Abstract
Levels of serum phosphate are controlled by the peptide hormone FGF23, secreted from bone osteocytes. Elevated levels of circulating FGF23 are a key factor in several hypophosphatemic disorders and play a role in chronic kidney disease. Posttranslational processing of FGF23 includes multi-site O-glycosylation, which reduces intracellular cleavage by proprotein convertases. The FGF23 protein also contains four serine phosphorylation consensus sequences (S-X-D/E); in this work, we asked whether FGF23 is a substrate for secretory phosphorylation. Both HEK cells as well as IDG-SW3 cells, an osteocyte model, incorporated radiolabeled orthophosphate into intact FGF23, as well as into the 14-kDa carboxy-terminal—but not the 17-kDa N-terminal—fragment. Sequential serine-to-alanine site-directed mutagenesis of four kinase consensus sites showed that labeling occurred on three serines within the carboxy-terminal fragment, Ser180 (adjacent to the cleavage site), Ser207, and Ser212. Liquid chromatography-coupled mass spectroscopy indicated the presence of phosphate at Ser212 in recombinant R&D mouse FGF23R179Q, confirming labeling results. A phosphopeptide-specific antibody was raised against phospho-Ser212 and exhibited immunoreactivity in osteocytes present in mouse long bone, providing further evidence that FGF23 is naturally phosphorylated in bone. Bone SIBLING proteins are serine-phosphorylated by the ubiquitous Golgi secretory kinase FAM20C. Cotransfection of HEK and MC3T3 cells with FGF23 and active, but not inactive, FAM20C kinase increased the storage and release of FGF23 in radiolabeling experiments, indicating potential effects of phosphorylation on FGF23 stability. Collectively, these data point to an important role for phosphorylation of FGF23 in bone.
Keywords: FGF23, PHOSPHATE HOMEOSTASIS, FAM20C, KINASE, BONE, OSTEOCYTE
Introduction
The circulating peptide hormone FGF23, derived largely from bone osteocytes, controls the levels of circulating phosphate via binding to FGFR1 in a ternary complex with the protein Klotho (reviewed in Liu and colleagues(1)); activation of this receptor in kidney tubules results in decreased phosphate retention. Unlike most peptide hormone precursors, the secretion of bioactive FGF23 is controlled degradatively rather than biosynthetically; cellular cleavage of FGF23 at Arg179, located within a proprotein convertase consensus cleavage site, results in the secretion of cleaved protein, which is inactive on FGF23 receptors.(2) However, circulating levels of cleaved FGF23 are high (especially in certain bone diseases), and recent data indicate that the C-terminus of FGF23 can act as an antagonist at FGF23 receptors to block effective binding of the intact hormone.(3)
There is as yet limited information on the posttranslational processing of FGF23. FGF23 is known to be multiply O-glycosylated, but only two O-glycosylation sites have been identified with certainty thus far.(4) O-glycosylation of FGF23 plays a critical role in controlling its secretion, as well as its proteolytic inactivation by convertases (reviewed in Gram Schjoldager and colleagues(5) and Kato and colleagues(6)); the identity of the proprotein convertase mediating FGF23 cleavage is still controversial.(7,8)
A new ubiquitously distributed secretory kinase, FAM20C, has recently been identified in many tissues, including bone.(9) Because it is the only kinase identified thus far that contains a signal peptide, FAM20C is thought to be responsible for most, if not all, phosphorylation of secretory proteins at serines located within S-X-E/D phosphorylation consensus sites.(9) The direct phosphorylation of many acidic secreted bone proteins in the SIBLING protein family is efficiently accomplished by this kinase, and mutations in this kinase are known to result in a human bone disease.(9,10) Interestingly, FGF23 itself contains several consensus sites for FAM20C phosphorylation. In this report, we present data showing that the carboxy-terminal fragment of FGF23 is multiply phosphorylated by an endogenous kinase in bone cells, likely FAM20C.
