Abstract
Objectives
To estimate the incidence of surgical complications and associated in-hospital morbidity and mortality following surgery for malignant brain tumors.
Patients and methods
The Nationwide Inpatient Sample (NIS) database was queried from 2002 to 2011. All adult patients who underwent elective brain surgery for a malignant brain tumor were included. Surgical complications included wrong side surgery, retention of a foreign object, iatrogenic stroke, meningitis, hemorrhage/hematoma complicating a procedure, and neurological complications. A regression model was conducted to estimate the odds ratios (OR) with their 95% confidence intervals (95% CI) of in-hospital mortality for each surgical complication.
Results
A total of 16,530 admissions were analyzed, with 601 (36.2 events per 1000 cases) surgical complications occurring in 567 patients. Over the examined 10-year period, the overall incidence of surgical complications did not change (P = 0.061) except for iatrogenic strokes, which increased in incidence from 14.1 to 19.8 events per 1000 between 2002 and 2011 (P = 0.023). Patients who developed a surgical complication had significantly longer lengths of stay, total hospital costs, and higher rates of other complications. Patients who experienced an iatrogenic stroke had a significantly increased risk of mortality (OR 9.6; 95% 6.3–14.8) and so were patients with a hemorrhage/hematoma (OR 3.3; 95% CI 1.6–6.6).
Conclusion
In this study of an administrative database, patients undergoing surgery for a malignant brain tumor who suffered from a surgical complication had significantly longer lengths of stay, total hospital charges, and complication rates. Having a surgical complication was also an independent risk factor for inhospital mortality. Nonetheless, it is unclear whether all surgical complications were clinically relevant, and further research is encouraged.
Keywords: Surgical complication, Neurosurgery, Malignant brain tumor, Nationwide inpatient sample, Sentinel event
1. Introduction
Malignant brain tumors are the most common primary brain tumor, with an estimated incidence in adults of 5.26 cases per 100,000 persons per year in the United States [1]. Although newer treatment modalities such as chemotherapy and immune therapy have emerged as potential adjuvants for management of these tumors, surgery continues to be first-line therapy [1,2]. The importance of this, however, is that newer evidence has supported more aggressive resections as a mode to improve survival [3,4]. Nonetheless, more aggressive resections carry an increased risk of potentially catastrophic surgical complications, including irreversible neurological deficits.
The purpose of this study is to estimate the incidence of surgical complications, (including sentinel events) in patients undergoing malignant brain tumor surgery using a large administrative database, and analyze their impact on in-hospital morbidity and mortality.
2. Patients and methods
2.1. Study design and data source
In this retrospective cohort study, the Nationwide Inpatient Sample (NIS) administrative database was queried for the years 2002–2011. The NIS, as part of the Healthcare Cost and Utilization Project (HCUP), is the largest inpatient administrative database in the United States, reporting approximately 8 million admissions from a 20% sample of all non-federal hospitals per year. The NIS database reports diagnoses, procedures and complications in the form of ICD-9-CM (International Classification of Disease-9th Edition-Clinical Modification) codes.
2.2. Inclusion and exclusion criteria
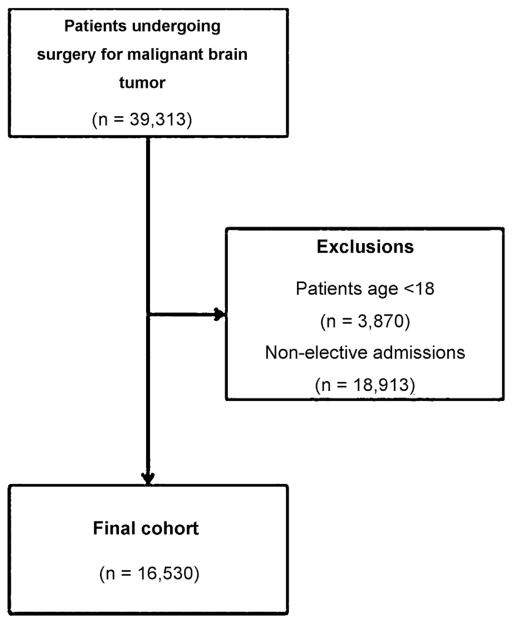
Patients with a primary diagnosis of a malignant brain tumor (codes 191.0–1.919) that underwent a craniotomy or craniectomy (01.20–01.29), incision of brain and cerebral meninges (01.31–01.39) or excision/destruction of lesion or tissue of brain (01.59) were included. Patients under the age of 18 years and with non-elective admissions were excluded from this study (Fig. 1).
