Abstract
The monocytic leukemic zinc finger (MOZ) histone acetyltransferase (HAT) plays a role in acute myeloid leukemia (AML). It functions as a quaternary complex with the bromodomain PHD finger protein 1 (BRPF1), the human Esa1-associated factor 6 homolog (hEAF6), and the inhibitor of growth 5 (ING5). Each of these subunits contain chromatin reader domains that recognize specific post-translational modifications (PTMs) on histone tails, and this recognition directs the MOZ HAT complex to specific chromatin substrates. The structure and function of these epigenetic reader modules has now been elucidated, and a model describing how the cooperative activity of these domains regulates HAT activity in response to the epigenetic landscape is proposed. The emerging role of epigenetic reader domains in disease, and their therapeutic potential for many types of cancer is also highlighted.
Keywords: MOZ, Chromatin reader domains, Epigenetics, Histone acetyltransferase, Acute myeloid leukemia
The monocytic leukemic zinc-finger (MOZ) histone acetyltransferase (HAT) functions as a quaternary complex with the bromodomain PHD finger protein 1 (BRPF1), the human Esa1-associated factor 6 homolog (hEAF6), and the inhibitor of growth 5 (ING5) subunits (Figure 1A) (Ullah et al., 2008). Interestingly, each subunit of the complex contains chromatin reader domains that recognize post-translational modifications (PTMs) on the histone tail. For example, MOZ contains a double plant homeodomain (PHD) that recognizes histone H3, while the ING5 subunit has a C-terminal PHD finger that binds to histone H3 tri-methylated at lysine 4 (H3K4me3) (Champagne et al., 2008; Qiu et al., 2012). Additionally, BRPF1 contains multiple epigenetic reader modules including a unique double PHD and zinc finger assembly (PZP), a bromodomain and a chromo/Tudor-related Pro-Tyr-Tyr-Pro (PWWP) domain (Laue et al., 2008; Qin et al., 2011; Vezzoli et al., 2010). In this review we summarize the current knowledge of how the MOZ HAT functions in normal biological processes as well as in disease progression. We propose a model of how epigenetic reader domains within the MOZ HAT target it to modified chromatin substrates, and highlight how our current knowledge of MOZ structure and function might lead to the development of a novel, effective treatment for acute myeloid leukemia.