Materials and Methods
Radiolabeling and immunoprecipitation
For analysis of phosphorylation, the pCAGEN-flag-hFGF23 vector, constructed by digesting the Flag-hFGF23 vector(11) with EcoR1 and XhoI restriction enzymes and transferring into the pCAGEN vector, was transiently transfected into either HEK293, MC3T3 osteoblasts, or IDG-SW3 osteocyte-like cells in a 6-well plate. MC3T3 cells were transfected with FGF23 and other cDNAs at a 3:1 ratio (FGF23 to GalNT3, FAM20C, or FAM20C-D478A); an equivalent amount of pcDNA3 encoding alpha 1-antitrypsin cDNA (AAT) was used in the FGF23 control sample to ensure that all wells received the same amount of protein-encoding cDNA. cDNAs encoding FAM20C and variants were obtained from S Ichikawa and M Econs,(11) whereas cDNAs encoding FAM20C kinase or its inactive D478A variant were obtained from Drs V Tagliabracci and J Dixon.(9) One day after transfection, wells were washed twice with phosphate-free medium (either DMEM or RPMI), starved for 60 minutes, and 0.5 to 1.0 mCi/mL of H2H32PO4 added to each well. After 3 to 4 hours of labeling, wells were washed with medium containing phosphate, the medium was replaced with normal DMEM containing 1% fetal bovine serum (FBS), 100 μg/mL aprotinin and levamisole (100 μM), and cells were incubated for a further 2 to 3 hours. Medium samples were diluted in 5× RIPA buffer with protease and phosphatase inhibitors (“Halt”; Roche Diagnostics, Mannheim, Germany) and immunoprecipitated with goat antibodies directed against recombinant human FGF23 (R&D Systems, Minneapolis, MN, USA; AF2604) or with a mixture of these antibodies with goat antibodies against the C-terminus of human FGF23 (residues 225 to 244; courtesy of Dr J Lavigne, Immutopics, San Clemente, CA, USA). For methionine labeling, a similar protocol was followed, except that 0.5 mCi of 35S-methionine was used in methionine- and cysteine-free medium (either DMEM or RPMI) supplemented with 10 mM HEPES, pH 7.4. Identification of phosphorylation sites in human FGF23 was examined by performing Ser-to-Ala Quikchange mutagenesis of residues within the four FAM20C consensus sequences, serines 77, 180, 207, and 212 (numbering is given for native human FGF23, including the initiating methionine; however, the construct we used contains an N-terminal Flag-tag sequence(11)). Mutagenesis reactions were carried out by GenScript (Piscataway, NJ, USA) and confirmed by sequencing of the entire insert. All radiolabeling experiments were carried out at least three times, except the analysis of the triple Ala mutant, which was carried out twice.
Western blotting
One day after Fugene-mediated transfection of MC3T3 cells, OptiMem containing 0.1% heat-treated FBS, 100 μg/mL recombinant aprotinin, 100 μM levamisole, and 1% penicillin-streptomycin was added to OptiMem-washed cells and the cells further incubated at 37°C for 18 to 24 hours. The conditioned medium was precipitated on ice with 10% trichloroacetic acid, centrifuged, and the pellets resuspended in Laemmli SDS-sample buffer containing 6 M urea, whereas cells were lysed directly in Laemmli sample buffer. Western blotting was carried out using a mixture of the goat anti-FGF23 antiserum mentioned above at 1:1000 and a goat anti-Flag antiserum (Acris, San Diego, CA, USA) at 1:2000 final dilution; 10% to 30% of the medium and cell samples were assayed.
Digestion and mass spectroscopy
Carrier-free and hexa-His-tagged mouse recombinant FGF23R179Q was purchased from R&D Systems. Two milligrams were resuspended in PBS and subjected to reduction-alkylation and a 5-hour trypsin digestion using the Pierce in-solution tryptic digestion and guanidation kit (Thermo Fisher Scientific, Pittsburgh, PA, USA). Samples were desalted over a 3-layer C-18 tip and digests analyzed by LC-MS/MS analysis using an LTQ- Orbitrap mass spectrometer. MS1 data were acquired in the profile mode in the Orbitrap with a resolution of 60,000 at 400 m/z, and the top 10 most intense ions in each MS1 scan were selected for collision-induced dissociation in the linear ion trap. Dynamic exclusion was enabled with a repeat count of 2 and exclusion duration of 15 seconds. Other mass spectrometry data generation parameters were as follows: collision energy, 35%; ion selection threshold for MS/MS, 1000 counts; isolation width 3; and default charge state, 3. Each sample was analyzed twice by LC- MS/MS for two technical replicates. For the identification of phosphopeptide, the systematic parent ion mass measurement errors were corrected by DtaRefinery. The resulting DTA files were searched against a UniProt human database using SEQUEST, and were filtered by the following criteria and validated manually: Xcorr ≥2.5, 3.0 and 3.5 for 2+, 3+, and 4+ peptides, respectively (1+ peptides were ignored) and ΔCn > 0.1. The candidate peptides were then validated manually by visual inspection.
