Abstract
Introduction
One of the most effective interventions for intractable major depressive episodes is electroconvulsive therapy (ECT). Because ECT is also relatively fast-acting, longitudinal study of its neurobiological effects offers critical insight into the mechanisms underlying depression and antidepressant response. Here we assessed modulation of intrinsic brain activity in corticolimbic networks associated with ECT and clinical response.
Methods
We measured resting-state functional connectivity (RSFC) in patients with treatment-resistant depression (n=30), using functional magnetic resonance imaging (fMRI) acquired before and after completing a treatment series with right-unilateral ECT. Using independent component analysis, we assessed changes in RSFC with 1) symptom improvement and 2) ECT regardless of treatment outcome in patients, with reference to healthy controls (n=33, also scanned twice).
Results
After ECT, consistent changes in RSFC within targeted depression-relevant functional networks were observed in the dorsal anterior cingulate (ACC), mediodorsal thalamus (mdTh), hippocampus, and right anterior temporal, medial parietal, and posterior cingulate cortex in all patients. In a separate analysis, changes in depressive symptoms were associated with RSFC changes in the dorsal ACC, mdTh, putamen, medial prefrontal, and lateral parietal cortex. RSFC of these regions did not change in healthy controls.
Conclusions
Neuroplasticity underlying clinical change was in part separable from changes associated with the effects of ECT observed in all patients. However, both ECT and clinical change were associated with RSFC modulation in dorsal ACC, mdTh and hippocampus, which may indicate that these regions underlie the mechanisms of clinical outcome in ECT and may be effective targets for future neurostimulation therapies.
Keywords: depression, ECT, connectivity, fMRI, anterior cingulate, thalamus
Introduction
Current theories describe major depression as a brain network disorder, manifesting as hyperactivity in ventral limbic structures together with dysregulation by hypoactive dorsal ACC, lateral prefrontal cortex, and/or related structures (1–4). However, the interrelationships amongst these structures as related to treatment and clinical response to treatment have yet to be empirically determined. Electroconvulsive therapy (ECT) is an effective intervention for patients with major depressive episodes (MDEs), and occurs by eliciting controlled transient seizures every 2-3 days over 2-4 weeks, sometimes followed by maintenance sessions (5). Because both response rates (50-80%) and response times (≤1 month) are better for ECT than for other currently available treatments (6–8), longitudinal neuroimaging research of ECT-induced treatment response offers a pivotal opportunity to improve our understanding of the role of corticolimbic networks in depression and antidepressant response to treatment.
Previous studies have demonstrated that ECT elicits changes in specific brain regions as impacted by electrode placement (9; 10). Some of the brain structures affected by ECT, including the ACC (11–13) and hippocampus (14; 15), are also frequently implicated in the pathophysiology of major depression by other studies. However, not all patients respond to ECT; for example, just over half (55-65%) experience remission when using right-unilateral ECT with optimal parameters (16; 17). Therefore, brain networks affected by ECT-induced seizures in all patients may differ or only partially overlap with networks supporting improved depressive symptoms in patients that respond to ECT. To date, very few neuroimaging studies address the contributions of ECT-induced seizures and symptom improvement to structural or functional neuroplasticity, relying instead on post hoc analyses of symptoms in regions already showing ECT effects (11; 18), or restricting analyses to treatment responders (12; 19; 20). Therefore, some ECT-related effects reported previously may not underlie clinical outcome, but instead reflect nonspecific physiological effects of ECT unrelated to depressive symptoms. ECT research is further complicated by the challenges in recruiting a sufficiently large and homogeneous study sample, as ECT is typically reserved for more severe or treatment-resistant depression and may be avoided due to its potential cognitive side effects and lingering stigma. Thus, neuroimaging research has yet to form a coherent understanding of the neurobiology of ECT.
In the current study, we used functional magnetic resonance imaging (fMRI) to examine changes in resting state functional connectivity (RSFC) associated both with ECT itself (ΔECT) and with changes in depressive symptoms during ECT (ΔMD). We measured RSFC during fMRI scans in patients before right-unilateral ECT and after 2-4 weeks of index treatments, and in healthy volunteers assessed 2-4 weeks apart to quantify normative values and variance. We used independent component analysis (ICA) to define resting-state networks (RSNs), which are comprised of brain regions that share temporally coherent (i.e., correlated) intrinsic brain activity while participants are at rest. In particular, we targeted well-characterized RSNs (21–24) overlapping medial fronto-limbic and temporal regions previously implicated in depression and ECT response, specifically medial prefrontal cortex, ACC, and associated fronto-thalamo-striatal networks, and hippocampus. However, because we hypothesized that ΔECT and ΔMD effects would be unlikely to be captured by a single RSN, we measured RSFC changes both 1) within each RSN, and 2) overlapping across RSNs in partial conjunction analyses.
Methods and Materials
Participants
Thirty patients (16 female, age mean/STD = 40.90/12.45 years) and 33 demographically similar healthy controls (16 female, age mean/STD = 39.66/12.54 years) gave informed consent to participate in this UCLA IRB-approved study. All patients were characterized as treatment refractory and were experiencing a DSM-IV TR defined MDE; 24 were diagnosed with major depressive disorder and 6 with bipolar disorder, compatible with recent support for reframing mental disorders in terms of shared symptomatology and neurobiology rather than binary diagnoses (25). Depressive symptoms were assessed in patients using the Hamilton Depression Inventory and duration of illness measured from first MDE was variable. Additional participant information is given in Table 1, and inclusion/exclusion criteria and additional clinical information is provided in the attached Supplementary Methods.