Figure 1.
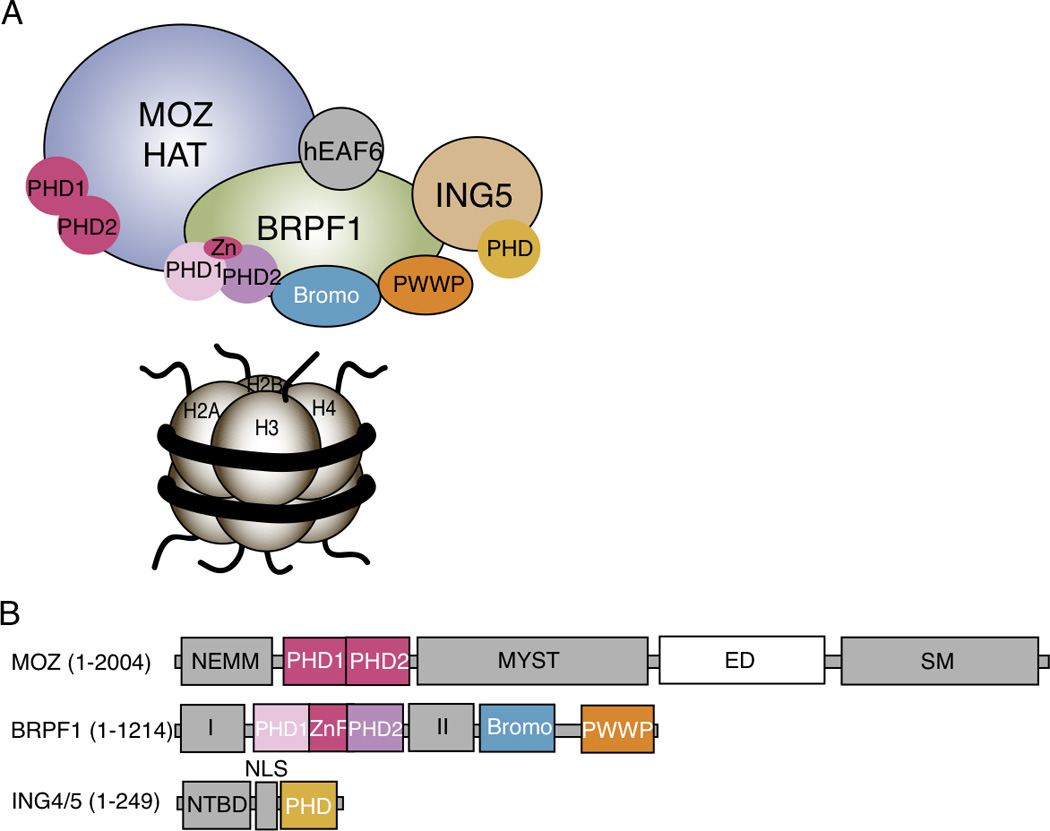
The MOZ HAT complex and subunit architecture. A. The MOZ HAT complex is a hetero-tetramer composed of the monocytic leukemic zinc-finger (MOZ) catalytic subunit, the bromodomain-PHD finger protein 1 (BRPF1), inhibitor of growth 5 (ING5) and hEaf6 subunits. B. The MOZ catalytic domain is a large, multi-domain protein (~250 kDa) possessing HAT activity via its MYST domain, in addition to multiple protein interaction domains including a N-terminal part of Enok, MOZ, or MORF (NEMM) domain, a tandem PHD finger region, a Glu/Asp rich region (ED) and a Ser/Met rich region (SM). The BRPF1 subunit contains multiple chromatin reader domains including a double PHD and zinc finger (ZnF) assembly (PZP), a bromodomain and a chromo/Tudor-related PWWP domain. BRPF1 also includes two protein interaction domains (I and II), which contact MOZ and ING5/Eaf6, respectively. The ING5 subunit contains an N-terminal binding domain (NTBD) that contacts BRPF1, a nuclear localization signal (NLS) and a C-terminal PHD finger.
Functional Significance of the MOZ HAT
The MOZ HAT forms a multi-subunit HAT complex that acetylates free histones H3, H4, H2A and H2B in vitro (Champagne et al., 2001; Holbert et al., 2007; Kitabayashi et al., 2001a; Laue et al., 2008; Ullah et al., 2008; Voss et al., 2009). Acetylation of histones located near gene promoters is associated with up-regulation of gene transcription, and the acetylation activity of MOZ has been shown to control expression of HOX genes (Camos et al., 2006). The MOZ HAT plays a direct role in hematopoiesis and is essential for the development and maintenance of hematopoietic stem cells (HSCs) (Perez-Campo et al., 2009). As such, MOZ has also been shown to be a strong co-activator of the RUNX1 transcription factor, the master regulator of hematopoiesis (Bristow and Shore, 2003; Kitabayashi et al., 2001a). This critical role of MOZ was highlighted by a study showing that MOZ mutations introduced in mice resulted in their death at birth, along with impaired hematopoiesis and stem cell development (Katsumoto et al., 2006; Thomas and Voss, 2004; Yang and Ullah, 2007). Furthermore, disruption of the MOZ HAT activity has been linked to the development of disease, particularly leukemias (Carapeti et al., 1998; Crowley et al., 2005; Esteyries et al., 2008).