Immunocytochemistry and phosphate-specific antiserum production and validation
A phosphorylated human FGF23 peptide, CSQELP[pS]AE, was conjugated to keyhole limpet hemocyanin with maleimide and the conjugate used to immunize rabbits (Covance, Denver, PA, USA). An IgG fraction was prepared using a Protein A column and used for all experiments. The specificity of this IgG preparation (final concentration, 1 μg/mL) for phosphorylated peptide was tested by preferential Western blot reaction for FGF23R176Q obtained from the conditioned medium of HEK cells cotransfected with FAM20C versus inactive kinase cDNAs, as well as by phosphopeptide ELISA, as described in Results. Immunocytochemistry was performed on paraffin-embedded sections of a decalcified mouse tibia using the Vectastain Elite ABC kit and NovaRed Peroxidase substrate (Vector Laboratories, Burlingame, CA, USA) in the presence or absence of phospho-FGF23 IgG (1:50 dilution). Specificity was confirmed by incubating the antibody solution with a serial tibial section in the presence of excess phosphorylated human FGF23 immunogen peptide.
Results
The primary structure of human FGF23 contains four Ser-X-D/E consensus FAM20C kinase sites;(9) one serine (S77) is present in the amino-terminal domain and three serines (S180, S207, and S212) in the carboxy-terminal domain. All of these sites are conserved between mouse and human FGF23 and in the seven mammalian species cloned to date.
Site-directed mutagenesis of FGF23 shows phosphorylation of Ser180, Ser207, and Ser212 within the C-terminal fragment
To identify the particular residue involved in FGF23 phosphorylation, site-directed mutagenesis was performed on four serines within known FAM20C consensus sequences and these cDNAs transfected into HEK cells. These data (shown in Fig. 1A) clearly show that Ser207 and Ser212 represent major phosphorylation loci by an endogenous HEK kinase. However, even when these two sites were mutated to alanine, C-terminal peptide phosphorylation persisted, indicating usage of another site.
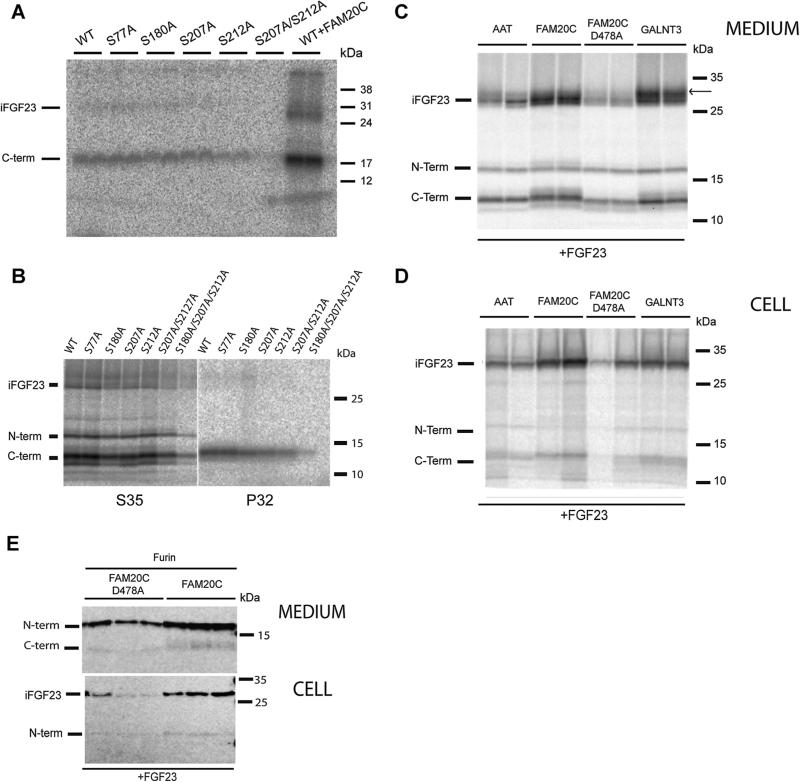
Fig. 1.
