 2001;33:453–458. doi: 10.1023/a:1012866803188. [DOI] [PubMed] [Google Scholar]
- 16.Seeger MA, van Veen HW. Molecular basis of multidrug transport by ABC transporters. Biochim. Biophys. Acta. 2009;1794:725–737. doi: 10.1016/j.bbapap.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- 18.Aller SG, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int. J. Toxicol. 2006;25:231–259. doi: 10.1080/10915810600746023. [DOI] [PubMed] [Google Scholar]
- 20.Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol. Sci. 2010;31:246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney DE, et al. Functional maturation of drug transporters in the developing, neonatal, and postnatal kidney. Mol. Pharmacol. 2011;80:147–154. doi: 10.1124/mol.110.070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen QX, Hu HH, Zhou Q, Yu AM, Zeng S. An overview of ABC and SLC drug transporter gene regulation. Curr. Drug Metab. 2013;14:253–264. [PubMed] [Google Scholar]
- 23.de Jonge-Peeters SD, Kuipers F, de Vries EG, Vellenga E. ABC transporter expression in hematopoietic stem cells and the role in AML drug resistance. Crit. Rev. Oncol. Hematol. 2007;62:214–226. doi: 10.1016/j.critrevonc.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Burckhardt G, Burckhardt BC. In: Drug Transporters. Fromm MF, Kim RB, editors. Springer; 2011. pp. 29–104. [Google Scholar]
- 25.Choi YH, Yu AM. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2013;20:793–807. doi: 10.2174/138161282005140214165212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagenbuch B, Stieger B. The SLCO (former SLC21) superfamily of transporters. Mol. Aspects Med. 2013;34:396–412. doi: 10.1016/j.mam.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. Renal transporters in drug development. Annu. Rev. Pharmacol. Toxicol. 2013;53:503–529. doi: 10.1146/annurev-pharmtox-011112-140317. [DOI] [PubMed] [Google Scholar]
- 28.Ahn SY, Bhatnagar V. Update on the molecular physiology of organic anion transporters. Curr. Opin. Nephrol. Hypertens. 2008;17:499–505. doi: 10.1097/MNH.0b013e32830b5d5d. [DOI] [PubMed] [Google Scholar]
- 29.Nigam SK, Bhatnagar V. How much do we know about drug handling by SLC and ABC drug transporters in children? Clin. Pharmacol. Ther. 2013;94:27–29. doi: 10.1038/clpt.2013.82. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Lopez E, et al. Polymorphisms in the methotrexate transport pathway: a new tool for MTX plasma level prediction in pediatric acute lymphoblastic leukemia. Pharmacogenet. Genom. 2013;23:53–61. doi: 10.1097/FPC.0b013e32835c3b24. [DOI] [PubMed] [Google Scholar]
- 31.Maeda A, et al. Drug interaction between celecoxib and methotrexate in organic anion transporter 3-transfected renal cells and in rats. in vivo. Eur. J. Pharmacol. 2010;640:168–171. doi: 10.1016/j.ejphar.2010.04.051. [DOI] [PubMed] [Google Scholar]
- 32.VanWert AL, Sweet DH. Impaired clearance of methotrexate in organic anion transporter 3 (Slc22a8) knockout mice: a gender specific impact of reduced folates. Pharm. Res. 2008;25:453–462. doi: 10.1007/s11095-007-9407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlaming ML, et al. Abcc2 (Mrp2), Abcc3 (Mrp3), and Abcg2 (Bcrp1) are the main determinants for rapid elimination of methotrexate and its toxic metabolite 7-hydroxymethotrexate in vivo. Mol. Cancer Ther. 2009;8:3350–3359. doi: 10.1158/1535-7163.MCT-09-0668. [DOI] [PubMed] [Google Scholar]
- 34.Kaler G, et al. Structural variation governs substrate specificity for organic anion transporter (OAT) homologs. Potential remote sensing by OAT family members. J. Biol. Chem. 2007;282:23841–23853. doi: 10.1074/jbc.M703467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagle MA, et al. Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J. Biol. Chem. 2011;286:243–251. doi: 10.1074/jbc.M110.139949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vlaming ML, et al. Functionally overlapping roles of Abcg2 (Bcrp1) and Abcc2 (Mrp2) in the elimination of methotrexate and its main toxic metabolite 7-hydroxymethotrexate in vivo. Clin. Cancer Res. 2009;15:3084–3093. doi: 10.1158/1078-0432.CCR-08-2940. [DOI] [PubMed] [Google Scholar]
- 37.Vlaming ML, et al. Bcrp1;Mdr1a/b;Mrp2 combination knockout mice: altered disposition of the dietary carcinogen PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) and its genotoxic metabolites. Mol. Pharmacol. 2014;85:520–530. doi: 10.1124/mol.113.088823. [DOI] [PubMed] [Google Scholar]
- 38.Eraly SA, et al. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J. Biol. Chem. 2006;281:5072–5083. doi: 10.1074/jbc.M508050200. [DOI] [PubMed] [Google Scholar]
- 39.Han YF, et al. Association of intergenic polymorphism of organic anion transporter 1 and 3 genes with hypertension and blood pressure response to hydrochlorothiazide. Am. J. Hypertens. 2011;24:340–346. doi: 10.1038/ajh.2010.191. [DOI] [PubMed] [Google Scholar]
- 40.Vallon V, et al. Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am. J. Physiol. Renal Physiol. 2008;294:F867–F873. doi: 10.1152/ajprenal.00528.2007. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, et al. Effect of genetic variation in the organic cation transporter 2 on the renal elimination of metformin. Pharmacogenet. Genom. 2009;19:497–504. doi: 10.1097/FPC.0b013e32832cc7e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christensen MM, et al. A gene-gene interaction between polymorphisms in the OCT2 and MATE1 genes influences the renal clearance of metformin. Pharmacogenet. Genom. 2013;23:526–534. doi: 10.1097/FPC.0b013e328364a57d. [DOI] [PubMed] [Google Scholar]
- 43.Stocker SL, et al. The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin. Pharmacol. Ther. 2013;93:186–194. doi: 10.1038/clpt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez-Nieto CE, You G, Barros EJG, Beier DR, Nigam SK. Molecular cloning and characterization of a novel transporter protein with very high expression in the kidney (abstract) J. Am. Soc. Nephrol. 1996;7:1301. [Google Scholar]
- 45.Lopez-Nieto CE, et al. Mus musculus kidney-specific transport protein mRNA (complete cds), accession number U52842.1. NCBI GenBank. 1996 [online], http://www.ncbi.nlm.nih.gov/gene/?term=U52842.1.