Table 1. Participant Characteristics.
| Sex | Age | Age at 1st MDD Episode | Total # ECT Sessions | HAMD Scores | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 2 | 3 | |||||
| MDE Patients (n = 30) | 14m,16f | 40.9 (12.5) | 24.5 (12.5) | 9.4 (3.3) | 26.3 (5.8) | 20.4 (6.4) | 9.3 (5.5) |
| Healthy Controls (n = 32) | 16m,16f | 39.7 (12.6) | n/a | n/a | n/a | n/a | n/a |
| t or χ2* (p-value) | 0.0 (1.0) | 0.39 (0.70) | n/a | n/a | n/a | 5.6 (<0.0001) | 10.7 (<0.0001) |
Abbreviations: Hamilton Depression Rating Scale (HAMD), not applicable (n/a).
Chi-squared (χ2) test was applied to sex data, unpaired t-test compared age data, and paired t-tests compared changes in HAMD score from baseline with ECT.
Electroconvulsive Therapy (ECT) and Research Sessions
Patients volunteered for this research study before initiating a clinically prescribed course of ECT at the UCLA Resnick Neuropsychiatric Hospital. Right-unilateral ECT was administered using standard protocols (Suppl. Methods) after patients were tapered off all psychotropic medications for a minimum of 48-72 hrs and for the duration of the 2-4 week index series. Research sessions included inventories to assess depressive symptoms and MRI scanning at: 1) baseline before starting ECT (MD1), 2) before the third treatment (MD2), and 3) after 2-4 weeks of treatment (MD3) when clinical decisions indicated transition to a maintenance therapy. Controls were also scanned twice, approximately 2-4 weeks apart (CO1 and CO3). Research sessions occurred in the morning prior to patients' ECT sessions; therefore, any changes in functional connectivity measured could be considered “lasting” or cumulative effects of prior treatments.
Image Acquisition and Preprocessing
Using a 3T Siemens Allegra scanner, functional images were acquired: TR = 2.0 s, TE = 30 ms, flip angle = 70°, 34 axial slices, 3.4 × 3.4 × 5 mm3 resolution, 180 volumes. A high-resolution T1-weighted anatomical scan (MPRAGE) was also collected at each session. Preprocessing and normalization procedures are described in detail in the Supplementary Methods.
Statistical Analyses
Independent component analysis (ICA) were executed in FSL (Suppl. Methods) and established cross-subject RSNs. Eight canonical RSNs that a) cover medial cortico-limbic areas previously implicated in depression and emotional processing and b) have been reliably demonstrated in healthy controls in previous research (21–24) were targeted as networks of interest. Two approaches were taken for group-level statistics: one model measured changes in resting state functional connectivity (RSFC) resulting from ECT (ΔECT), and the other measured changes in RSFC associated with changes in depressive symptoms (ΔMD).
ΔECT Analysis
We constructed linear mixed-effects (LME) models implemented in R version 3.0.3 (The R Project for Statistical Computing), with subject as a random factor and the hypothesized ΔECT effect as a fixed factor, which modeled a change in patients and no change in controls (i.e., MD1 ≠ MD3 = CO1 = CO3 or MD3 ≠ MD1 = CO1 = CO3). First, this LME model identified regions that exhibited ΔECT effects within each of the eight RSNs separately, with FDR-correction for voxelwise threshold (q < 0.05) and cluster correction using random field theory (26) (pcorr < 0.05, reflecting a further Bonferroni correction for 8 RSNs). We also performed a partial conjunction analysis to isolate regions exhibiting modest ΔECT effects (voxelwise p < 0.05) in at least 3 RSNs (k > 50 voxels). The probability of achieving this degree of overlap was calculated at pcorr < 0.0001 using a permutation method to assess the rate of false positives (see Suppl. Methods).
ΔMD Analysis
We performed voxelwise correlation analyses (Pearson's r) between the change in RSFC in patients (i.e., MD3 vs. MD1) and the corresponding proportional change in depressive symptoms as measured by the HAMD. We considered both positive and negative correlations. As with the ΔECT analysis described above, we measured RSN-specific ΔMD effects (FDR correction q < 0.05, cluster correction with Bonferroni correction for 8 RSNs pcorr < 0.05), as well as overlapping ΔMD effects (p < 0.05 voxelwise in at least 3 RSNs, k > 50) in a partial conjunction analysis. To estimate the rate of false positives in this latter analysis, we used permutation testing to calculate the probability of achieving this degree of overlap with our chosen parameters, which was less than 0.01%, as above (pcorr < 0.0001; Suppl. Methods).
Region of interest (ROI) analyses
We performed ROI analyses on regions exhibiting ΔECT and ΔMD effects in partial conjunction analyses. While analyses described thus far measured functional relationships between ROIs and RSNs, these analyses examined the extent to which direct ROI-to-ROI relationships were affected by ΔECT and ΔMD effects. Graph theory analyses performed in Matlab using the Brain Connectivity Toolbox (27) established three network metrics (strength, efficiency, and clustering coefficient) and single-node calculations were performed for every ROI (Suppl. Methods) (27; 28). Graph theory metrics and pairwise ROI correlation values (Fisher's z) were subjected to the same ΔECT and ΔMD analyses described above. Effects were considered significant at pcorr < 0.05, Bonferroni-corrected for the number of tests performed for each metric.
Results
Clinical Scores
Depressive symptoms (HAMD scores) significantly decreased after an index series of right-unilateral ECT (MD1 vs. MD3; t = 10.55, p < 1×10-8). In these patients, 76.2% exhibited at least a 50% reduction in HAMD scores after index, and 33.3% remitted (HAMD score ≤ 7, (29)) (Figure 1).
Figure 1.