FGF23 is phosphorylated at Ser180, Ser207, and Ser212 within the carboxyl-terminal domain. (A) Endogenous phosphorylation of FGF23 by HEK cells. The C-terminal fragment is the major phosphorylated species. HEK cells were transfected with FGF23 cDNAs containing the mutations indicated, with FAM20C cDNA added to the two wells in the last lane. Cells were labeled with 32P-orthophosphate, chased in medium containing cold phosphate, and the medium immunoprecipitated with FGF23 antiserum. Samples were separated using SDS-PAGE, dried, and radioactivity visualized by phosphoimaging. iFGF23 = intact FGF23; C-term = C-terminal fragment of FGF23. (B) Endogenous phosphorylation of FGF23 in IDG-SW3 cells. Site identification. IDG-SW3 cells were radiolabeled with either 35S-methionine (left) or 32P-orthophosphate (right) and chased in medium containing either cold methionine or phosphate. iFGF23 = intact FGF23; N-term = N-terminal fragment; C-term = C-terminal fragment of FGF23. (C) Enhanced secretion of FGF23 by FAM20C kinase co-expression in MC3T3 cells. MC3T3 cells were transfected with cDNAs encoding FGF23 and either AAT, FAM20C, FAM20C/D478A, or GalNT3 at a 3:1 ratio, radiolabeled with 35S-methionine for 3 hours, and the 3-hour chase medium immunoprecipitated with FGF23 antiserum in the presence of protease and phosphatase inhibitors. The arrow shows an upper band present in GalNT3-transfected cells that is not present in FAM20C-transfected cells, potentially indicating the presence of an additional sugar chain in GalNT3-treated cells. iFGF23 = intact FGF23; N-term = N-terminal fragment; C-term = C-terminal fragment of FGF23. (D) Increased cellular expression of FGF23 by FAM20C kinase co-expression in MC3T3 cells. Cells from the experiment described in (C) were extracted with RIPA buffer and the cleared supernatant subjected to FGF23 immunoprecipitation in the presence of protease and phosphatase inhibitors. iFGF23 = intact FGF23; N-term = N-terminal fragment; C-term = C-terminal fragment of FGF23. (E) Effect of FAM20C on FGF23 expression and secretion in furin-transfected MC3T3 cells. A 12-well plate of MC3T3 cells was transfected in triplicate with FGF23 and furin and either FAM20C or the inactive D478A mutant at a 2.5 to 1 ratio of FGF23 to other cDNAs. The conditioned medium was concentrated by TCA precipitation and subjected to Western blotting. No intact secreted FGF23 was detected. Cell lysates are shown in the bottom half of this panel.
Bone osteocytes are the known source of most circulating FGF23; we, therefore, examined the sites at which IDG-SW3 cells, a bone cell line that models osteocyte differentiation,(20) are able to phosphorylate FGF23. Fig. 1B shows that an endogenous kinase present in IDG-SW3 cells carries out efficient phosphorylation of FGF23. Consistent with the pattern observed in HEK cells, in IDG-SW3 cells the Ser207/212Ala mutant was phosphorylated to a much lesser extent, but phosphorylation was again not eliminated. We then constructed a triple mutant with no remaining C-terminal kinase consensus sequences; this totally eliminated phosphorylation. These results support the idea that all three C-terminal serine consensus sites within FGF23 are phosphorylated by an endogenous kinase in bone cells. The lack of phosphate labeling in the N-terminal fragment clearly indicates the absence of phosphorylation at Ser77.