- 46.Lopez-Nieto CE, et al. Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. J. Biol. Chem. 1997;272:6471–6478. doi: 10.1074/jbc.272.10.6471. [DOI] [PubMed] [Google Scholar]
- 47.Smith HW, Finkelstein N, Aliminosa L, Crawford B, Graber M. The renal clearances of substituted hippuric acid derivatives and other aromatic acids in dog and man. J. Clin. Invest. 1945;24:388–404. doi: 10.1172/JCI101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burnell JM, Kirby WM. Effectiveness of a new compound, benemid, in elevating serum penicillin concentrations. J. Clin. Invest. 1951;30:697–700. doi: 10.1172/JCI102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pritchard JB. Luminal and peritubular steps in renal transport of p-aminohippurate. Biochim. Biophys. Acta. 1987;906:295–308. doi: 10.1016/0304-4157(87)90015-3. [DOI] [PubMed] [Google Scholar]
- 50.Nigam SK, et al. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. doi: 10.1152/physrev.00025.2013. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahn SY, Eraly SA, Tsigelny I, Nigam SK. Interaction of organic cations with organic anion transporters. J. Biol. Chem. 2009;284:31422–31430. doi: 10.1074/jbc.M109.024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Truong DM, Kaler G, Khandelwal A, Swaan PW, Nigam SK. Multi-level analysis of organic anion transporters 1, 3, and 6 reveals major differences in structural determinants of antiviral discrimination. J. Biol. Chem. 2008;283:8654–8663. doi: 10.1074/jbc.M708615200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Windass AS, Lowes S, Wang Y, Brown CD. The contribution of organic anion transporters OAT1 and OAT3 to the renal uptake of rosuvastatin. J. Pharmacol. Exp. Ther. 2007;322:1221–1227. doi: 10.1124/jpet.107.125831. [DOI] [PubMed] [Google Scholar]
- 54.Bahn A, et al. Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am. J. Physiol. Cell Physiol. 2005;289:C1075–1084. doi: 10.1152/ajpcell.00619.2004. [DOI] [PubMed] [Google Scholar]
- 55.Khamdang S, et al. Interactions of human- and rat-organic anion transporters with pravastatin and cimetidine. J. Pharmacol. Sci. 2004;94:197–202. doi: 10.1254/jphs.94.197. [DOI] [PubMed] [Google Scholar]
- 56.Pavlova A, et al. Developmentally regulated expression of organic ion transporters NKT (OAT1), OCT1, NLT (OAT2), and Roct. Am. J. Physiol. Renal Physiol. 2000;278:F635–643. doi: 10.1152/ajprenal.2000.278.4.F635. [DOI] [PubMed] [Google Scholar]
- 57.Zalups RK, Ahmad S. Handling of cysteine S-conjugates of methylmercury in MDCK cells expressing human OAT1. Kidney Int. 2005;68:1684–1699. doi: 10.1111/j.1523-1755.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 58.Wong CC, Botting NP, Orfila C, Al-Maharik N, Williamson G. Flavonoid conjugates interact with organic anion transporters (OATs) and attenuate cytotoxicity of adefovir mediated by organic anion transporter 1 (OAT1/SLC22A6) Biochem. Pharmacol. 2011;81:942–949. doi: 10.1016/j.bcp.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 59.Rizwan AN, Burckhardt G. Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm. Res. 2007;24:450–470. doi: 10.1007/s11095-006-9181-4. [DOI] [PubMed] [Google Scholar]
- 60.Mizuno N, et al. Human organic anion transporters 1 (hOAT1/SLC22A6) and 3 (hOAT3/SLC22A8) transport edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one) and its sulfate conjugate. Drug Metab. Dispos. 2007;35:1429–1434. doi: 10.1124/dmd.106.013912. [DOI] [PubMed] [Google Scholar]
- 61.Wikoff WR, Nagle MA, Kouznetsova VL, Tsigelny IF, Nigam SK. Untargeted metabolomics identifies enterobiome metabolites and putative uremic toxins as substrates of organic anion transporter 1 (Oat1) J. Proteome Res. 2011;10:2842–2851. doi: 10.1021/pr200093w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagle MA, Wu W, Eraly SA, Nigam SK. Organic anion transport pathways in antiviral handling in choroid plexus in Oat1 (Slc22a6) and Oat3 (Slc22a8) deficient tissue. Neurosci. Lett. 2013;534:133–138. doi: 10.1016/j.neulet.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torres AM, Dnyanmote AV, Bush KT, Wu W, Nigam SK. Deletion of multispecific organic anion transporter Oat1/Slc22a6 protects against mercury-induced kidney injury. J. Biol. Chem. 2011;286:26391–26395. doi: 10.1074/jbc.M111.249292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Engstrom K, et al. Polymorphisms in genes encoding potential mercury transporters and urine mercury concentrations in populations exposed to mercury vapor from gold mining. Environ. Health Perspect. 2013;121:85–91. doi: 10.1289/ehp.1204951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weaver YM, Ehresman DJ, Butenhoff JL, Hagenbuch B. Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicol. Sci. 2010;113:305–314. doi: 10.1093/toxsci/kfp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brady KP, et al. A novel putative transporter maps to the osteosclerosis (oc) mutation and is not expressed in the oc mutant mouse. Genomics. 1999;56:254–261. doi: 10.1006/geno.1998.5722. [DOI] [PubMed] [Google Scholar]
- 67.Bakhiya N, et al. Molecular evidence for an involvement of organic anion transporters (OATs) in aristolochic acid nephropathy. Toxicology. 2009;264:74–79. doi: 10.1016/j.tox.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 68.Vallon V, et al. Organic anion transporter 3 contributes to the regulation of blood pressure. J. Am. Soc. Nephrol. 2008;19:1732–1740. doi: 10.1681/ASN.2008020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vallon V, et al. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am. J. Physiol. Renal Physiol. 2012;302:F1293–F1299. doi: 10.1152/ajprenal.00013.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ciarimboli G, et al. Proximal tubular secretion of creatinine by organic cation transporter OCT2 in cancer patients. Clin. Cancer Res. 2012;18:1101–1108. doi: 10.1158/1078-0432.CCR-11-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eisner C, et al. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int. 2010;77:519–526. doi: 10.1038/ki.2009.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lepist EI, et al. Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int. 2014;86:350–357. doi: 10.1038/ki.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sveinbjornsson G, et al. Rare mutations associating with serum creatinine and chronic kidney disease. Hum. Mol. Genet. 2014 doi: 10.1093/hmg/ddu399. http://dx.doi.org/10.1093/hmg/ddu399. [DOI] [PubMed]
