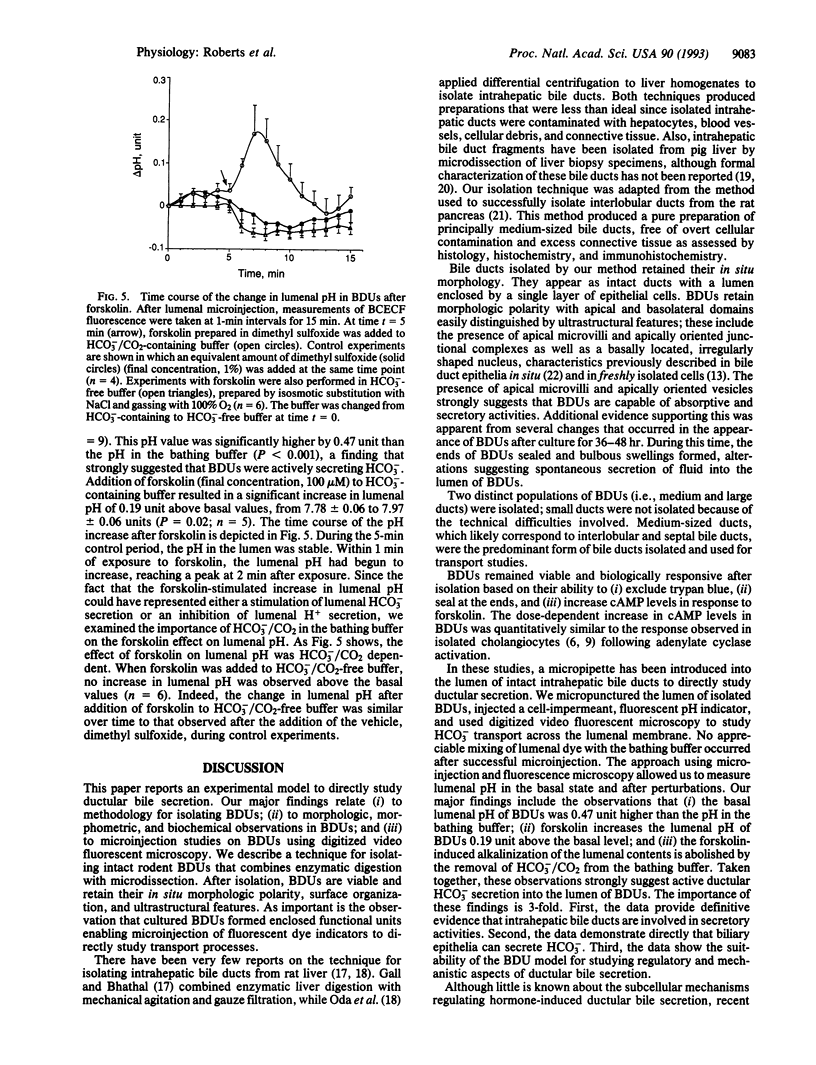
Abstract
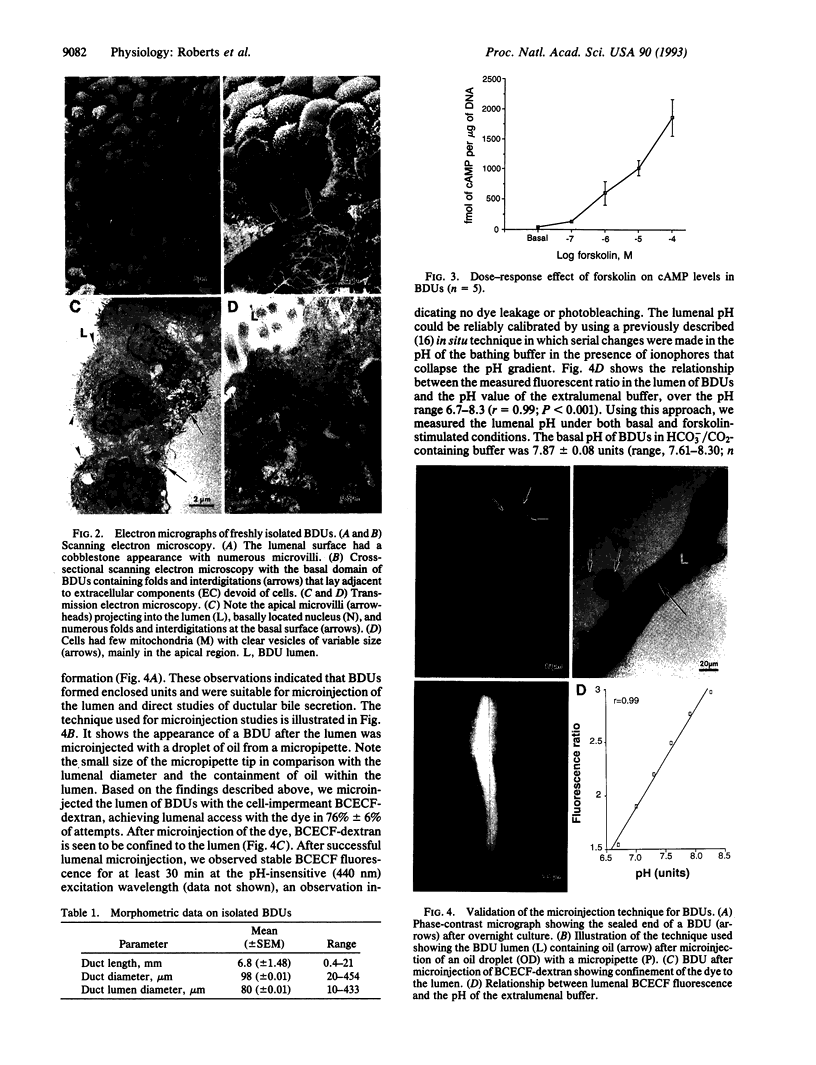
While intrahepatic bile duct epithelial cells secrete bile through transport of ions and water, the physiological mechanisms regulating ductular bile secretion are obscure, in part because of the lack of suitable experimental models. We report here the successful micropuncture of the lumen of isolated intrahepatic bile ducts and direct measurements of ductular ion secretion. Intact, polarized bile duct units (BDUs) were isolated from livers of normal rats by enzymatic digestion and microdissection. BDUs were cultured and mounted on a microscope in bicarbonate-containing buffer, and the lumens were microinjected with 2',7'-bis(2-carboxyethyl)-5-(and -6)carboxyfluorescein (BCECF)-dextran. Lumenal pH was measured by ratio imaging of BCECF fluorescence using digitized video fluorescent microscopy. After 36 hr in culture, the ends of BDUs sealed, forming closed compartments. After lumenal microinjection of BCECF-dextran, fluorescence was stable at the pH-insensitive wavelength, indicating no dye leakage. Serial changes in pH of extralumenal buffers containing pH-gradient collapsing ionophores allowed us to establish reliable standard curves relating fluorescence ratio to lumenal pH (r = 0.99; P < 0.001). By this approach, the basal pH inside the lumen of BDUs was 7.87 +/- 0.08 units (n = 9), 0.47 unit higher (P < 0.001) than the bathing buffer pH. Addition of 100 microM forskolin increased (P = 0.02) the lumenal pH from 7.78 +/- 0.06 to 7.97 +/- 