The FAM20C kinase was recently shown to phosphorylate a host of different bone proteins.(9) We observed a strong increase in incorporation of radiolabeled phosphate into FGF23 in the presence of cotransfected FAM20C kinase (Fig. 1A, last lane), supportive of FAM20C-mediated phosphorylation. Interestingly, FAM20C transfection also consistently increased FGF23 secretion, suggesting that phosphorylation may enhance FGF23 stability and/or secretion in a manner similar to O-glycosylation.(6,12) We note that the identity of the endogenous HEK cell kinase as FAM20C is not yet proven; it is possible that an unidentified secretory kinase performs this reaction. However, no other secretory kinase has been identified to date.
We further assessed the contribution of phosphorylation to FGF23 secretion using radiolabeling of transiently transfected MC3T3 cells, which produces similarly sized FGF23 cleavage products as those generated by IDG-SW3 cells.(7,8) Increased secretion of FGF23 and cleavage products was consistently observed in the presence of the active but not the inactive form of the FAM20C kinase, as detected by radiolabeling of MC3T3 cells with 35S-methionine and immunoprecipitation (Fig. 1C). Interestingly, an upper band is present in GalNT3-transfected cells that is largely absent in FAM20C-transfected cells, implying differential glycosylation in the presence of kinase versus sugar transferase. However, differential cleavage of FGF23 in the presence of active FAM20C was never observed in radiolabeling experiments. Fig. 1D depicts the cell data from the same experiment shown in Fig. 1C, which shows that FGF23- transfected cells cotransfected with active FAM20C and GalNT3 contain more intact FGF23 than control cells cotransfected with expression vectors encoding alpha-1 antitrypsin or with an inactive FAM20C variant.
In transfected MC3TC3 cells, we detected increased production and secretion of cleaved N-terminal product in the presence of furin and FAM20C compared with inactive FAM20C (Fig. 1E).
Commercial recombinant FGF23 contains phosphate at Ser212
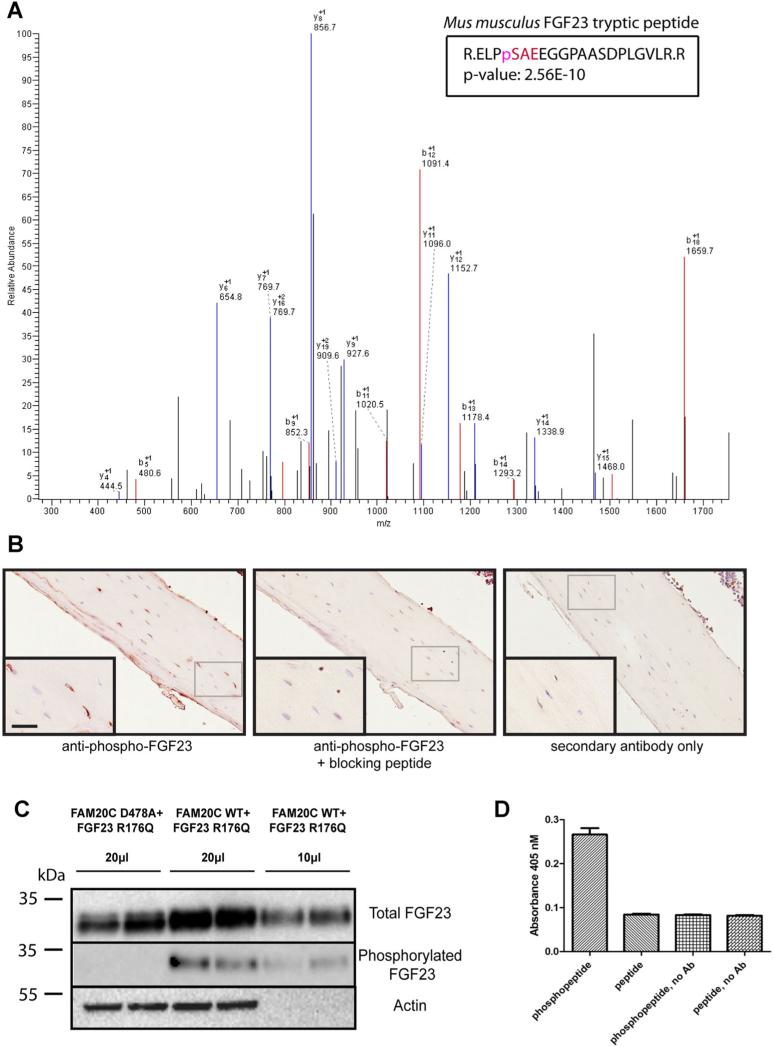
Because FAM20C is ubiquitously expressed,(9) we asked whether commercially available FGF23, made in NSO cells and obtained from R&D, also contains phosphorylated residues. Fig. 2A shows the mass spectrum of a peptide containing phosphorylated Ser212 present in commercial mouse FGF23R179Q. These data show multiple phosphorylated fragments corresponding to the tryptic peptide expected from digestion of recombinant FGF23 phosphorylated Ser212; the confidence level for detection of this fragment is 2.56 × e−10. The fact that no other phosphopeptides were found may imply their lesser abundance in this particular recombinant protein preparation.