Fig. 1.
Framework for patient selection shows inclusion/exclusion criteria.
2.3. Data collection
Patients were divided into two cohorts: non-surgical complication and surgical complication. In accordance with the Joint Commission guidelines, patients with retention of a foreign object (codes E870.1 and 998.4) and patients who received wrong side surgery (E876.5–E876.9) were classified as having a surgical complication [5]. Additionally, patients who experienced an iatrogenic stroke (997.02), meningitis (320.0–320.9) or hemorrhage/hematoma complicating a procedure (998.1–998.13) were included in the surgical complication cohort [6]. These complications were chosen based on the work by Landriel-Ibañez et al., who proposed a new classification for complications in neurosurgery. In the proposed system, the aforementioned complications are considered “Grade III” surgical complications, and refer to “life-threatening adverse events requiring treatment and care in a more complex hospital area” [6]. Patients with codes 997.00–997.09 were also classified as having a neurosurgical complication, in accordance to a recent study utilizing the NIS to examine outcomes after brain tumor resection [7].
Demographic variables such as age, sex, hospital location, and teaching status were collected for each admission. Hospital location is determined via use of Core Based Statistical Area (CBSA) codes. Hospitals located in counties with a CBSA type of metropolitan were considered “urban” hospitals, whereas hospitals with a CBSA type of micropolitan were classified as “rural” hospital. Hospitals are considered “teaching hospitals” if they meet any of the following criteria: (1) an approved residency program, (2) is member of the Council of Teaching Hospitals, or (3) has a ratio of interns/residents to beds of 0.25 or higher. Overall preoperative comorbidity was calculated via the Elixhauser comorbidity score, an established method to calculate a score from administrative databases; this method adds 1 point per comorbidity (from a list of 30 comorbidities) to produce a final score [8].
2.4. Outcomes
Both cohorts were analyzed to compare outcomes in the form of other complication development, average length of stay, total hospital charges and mortality. Other complications included: pneumonia (481, 482, 483), myocardial infarction (410.0–410.91), acute kidney injury (584.5–584.9), pulmonary complications (518.5–518.53), pancreatitis (577.0), urinary tract infection (UTI; 595.0, 595.9, 599.0), deep vein thrombosis (453.4–453.42, 453.8, 453.9) pulmonary embolism (415.22, 415.13, 415.19) and surgical site complication (998.83, 998.32, 998.51, 998.59, 998.6) [6].
2.5. Statistical analysis
Descriptive statistical analyses were performed to compare demographic variables between cohorts. Results are presented as mean ± standard deviation when applicable or as mean with interquartile ranges (IQR) for non-parametric data. Continuous data was compared via the Student’s T-test and non-continuous data was compared via the χ2 test. A linear regression analysis was used to analyze trends of surgical complications, length of stay and total hospital charges over time. A multivariable logistical regression analysis was conducted to estimate odds ratios (OR) of mortality with their 95% confidence intervals (CI). OR analyses were controlled for patient age, sex and comorbidities. P-values <0.05 were considered significant. Statistical analyses were performed using STATA SE 12 (StataCorp LP, College Station, Texas).
3. Results
A total of 16,530 patients who underwent surgery for a malignant brain tumor between 2002 and 2011 were identified, with 567 patients (3.4%) in the surgical complication cohort [Table 1]. Patients in the surgical complication and non-surgical complication cohorts were similar in terms of age and sex. However, the median number of comorbidities in the surgical complication cohort was two, compared to one in the non-surgical complication cohort (P < 0.001). The proportion of patients treated at urban and teaching hospitals was not statistically different between cohorts.
Table 1.