- 74.Cimoch PJ, et al. Pharmacokinetics of oral ganciclovir alone and in combination with zidovudine, didanosine, and probenecid in HIV-infected subjects. J. Acquir. Immune Def. Syndr. Hum. Retrovirol. 1998;17:227–234. doi: 10.1097/00042560-199803010-00007. [DOI] [PubMed] [Google Scholar]
- 75.Eraly SA, Blantz RC, Bhatnagar V, Nigam SK. Novel aspects of renal organic anion transporters. Curr. Opin. Nephrol. Hypertens. 2003;12:551–558. doi: 10.1097/00041552-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 76.Maeda A, et al. Evaluation of the interaction between nonsteroidal anti-inflammatory drugs and methotrexate using human organic anion transporter 3-transfected cells. Eur. J. Pharmacol. 2008;596:166–172. doi: 10.1016/j.ejphar.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 77.Takeda M, et al. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J. Pharmacol. Exp. Ther. 2002;302:666–671. doi: 10.1124/jpet.102.034330. [DOI] [PubMed] [Google Scholar]
- 78.Yee SW, et al. Reduced renal clearance of cefotaxime in Asians with a low-frequency polymorphism of OAT3 (SLC22A8) J. Pharm. Sci. 2013;102:3451–3457. doi: 10.1002/jps.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol. Rev. 2011;63:157–181. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- 80.Carr DF, et al. SLCO1B1 genetic variant associated with statin-induced myopathy: a proof of concept study using the clinical practice research datalink (CPRD) Clin. Pharmacol. Ther. 2013;94:695–701. doi: 10.1038/clpt.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Link E, et al. SLCO1B1 variants and statin-induced myopathy — a genomewide study. N. Engl. J. Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 82.Voora D, et al. The SLCO1B1*5 genetic variant is associated with statin-induced side effects. J. Am. Coll. Cardiol. 2009;54:1609–1616. doi: 10.1016/j.jacc.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang MJ, et al. Risk factors for severe hyperbilirubinemia in neonates. Pediatr. Res. 2004;56:682–689. doi: 10.1203/01.PDR.0000141846.37253.AF. [DOI] [PubMed] [Google Scholar]
- 84.Zhang W, et al. OATP1B1 polymorphism is a major determinant of serum bilirubin level but not associated with rifampicin-mediated bilirubin elevation. Clin. Exp. Pharmacol. Physiol. 2007;34:1240–1244. doi: 10.1111/j.1440-1681.2007.04798.x. [DOI] [PubMed] [Google Scholar]
- 85.Choi JH, et al. A common 5′-UTR variant in MATE2-K is associated with poor response to metformin. Clin. Pharmacol. Ther. 2011;90:674–684. doi: 10.1038/clpt.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lepper ER, et al. Mechanisms of resistance to anticancer drugs: the role of the polymorphic ABC transporters ABCB1 and ABCG2. Pharmacogenomics. 2005;6:115–138. doi: 10.1517/14622416.6.2.115. [DOI] [PubMed] [Google Scholar]
- 87.Noguchi K, Katayama K, Mitsuhashi J, Sugimoto Y. Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv. Drug Deliv. Rev. 2009;61:26–33. doi: 10.1016/j.addr.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Bosch TM, Meijerman I, Beijnen JH, Schellens JH. Genetic polymorphisms of drug-metabolising enzymes and drug transporters in the chemotherapeutic treatment of cancer. Clin. Pharmacokinet. 2006;45:253–285. doi: 10.2165/00003088-200645030-00003. [DOI] [PubMed] [Google Scholar]
- 89.Campa D, et al. A comprehensive study of polymorphisms in ABCB1, ABCC2 and ABCG2 and lung cancer chemotherapy response and prognosis. Int. J. Cancer. 2012;131:2920–2928. doi: 10.1002/ijc.27567. [DOI] [PubMed] [Google Scholar]
- 90.Kivisto KT, Niemi M. Influence of drug transporter polymorphisms on pravastatin pharmacokinetics in humans. Pharm. Res. 2007;24:239–247. doi: 10.1007/s11095-006-9159-2. [DOI] [PubMed] [Google Scholar]
- 91.Eraly SA, et al. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol. Genom. 2008;33:180–192. doi: 10.1152/physiolgenomics.00207.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu G, et al. Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)] Kidney Int. 2005;68:1491–1499. doi: 10.1111/j.1523-1755.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 93.Bhatnagar V, et al. Analyses of 5’ regulatory region polymorphisms in human SLC22A6 (OAT1) and SLC22A8 (OAT3) J. Hum. Genet. 2006;51:575–580. doi: 10.1007/s10038-006-0398-1. [DOI] [PubMed] [Google Scholar]
- 94.Kikuchi R, et al. Regulation of the expression of human organic anion transporter 3 by hepatocyte nuclear factor 1alpha/beta and DNA methylation. Mol. Pharmacol. 2006;70:887–896. doi: 10.1124/mol.106.025494. [DOI] [PubMed] [Google Scholar]
- 95.Martovetsky G, Tee JB, Nigam SK. Hepatocyte nuclear factors 4a and 1a (Hnf4a and Hnf1a) regulate kidney developmental expression of drug-metabolizing enzymes and drug transporters. Mol. Pharmacol. 2013;84:808–823. doi: 10.1124/mol.113.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saito H. Pathophysiological regulation of renal SLC22A organic ion transporters in acute kidney injury: pharmacological and toxicological implications. Pharmacol. Ther. 2010;125:79–91. doi: 10.1016/j.pharmthera.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 97.Schlessinger A, Yee SW, Sali A, Giacomini KM. SLC classification: an update. Clin. Pharmacol. Ther. 2013;94:19–23. doi: 10.1038/clpt.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaler G, et al. Olfactory mucosa-expressed organic anion transporter, Oat6, manifests high affinity interactions with odorant organic anions. Biochem. Biophys. Res. Commun. 2006;351:872–876. doi: 10.1016/j.bbrc.2006.10.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Monte JC, Nagle MA, Eraly SA, Nigam SK. Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochem. Biophys. Res. Commun. 2004;323:429–436. doi: 10.1016/j.bbrc.2004.08.112. [DOI] [PubMed] [Google Scholar]
- 100.Schnabolk GW, Youngblood GL, Sweet DH. Transport of estrone sulfate by the novel organic anion transporter Oat6 (Slc22a20) Am. J. Physiol. Renal Physiol. 2006;291:F314–F321. doi: 10.1152/ajprenal.00497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Enomoto A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 102.Shiraya K, et al. A novel transporter of SLC22 family specifically transports prostaglandins and co-localizes with 15-hydroxyprostaglandin dehydrogenase in renal proximal tubules. J. Biol. Chem. 2010;285:22141–22151. doi: 10.1074/jbc.M109.084426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cha SH, et al. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J. Biol. Chem. 2000;275:4507–4512. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- 104.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab. Pharmacokinet. 2005;20:452–477. doi: 10.2133/dmpk.20.452. [DOI] [PubMed] [Google Scholar]