Fig. 2.
(A) Commercial FGF23 is phosphorylated at Ser212. Mass spectroscopic identification of phosphorylation sites in recombinant mouse FGF23R179Q indicates the presence of a single phosphate within multiple peptide peaks (shown in red), all of which have masses consistent with peptides phosphorylated at Ser212. (B) Mouse long bone contains phospho-Ser212-immunoreactive FGF23. Staining of osteocytes in mouse tibial slices using phospho-Ser212 antibodies. The left panel shows staining in the absence of blocking peptide; the middle panel shows staining in the presence of blocking peptide; and the right panel shows staining in the absence of primary antibody. (C) Increased release of FGF23 from FAM20C-transfected cells; specificity of the phospho-Ser212 antibody. FGF23R176Q cDNA was transfected in the presence of either active FAM20C or inactive D478A FAM20C kinase cDNAs in duplicate. Western blotting was performed using the R&D pan-specific FGF23 antibody or the phospho-Ser212 antibody. Actin blotting of cells corresponding to the medium samples is shown below only for the 20-μL sample; the 10-μL sample was taken from the same plate. Quantitation of these bands indicated that the phospho-Ser212 antibody was 15-fold more reactive toward phosphorylated FGF23. (D) The phospho-212 antiserum preferentially detects phosphorylated peptide in ELISA. An ELISA was performed using a 1:500 dilution of protein A-purified phospho-212 antibodies on plates coated with either phosphopeptide (CSQELP{pS}AE) or a peptide lacking phosphate (CSQELPSAEDNSPMASDP), both at 0.5 μM. Results are the mean ± the SD, n = 3. The experiment was repeated once with an independent IgG preparation with similar results.
Phospho212-FGF23 immunoreactivity is present in mouse bone
To determine whether FGF23 is phosphorylated in bone, we raised an antiserum against the Ser212 phosphosite by using a synthetic phosphorylated peptide as an antigen. Fig. 2B shows that the phospho212-specific antiserum MD283 was able to recognize an antigen within osteocytes in mouse long bones; immunoreactivity was blocked by the addition of immunogen phosphopeptide and was not observed when the primary antiserum was omitted, suggesting endogenous phosphorylation of FGF23 in bone. The specificity of the phospho-Ser212 IgG preparation used in this immunocytochemistry experiment is illustrated in Fig. 2C, which indicates 15-fold lower reaction with FGF23 synthesized in the absence of active kinase. These data also show increased FGF23 in the medium after cotransfection of FGF23R176Q with active but not inactive FAM20C. In four independent HEK cell experiments, the average fold-increase in FGF23R176Q secretion conferred by active versus inactive FAM20C cotransfection was 1.62 ± 0.31 (mean ± SD). Fig. 2D further confirms the specificity of the phospho212 antiserum against FGF23 phosphopeptide (versus unphosphorylated peptide) in an ELISA reaction.
Discussion
The circulating peptide hormone FGF23 is critical to phosphate homeostasis because of its controlling role in kidney mineral exchange. Posttranslational modification is known to control the bioactivity of this hormone: O-glycosylation blocks convertase cleavage and results in enhanced secretion of intact bioactive hormone, whereas inactivating mutations in the GalNT3 enzyme responsible for O-glycosylation result in increased cleavage of FGF23 concomitant with secretion of inactive cleavage products.(4,6,11) In this report, we describe a second type of posttranslational modification of FGF23, that of serine phosphorylation. The data presented here provide novel evidence for the physiological phosphorylation of the C-terminal peptide of FGF23, generating a 14-kDa phosphorylated fragment secreted from both MC3T3 and IDG-SW3 bone cells.