Role of MOZ in Disease Progression
The MOZ HAT is involved in chromosomal translocations found in a subtype of acute myeloid leukemia (AML) associated with a poor prognosis, and a median survival of only 6 months (Borrow et al., 1996; Brown et al., 2012; Panagopoulos et al., 2001). According to the CDC website, each year in the United States, 100,000 blood cancer cases are diagnosed, and more than 50,000 people die from these cancers. AML accounts for 15% of childhood and 80% of adult leukemia cases, and the long-term survival rates for these patients are poor (Brown et al., 2012). MOZ was first identified as a fusion partner with the CREB binding protein (CBP) HAT in a t(8;16)(p11;p13) translocation found in AML, and disruption of the normal acetylation activity of MOZ leads to leukemogenic transformations and oncogenesis (Borrow et al., 1996). MOZ has also been found translocated to the CBP homolog p300 (Kitabayashi et al., 2001b), to the transcriptional intermediary binding factor 2 (TIF2) (Carapeti et al., 1998) and to the nuclear receptor co-activator 3 (NcoA3) transcription factor (Esteyries et al., 2008). The t(8;16)(p11;p13) translocation of MOZ to CBP produces a characteristic gene expression pattern with elevated levels of HOXA9, HOXA10 and MEIS1 (Camos et al., 2006). CBP is another HAT that acetylates histone and non-histone proteins, and acts as a transcriptional co-activator important for many cellular processes (Bannister and Kouzarides, 1996). The BRPF1 subunit in the multi-protein HAT complex functions as a platform of assembly linking the MOZ catalytic subunit to the ING5 and hEaf6 subunits, promoting activity (Ullah et al., 2008). Fusion with the CBP HAT leaves the MOZ MYST domain intact and likely allows for formation of the tetrameric hEaf6/BRPF1/ING5 complex (Yang and Ullah, 2007). In the MOZ fusion, most of the CBP HAT also remains functional and leukemic transformations could be the result of aberrant HAT activity contributed by deregulation of both HAT complexes (Borrow et al., 1996). AML patients with the MOZ-CBP translocation are rare, but they exhibit a unique gene expression pattern distinct from other types of AML that have a better prognosis and outcomes (Brown et al., 2012). MOZ is also found in a t(8;22)(p11;q13) chromosome translocation with the p300 protein (Kitabayashi et al., 2001b). Like the translocation with CBP, in this translocation, the p300 HAT and the MOZ acetyltransferase domains remain largely intact, resulting in aberrant HAT activity that contributes to leukemogenesis and the development of AML.
MOZ Epigenetic Reader Domains
Epigenetic reader domains are found in chromatin-associated proteins that control fundamental cellular processes, including gene transcription, DNA replication, and recombination, and their disruption is often linked to disease. Interaction of chromatin reader domains with histones appears to regulate the substrate specificity of MOZ HAT in normal and disease states. Recent research has elucidated the function of the chromatin reader modules within the MOZ complex (Figure 1B). The MOZ catalytic subunit contains a duo of PHD fingers that work in tandem (PHD12). PHD fingers are a conserved C3HC4 zinc finger motif commonly found in nuclear proteins that regulate transcription and chromatin remodeling. PHD fingers can be divided into different subsets based upon their binding affinity towards various post-translational modifications (Champagne and Kutateladze, 2009; Li and Li, 2012; Slama and Geman, 2011). The MOZ tandem PHD12 finger domain recognizes histone H3 when it is acetylated at lysine 14 (H3K14ac) (23 μM) (Qiu et al., 2012). Removing the acetylation on H3K14 reduced the binding affinity of the histone tail peptide (unmodified H3, 65 μM), and methylation of H3R2 abolishes the binding interaction (Qiu et al., 2012) It was also shown that MOZ acetylates the HOXA9 promoter, and that activation of HOXA9 transcription is dependent on the recognition of H3K14ac by the MOZ tandem PHD12 finger (Qiu et al., 2012).
The C-terminal PHD finger of ING5 has been shown to bind to histone H3 tri-methylated at lysine 4 (K3K4me3) with low micromolar affinity (2.4 μM), while the N-terminal binding domain (NTBD) of ING5 is important for linking this subunit to the MOZ HAT complex (Champagne et al., 2008; Ullah et al., 2008). ING5 is necessary for the HAT activity of the MOZ complex, and its presence results in the preferential acetylation of histone peptides tri-methylated at H3K4 (Champagne et al., 2008). Thus, ING5 appears to act as an adapter molecule targeting the MOZ HAT complex to chromatin via histone recognition of its PHD finger domain.