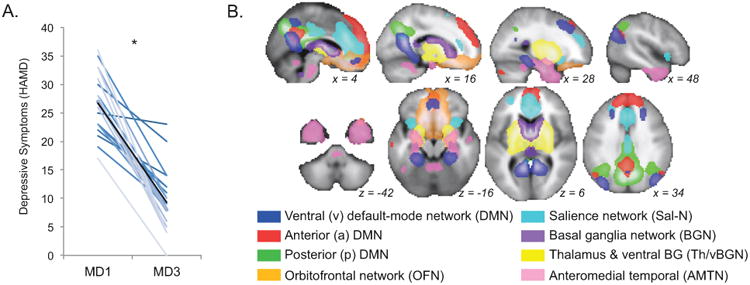
A. Symptoms of depression significantly improved after ECT (p = 1×10-8). Depressive symptoms as assessed with the Hamilton Depression Rating Scale (HAMD) are plotted (y-axis) for each patient in shades of blue for baseline (MD1) and follow-up measurements after the ECT index series (MD3). A black line marks mean HAMD scores across all patients for MD1 an MD3; asterisk indicates a significant difference in these means (paired t-test). Note that although there is variability in clinical response, the great majority of patients improved. B. Analyses of resting-state functional connectivity were restricted to networks of interest (RSNs) overlapping with fronto-limbic and medial temporal regions previously implicated in depression and ECT. Group maps including data from both patients and controls are displayed, overlaid on a group-average template brain. Color of voxels indicates the corresponding RSN (key at bottom).
Corticolimbic RSNs
Three default-mode networks (DMNs) were identified: 1) ventral DMN (vDMN) including ventromedial prefrontal cortex (PFC), posterior cingulate cortex (PCC) and adjacent precuneus, bilateral hippocampus, and dorsolateral PFC, 2) anterior DMN (aDMN) including medial PFC and PCC, and 3) posterior DMN (pDMN) including PCC and precuneus. Additional networks chosen were: 4) Salience Network including anterior cingulate cortex (ACC) and adjacent medial PFC, mid-cingulate cortex, bilateral insula, and dorsolateral PFC, 5) an orbitofrontal network including orbitofrontal cortex, ventral striatum, and basal forebrain, and RSNs including 6) dorsal basal ganglia, 7) ventral basal ganglia and thalamus, and 8) antero-medial temporal lobe structures. All eight RSNs have been reliably demonstrated in healthy volunteers in previous research (21–24).
ΔECT effects within single RSNs
We identified several changes in RSFC associated with treatment (ΔECT) within individual RSNs (pcorr < 0.05, Figure 2, Table S1). In the RSN including thalamus and ventral basal ganglia (Th/vBGN), functional connectivity with the dorsal ACC increased with ECT in patients to a level similar to that seen in controls. Correspondingly, RSFC was also restored between the Salience RSN (which includes dorsal ACC) and mediodorsal thalamus. Together, these results indicate that ECT strengthens connectivity between the thalamus and ACC, and of the mediodorsal thalamus and dorsal ACC specifically.
Figure 2.
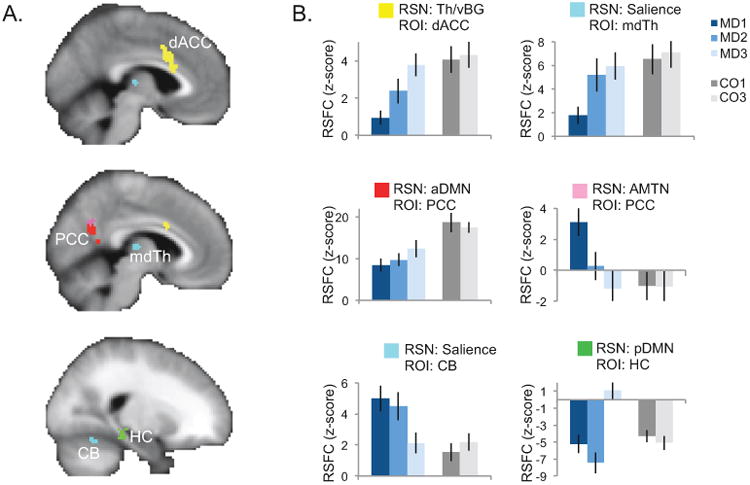
Response to right-unilateral electroconvulsive therapy (ΔECT) within individual resting-state networks of interest (RSNs). A. The results of a linear mixed-effects analysis of ΔECT within each RSN are displayed, restricted to voxels belonging to any RSN (abbreviations given in Figure 1B). Significant clusters are displayed on an average-template brain (pcorr < 0.00625); color indicates the RSN for which the ΔECT effects (described in Figure 1B) were significant (key in B). Effects were significant in dorsal anterior cingulate (dACC), posterior cingulate (PCC), mediodorsal thalamus (mdTh), hippocampus (HC), and cerebellum (CB). B. Mean resting-state functional connectivity (RSFC, z-score) in significant clusters is plotted for each group and time-point at right, where color indicates group and shade indicates time-point (key displayed at right). Error bars mark the standard error of the mean across subjects.
RSFC in PCC was also restored to normative patterns with both the anterior DMN and the anteromedial temporal network. Complementary to this latter effect, RSFC decreased between a cluster overlapping the left hippocampus and the posterior DMN (which includes the PCC), though this effect was compensatory, i.e. diverged from normal. Together, these results indicate that PCC connectivity with medial prefrontal areas (anterior DMN) and anteromedial temporal regions change with ECT, and that functional connectivity in the PCC and medial parietal cortex more generally (i.e., posterior DMN) may also change in relation to the hippocampus (part of the anteromedial temporal network).
Connectivity was also reduced with ECT between the Salience RSN and a cluster in the left lateral cerebellum. No ΔECT effects were seen for other RSNs.
Partial conjunction of ΔECT effects across RSNs
Next, we considered effects of ECT on RSFC that were robust across multiple RSNs (pcorr < 0.0001), hypothesizing that these may reflect more general changes in functional connectivity not specific to any single RSN (Figure 3A, Table S1). Using a voxelwise criterion of p < 0.05 for three or more RSNs, many more than three RSNs were often represented in each cluster (Figure 3B). Regions exhibiting significant overlapping ΔECT effects included the dorsal ACC, mediodorsal thalamus, PCC, right anterior temporal cortex (R ATL), and precuneus. Three of these regions, dorsal ACC, mediodorsal thalamus, and PCC, also demonstrated significant ΔECT effects in the within-RSN analysis (Figure 2), indicating that the effects of ECT on RSFC in these regions is perhaps more complex and/or involves more regions of the brain than demonstrated by the within-RSN analysis.