Demographics of patients undergoing elective cranial neurosurgery for malignant brain tumors between 2002 and 2011.
| Variable | No surgical complication | Surgical complication | P-value |
|---|---|---|---|
| No. cases | 15,963 | 567 | |
| Age | 53.9 ± 16.3 | 54.7 ± 17.3 | 0.126 |
| Sex (male, %) | 57.5 | 59.8 | 0.286 |
| Elixhauser comorbidity score (median, IQR) | 1 (0–8) | 2 (0–6) | <0.001 |
| Urban hospital (%) | 97.6 | 96.7 | 0.234 |
| Teaching hospital (%) | 84.2 | 82.6 | 0.394 |
| Complications (%) | 5.4 | 19.2 | <0.001 |
| Pneumonia (%) | 0.6 | 3.5 | <0.001 |
| Myocardial infarction (%) | 0.1 | 1.9* | 0.059 |
| Acute kidney injury (%) | 0.5 | 1.9* | <0.001 |
| Respiratory complication (%) | 1.1 | 9.0 | <0.001 |
| Pancreatitis (%) | 0.1* | 1.9* | 0.075 |
| UTI (%) | 2.4 | 9.9 | <0.001 |
| DVT (%) | 1.0 | 5.1 | <0.001 |
| PE (%) | 0.2 | 1.9* | 0.034 |
| Surgical site complication (%) | 0.3 | 3.9 | <0.001 |
| Length of stay (mean) | 4.4 ± 4.7 | 11.8 ± 11.8 | <0.001 |
| Mortality (%) | 0.7 | 9.0 | <0.001 |
| Total charges ($) | 53,638 ± 41,801 | 111,518 ± 105,304 | <0.001 |
IRQ: interquartile range, UTI: urinary tract infection, DVT: deep vein thrombosis, PE: pulmonary embolism.
Boldface denotes statistically significant results.
NIS does not allow the publication of values that are less than or equal to 10 observations, so the number 11 was used to calculate percentages.
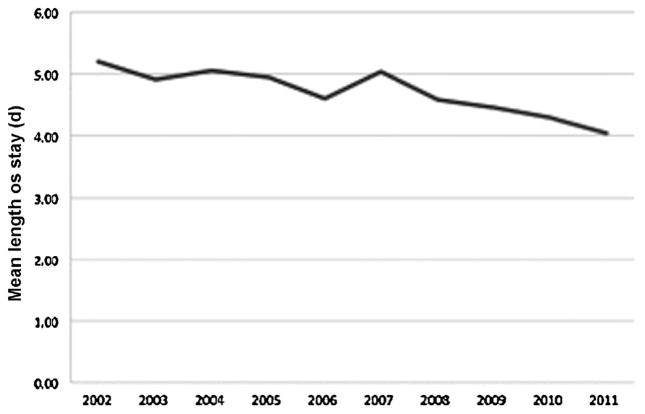
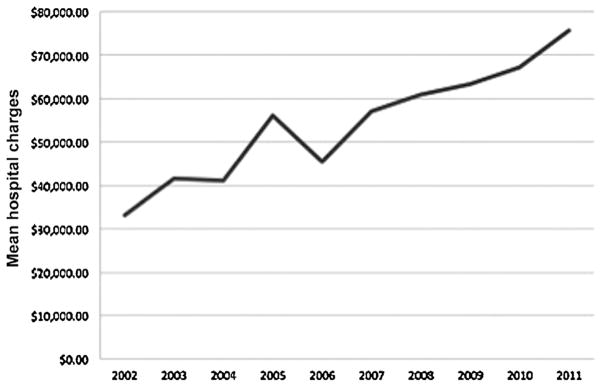
When analyzing hospital resource utilization, patients with a surgical complication had significantly longer average lengths of stay (11.8 vs. 4.4 days, P < 0.001) and double the total hospital charges ($111,518 vs. $53,638, P < 0.001). Length of stay significantly decreased over time from an average of 5.2 days in 2002 to 4.0 days in 2011 (P < 0.001) [Fig. 2]. On the other hand, total hospital charges increased from a mean of $33,210 in 2002 to $75,774 in 2011 (P < 0.001) [Fig. 3].
Fig. 2.
Average length of stay for patients undergoing malignant brain tumor surgery between 2002 and 2011. Length of stay significantly decreased over time (P < 0.001).
Fig. 3.
Mean total hospital charges for patients undergoing malignant brain tumor surgery between 2002 and 2011. Hospital charges increased significantly over time (P < 0.001).
Additionally, patients in the surgical complication cohort had significantly higher rates of other complications (19.2% vs. 5.4%, P < 0.001), including pneumonia, acute kidney injury, respiratory complications, UTI, DVT, PE and surgical site complications (including wound infection).