BRPF1 is an important regulator of MOZ’s enzymatic activity, and acts as a platform for assembly of the MOZ tetrameric complex (Saksouk et al., 2009). BRPF1 also contains multiple epigenetic reader domains, including a unique double PHD and zinc finger assembly (PZP), a bromodomain, and a PWWP domain. The PWWP domain is necessary for the association of BRPF1 with condensed chromatin and is able to recognize H3K36me3 with an affinity of 2.7mM (Laue et al., 2008; Vezzoli et al., 2010). Similarly to other bromodomains the BRPF1 bromodomain recognizes multiple acetyl lysine residues on the histone tail including H2AK5ac, H3K14ac, H4K5ac, H4K8ac and H4K12ac (Poplawski et al., 2013). The H2AK5ac histone peptide binds to the BRPF1 bromodomain with the highest affinity (48.5 μM), followed by H4K12ac (86.5 μM), and H3K14ac (626 μM) (Poplawski et al., 2013). Interestingly, the extended PHD1-ZnF-PHD2 (PZP) region in BRPF1/3 is conserved in a number of proteins. In BPRF1, the PZP module binds the unmodified histone H3 tail via PHD1 with an affinity of 1.8 μM, while PHD2 does not appear to interact directly with the histone tail (Lalonde et al., 2013) Additionally, the atypical PHD2 finger in BRPF2 was shown to interact non-specifically with DNA (Liu et al., 2012).
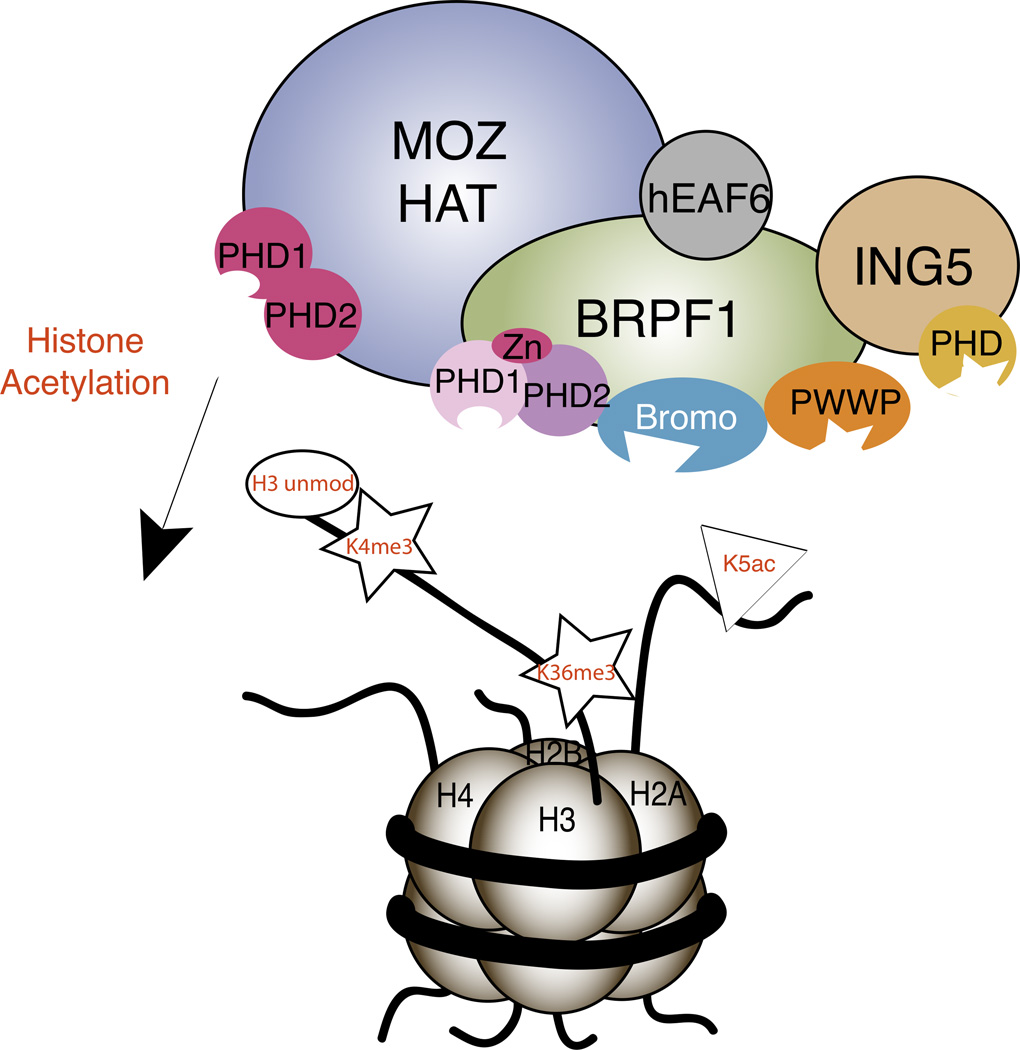
These results suggest that it is the combinatorial action of multiple epigenetic reader domains within the MOZ HAT complex that direct it to chromatin substrates and regulate its acetylation activity. Figure 2 illustrates a model of this process. First, ING5 targets H3K4me3 present at the promoters of actively transcribed regions, recruiting the MOZ HAT complex. Then the tandem PHD12 fingers in the MOZ catalytic subunit recognize the unmodified histone H3 solidifying the interaction, while the PWWP domain of BRPF1 also binds to any H3K36me3 marks on the nucleosome. Once bound to the chromatin, MOZ acetylates histones H3 and H4 at H3K14ac H4K5ac, H4K8ac, H4K12ac, and H4K16ac (Kitabayashi et al., 2001a). This in turn increases the affinity of the MOZ HAT complex for chromatin through binding of the BRPF1 bromodomain and MOZ PHD12 to the acetyllysine modifications. Ultimately, this creates a positive feedback loop where increased acetylation activity of the MOZ HAT complex increases the complex’s affinity for chromatin substrates, spreading the acetylation of chromatin and up-regulating gene expression. The localization of MOZ HAT activity is regulated by the absence and presence of particular histone modifications. For example, it is known that H3K4me3, H3K36me3 and acetylation are associated with actively transcribed genes, and these modifications increase the interaction of MOZ with chromatin. However, the presence of H3R2me2 would abrogate binding of MOZ PHD12 and inhibit tri-methylation of H3K4 by the Set1 methyltransferase (Kirmizis et al., 2007; Qiu