Figure 3.
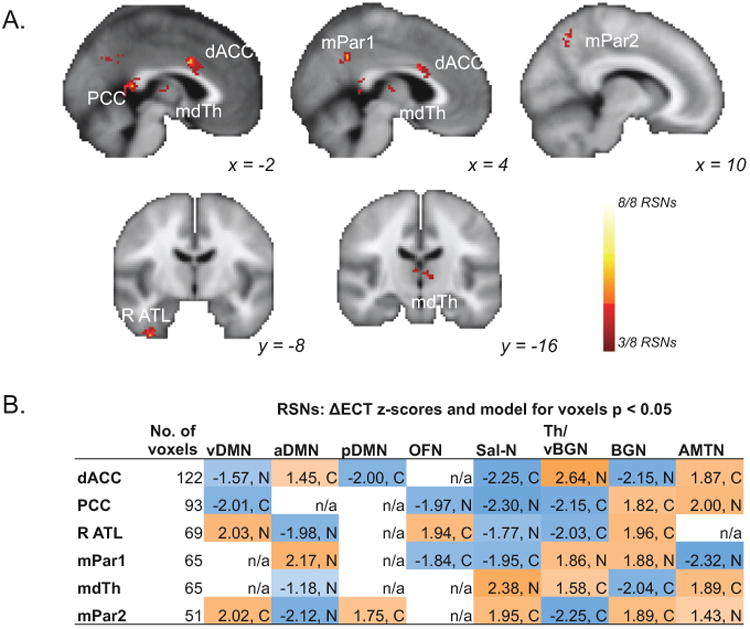
Response to electroconvulsive therapy (ΔECT) overlapping across multiple networks of interest (RSNs). A. Voxels displayed exhibited significant partial conjunction (pcorr < 0.0001) of voxelwise ΔECT effects (p < 0.05) in at least 3 different resting-state networks (RSNs; k > 50). Color indicates the number of RSNs significant for each voxel (key at lower right). Significant effects are shown in dorsal anterior cingulate (dACC), posterior cingulate (PCC), mediodorsal thalamus (mdTh), precuneus (mPar), and right anterior temporal cortex (R ATC). B. For each region of interest (ROI) in A, the mean z-score for each RSN is displayed (for voxels p < 0.05; abbreviations given in Figure 1C), along with the corresponding model direction (C, compensation, MD3 ≠ MD1 = CO1 = CO3; N, normalization, MD1 ≠ MD3 = CO1 = CO3). ROIs with no significant voxels in a given RSN are indicated with “n/a.” Color and z-score sign indicate the direction of change from MD1 to MD3, either an increase in functional connectivity (positive, orange) or a decrease (negative, blue).
ΔMD effects within single RSNs
We used voxelwise correlation analyses (Pearson's r) to assess the relationship between changes in RSFC within each RSN and changes in depressive symptoms (HAMD) over the course of ECT (ΔMD, Figure 1C). At our chosen thresholds (voxelwise FDR correction at q < 0.05 and cluster correction at pcorr < 0.05), no brain regions exhibited significant ΔMD effects with any of our eight RSNs.
Partial conjunction of ΔMD effects across RSNs
Effects of symptom change (ΔMD) overlapping across multiple RSNs were next considered, using the same thresholds as for ΔECT analyses (pcorr < 0.0001). Again, we reasoned that these effects may reflect more general changes in RSFC not specific to any single RSN. Five frontal and ACC regions exhibited significant overlapping ΔMD effects (Figure 4A). These included the subgenual ACC (sgACC), ventromedial prefrontal cortex (vmPFC), dorsomedial prefrontal cortex (dmPFC), dorsolateral prefrontal cortex (dlPFC), and dorsal ACC. Other regions showing these effects were the mediodorsal thalamus, left hippocampus, putamen, and two clusters in bilateral lateral parietal cortex (lPar). Three of these regions - dorsal ACC, mediodorsal thalamus, and hippocampus -also demonstrated ΔECT effects as described above. Although the voxelwise criterion included overlap with three or more networks, all clusters exhibited modest ΔMD effects in 6 or more RSNs (Figure 4B, Table S1).
Figure 4.
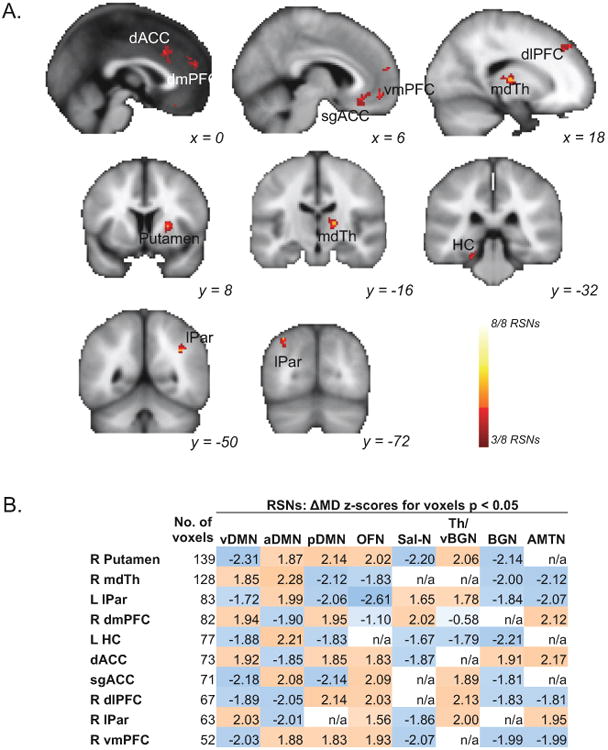
Changes in depressive symptoms (ΔMD) in patients receiving right-unilateral electroconvulsive therapy (ECT) correspond with changes in resting-state functional connectivity (RSFC) in regions overlapping across resting-state networks (RSNs). A. Statistical maps display voxels exhibiting significant partial conjunction (pcorr < 0.0001) of voxelwise ΔMD effects (p < 0.05) in at least 3 different resting-state networks (RSNs; k > 50). Color indicates the number of RSNs significant for each voxel (key at lower right). Significant effects are shown for dorsal and subgenual anterior cingulate (dACC and sgACC respectively), dorsomedial, ventromedial, and dorslateral prefrontal cortex (dmPFC, vmPFC, and dlPFC respectively), mediodorsal thalamus (mdTh), hippocampus (HC), and lateral parietal cortex (lPar). B. A matrix shows the mean ΔMD z-score, representing the relationship between changes in RSFC and changes in depressive symptoms (HAMD score) in regions of interest (ROIs) displayed in A. Values are listed for each RSN (columns; abbreviations given in Figure 1B) and significant region (rows). Color indicates the direction of the effect; positive values in orange reflect instances where larger changes in ROI-to-RSN RSFC correlate with larger improvements in HAMD scores, while negative values in blue reflect larger changes in ROI-to-RSN RSFC correlating with minimal improvements in HAMD. ROIs with no significant voxels in a given RSN are indicated with “n/a.”