During the examined period, there were a total of 601 documented surgical complications (36.2 per 1000 cases) occurring in 567 patients; 34 patients had two surgical complications. There were less than 10 cases of foreign object retention (0.7 per 1000 cases), less than 10 wrong-side surgeries (0.7 per 1000 cases), 269 cases of iatrogenic stroke (16.3 per 1000), 19 cases of meningitis (1.1 per 1000), 170 cases of hemorrhage/hematoma complicating a procedure (10.3 per 1000), and 137 cases of other neurological complications (8.2 per 1000) [Table 2]. During this period, the only event that increased in incidence was iatrogenic stroke, with a 2002 incidence of 14.1 per 1000 compared to 19.8 per 1000 cases in 2011 (P = 0.023); the overall incidence of surgical complications did not significantly change over the 10-year period (P = 0.061). A comparison between surgical complications between patients who underwent surgery for a malignant brain tumor and patients who underwent surgery for a benign tumor (from a cohort of patients operated on between 2002 and 2011 from the NIS) revealed that the complication rate was higher in the benign tumor group (4.5% vs. 3.4%, P < 0.001). Nevertheless, it is unclear whether this 1.1% absolute difference would be clinically-relevant, and warrants further investigation.
Table 2.
Incidence of surgical complications in elective cranial neurosurgery for malignant brain tumors between 2002 and 2011.
| Year | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | Total | P – value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | 728 | 1,588 | 1,466 | 1,461 | 1,769 | 1,891 | 1,874 | 1,580 | 2,056 | 2,117 | 16,530 | |
| Object retention | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | * | 0.7 (n < 10)* | 0.149 |
| Wrong-side surgery | 0.0 | * | 0.0 | 0.0 | 0.0 | 0.0 | * | 0.0 | 0.0 | 0.0 | 0.7 (n < 10)* | 0.400 |
| Iatrogenic stroke | 14.1 | 15.1 | 10.2 | 13.0 | 17.0 | 13.7 | 19.7 | 15.8 | 19.5 | 19.8 | 16.3 (n = 269) | 0.023 |
| Meningitis | 0.0 | * | * | * | * | * | * | * | * | * | 1.1 (n = 19) | 0.266 |
| Hemorrhage or hematoma | 6.4 | 10.0 | 10.2 | 15.1 | 13.0 | 8.5 | 8.0 | 11.4 | 12.6 | 6.6 | 10.3 (n = 170) | 0.536 |
| Other nervous system complication | * | 6.9 | 10.2 | 8.9 | 7.3 | 7.4 | * | 10.8 | 11.2 | 9.4 | 8.2 (n = 137) | 0.126 |
| Total incidence | 23.0 (n = 18) | 34.0 (n = 54) | 31.4 (n = 46) | 37.6 (n = 55) | 39.0 (n = 69) | 30.0 (n = 57) | 36.3 (n = 68) | 40.5 (n = 64) | 44.3 (n = 91) | 37.3 (n = 79) | 36.4 (n = 601) | 0.061 |
Incidence is per 1000 cases.
Boldface denotes statistically significant results (linear regression analysis).
NIS does not allow the publication of values that are less than or equal to 10 observations.
Patients in the surgical complication cohort had a 9.0% mortality compared to only 0.7% in the non-surgical complication t cohort (P < 0.001). Following multivariable analysis, it was found that patients with any surgical complication were 4.4 times more likely to die during their hospital stay (OR 4.4; 95% CI, 2.9–6.6), and so were patients with an iatrogenic stroke (OR 9.6; 95% CI 6.3–14.8) and hemorrhage/hematoma (OR 3.3; 95% CI 1.6–6.6) [Table 3].
Table 3.
Multivariable analysis showing odds ratios of in-hospital mortality stratified by surgical complication.
| Sentinel event | OR | 95% confidence interval | P-value |
|---|---|---|---|
| Object retention | 0.00 | 0.0–0.0 | – |
| Wrong-side surgery | 0.00 | 0.0–0.0 | – |
| Iatrogenic stroke | 9.6 | 6.3–14.8 | <0.001 |
| Meningitis | 0.6 | 0.1–5.4 | 0.688 |
| Hemorrhage or hematoma | 3.3 | 1.6–6.6 | <0.001 |
| Other neurological complication | 0.00 | 0.0–0.0 | – |
| Any surgical complication | 4.4 | 2.9–6.6 | <0.001 |
OR: odds ratio.