et al., 2012), weakening the interaction of the complex with its histone substrates. Also, since the BRPF1 subunit is still able to interact with the MOZ HAT after leukemic translocations of MOZ with CBP, p300 and TIF2, it is likely that recognition of histone modifications by chromatin reader domains in BRPF1 and ING5 contribute to the aberrant acetylation activity of MOZ in the development of disease (Yang and Ullah, 2007).
Figure 2.
Epigenetic reader domains in the MOZ HAT complex recognize histone post-translational modifications. Model illustrating how recognition of histone modifications by various subunits within the MOZ HAT directs the complex to chromatin and regulate enzymatic activity.
Potential of Epigenetic Drug Targets
Cancer is a genetic disease as well as an epigenetic disease. Epigenetics describes the study of heritable changes in genome function that occur without a change in the DNA sequence. Genome function and integrity depends on epigenetic factors including modifications to DNA and chromatin that bring functional conformation to chromosomes. The importance of epigenetic integrity is evident by the growing list of chromatin regulating genes, including the MOZ HAT, which are disrupted in various cancers (Cengiz et al., 2007; Doyon et al., 2006; Garkavtsev et al., 2004; Gunduz et al., 2002; Kitabayashi et al., 2001b; Shiseki et al., 2003). These genes code for proteins involved in DNA methylation, histone modifications, and ATP dependent chromatin remodeling. Unlike genetic changes in cancer, epigenetic changes are potentially reversible. This has led to the design of DNA-demethylating agents and HDAC inhibitors, which have shown significant antitumor activity (Mack, 2006; Thompson, 2006). More recently, it has become evident that chromatin reader domains, such as the PHD fingers and bromodomain found in the MOZ HAT complex, are druggable with small molecules (Santiago et al., 2011; Vidler et al., 2012; Wagner et al., 2012), and selective inhibition of epigenetic regulators is now recognized as a valuable therapeutic avenue (Zhao et al., 2013). Modulation of MOZ HAT enzyme activity could be an effective therapeutic strategy for leukemia patients, and the chromatin-binding subunit BRPF1 or protein-protein interactions between subunits in the MOZ HAT complex may become important potential targets. Further investigation into the development of small-molecule inhibitors for the MOZ HAT presents a promising new avenue of therapy that will provide more specific treatment strategies, and better overall outcomes for patients with leukemia.
Acknowledgments
This publication was supported by an award from the National Institute of General Medical Sciences of the National Institutes of Health under award numbers R15GM104865 to KCG
Footnotes
Conflict of Interest Disclosure:
The authors have no conflict of interest to declare.