ROI-to-ROI analyses
In regions exhibiting ΔECT and ΔMD effects in multiple RSNs, we examined direct ROI-to-ROI connectivity in relation to ΔECT and ΔMD effects. In a connectivity graph based on mean ROI-to-ROI correlations (Figure 5A), ROIs exhibiting ΔECT effects were mostly central, demonstrating their relatively higher connectivity with other network ROIs. No significant ΔECT and ΔMD effects were identified for graph theory metrics (27; 28) or pairwise ROI-to-ROI correlation values (pcorr > 0.05). Thus, direct ROI-to-ROI relationships were similar across groups and time-points (Figure 5B) and seemed not to drive ΔECT and ΔMD effects within RSNs reported above.
Figure 5.
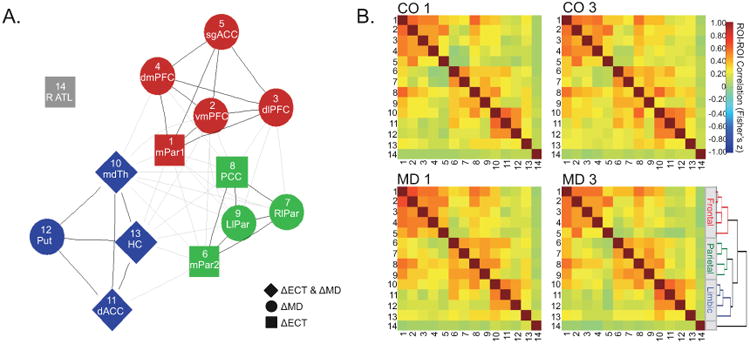
Cross-correlation analyses between regions of interest (ROIs). A. A graph plots the mean ROI-to-ROI relationships across all groups (MD1, MD3, CO1, CO3). Nodes represent ROIs resulting from analyses of ΔECT and ΔMD effects in multiple RSNs (Figure 3A, Figure 5A), and edges or lines represent ROI-to-ROI correlations significant in each time-point (pcorr < 0.005 for each of MD1, MD3, CO1, CO3). Node colors reflect the results of a hierarchical clustering analysis applied for easier visualization of graph connectivity, the dendogram for which is displayed in B. Black edges mark connections, and grey lines mark inter-cluster connections. B. Similarity matrices are plotted for each group, where color indicates the strength of functional connectivity (key at right). Numbers along rows and columns indicate the ROI plotted in A. Dendrogram is shown from hierarchical cluster analysis for visualization of ROI-ROI relationships described in A. ROIs include dorsal and subgenual anterior cingulate (dACC and sgACC respectively), dorsomedial, ventromedial, and dorslateral prefrontal cortex (dmPFC, vmPFC, and dlPFC), medial and lateral parietal cortex (mPar and lPar), right anterior temporal cortex (R ATC), mediodorsal thalamus (mdTh), putamen (Put), and hippocampus (HC).
Discussion
The current study targeted functional plasticity associated with ECT in patients with severe, refractory depression. We first identified brain regions that were similarly affected by ECT across all patients regardless of clinical outcome (ΔECT), including PCC, precuneus, cerebellum, and anteromedial temporal cortex. Because RSFC did not change in these same regions in healthy volunteers (as chosen by design), we can assume these effects were not due to habituation to MRI. In a complementary analysis, we identified a largely separate set of regions associated with changes in depressive symptoms (ΔMD) over the course of ECT, including medial and dorsolateral PFC, subgenual ACC, basal ganglia, and lateral parietal cortex. Notably, some structures exhibited both ΔECT and ΔMD effects: mediodorsal thalamus, dorsal ACC, and hippocampus. Modulation of these three regions by ECT-induced seizure activity may ultimately confer functional changes underlying improved depressive symptoms. In the following, we discuss our findings in relation to previous research examining the effects of ECT, depression, and seizures on the brain, ending with a discussion of challenges facing ECT research.
Deriving ECT's mechanism of action from overlapping ΔECT and ΔMD effects
Two partially separable groups of regions were associated with ECT (ΔECT) and with changes in depressive symptoms (ΔMD). We hypothesized that interactions between these two ΔECT and ΔMD “networks” might elucidate how the cumulative effects of ECT-induced seizures elicit brain changes necessary to support antidepressant response. Our study addressed this in two ways: by identifying regions exhibiting both ΔECT and ΔMD effects, and through an ROI-ROI network analysis directly measuring changes in RSFC between regions (i.e., rather than between ROIs and RSNs). The latter ROI-ROI approach did not yield significant results, suggesting that direct interactions between specific regions may not be as important to ECT's effectiveness as more global interactions between ROIs and larger networks like RSNs.