All analyses were adjusted for patient age, sex and comorbidities.
Boldface denotes statistically significant results.
4. Discussion
Malignant brain tumors (most commonly high-grade astrocytomas such as glioblastoma multiforme) are the most common primary brain tumor, and incidence rates have increased over time [9]. Surgery is considered first-line therapy for these patients [10–12], but attempting resection of these tumors carries inherent risks such as neurological deficits and subsequent decreases in overall survival [13].
The present study analyzed 16,530 admissions of patients undergoing surgery for malignant brain tumors, demonstrating a 3.4% risk of having at least one surgical complication. The most common surgical complication was iatrogenic stroke, with an estimated incidence of 16.3 per 1000 cases. Notably, this event was the only event to significantly increase over the examined 10-year period. Moreover, suffering from an iatrogenic stroke increased the risk of in-hospital mortality by 9-fold. In a study by Gempt et al. analyzing the incidence of infarctions following glioma resection, 31% of patients were found to have new postoperative ischemic lesions (assessed by diffusion-weighted imaging) with an associated decline in neurological function [14]. This group of authors identified the proximity of the tumor to central arteries as the greatest risk factor for postoperative ischemic damage [14].
The second most common surgical complication was hemorrhage or hematoma complicating a procedure, with an incidence of 10.3 per 1000 cases. Similar to iatrogenic strokes, having a hemorrhage/hematoma carried a significantly increased risk of in-hospital mortality (OR 3.3; 95% CI 1.6–6.6). Postoperative hemorrhage has been also recognized as a complication following glioma surgery, and a study by Tanaka et al. found a rate of 5.6%, with one case of fatal hemorrhage following surgery [15].
Retention of a foreign object and wrong side surgery were relatively rare events, with reported incidences of less than 0.7 per 1000 cases. Notably, these cases were not found to carry an increased risk of mortality. Postoperative meningitis occurred in 1.1 per 1000 cases, and also did not increase the risk of mortality.
Both retention of a foreign object and wrong side surgery are considered “sentinel events” by the Joint Commission, an organization that evaluates, accredits and certifies more than 20,000 health care organizations and programs in the United States according to certain performance standards. According to the Joint Commission, these events are preventable, and the cause is often attributed to systems and processes rather than to an individual person [16]. On the other hand, iatrogenic strokes, hemorrhage/hematoma and meningitis are more related to the procedure itself and postoperative care. Of note, the less than 10 cases of wrong-side surgery in the present study occurred prior to the year 2008, when the surgical safety checklist was implemented [17].
A study published by Landriel Ibanez et al. encouraged a new classification for complications following neurosurgical procedures [6]. This classification consists of three grades, with Grade III surgical complications implying “life-threatening complications requiring management in Intensive Care Unit” [6]. According to this proposed classification, iatrogenic strokes, hemorrhage/hematoma and meningitis are all considered surgical complications and thus were included in this cohort.
The significant increase in iatrogenic strokes in the examined 10-year period warrants special attention. During the last decade, numerous studies have supported gross total resection as a means to improve survival [12,18], with recent reports encouraging at least a 70% resection and less than 5 mL of residual tumoral volume [3,4]. As stated by Gulati et al., “glioblastoma surgery is a delicate balance between achieving maximal tumor resection and inducing new deficits” [19]. While both surgeons and patients may be tempted to resect as much as possible, a thorough discussion with the patient should be made prior to surgery, and benefits of a potential increased survival with the associated risk of neurological deficits should be discussed. Currently, it is unclear whether all iatrogenic strokes and hemorrhages/hematomas in this study were merely incidental postoperative imaging findings (not uncommon in the setting of brain tumor resection) or associated with neurological deficits. While the increase in iatrogenic strokes may be a result of the shift toward more aggressive resections in recent years, more sensitive postoperative imaging techniques might have also played a significant role in increasing the diagnosis of postoperative ischemia. Nevertheless, extensive adjustment analysis revealed both of these vascular complications to be independent risk factors for in-hospital morbidity, which may indicate that they could have been clinically relevant to some degree.