The FAM20C kinase recently identified by Dixon and colleagues(9) is robustly expressed in both osteoblasts and osteocytes,(13) and transfection of cDNA encoding this kinase greatly increases the phosphorylation of the C-terminal fragment of FGF23. This Golgi kinase has recently been genetically associated with many bone disorders, now thought to be mediated via improperly phosphorylated bone proteins.(9,10,14) Our data indicate that FAM20C disorders may also impact bone secretory biology via interruption of multiple C-terminal FGF23 fragment phosphorylation. Whether FAM20C activity can be regulated is as yet unknown.
The functional significance of serine phosphorylation of many secreted proteins is thought to lie partly in the additional negative charges conferred, which offer protection against degradation and increase solubility. Serine phosphorylation of secreted peptide hormones is not common, although pro- opiomelanocortin, proenkephalin, and progastrin have all previously been shown to be phosphorylated.(15–18) The bone SIBLING proteins, a group of acidic secreted proteins with conserved ASARM motifs, require phosphorylation for functional interaction with bone hydroxyapatite and with the PHEX metalloprotease (reviewed in Rowe(19)), and many are efficiently phosphorylated by FAM20C.(9) The C-terminal portion of FGF23 is involved in FGF23-Klotho interactions,(21) interactions that ultimately determine FGF23 signaling efficacy;(3) phosphorylation may also play a role in receptor activation.
Our finding of Ser180 phosphorylation is interesting in that this site flanks Arg179, the residue within the convertase consensus site at which intracellular degradation occurs. O-glycosylation in the vicinity of this site is known to block convertase cleavage.(11) Although our radiolabeling data showing similar secretion of cleaved C-terminal peptides in wild-type and Ser180Ala- substituted FGF23 do not support the idea that phosphorylation of this residue strongly affects convertase-mediated proteolytic cleavage within MC3T3 or IDG-SW3 cells, preliminary results using bacterially expressed FGF23 indicate that furin is able to cleave FAM20C-phosphorylated FGF23 (data not shown).
In summary, the data presented here show multiple phosphorylation of the C-terminal fragment of FGF23 in bone cell lines, a posttranslational modification that impacts FGF23 secretion and stability.
Note: While this article was in preparation, a paper was published demonstrating FAM20C-mediated phosphorylation of FGF23 at Ser180, which inhibits O-glycosylation at Thr178.(22)
Acknowledgments
This work was supported by NIH grant DK49703-16 to IL. We thank Drs S Ichikawa and M Econs for the gift of FGF23-encoding and GalNT3-encoding cDNAs; Drs V Tagliabracci and J Dixon for the gift of FAM20C-encoding vectors; Dr E Blanco for helpful suggestions; and R Bittman for early experiments on FGF23 phosphorylation.
Authors’ roles: Study design: IL, JPS, and AJY. Study conduct: IL, HWP, JPS, KZL, AJY, and DC. Data collection: IL, JPS, AJY, DC, and KZL. Data analysis: IL, JPS, AJY, and DC. Data interpretation: IL, JPS, AJY, DC, and KZL. Drafting manuscript: IL, JPS, and AJY. Revising manuscript content: IL, JPS, and LB. Approving final version of manuscript: IL. IL takes responsibility for the integrity of the data analysis.