Changes in three regions were associated with both ΔECT and ΔMD effects: mediodorsal thalamus, dorsal ACC, and hippocampus. ΔECT effects in these three regions were strong in relation to specific RSNs (Figure 2), and consistently present, though less pronounced in some cases, for multiple RSNs (Figure 3). By contrast, no ΔMD effects survived the strict thresholds used in within-RSN analyses; instead, more modest but consistent ΔMD effects were present between dorsal ACC, mediodorsal thalamus, and hippocampus and multiple RSNs (Figure 4). Thus, treatment efficacy appears associated with more widespread changes in functional connectivity (i.e., rather than between specific regions). Although the spatial extent of clusters may not overlap in these regions (see Table S1 for coordinates), nevertheless these results suggest that RSFC modulation of hippocampus, thalamus, and dorsal ACC may be critical for ECT's efficacy, supporting and extending current theories of ECT and depression.
The hippocampus has been consistently linked to both depression and ECT response. Neuroimaging studies have shown decreased hippocampal volume in depressed patients that is restored with ECT (14; 18), which could reflect increases in synapses, dendrites, or glial cells and/or neurogenesis (30) as demonstrated in studies using electroshock to simulate ECT in mice and nonhuman primates (31; 32). Our data are among the first to lend insight into the functional consequences of these morphological changes. In the current study, ECT associated with normalization of RSFC between the hippocampus and medial parietal and posterior cingulate cortices, as evidenced by reduced correlations between 1) an hippocampal region and the posterior DMN (which contains precuneus and PCC) and 2) a PCC region and the anteromedial temporal RSN (which contains hippocampus). These results confirm a previous report of normalized RSFC of right hippocampus with ECT, though our methodological approaches differed (33). PCC and precuneus share direct anatomical connections with the hippocampus (34; 35), and together with medial PFC form the default mode RSN widely implicated in self-referential processing and depression (36–38), and aspects of epilepsy (39). The hippocampus is also a key structure in the pathophysiology of seizure disorders (e.g., temporal lobe epilepsy) (40), perhaps indicating that this structure is vulnerable to (and may even help to propagate) seizure activity elicited during ECT.
Some have also hypothesized that, in order for ECT to be effective, seizure activity must reach subcortical structures, specifically the thalamus (41; 42). In our data, ECT was associated with restored connectivity between mediodorsal thalamus and the Salience RSN (containing dorsal ACC) and between dorsal ACC and the RSN containing thalamus and ventral basal ganglia. The thalamus is thought to play a major role in cortico-cortical interactions and feedback (43), and the mediodorsal thalamus has direct anatomical connections with hippocampus, ACC, and mPFC (44–46). Dorsal ACC and mediodorsal thalamus have been associated with ECT-related seizure activity in PET/SPECT research (9; 42; 47; 48), and more recent studies further highlight the relevance of dorsal ACC to ECT. Our own work has indicated that ECT restores white-matter microstructure in tracts adjacent to dorsal ACC (11), and other RSFC studies have reported that baseline RSFC in RSNs overlapping dorsal ACC may help predict clinical response to ECT (13), and that ECT alters functional relationships between an RSN overlapping dorsal ACC and other RSNs. Our current results extend these findings by highlighting the importance of dorsal ACC in relation to the thalamus. Indeed, mediodorsal thalamus serves a role in depression (49; 50), perhaps particularly as a major interface between the hippocampus and mPFC mediating regulatory control of emotional and cognitive aspects of behavior and depression (51; 52). Future research cutting across imaging modalities and model systems will be most helpful in integrating these and other findings to inform and improve hippocampal neurotrophic theories (53; 54) and other models (41; 42) of ECT and of depression.
Functional plasticity in medial prefrontal circuits underlying changes in depressive symptoms
To parse effects relating to antidepressant response from the effects of seizure activity, we directly assessed changes in RSFC associated with changes in symptoms (ΔMD). There were no significant ΔMD effects within single RSNs; however, partial conjunction analyses identified a set of regions that were in part separate from ΔECT analysis, particularly in medial and lateral PFC, sgACC, and associated regions like the putamen, mediodorsal thalamus, and dorsolateral prefrontal cortex. Medial PFC regions form well-recognized circuits with thalamic and striatal nuclei (55; 56), and these thalamo-cortico-striatal loops factor prominently in current models of depression (1; 37; 52; 57). The mPFC may play a particular role in depression and treatment response (3); the sgACC exhibits volume loss and hyperconnectivity in MD (36; 58; 59), and BA 25 is a current target of deep brain stimulation (DBS) in clinical trials (60). The vmPFC is also modulated by pharmacological treatment of MDD (60; 61), and the ventral striatal loop and mesolimbic reward pathway are implicated in depression (37; 49; 50; 62), including ECT response (42; 62).
Previous studies have reported ECT-related changes in RSFC in dorsolateral PFC (19; 20). In the current study, we identified ΔMD effects in dorsolateral PFC in partial conjunction analyses; however, dorsolateral PFC did not exhibit ΔECT effects, meaning that RSFC did not change on average across all patients in this region. One explanation for this discrepancy is that all patients in these previous studies remitted to ECT (19; 20), while our study included patients with varied clinical response. Thus, these previous studies were unable separate the impact of remitted depressive symptoms from the effects of ECT itself. Alternative explanations are possible, including differences in ECT administration, sample size, and statistical approach between studies. However, this further illustrates the importance of including nonresponders in ECT neuroimaging research where possible.