- 105.Cropp CD, et al. Organic anion transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Mol. Pharmacol. 2008;73:1151–1158. doi: 10.1124/mol.107.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kimura H, et al. Human organic anion transporters and human organic cation transporters mediate renal transport of prostaglandins. J. Pharmacol. Exp. Ther. 2002;301:293–298. doi: 10.1124/jpet.301.1.293. [DOI] [PubMed] [Google Scholar]
- 107.Kobayashi Y, et al. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]) J. Pharm. Pharmacol. 2005;57:573–578. doi: 10.1211/0022357055966. [DOI] [PubMed] [Google Scholar]
- 108.Kobayashi Y, et al. Renal transport of organic compounds mediated by mouse organic anion transporter 3 (mOat3): further substrate specificity of mOat3. Drug Metab. Dispos. 2004;32:479–483. doi: 10.1124/dmd.32.5.479. [DOI] [PubMed] [Google Scholar]
- 109.Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol. Cell. Biol. 2003;23:7902–7908. doi: 10.1128/MCB.23.21.7902-7908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Farthing CA, Sweet DH. Expression and function of organic cation and anion transporters (SLC22 family) in the CNS. Curr. Pharm. Des. 2014;20:1472–1486. doi: 10.2174/13816128113199990456. [DOI] [PubMed] [Google Scholar]
- 111.Massmann V, et al. The organic cation transporter 3 (OCT3) as molecular target of psychotropic drugs: transport characteristics and acute regulation of cloned murine OCT3. Pflugers Arch. 2014;466:517–527. doi: 10.1007/s00424-013-1335-8. [DOI] [PubMed] [Google Scholar]
- 112.Gilchrist SE, Alcorn J. Lactation stage-dependent expression of transporters in rat whole mammary gland and primary mammary epithelial organoids. Fundam. Clin. Pharmacol. 2010;24:205–214. doi: 10.1111/j.1472-8206.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- 113.Lamhonwah AM, et al. Upregulation of mammary gland OCTNs maintains carnitine homeostasis in suckling infants. Biochem. Biophys. Res. Commun. 2011;404:1010–1015. doi: 10.1016/j.bbrc.2010.12.100. [DOI] [PubMed] [Google Scholar]
- 114.Crill CM, Helms RA. The use of carnitine in pediatric nutrition. Nutr. Clin. Pract. 2007;22:204–213. doi: 10.1177/0115426507022002204. [DOI] [PubMed] [Google Scholar]
- 115.Shekhawat PS, et al. Spontaneous development of intestinal and colonic atrophy and inflammation in the carnitine-deficient jvs (OCTN2−/−) mice. Mol. Genet. Metab. 2007;92:315–324. doi: 10.1016/j.ymgme.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alcorn J, Lu X, Moscow JA, McNamara PJ. Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction. J. Pharmacol. Exp. Ther. 2002;303:487–496. doi: 10.1124/jpet.102.038315. [DOI] [PubMed] [Google Scholar]
- 117.Riordan JR, Ling V. Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability. J. Biol. Chem. 1979;254:12701–12705. [PubMed] [Google Scholar]
- 118.Li Y, Paxton JW. The effects of flavonoids on the ABC transporters: consequences for the pharmacokinetics of substrate drugs. Expert Opin. Drug Metab. Toxicol. 2013;9:267–285. doi: 10.1517/17425255.2013.749858. [DOI] [PubMed] [Google Scholar]
- 119.Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 2006;580:998–1009. doi: 10.1016/j.febslet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 120.He SM, Li R, Kanwar JR, Zhou SF. Structural and functional properties of human multidrug resistance protein 1 (MRP1/ABCC1) Curr. Med. Chem. 2011;18:439–481. doi: 10.2174/092986711794839197. [DOI] [PubMed] [Google Scholar]
- 121.Poguntke M, Hazai E, Fromm MF, Zolk O. Drug transport by breast cancer resistance protein. Expert Opin. Drug Metab. Toxicol. 2010;6:1363–1384. doi: 10.1517/17425255.2010.519700. [DOI] [PubMed] [Google Scholar]
- 122.Hu S, et al. Interaction of the multikinase inhibitors sorafenib and sunitinib with solute carriers and ATP-binding cassette transporters. Clin. Cancer Res. 2009;15:6062–6069. doi: 10.1158/1078-0432.CCR-09-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ose A, et al. Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxyl ate phosphate (Ro 64–0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4) Drug Metab. Dispos. 2009;37:315–321. doi: 10.1124/dmd.108.024018. [DOI] [PubMed] [Google Scholar]
- 124.Chu XY, et al. Characterization of mice lacking the multidrug resistance protein MRP2 (ABCC2) J. Pharmacol. Exp. Ther. 2006;317:579–589. doi: 10.1124/jpet.105.098665. [DOI] [PubMed] [Google Scholar]
- 125.Lagas JS, et al. P-glycoprotein (P-gp/Abcb1), Abcc2, and Abcc3 determine the pharmacokinetics of etoposide. Clin. Cancer Res. 2010;16:130–140. doi: 10.1158/1078-0432.CCR-09-1321. [DOI] [PubMed] [Google Scholar]
- 126.Zelcer N, et al. Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and morphine-6-glucuronide antinociception. Proc. Natl Acad. Sci. USA. 2005;102:7274–7279. doi: 10.1073/pnas.0502530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lagas JS, Vlaming ML, Schinkel AH. Pharmacokinetic assessment of multiple ATP-binding cassette transporters: the power of combination knockout mice. Mol. Interv. 2009;9:136–145. doi: 10.1124/mi.9.3.7. [DOI] [PubMed] [Google Scholar]
- 128.Kruh GD, Belinsky MG, Gallo JM, Lee K. Physiological and pharmacological functions of Mrp2, Mrp3 and Mrp4 as determined from recent studies on gene-disrupted mice. Cancer Metastasis Rev. 2007;26:5–14. doi: 10.1007/s10555-007-9039-1. [DOI] [PubMed] [Google Scholar]
- 129.Keppler D. Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. Handb. Exp. Pharmacol. 2011;201:299–323. doi: 10.1007/978-3-642-14541-4_8. [DOI] [PubMed] [Google Scholar]
- 130.Tang SC, Hendrikx JJ, Beijnen JH, Schinkel AH. Genetically modified mouse models for oral drug absorption and disposition. Curr. Opin. Pharmacol. 2013;13:853–858. doi: 10.1016/j.coph.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 131.Mori K, et al. Kidney-specific expression of a novel mouse organic cation transporter-like protein. FEBS Lett. 1997;417:371–374. doi: 10.1016/s0014-5793(97)01325-2. [DOI] [PubMed] [Google Scholar]
- 132.Kolz M, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mount DB. The kidney in hyperuricemia and gout. Curr. Opin. Nephrol. Hypertens. 2013;22:216–223. doi: 10.1097/MNH.0b013e32835ddad2. [DOI] [PubMed] [Google Scholar]
- 134.Tin A, et al. Genome-wide association study for serum urate concentrations and gout among African Americans identifies genomic risk loci and a novel URAT1 loss-of-function allele. Hum. Mol. Genet. 2011;20:4056–4068. doi: 10.1093/hmg/ddr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vazquez-Mellado J, et al. Molecular analysis of the SLC22A12 (URAT1) gene in patients with primary gout. Rheumatology. 2007;46:215–219. doi: 10.1093/rheumatology/kel205. [DOI] [PubMed] [Google Scholar]