The postoperative course of patients who experienced a surgical complication was significantly different from patients without a surgical complication. The former group had significantly higher in-hospital complications and stayed on average 11.8 days in the hospital, compared to only 4.4 in the latter cohort. Additionally, hospital costs almost doubled at an estimated of $111,518 U.S. dollars. The impact of surgical complications on hospital resource utilization is a potential incentive for quality of care improvement, and both the hospital staff and surgeons must cooperate to decrease the incidence of this events [16].
In a study by Zacharia et al., the authors utilized the NIS to investigate the incidence of Hospital-Acquired Conditions (HACs) following resection of benign and malignant brain tumors [7]. HACs included retention of a foreign object, air embolism, blood incompatibility, pressure ulcers, falls, urinary tract infection, surgical site infection, and others. The authors found a HAC incidence of 5.4%, and having a HAC was an independent risk factor for in-hospital mortality and increased hospital costs [7]. However, that study did not examine trends over time, nor did it examine the independent impact of each HAC on in-hospital mortality.
4.1. Strengths and limitations
The findings in the present study provide several useful insights to the neurosurgical community. First, an analysis over time revealed that while the rate of all surgical complications remained steady over time, only iatrogenic strokes increased, most likely due to the increased aggressiveness of tumor resection. Second, it was demonstrated via extensive adjusted analyses that patients who developed a surgical complication had a significantly increased risk of mortality, providing further evidence to further analyze the risks and benefits of overly-aggressive surgery.
This study however, has some limitations. The use of an administrative database carries the risk of coding or reporting bias. Identification of vascular complications such as iatrogenic stroke and hemorrhage/hematoma was done entirely via ICD-9 codes, and it is unclear what proportion of these complications were clinically relevant, or asymptomatic procedure-related radiographic findings. It is not uncommon to find small hemorrhages/hematomas on imaging following brain tumor resection, and more extensive investigation into this matter is warranted. The lower surgical complication rate (3.4%) found in this study when compared to other clinical series may be explained by the fact that other common surgical complications are not captured by the NIS. For example, adverse events such as transient new neurological deficits, transient diabetes insipidus, and postoperative seizures requiring anticonvulsant do not have such specific ICD-9 codes [6], and thus are difficult to capture in an administrative database. Although in-hospital complications could be identified via ICD9-CM codes, complications occurring outside the hospital could not be assessed. Likewise, neurological status or other functional long-term outcomes cannot be assessed using the NIS.
Overall, it is difficult to translate administrative data to the clinical setting. However, the fact remains that studies have advocated for more aggressive tumor resection [3,12], in an attempt to improve survival. Whether the increase in iatrogenic strokes observed in our study is a consequence of these studies cannot be proven just yet, and more evidence is needed. Likewise, though it appears that iatrogenic strokes were an independent risk factor for mortality, it may also be possible that unmeasured covariates (such as preoperative performance status, tumor location, tumor size, etc.) had an influence on inpatient death, and we believe prospective multi-center collaborations are the most desired for this type of question.
5. Conclusion
In this study of an administrative database, the incidence of surgical complications following malignant brain tumor surgery was estimated at 36.2 per 1000 cases. Patients with a surgical complication experienced higher complication rates, longer hospitalizations, increased hospital costs and a significantly increased risk of in-hospital mortality. Iatrogenic strokes were the only surgical complication to significantly increase in the examined 10-year period, potentially due to the increasing evidence that more aggressive tumor resection leads to increased survival. Nonetheless, the risks and benefits of overly aggressive surgery warrant further investigation, inasmuch as patients who experienced an iatrogenic stroke were nine times more likely to die during their hospital stay.
Footnotes
Competing interests disclosures
None.
Funding
No funding.
Conception and design: MB, RDLGR; acquisition of data: RDLGR, PK, MB; analysis and interpretation of data: RDLGR, PK, MB; drafting the article: RDLGR, PK, RJT, HB, JH, MB; critically revising the article: MB; reviewed submitted version of manuscript: all authors; approved the final version of the manuscript on behalf of all authors: PK, MB; administrative/technical/material support: RDLGR, PK, MB; study supervision: RJT, HB, JH, MB.