Parsing the lasting effects of seizure therapy
Generalized seizures associated with ECT and epilepsy preferentially affect certain parts of the brain, presumably influenced by electrode placement or seizure focus, respectively (42; 47). Our study supports this notion: right-unilateral ECT affected right-lateralized and midline structures in our ΔECT model. Two clusters identified, right anterior temporal cortex and dorsal ACC, were even located relatively close to electrode sites and have been previously associated with ECT (9; 11–13). Epilepsy research suggests these regions are impacted during seizures (63). Notably, the cerebellum exhibits increased activity during post-ictal phase (i.e., a period of altered consciousness following a seizure) (39; 63), and may influence thalamocortical circuits to aid in seizure termination and suppression (39; 64). In our study, functional plasticity in the cerebellum and other regions exhibiting ΔECT effects in the absence of ΔMD effects may reflect nonspecific physiological effects of seizure-related processes. However, cerebellar function has also been linked depression, for example (65), and other aspects of therapy may influence the brain and clinic outcome as well (e.g., anesthesia, increased care). More focal neurostimulation approaches (e.g., animal models, deep brain stimulation in humans) will be useful in determining precisely which regions are critical for clinical response, and which are epiphenomena of ECT and related factors.
Conclusions and additional considerations
Our study results add new understanding with respect to how the brain changes both with ECT and with clinical response. Separate consideration of ΔECT and ΔMD effects, combined with the longitudinal examination of other biomarkers of treatment-related neuroplasticity (e.g., dorsal ACC connectivity changes, hippocampal volume increases, functional genomics, immune system response, etc.) may ultimately lead to more targeted and fast-acting therapies for depression. However, there are several additional challenges facing ECT research, some of which are also relevant for the current study.
Heterogeneity within patient groups presents an ongoing issue in clinical and translational research. Symptoms vary within diagnostic categories, leading some to argue that identifying the shared neurobiology underlying common symptoms across disease categories may be more effective for translation and more personalized treatments (25). In the current study, we targeted functional plasticity associated with ECT and with improvements in severe depressive symptoms. Therefore, our study sample included patients that underwent right-unilateral ECT and shared a diagnosis of treatment-refractory MDEs, while taking care to match patients and healthy volunteers in age and sex. So, although unipolar/bipolar diagnosis, duration of illness, past medication history, clinical outcome, psychosis, and other factors may also affect the brain, we reasoned that ΔECT and ΔMD effects would have similar neurobiological underpinnings regardless of inter-subject variability. Indeed, the fact that our longitudinal analyses identified these effects consistently across subjects makes our results more generalizable than if we had restricted our sample further (e.g., to include only unipolar patients). Multi-site studies will be better able to leverage large and diverse samples to determine how the neurobiology of ECT and MDEs are differentially affected in patients with heterogeneous features. These larger studies will also have greater power to address the problem of attrition, which is a challenge for this and other clinical studies.
Finally, although we report our findings as “lasting” effects of seizure therapy, many patients relapse during a variable period following the end of treatment (5). This suggests that the short-term therapeutic effects of ECT may be different from changes integral to maintaining response long term. However, most ECT research, including the current study, focuses on short-term response. Future studies targeting long-term outcomes are thus needed to fully address the global burden of depression. Furthermore, patients who do not respond to treatment quickly may end treatment, making attrition both a clinical and research issue. Specifically targeting regions most associated with symptom change in future neurostimulation therapies, especially regions showing both ΔMD and ΔECT effects, may improve treatment efficacy while minimizing unwanted cognitive side effects. Though largely effective, ECT can be improved; we hope that this and other work will ultimately support that goal.
Supplementary Material
Acknowledgments
This work was supported by the NIH, including R01 MH092301 to Drs. Katherine Narr and Randall Espinoza and K24 MH102743 to Dr. Katherine Narr. These data were previously reported at the 2014 Annual Meeting of the Organization for Human Brain Mapping (Hamburg, Germany).
Footnotes
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 2.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–31. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 5.Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357:1939–45. doi: 10.1056/NEJMct075234. [DOI] [PubMed] [Google Scholar]
- 6.Dierckx B, Heijnen WT, van den Broek WW, Birkenhäger TK. Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord. 2012;14:146–50. doi: 10.1111/j.1399-5618.2012.00997.x. [DOI] [PubMed] [Google Scholar]
- 7.Fink M. What was learned: studies by the consortium for research in ECT (CORE) 1997-2011. Acta Psychiatr Scand. 2014;129:417–26. doi: 10.1111/acps.12251. [DOI] [PubMed] [Google Scholar]
- 8.Husain MM, Rush AJ, Fink M, Knapp R, Petrides G, Rummans T, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry. 2004;65:485–91. doi: 10.4088/jcp.v65n0406. [DOI] [PubMed] [Google Scholar]
- 9.Blumenfeld H, Westerveld M, Ostroff RB, Vanderhill SD, Freeman J, Necochea A, et al. Selective frontal, parietal, and temporal networks in generalized seizures. Neuroimage. 2003;19:1556–1566. doi: 10.1016/s1053-8119(03)00204-0. [DOI] [PubMed] [Google Scholar]
- 10.Nobler MS. Regional Cerebral Blood Flow in Mood Disorders, III. Arch Gen Psychiatry. 1994;51:884. doi: 10.1001/archpsyc.1994.03950110044007. [DOI] [PubMed] [Google Scholar]
- 11.Lyden H, Espinoza RT, Pirnia T, Clark K, Joshi SH, Leaver AM, et al. Electroconvulsive therapy mediates neuroplasticity of white matter microstructure in major depression. Transl Psychiatry. 2014;4:e380. doi: 10.1038/tp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott CC, Lemke NT, Gopal S, Thoma RJ, Bustillo J, Calhoun VD, Turner JA. Electroconvulsive therapy response in major depressive disorder: a pilot functional network connectivity resting state FMRI investigation. Front psychiatry. 2013;4:10. doi: 10.3389/fpsyt.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Waarde JA, Scholte HS, van Oudheusden LJB, Verwey B, Denys D, van Wingen GA. A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment-resistant depression. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.78. [DOI] [PubMed] [Google Scholar]