- 136.Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleos. Nucleot. Nucl. 2008;27:608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mazzali M, et al. Uric acid and hypertension: cause or effect? Curr. Rheumatol Rep. 2010;12:108–117. doi: 10.1007/s11926-010-0094-1. [DOI] [PubMed] [Google Scholar]
- 138.van Herwaarden AE, et al. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B ) into milk. Mol. Cell. Biol. 2007;27:1247–1253. doi: 10.1128/MCB.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ichida K, et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nature Commun. 2012;3:764. doi: 10.1038/ncomms1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hosomi A, Nakanishi T, Fujita T, Tamai I. Extra-renal elimination of uric acid via intestinal efflux transporter BCRP/ABCG2. PLoS ONE. 2012;7:e30456. doi: 10.1371/journal.pone.0030456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matsuo H, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci. Transl. Med. 2009;1:5ra11. doi: 10.1126/scitranslmed.3000237. [DOI] [PubMed] [Google Scholar]
- 142.Sakurai H. Urate transporters in the genomic era. Curr. Opin. Nephrol. Hypertens. 2013;22:545–550. doi: 10.1097/MNH.0b013e328363ffc8. [DOI] [PubMed] [Google Scholar]
- 143.Woodward OM, et al. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl Acad. Sci. USA. 2009;106:10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yano H, Tamura Y, Kobayashi K, Tanemoto M, Uchida S. Uric acid transporter ABCG2 is increased in the intestine of the 5/6 nephrectomy rat model of chronic kidney disease. Clin. Exp. Nephrol. 2014;18:50–55. doi: 10.1007/s10157-013-0806-8. [DOI] [PubMed] [Google Scholar]
- 145.Dehghan A, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372:1953–1961. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nezu J, et al. Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nature Genet. 1999;21:91–94. doi: 10.1038/5030. [DOI] [PubMed] [Google Scholar]
- 147.Tang NL, et al. Mutations of OCTN2, an organic cation/carnitine transporter, lead to deficient cellular carnitine uptake in primary carnitine deficiency. Hum. Mol. Genet. 1999;8:655–660. doi: 10.1093/hmg/8.4.655. [DOI] [PubMed] [Google Scholar]
- 148.Tamai I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21) Biopharm. Drug Dispos. 2013;34:29–44. doi: 10.1002/bdd.1816. [DOI] [PubMed] [Google Scholar]
- 149.Tokuhiro S, et al. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nature Genet. 2003;35:341–348. doi: 10.1038/ng1267. [DOI] [PubMed] [Google Scholar]
- 150.Peltekova VD, et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nature Genet. 2004;36:471–475. doi: 10.1038/ng1339. [DOI] [PubMed] [Google Scholar]
- 151.Waller S, et al. Evidence for association of OCTN genes and IBD5 with ulcerative colitis. Gut. 2006;55:809–814. doi: 10.1136/gut.2005.084574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aouida M, Poulin R, Ramotar D. The human carnitine transporter SLC22A16 mediates high affinity uptake of the anticancer polyamine analogue bleomycin-A5. J. Biol. Chem. 2010;285:6275–6284. doi: 10.1074/jbc.M109.046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Eraly SA, Nigam SK. Novel human cDNAs homologous to Drosophila Orct and mammalian carnitine transporters. Biochem. Biophys. Res. Commun. 2002;297:1159–1166. doi: 10.1016/s0006-291x(02)02343-4. [DOI] [PubMed] [Google Scholar]
- 154.Toh S, et al. Genomic structure of the canalicular multispecific organic anion-transporter gene (MRP2/ cMOAT) and mutations in the ATP-binding-cassette region in Dubin-Johnson syndrome. Am. J. Hum. Genet. 1999;64:739–746. doi: 10.1086/302292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schuetz JD, Swaan PW, Tweedie DJ. The role of transporters in toxicity and disease. Drug Metab. Dispos. 2014;42:541–545. doi: 10.1124/dmd.114.057539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Keebaugh AC, Thomas JW. The evolutionary fate of the genes encoding the purine catabolic enzymes in hominoids, birds, and reptiles. Mol. Biol. Evol. 2010;27:1359–1369. doi: 10.1093/molbev/msq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jonker JW, et al. Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Mol. Cell. Biol. 2001;21:5471–5477. doi: 10.1128/MCB.21.16.5471-5477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sweet DH, et al. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J. Biol. Chem. 2002;277:26934–26943. doi: 10.1074/jbc.M203803200. [DOI] [PubMed] [Google Scholar]
- 159.Wu W, et al. Multispecific drug transporter slc22a8 (oat3) regulates multiple metabolic and signaling pathways. Drug Metab. Dispos. 2013;41:1825–1834. doi: 10.1124/dmd.113.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kouznetsova VL, Tsigelny IF, Nagle MA, Nigam SK. Elucidation of common pharmacophores from analysis of targeted metabolites transported by the multispecific drug transporter-Organic anion transporter1 (Oat1) Bioorg. Med. Chem. 2011;19:3320–3340. doi: 10.1016/j.bmc.2011.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol. Rev. 2004;84:987–1049. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]
- 162.Gong L, et al. Characterization of organic anion-transporting polypeptide (Oatp) 1a1 and 1a4 null mice reveals altered transport function and urinary metabolomic profiles. Toxicol. Sci. 2011;122:587–597. doi: 10.1093/toxsci/kfr114. [DOI] [PubMed] [Google Scholar]
- 163.Toyohara T, et al. SLCO4C1 transporter eliminates uremic toxins and attenuates hypertension and renal inflammation. J. Am. Soc. Nephrol. 2009;20:2546–2555. doi: 10.1681/ASN.2009070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van de Steeg E, et al. Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J. Clin. Invest. 2010;120:2942–2952. doi: 10.1172/JCI42168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Iusuf D, van de Steeg E, Schinkel AH. Functions of OATP1A and 1B transporters in vivo: insights from mouse models. Trends Pharmacol. Sci. 2012;33:100–108. doi: 10.1016/j.tips.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 166.Hagenbuch B. Cellular entry of thyroid hormones by organic anion transporting polypeptides. Best Pract. Res. Clin. Endocrinol. Metab. 2007;21:209–221. doi: 10.1016/j.beem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 167.Mayerl S, Visser TJ, Darras VM, Horn S, Heuer H. Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology. 2012;153:1528–1537. doi: 10.1210/en.2011-1633. [DOI] [PubMed] [Google Scholar]