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Liu S, Tang W, Fang J, et al. Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol Endocrinol. 2009;23(9):1505–18. doi: 10.1210/me.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christov M, Juppner H. Insights from genetic disorders of phosphate homeostasis. Semin Nephrol. 2013;33(2):143–57. doi: 10.1016/j.semnephrol.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goetz R, Nakada Y, Hu MC, et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci USA. 2010;107(1):407–12. doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergwitz C, Banerjee S, Abu-Zahra H, et al. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J Clin Endocrinol Metab. 2009;94(11):4267–74. doi: 10.1210/jc.2009-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gram Schjoldager KT, Vester-Christensen MB, Goth CK, et al. A systematic study of site-specific GalNAc-type O-glycosylation modulating proprotein convertase processing. J Biol Chem. 2011;286(46):40122–32. doi: 10.1074/jbc.M111.287912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato K, Jeanneau C, Tarp MA, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281(27):18370–7. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 7.Yuan B, Feng JQ, Bowman S, et al. Hexa-D-arginine treatment increases 7B2*PC2 activity in hyp-mouse osteoblasts and rescues the HYP phenotype. J Bone Miner Res. 2013;28(1):56–72. doi: 10.1002/jbmr.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benet-Pages A, Lorenz-Depiereux B, Zischka H, White KE, Econs MJ, Strom TM. FGF23 is processed by proprotein convertases but not by PHEX. Bone. 2004;35(2):455–62. doi: 10.1016/j.bone.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Tagliabracci VS, Engel JL, Wen J, et al. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science. 2012;336(6085):1150–3. doi: 10.1126/science.1217817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa HO, Xu A, Ogura E, Manning G, Irvine KD. The Raine syndrome protein FAM20C is a Golgi kinase that phosphorylates bio- mineralization proteins. PLoS One. 2012;7(8):e42988. doi: 10.1371/journal.pone.0042988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichikawa S, Lyles KW, Econs MJ. A novel GALNT3 mutation in a pseudoautosomal dominant form of tumoral calcinosis: evidence that the disorder is autosomal recessive. J Clin Endocrinol Metab. 2005;90(4):2420–3. doi: 10.1210/jc.2004-2302. [DOI] [PubMed] [Google Scholar]
- 12.Larsson T, Davis SI, Garringer HJ, et al. Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology. 2005;146(9):3883–91. doi: 10.1210/en.2005-0431. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Hao J, Xie Y, et al. Expression of FAM20C in the osteogenesis and odontogenesis of mouse. J Histochem Cytochem. 2010;58(11):957–67. doi: 10.1369/jhc.2010.956565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rafaelsen SH, Raeder H, Fagerheim AK, et al. Exome sequencing reveals FAM20c mutations associated with FGF23-related hypophosphatemia, dental anomalies and ectopic calcification. J Bone Miner Res. 2013;28(6):1378–85. doi: 10.1002/jbmr.1850. [DOI] [PubMed] [Google Scholar]
- 15.Bennett HPJ, Browne CA, Solomon S. Biosynthesis of phosphorylated forms of corticotropin-related peptides. Proc Natl Acad Sci USA. 1981;78(8):4713–7. doi: 10.1073/pnas.78.8.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Souza NB, Lindberg I. Evidence for the phosphorylation of a proenkephalin-derived peptide, peptide B. J Biol Chem. 1988;263(5):2548–52. [PubMed] [Google Scholar]
- 17.Watkinson A, Dockray GJ, Young J, Gregory H. Proenkephalin A processing in the upper digestive tract: isolation and characterisation of phosphorylated N-terminally extended Met-enkephalin Arg6Phe7 variants. J Neurochem. 1988;51(4):1252–7. doi: 10.1111/j.1471-4159.1988.tb03094.x. [DOI] [PubMed] [Google Scholar]
- 18.Thibault G, Lazure C, Chretien M, Cantin M. Molecular heterogeneity of pro-atrial natriuretic factor. J Biol Chem. 1989;264(31):18796–02. [PubMed] [Google Scholar]
- 19.Rowe PS. The chicken or the egg: PHEX, FGF23 and SIBLINGs unscrambled. Cell Biochem Funct. 2012;30(5):355–75. doi: 10.1002/cbf.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res. 2011;26(11):2634–46. doi: 10.1002/jbmr.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garringer HJ, Malekpour M, Esteghamat F, et al. Molecular genetic and biochemical analyses of FGF23 mutations in familial tumoral calcinosis. Am J Physiol Endocrinol Metab. 2008;295(4):E929–37. doi: 10.1152/ajpendo.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tagliabracci VS, Engel JL, Wiley SE, et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosyltion, and furin proteolysis. Proc Natl Acad Sci USA. 2014;111(15):5520–5. doi: 10.1073/pnas.1402218111. [DOI] [PMC free article] [PubMed] [Google Scholar]