References
- 1.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 2.Sherman JH, Hoes K, Marcus J, Komotar RJ, Brennan CW, Gutin PH. Neurosurgery for brain tumors: update on recent technical advances. Curr Neurol Neurosci Rep. 2011;11:313–319. doi: 10.1007/s11910-011-0188-9. [DOI] [PubMed] [Google Scholar]
- 3.Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I, Wijesekera O, Olivi A, Rahman M, Quinones-Hinojosa A. When gross total resection of a glioblastoma is possible. How much resection should be achieved? World Neurosurg. 2014;82:257–265. doi: 10.1016/j.wneu.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, Raza SM, Pascual-Gallego M, Ibrahim A, Hernandez-Hermann M, Gomez L, Ye X, Weingart JD, Olivi A, Blakeley J, Gallia GL, Lim M, Brem H, Quinones-Hinojosa A. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro-oncology. 2014;16:113–122. doi: 10.1093/neuonc/not137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comission TJ. Sentinel Event. 2014;2014 [Google Scholar]
- 6.Landriel Ibanez FA, Hem S, Ajler P, Vecchi E, Ciraolo C, Baccanelli M, Tramontano R, Knezevich F, Carrizo A. A new classification of complications in neurosurgery. World Neurosurg. 2011;75:709–715. doi: 10.1016/j.wneu.2010.11.010. discussion 604–711. [DOI] [PubMed] [Google Scholar]
- 7.Zacharia BE, Deibert C, Gupta G, Hershman D, Neugut AI, Bruce JN, Spencer BA. Incidence, cost, and mortality associated with hospital-acquired conditions after resection of cranial neoplasms. Neurosurgery. 2014;74:638–647. doi: 10.1227/NEU.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 8.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Fischer JL, Wrensch M, Wiemels JL, Schwartzbaum JA. Epidemiology of brain tumors. In: Winn R, editor. Youmans Neurological Surgery. Saunders; 2011. pp. 1179–1187. [Google Scholar]
- 10.Chaichana KL, Chaichana KK, Olivi A, Weingart JD, Bennett R, Brem H, Quinones-Hinojosa A. Surgical outcomes for older patients with glioblastoma multiforme: preoperative factors associated with decreased survival. Clinical article. J Neurosurg. 2011;114:587–594. doi: 10.3171/2010.8.JNS1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaichana KL, Garzon-Muvdi T, Parker S, Weingart JD, Olivi A, Bennett R, Brem H, Quinones-Hinojosa A. Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Ann Surg Oncol. 2011;18:239–245. doi: 10.1245/s10434-010-1242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 13.McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–469. doi: 10.1227/01.NEU.0000349763.42238.E9. discussion 469–470. [DOI] [PubMed] [Google Scholar]
- 14.Gempt J, Forschler A, Buchmann N, Pape H, Ryang YM, Krieg SM, Zimmer C, Meyer B, Ringel F. Postoperative ischemic changes following resection of newly diagnosed and recurrent gliomas and their clinical relevance. J Neurosurg. 2013;118:801–808. doi: 10.3171/2012.12.JNS12125. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka S, Meyer FB, Buckner JC, Uhm JH, Yan ES, Parney IF. Presentation, management, and outcome of newly diagnosed glioblastoma in elderly patients. J Neurosurg. 2013;118:786–798. doi: 10.3171/2012.10.JNS112268. [DOI] [PubMed] [Google Scholar]
- 16.Marquez-Lara A, Nandyala SV, Hassanzadeh H, Sundberg E, Jorgensen A, Singh K. Sentinel events in lumbar spine surgery. Spine. 2014 doi: 10.1097/BRS.0000000000000247. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, Herbosa T, Joseph S, Kibatala PL, Lapitan MC, Merry AF, Moorthy K, Reznick RK, Taylor B, Gawande AA. Safe Surgery Saves Lives Study G, A surgical safety checklist to reduce morbidity and mortality in a global population. New Engl J Med. 2009;360:491–499. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 18.Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, Rohde V, Oppel F, Turowski B, Woiciechowsky C, Franz K, Pietsch T Group AL-GS. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62:564–576. doi: 10.1227/01.neu.0000317304.31579.17. discussion 564–576. [DOI] [PubMed] [Google Scholar]
- 19.Gulati S, Jakola AS, Nerland US, Weber C, Solheim O. The risk of getting worse: surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 2011;76:572–579. doi: 10.1016/j.wneu.2011.06.014. [DOI] [PubMed] [Google Scholar]