- 168.van de Wetering K, Feddema W, Helms JB, Brouwers JF, Borst P. Targeted metabolomics identifies glucuronides of dietary phytoestrogens as a major closs of Mrp3 substrates in vivo. Gastroenterology. 2009;137:1725–1735. doi: 10.1053/j.gastro.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 169.Tiwari AK, Zhang R, Gallo JM. Overlapping functions of ABC transporters in topotecan disposition as determined in gene knockout mouse models. Mol. Cancer Ther. 2013;12:1343–1355. doi: 10.1158/1535-7163.MCT-13-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jonker JW, et al. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nature Med. 2005;11:127–129. doi: 10.1038/nm1186. [DOI] [PubMed] [Google Scholar]
- 171.Merino G, et al. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab. Dispos. 2006;34:690–695. doi: 10.1124/dmd.105.008219. [DOI] [PubMed] [Google Scholar]
- 172.Wang L, Leggas M, Goswami M, Empey PE, McNamara PJ. N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918) as a chemical ATP-binding cassette transporter family G member 2 (Abcg2) knockout model to study nitrofurantoin transfer into milk. Drug Metab. Dispos. 2008;36:2591–2596. doi: 10.1124/dmd.108.021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lagas JS, et al. P-glycoprotein, multidrug-resistance associated protein 2, Cyp3a, and carboxylesterase affect the oral availability and metabolism of vinorelbine. Mol. Pharmacol. 2012;82:636–644. doi: 10.1124/mol.111.077099. [DOI] [PubMed] [Google Scholar]
- 174.Szallasi Z, Stelling J, Periwal V. System Modeling in Cell Biology: From Concepts to Nuts and Bolts. The MIT Press; 2006. [Google Scholar]
- 175.Duarte NC, et al. Global reconstruction of the human metabolic network based on genomic and bibliomic data. Proc. Natl Acad. Sci. USA. 2007;104:1777–1782. doi: 10.1073/pnas.0610772104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Thiele I, et al. A community-driven global reconstruction of human metabolism. Nature Biotech. 2013;31:419–425. doi: 10.1038/nbt.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ahn SY, et al. Linkage of organic anion transporter-1 to metabolic pathways through integrated ‘omics’-driven network and functional analysis. J. Biol. Chem. 2011;286:31522–31531. doi: 10.1074/jbc.M111.272534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Weiss J, Haefeli WE. Impact of ATP-binding cassette transporters on human immunodeficiency virus therapy. Int. Rev. Cell. Mol. Biol. 2010;280:219–279. doi: 10.1016/S1937-6448(10)80005-X. [DOI] [PubMed] [Google Scholar]
- 179.Minuesa G, et al. Drug uptake transporters in antiretroviral therapy. Pharmacol. Ther. 2011;132:268–279. doi: 10.1016/j.pharmthera.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 180.Nix LM, Tien PC. Metabolic syndrome, diabetes, and cardiovascular risk in HIV. Curr. HIV/AIDS Rep. 2014;11:271–278. doi: 10.1007/s11904-014-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Buscemi S, et al. Impact of chronic diuretic treatment on glucose homeostasis. Diabetol Metab. Syndr. 2013;5:80. doi: 10.1186/1758-5996-5-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Toyohara T, et al. Metabolomic profiling of uremic solutes in CKD patients. Hypertens. Res. 2010;33:944–952. doi: 10.1038/hr.2010.113. [DOI] [PubMed] [Google Scholar]
- 183.Jourde-Chiche N, et al. Protein-bound toxins — update 2009. Semin. Dial. 2009;22:334–339. doi: 10.1111/j.1525-139X.2009.00576.x. [DOI] [PubMed] [Google Scholar]
- 184.Vanholder R, Van Laecke S, Glorieux G. What is new in uremic toxicity? Pediatr. Nephrol. 2008;23:1211–1221. doi: 10.1007/s00467-008-0762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Banoglu E, King RS. Sulfation of indoxyl by human and rat aryl (phenol) sulfotransferases to form indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 2002;27:135–140. doi: 10.1007/BF03190428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vecsei L, Szalardy L, Fulop F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nature Rev. Drug Discov. 2013;12:64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 187.Wang J, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006;281:22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 188.Meyer TW, Hostetter TH. Uremic solutes from colon microbes. Kidney Int. 2012;81:949–954. doi: 10.1038/ki.2011.504. [DOI] [PubMed] [Google Scholar]
- 189.Geisler M, Murphy AS. The ABC of auxin transport: the role of p-glycoproteins in plant development. FEBS Lett. 2006;580:1094–1102. doi: 10.1016/j.febslet.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 190.Zazimalova E, Murphy AS, Yang H, Hoyerova K, Hosek P. Auxin transporters—why so many? Cold Spring Harb. Perspect. Biol. 2010;2:a001552. doi: 10.1101/cshperspect.a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sparks E, Wachsman G, Benfey PN. Spatiotemporal signalling in plant development. Nature Rev. Genet. 2013;14:631–644. doi: 10.1038/nrg3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Duranton F, et al. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012;23:1258–1270. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sharma K, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J. Am. Soc. Nephrol. 2013;24:1901–1912. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Beyoglu D, Idle JR. The metabolomic window into hepatobiliary disease. J. Hepatol. 2013;59:842–858. doi: 10.1016/j.jhep.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Prentice KJ, et al. The furan fatty acid metabolite CMPF is elevated in diabetes and induces β cell dysfunction. Cell. Metab. 2014;19:653–666. doi: 10.1016/j.cmet.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 196.Alebouyeh M, et al. Expression of human organic anion transporters in the choroid plexus and their interactions with neurotransmitter metabolites. J. Pharmacol. Sci. 2003;93:430–436. doi: 10.1254/jphs.93.430. [DOI] [PubMed] [Google Scholar]
- 197.Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS. 2011;8:3. doi: 10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brandoni A, Hazelhoff MH, Bulacio RP, Torres AM. Expression and function of renal and hepatic organic anion transporters in extrahepatic cholestasis. World J. Gastroenterol. 2012;18:6387–6397. doi: 10.3748/wjg.v18.i44.6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hosseini E, Grootaert C, Verstraete W, Van de Wiele T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011;69:245–258. doi: 10.1111/j.1753-4887.2011.00388.x. [DOI] [PubMed] [Google Scholar]