Literature Cited
- Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384(6610):641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- Borrow J, Stanton VP, Jr, Andresen JM, Becher R, Behm FG, Chaganti RS, Civin CI, Disteche C, Dube I, Frischauf AM, Horsman D, Mitelman F, Volinia S, Watmore AE, Housman DE. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14(1):33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- Bristow CA, Shore P. Transcriptional regulation of the human MIP-1alpha promoter by RUNX1 and MOZ. Nucleic Acids Res. 2003;31(11):2735–2744. doi: 10.1093/nar/gkg401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T, Swansbury J, Taj MM. Prognosis of patients with t(8;16)(p11;p13) acute myeloid leukemia. Leuk Lymphoma. 2012;53(2):338–341. doi: 10.3109/10428194.2011.614703. [DOI] [PubMed] [Google Scholar]
- Camos M, Esteve J, Jares P, Colomer D, Rozman M, Villamor N, Costa D, Carrio A, Nomdedeu J, Montserrat E, Campo E. Gene expression profiling of acute myeloid leukemia with translocation t(8;16)(p11;p13) and MYST3-CREBBP rearrangement reveals a distinctive signature with a specific pattern of HOX gene expression. Cancer Res. 2006;66(14):6947–6954. doi: 10.1158/0008-5472.CAN-05-4601. [DOI] [PubMed] [Google Scholar]
- Carapeti M, Aguiar RC, Goldman JM, Cross NC. A novel fusion between MOZ and the nuclear receptor coactivator TIF2 in acute myeloid leukemia. Blood. 1998;91(9):3127–3133. [PubMed] [Google Scholar]
- Cengiz B, Gunduz M, Nagatsuka H, Beder L, Gunduz E, Tamamura R, Mahmut N, Fukushima K, Ali MA, Naomoto Y, Shimizu K, Nagai N. Fine deletion mapping of chromosome 2q21-37 shows three preferentially deleted regions in oral cancer. Oral Oncol. 2007;43(3):241–247. doi: 10.1016/j.oraloncology.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Champagne KS, Kutateladze TG. Structural insight into histone recognition by the ING PHD fingers. Curr Drug Targets. 2009;10(5):432–441. doi: 10.2174/138945009788185040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne KS, Saksouk N, Pena PV, Johnson K, Ullah M, Yang XJ, Cote J, Kutateladze TG. The crystal structure of the ING5 PHD finger in complex with an H3K4me3 histone peptide. Proteins. 2008;72(4):1371–1376. doi: 10.1002/prot.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne N, Pelletier N, Yang XJ. The monocytic leukemia zinc finger protein MOZ is a histone acetyltransferase. Oncogene. 2001;20(3):404–409. doi: 10.1038/sj.onc.1204114. [DOI] [PubMed] [Google Scholar]
- Crowley JA, Wang Y, Rapoport AP, Ning Y. Detection of MOZ-CBP fusion in acute myeloid leukemia with 8;16 translocation. Leukemia. 2005;19(12):2344–2345. doi: 10.1038/sj.leu.2403971. [DOI] [PubMed] [Google Scholar]
- Doyon Y, Cayrou C, Ullah M, Landry AJ, Cote V, Selleck W, Lane WS, Tan S, Yang XJ, Cote J. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Moll Cell. 2006;21(1):51–64. doi: 10.1016/j.molcel.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Esteyries S, Perot C, Adelaide J, Imbert M, Lagarde A, Pautas C, Olschwang S, Birnbaum D, Chaffanet M, Mozziconacci MJ. NCOA3, a new fusion partner for MOZ/MYST3 in M5 acute myeloid leukemia. Leukemia. 2008;22(3):663–665. doi: 10.1038/sj.leu.2404930. [DOI] [PubMed] [Google Scholar]
- Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E, Barnett GH, Jain RK. The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature. 2004;428(6980):328–332. doi: 10.1038/nature02329. [DOI] [PubMed] [Google Scholar]
- Gunduz M, Ouchida M, Fukushima K, Ito S, Jitsumori Y, Nakashima T, Nagai N, Nishizaki K, Shimizu K. Allelic loss and reduced expression of the ING3, a candidate tumor suppressor gene at 7q31, in human head and neck cancers. Oncogene. 2002;21(28):4462–4470. doi: 10.1038/sj.onc.1205540. [DOI] [PubMed] [Google Scholar]