- 14.Tendolkar I, van Beek M, van Oostrom I, Mulder M, Janzing J, Voshaar RO, van Eijndhoven P. Electroconvulsive therapy increases hippocampal and amygdala volume in therapy refractory depression: a longitudinal pilot study. Psychiatry Res. 2013;214:197–203. doi: 10.1016/j.pscychresns.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.02.029. Epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry. 2010;196:226–34. doi: 10.1192/bjp.bp.109.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57:425–34. doi: 10.1001/archpsyc.57.5.425. [DOI] [PubMed] [Google Scholar]
- 18.Dukart J, Regen F, Kherif F, Colla M, Bajbouj M, Heuser I, et al. Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc Natl Acad Sci U S A. 2014;111:1156–61. doi: 10.1073/pnas.1321399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, Schwarzbauer C. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci U S A. 2012;109:5464–8. doi: 10.1073/pnas.1117206109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei Q, Tian Y, Yu Y, Zhang F, Hu X, Dong Y, et al. Modulation of interhemispheric functional coordination in electroconvulsive therapy for depression. Transl Psychiatry. 2014;4:e453. doi: 10.1038/tp.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, et al. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–37. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 26.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 27.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–69. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 28.He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, et al. Uncovering intrinsic modular organization of spontaneous brain activity in humans. In: Sporns O, editor. PLoS One. Vol. 4. 2009. p. e5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerman M, Martinez J, Attiullah N, Friedman M, Toba C, Boerescu DA, Rahgeb M. Further evidence that the cutoff to define remission on the 17-item Hamilton Depression Rating Scale should be lowered. Depress Anxiety. 2012;29:159–65. doi: 10.1002/da.20870. [DOI] [PubMed] [Google Scholar]
- 30.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–36. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingström A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 32.Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbott CC, Jones T, Lemke NT, Gallegos P, McClintock SM, Mayer AR, et al. Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl Psychiatry. 2014;4:e483. doi: 10.1038/tp.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- 35.Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 36.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry. 2011;16:763–72. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danielson NB, Guo JN, Blumenfeld H. The default mode network and altered consciousness in epilepsy. Behav Neurol. 2011;24:55–65. doi: 10.3233/BEN-2011-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bromfield EB, Cavazos JE, Sirven JI. An Introduction to Epilepsy. West Hartford, CT: American Epilepsy Society; 2006. [PubMed] [Google Scholar]
- 41.Bolwig TG. How does electroconvulsive therapy work? Theories on its mechanism. Can J Psychiatry. 2011;56:13–8. doi: 10.1177/070674371105600104. [DOI] [PubMed] [Google Scholar]
- 42.McNally KA, Blumenfeld H. Focal network involvement in generalized seizures: new insights from electroconvulsive therapy. Epilepsy Behav. 2004;5:3–12. doi: 10.1016/j.yebeh.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Guillery RW, Sherman SM. Thalamic Relay Functions and Their Role in Corticocortical Communication. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 44.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 45.Ray JP, Price JL. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1993;337:1–31. doi: 10.1002/cne.903370102. [DOI] [PubMed] [Google Scholar]
- 46.Russchen FT, Amaral DG, Price JL. The afferent input to the magnocellular division of the mediodorsal thalamic nucleus in the monkey, Macaca fascicularis. J Comp Neurol. 1987;256:175–210. doi: 10.1002/cne.902560202. [DOI] [PubMed] [Google Scholar]
- 47.Bolwig TG. Neuroimaging and electroconvulsive therapy: a review. J ECT. 2014;30:138–42. doi: 10.1097/YCT.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 48.Enev M, McNally KA, Varghese G, Zubal IG, Ostroff RB, Blumenfeld H. Imaging onset and propagation of ECT-induced seizures. Epilepsia. 2007;48:238–44. doi: 10.1111/j.1528-1167.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 49.Salomons TV, Dunlop K, Kennedy SH, Flint A, Geraci J, Giacobbe P, Downar J. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology. 2014;39:488–98. doi: 10.1038/npp.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li CT, Chen LF, Tu PC, Wang SJ, Chen MH, Su TP, Hsieh JC. Impaired prefronto-thalamic functional connectivity as a key feature of treatment-resistant depression: a combined MEG, PET and rTMS study. PLoS One. 2013;8:e70089. doi: 10.1371/journal.pone.0070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 52.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12:1079–88. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- 54.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–64. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 55.Divac I, Mogensen J, Marinkovic S, Mårtensson R. On the projections from the neostriatum to the cerebral cortex: the “displaced” neurons. Neuroscience. 1987;21:197–205. doi: 10.1016/0306-4522(87)90333-2. [DOI] [PubMed] [Google Scholar]
- 56.Ferry AT, Ongur D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425:447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 57.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 59.Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, et al. Subgenual Cingulate Cortex Volume in First-Episode Psychosis. Am J Psychiatry. 1999;156:1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Li X, Nahas Z, Kozel FA, Anderson B, Bohning DE, George MS. Acute left prefrontal transcranial magnetic stimulation in depressed patients is associated with immediately increased activity in prefrontal cortical as well as subcortical regions. Biol Psychiatry. 2004;55:882–90. doi: 10.1016/j.biopsych.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 62.Leaver A, Espinoza R, Joshi S, Vasavada M, Njau S, Pirnia T, et al. Desynchronization and plasticity of striato-frontal connectivity in major depressive disorder. Cereb Cortex. doi: 10.1093/cercor/bhv207. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salgado-Benftez A, Briones R, Fernandez-Guardiola A. Purkinje Cell Responses to a Cerebral Penicillin-Induced Epileptogenic Focus in the Cat. Epilepsia. 1982;23:597–606. doi: 10.1111/j.1528-1157.1982.tb05074.x. [DOI] [PubMed] [Google Scholar]
- 65.Liu L, Zeng LL, Li Y, Ma Q, Li B, Shen H, Hu D. Altered cerebellar functional connectivity with intrinsic connectivity networks in adults with major depressive disorder. PLoS One. 2012;7:e39516. doi: 10.1371/journal.pone.0039516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