- 200.Hanafy S, El-Kadi AO, Jamali F. Effect of inflammation on molecular targets and drug transporters. J. Pharm. Pharm. Sci. 2012;15:361–375. doi: 10.18433/j30300. [DOI] [PubMed] [Google Scholar]
- 201.Ikemura K, Nakagawa E, Kurata T, Iwamoto T, Okuda M. Altered pharmacokinetics of cimetidine caused by down-regulation of renal rat organic cation transporter 2 (rOCT2) after liver ischemia-reperfusion injury. Drug Metab. Pharmacokinet. 2013;28:504–509. doi: 10.2133/dmpk.dmpk-13-rg-021. [DOI] [PubMed] [Google Scholar]
- 202.Lu H, Klaassen C. Gender differences in mRNA expression of ATP-binding cassette efflux and bile acid transporters in kidney, liver, and intestine of 5/6 nephrectomized rats. Drug Metab. Dispos. 2008;36:16–23. doi: 10.1124/dmd.107.014845. [DOI] [PubMed] [Google Scholar]
- 203.Naud J, Nolin TD, Leblond FA, Pichette V. Current understanding of drug disposition in kidney disease. J. Clin. Pharmacol. 2012;52:10S–22S. doi: 10.1177/0091270011413588. [DOI] [PubMed] [Google Scholar]
- 204.Chahine S, Seabrooke S, O’Donnell MJ. Effects of genetic knock-down of organic anion transporter genes on secretion of fluorescent organic ions by Malpighian tubules of Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2012;81:228–240. doi: 10.1002/arch.21066. [DOI] [PubMed] [Google Scholar]
- 205.Korolnek T, Zhang J, Beardsley S, Scheffer GL, Hamza I. Control of metazoan heme homeostasis by a conserved multidrug resistance protein. Cell. Metab. 2014;19:1008–1019. doi: 10.1016/j.cmet.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shipp LE, Hamdoun A. ATP-binding cassette (ABC) transporter expression and localization in sea urchin development. Dev. Dyn. 2012;241:1111–1124. doi: 10.1002/dvdy.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hook JB, Hewitt WR. Development of mechanisms for drug excretion. Am. J. Med. 1977;62:497–506. doi: 10.1016/0002-9343(77)90404-1. [DOI] [PubMed] [Google Scholar]
- 208.Zhou F, Hong M, You G. Regulation of human organic anion transporter 4 by progesterone and protein kinase C in human placental BeWo cells. Am. J. Physiol. Endocrinol. Metab. 2007;293:E57–E61. doi: 10.1152/ajpendo.00696.2006. [DOI] [PubMed] [Google Scholar]
- 209.Zhang Q, et al. Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J. Biol. Chem. 2008;283:32570–32579. doi: 10.1074/jbc.M800298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chow EC, Sun H, Khan AA, Groothuis GM, Pang KS. Effects of 1α, 25-dihydroxyvitamin D3 on transporters and enzymes of the rat intestine and kidney in vivo. Biopharm. Drug Dispos. 2010;31:91–108. doi: 10.1002/bdd.694. [DOI] [PubMed] [Google Scholar]
- 211.Physicians’ Desk Reference. Thomson PDR; 2014. [Google Scholar]
- 212.Zheng WH, et al. Evolutionary relationships of ATP-Binding Cassette (ABC) uptake porters. BMC Microbiol. 2013;13:98. doi: 10.1186/1471-2180-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lorca GL, et al. Transport capabilities of eleven gram-positive bacteria: comparative genomic analyses. Biochim. Biophys. Acta. 2007;1768:1342–1366. doi: 10.1016/j.bbamem.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang X, et al. Twelve transmembrane helices form the functional core of mammalian MATE1 (multidrug and toxin extruder 1) protein. J. Biol. Chem. 2012;287:27971–27982. doi: 10.1074/jbc.M112.386979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Eraly SA, Hamilton BA, Nigam SK. Organic anion and cation transporters occur in pairs of similar and similarly expressed genes. Biochem. Biophys. Res. Commun. 2003;300:333–342. doi: 10.1016/s0006-291x(02)02853-x. [DOI] [PubMed] [Google Scholar]
- 216.Wu W, Baker ME, Eraly SA, Bush KT, Nigam SK. Analysis of a large cluster of SLC22 transporter genes, including novel USTs, reveals species-specific amplification of subsets of family members. Physiol. Genom. 2009;38:116–124. doi: 10.1152/physiolgenomics.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kent WJ, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Konieczna A, et al. Differential expression of ABC transporters (MDR1, MRP1, BCRP) in developing human embryos. J. Mol. Histol. 2011;42:567–574. doi: 10.1007/s10735-011-9363-1. [DOI] [PubMed] [Google Scholar]
- 219.Ricardo S, Lehmann R. An ABC transporter controls export of a Drosophila germ cell attractant. Science. 2009;323:943–946. doi: 10.1126/science.1166239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Eraly SA, Monte JC, Nigam SK. Novel slc22 transporter homologs in fly, worm, and human clarify the phylogeny of organic anion and cation transporters. Physiol. Genom. 2004;18:12–24. doi: 10.1152/physiolgenomics.00014.2004. [DOI] [PubMed] [Google Scholar]
- 221.Strader LC, Bartel B. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Mol. Plant. 2011;4:477–486. doi: 10.1093/mp/ssr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Good JR, et al. TagA, a putative serine protease/ABC transporter of Dictyostelium that is required for cell fate determination at the onset of development. Development. 2003;130:2953–2965. doi: 10.1242/dev.00523. [DOI] [PubMed] [Google Scholar]
- 223.Mao Q, Ganapathy V, Unadkat JD. In: Drug Transporters: Molecular Characterization and Role in Drug Disposition. You G, Morris ME, editors. John Wiley & Sons; 2014. pp. 341–354. [Google Scholar]
- 224.Funk RS, Brown JT, Abdel-Rahman SM. Pediatric pharmacokinetics: human development and drug disposition. Pediatr. Clin. North Am. 2012;59:1001–1016. doi: 10.1016/j.pcl.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 225.Sweet DH, Eraly SA, Vaughn DA, Bush KT, Nigam SK. Organic anion and cation transporter expression and function during embryonic kidney development and in organ culture models. Kidney Int. 2006;69:837–845. doi: 10.1038/sj.ki.5000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gallegos TF, Martovetsky G, Kouznetsova V, Bush KT, Nigam SK. Organic anion and cation SLC22 ‘drug’ transporter (Oat1, Oat3, and Oct1) regulation during development and maturation of the kidney proximal tubule. PLoS ONE. 2012;7:e40796. doi: 10.1371/journal.pone.0040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gao B, St Pierre MV, Stieger B, Meier PJ. Differential expression of bile salt and organic anion transporters in developing rat liver. J. Hepatol. 2004;41:201–208. doi: 10.1016/j.jhep.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 228.Braunlich H. Postnatal development of kidney function in rats receiving thyroid hormones. Exp. Clin. Endocrinol. 1984;83:243–250. doi: 10.1055/s-0029-1210336. [DOI] [PubMed] [Google Scholar]