- Holbert MA, Sikorski T, Carten J, Snowflack D, Hodawadekar S, Marmorstein R. The human monocytic leukemia zinc finger histone acetyltransferase domain contains DNA-binding activity implicated in chromatin targeting. J Biol Chem. 2007;282(50):36603–36613. doi: 10.1074/jbc.M705812200. [DOI] [PubMed] [Google Scholar]
- Katsumoto T, Aikawa Y, Iwama A, Ueda S, Ichikawa H, Ochiya T, Kitabayashi I. MOZ is essential for maintenance of hematopoietic stem cells. Genes Dev. 2006;20(10):1321–1330. doi: 10.1101/gad.1393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmizis A, Santos-Rosa H, Penkett CJ, Singer MA, Vermeulen M, Mann M, Bahler J, Green RD, Kouzarides T. Arginine methylation at histone H3R2 controls deposition of H3K4 trimethylation. Nature. 2007;449(7164):928–932. doi: 10.1038/nature06160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabayashi I, Aikawa Y, Nguyen LA, Yokoyama A, Ohki M. Activation of AML1-mediated transcription by MOZ and inhibition by the MOZ-CBP fusion protein. EMBO J. 2001a;20(24):7184–7196. doi: 10.1093/emboj/20.24.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabayashi I, Aikawa Y, Yokoyama A, Hosoda F, Nagai M, Kakazu N, Abe T, Ohki M. Fusion of MOZ and p300 histone acetyltransferases in acute monocytic leukemia with a t(8;22)(p11;q13) chromosome translocation. Leukemia. 2001b;15(1):89–94. doi: 10.1038/sj.leu.2401983. [DOI] [PubMed] [Google Scholar]
- Lalonde ME, Avvakumov N, Glass KC, Joncas FH, Saksouk N, Holliday M, Paquet E, Yan K, Tong Q, Klein BJ, Tan S, Yang XJ, Kutateladze TG, Cote J. Exchange of associated factors directs a switch in HBO1 acetyltransferase histone tail specificity. Genes Dev. 2013;27(18):2009–2024. doi: 10.1101/gad.223396.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue K, Daujat S, Crump JG, Plaster N, Roehl HH, Kimmel CB, Schneider R, Hammerschmidt M. The multidomain protein Brpf1 binds histones and is required for Hox gene expression and segmental identity. Development. 2008;135(11):1935–1946. doi: 10.1242/dev.017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li H. Many keys to push: diversifying the 'readership' of plant homeodomain fingers. Acta Biochim Biophys Sin (Shanghai) 2012;44(1):28–39. doi: 10.1093/abbs/gmr117. [DOI] [PubMed] [Google Scholar]
- Liu L, Qin S, Zhang J, Ji P, Shi Y, Wu J. Solution structure of an atypical PHD finger in BRPF2 and its interaction with DNA. J Struct Biol. 2012;180(1):165–173. doi: 10.1016/j.jsb.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Mack GS. Epigenetic cancer therapy makes headway. J Natl Cancer Inst. 2006;98(20):1443–1444. doi: 10.1093/jnci/djj447. [DOI] [PubMed] [Google Scholar]
- Panagopoulos I, Fioretos T, Isaksson M, Samuelsson U, Billstrom R, Strombeck B, Mitelman F, Johansson B. Fusion of the MORF and CBP genes in acute myeloid leukemia with the t(10;16)(q22;p13) Hum Mol Genet. 2001;10(4):395–404. doi: 10.1093/hmg/10.4.395. [DOI] [PubMed] [Google Scholar]
- Perez-Campo FM, Borrow J, Kouskoff V, Lacaud G. The histone acetyl transferase activity of monocytic leukemia zinc finger is critical for the proliferation of hematopoietic precursors. Blood. 2009;113(20):4866–4874. doi: 10.1182/blood-2008-04-152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplawski A, Hu K, Lee W, Natesan S, Peng D, Carlson S, Shi X, Balaz S, Markley JL, Glass KC. Molecular Insights into the Recognition of N-Terminal Histone Modifications by the BRPF1 Bromodomain. J Mol Biol. 2013 doi: 10.1016/j.jmb.