- 229.Bush KT, et al. In: Drug Transporters: Molecular Characterization and Role in Drug Disposition. You G, Morris ME, editors. John Wiley & Sons; 2014. pp. 25–42. [Google Scholar]
- 230.Nozaki Y, et al. Species difference in the inhibitory effect of nonsteroidal anti-inflammatory drugs on the uptake of methotrexate by human kidney slices. J. Pharmacol. Exp. Ther. 2007;322:1162–1170. doi: 10.1124/jpet.107.121491. [DOI] [PubMed] [Google Scholar]
- 231.Shibayama Y, et al. Effect of methotrexate treatment on expression levels of multidrug resistance protein 2, breast cancer resistance protein and organic anion transporters Oat1, Oat2 and Oat3 in rats. Cancer Sci. 2006;97:1260–1266. doi: 10.1111/j.1349-7006.2006.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cheng Y, Vapurcuyan A, Shahidullah M, Aleksunes LM, Pelis RM. Expression of organic anion transporter 2 in the human kidney and its potential role in the tubular secretion of guanine-containing antiviral drugs. Drug Metab. Dispos. 2012;40:617–624. doi: 10.1124/dmd.111.042036. [DOI] [PubMed] [Google Scholar]
- 233.Staud F, Cerveny L, Ahmadimoghaddam D, Ceckova M. Multidrug and toxin extrusion proteins (MATE/SLC47); role in pharmacokinetics. Int. J. Biochem. Cell Biol. 2013;45:2007–2011. doi: 10.1016/j.biocel.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 234.Aslamkhan AG, et al. The flounder organic anion transporter fOat has sequence, function, and substrate specificity similarity to both mammalian Oat1 and Oat3. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R1773–R1780. doi: 10.1152/ajpregu.00326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cihlar T, et al. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol. Pharmacol. 1999;56:570–580. doi: 10.1124/mol.56.3.570. [DOI] [PubMed] [Google Scholar]
- 236.Mulato AS, Ho ES, Cihlar T. Nonsteroidal anti-inflammatory drugs efficiently reduce the transport and cytotoxicity of adefovir mediated by the human renal organic anion transporter 1. J. Pharmacol. Exp. Ther. 2000;295:10–15. [PubMed] [Google Scholar]
- 237.Hsiang B, et al. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J. Biol. Chem. 1999;274:37161–37168. doi: 10.1074/jbc.274.52.37161. [DOI] [PubMed] [Google Scholar]
- 238.Nakai D, et al. Human liver-specific organic anion transporter, LST-1, mediates uptake of pravastatin by human hepatocytes. J. Pharmacol. Exp. Ther. 2001;297:861–867. [PubMed] [Google Scholar]
- 239.Sasaki M, Suzuki H, Ito K, Abe T, Sugiyama Y. Transcellular transport of organic anions across a double-transfected Madin-Darby canine kidney II cell monolayer expressing both human organic anion-transporting polypeptide (OATP2/SLC21A6) and Multidrug resistance-associated protein 2 (MRP2/ABCC2) J. Biol. Chem. 2002;277:6497–6503. doi: 10.1074/jbc.M109081200. [DOI] [PubMed] [Google Scholar]
- 240.Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J. Pharmacol. Exp. Ther. 2004;308:438–445. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- 241.Takeda M, et al. Evidence for a role of human organic anion transporters in the muscular side effects of HMG-CoA reductase inhibitors. Eur. J. Pharmacol. 2004;483:133–138. doi: 10.1016/j.ejphar.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 242.Hirano M, Maeda K, Hayashi H, Kusuhara H, Sugiyama Y. Bile salt export pump (BSEP/ABCB11) can transport a nonbile acid substrate, pravastatin. J. Pharmacol. Exp. Ther. 2005;314:876–882. doi: 10.1124/jpet.105.084830. [DOI] [PubMed] [Google Scholar]
- 243.Kitamura S, Maeda K, Wang Y, Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab. Dispos. 2008;36:2014–2023. doi: 10.1124/dmd.108.021410. [DOI] [PubMed] [Google Scholar]
- 244.Keskitalo JE, et al. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin. Pharmacol. Ther. 2009;86:197–203. doi: 10.1038/clpt.2009.79. [DOI] [PubMed] [Google Scholar]
- 245.Ho RH, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130:1793–1806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 246.Ito S, et al. Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J. Pharmacol. Exp. Ther. 2012;340:393–403. doi: 10.1124/jpet.111.184986. [DOI] [PubMed] [Google Scholar]
- 247.Kurata T, Muraki Y, Mizutani H, Iwamoto T, Okuda M. Elevated systemic elimination of cimetidine in rats with acute biliary obstruction: the role of renal organic cation transporter OCT2. Drug Metab. Pharmacokinet. 2010;25:328–334. doi: 10.2133/dmpk.dmpk-10-rg-004. [DOI] [PubMed] [Google Scholar]
- 248.Ohta KY, Inoue K, Yasujima T, Ishimaru M, Yuasa H. Functional characteristics of two human MATE transporters: kinetics of cimetidine transport and profiles of inhibition by various compounds. J. Pharm. Pharm. Sci. 2009;12:388–396. doi: 10.18433/j3r59x. [DOI] [PubMed] [Google Scholar]
- 249.Tsuda M, et al. Involvement of human multidrug and toxin extrusion 1 in the drug interaction between cimetidine and metformin in renal epithelial cells. J. Pharmacol. Exp. Ther. 2009;329:185–191. doi: 10.1124/jpet.108.147918. [DOI] [PubMed] [Google Scholar]
- 250.Tsuda M, et al. Targeted disruption of the multidrug and toxin extrusion 1 (Mate1) gene in mice reduces renal secretion of metformin. Mol. Pharmacol. 2009;75:1280–1286. doi: 10.1124/mol.109.056242. [DOI] [PubMed] [Google Scholar]
- 251.Tzvetkov MV, et al. The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3 on the renal clearance of metformin. Clin. Pharmacol. Ther. 2009;86:299–306. doi: 10.1038/clpt.2009.92. [DOI] [PubMed] [Google Scholar]
- 252.Ahmadimoghaddam D, Staud F. Transfer of metformin across the rat placenta is mediated by organic cation transporter 3 (OCT3/SLC22A3) and multidrug and toxin extrusion 1 (MATE1/ SLC47A1) protein. Reprod. Toxicol. 2013;39:17–22. doi: 10.1016/j.reprotox.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 253.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin. Pharmacol. Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.More SS, et al. Organic cation transporters modulate the uptake and cytotoxicity of picoplatin, a third-generation platinum analogue. Mol. Cancer Ther. 2010;9:1058–1069. doi: 10.1158/1535-7163.MCT-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ho GT, et al. ABCB1/MDR1 gene determines susceptibility and phenotype in ulcerative colitis: discrimination of critical variants using a gene-wide haplotype tagging approach. Hum. Mol. Genet. 2006;15:797–805. doi: 10.1093/hmg/ddi494. [DOI] [PubMed] [Google Scholar]
- 256.Krupoves A, et al. Associations between ABCB1/MDR1 gene polymorphisms and Crohn’s disease: a gene-wide study in a pediatric population. Inflamm. Bowel Dis. 2009;15:900–908. doi: 10.1002/ibd.20849. [DOI] [PubMed] [Google Scholar]