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Jin L, Zhang J, Liu L, Ji P, Wu M, Wu J, Shi Y. Recognition of Unmodified Histone H3 by the First PHD Finger of Bromodomain-PHD Finger Protein 2 Provides Insights into the Regulation of Histone Acetyltransferases Monocytic Leukemic Zinc-finger Protein (MOZ) and MOZ-related factor (MORF) J Biol Chem. 2011;286(42):36944–36955. doi: 10.1074/jbc.M111.244400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Liu L, Zhao C, Han C, Li F, Zhang J, Wang Y, Li G, Mei Y, Wu M, Wu J, Shi Y. Combinatorial readout of unmodified H3R2 and acetylated H3K14 by the tandem PHD finger of MOZ reveals a regulatory mechanism for HOXA9 transcription. Genes Dev. 2012;26(12):1376–1391. doi: 10.1101/gad.188359.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksouk N, Avvakumov N, Champagne KS, Hung T, Doyon Y, Cayrou C, Paquet E, Ullah M, Landry AJ, Cote V, Yang XJ, Gozani O, Kutateladze TG, Cote J. HBO1 HAT complexes target chromatin throughout gene coding regions via multiple PHD finger interactions with histone H3 tail. Mol Cell. 2009;33(2):257–265. doi: 10.1016/j.molcel.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C, Nguyen K, Schapira M. Druggability of methyl-lysine binding sites. Journal of computer-aided molecular design. 2011;25(12):1171–1178. doi: 10.1007/s10822-011-9505-2. [DOI] [PubMed] [Google Scholar]
- Shiseki M, Nagashima M, Pedeux RM, Kitahama-Shiseki M, Miura K, Okamura S, Onogi H, Higashimoto Y, Appella E, Yokota J, Harris CC. p29ING4 and p28ING5 bind to p53 and p300, and enhance p53 activity. Cancer Res. 2003;63(10):2373–2378. [PubMed] [Google Scholar]
- Slama P, Geman D. Identification of family-determining residues in PHD fingers. Nucleic Acids Res. 2011;39(5):1666–1679. doi: 10.1093/nar/gkq947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Voss AK. Querkopf, a histone acetyltransferase, is essential for embryonic neurogenesis. Front Biosci. 2004;9:24–31. doi: 10.2741/1208. [DOI] [PubMed] [Google Scholar]
- Thompson CA. Vorinostat approved for rare lymphoma. Am J Health Syst Pharm. 2006;63(22):2168. doi: 10.2146/news060020. [DOI] [PubMed] [Google Scholar]
- Ullah M, Pelletier N, Xiao L, Zhao SP, Wang K, Degerny C, Tahmasebi S, Cayrou C, Doyon Y, Goh SL, Champagne N, Cote J, Yang XJ. Molecular architecture of quartet MOZ/MORF histone acetyltransferase complexes. Mol Cell Biol. 2008;28(22):6828–6843. doi: 10.1128/MCB.01297-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzoli A, Bonadies N, Allen MD, Freund SM, Santiveri CM, Kvinlaug BT, Huntly BJ, Gottgens B, Bycroft M. Molecular basis of histone H3K36me3 recognition by the PWWP domain of Brpf1. Nat Struct Mol Biol. 2010;17(5):617–619. doi: 10.1038/nsmb.1797. [DOI] [PubMed] [Google Scholar]
- Vidler LR, Brown N, Knapp S, Hoelder S. Druggability analysis and structural classification of bromodomain acetyl-lysine binding sites. J Med Chem. 2012;55(17):7346–7359. doi: 10.1021/jm300346w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss AK, Collin C, Dixon MP, Thomas T. Moz and retinoic acid coordinately regulate H3K9 acetylation, Hox gene expression, and segment identity. Dev Cell. 2009;17(5):674–686. doi: 10.1016/j.devcel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Wagner EK, Nath N, Flemming R, Feltenberger JB, Denu JM. Identification and characterization of small molecule inhibitors of a plant homeodomain finger. Biochemistry. 2012;51(41):8293–8306. doi: 10.1021/bi3009278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Ullah M. MOZ and MORF, two large MYSTic HATs in normal and cancer stem cells. Oncogene. 2007;26(37):5408–5419. doi: 10.1038/sj.onc.1210609. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yang CY, Wang S. The Making of I-BET762, a BET Bromodomain Inhibitor Now in Clinical Development. J Med Chem. 2013;56(19):7498–7500. doi: 10.1021/jm4014407. [DOI] [PubMed] [Google Scholar]