0.06 units (n = 5); the forskolin effect was completely abolished by incubation of BDUs in HCO3-/CO2-free buffer. Moreover, forskolin caused a 50-fold increase in cAMP levels in BDUs. The observations are consistent with cAMP-dependent, active lumenal HCO3- secretion by BDUs. Furthermore, they demonstrate the suitability of the BDU model for studying regulatory and mechanistic aspects of ductular bile secretion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpini G., Lenzi R., Zhai W. R., Slott P. A., Liu M. H., Sarkozi L., Tavoloni N. Bile secretory function of intrahepatic biliary epithelium in the rat. Am J Physiol. 1989 Jul;257(1 Pt 1):G124–G133. doi: 10.1152/ajpgi.1989.257.1.G124. [DOI] [PubMed] [Google Scholar]
- Argent B. E., Arkle S., Cullen M. J., Green R. Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture. Q J Exp Physiol. 1986 Oct;71(4):633–648. doi: 10.1113/expphysiol.1986.sp003023. [DOI] [PubMed] [Google Scholar]
- Arkle S., Lee C. M., Cullen M. J., Argent B. E. Isolation of ducts from the pancreas of copper-deficient rats. Q J Exp Physiol. 1986 Apr;71(2):249–265. doi: 10.1113/expphysiol.1986.sp002982. [DOI] [PubMed] [Google Scholar]
- Bronk S. F., Gores G. J. Efflux of protons from acidic vesicles contributes to cytosolic acidification of hepatocytes during ATP depletion. Hepatology. 1991 Oct;14(4 Pt 1):626–633. doi: 10.1016/0270-9139(91)90049-2. [DOI] [PubMed] [Google Scholar]
- Bronk S. F., Powers S. P., Gores G. J. Dynamic measurements of intracellular aminopeptidase activity in hepatocytes using multiparameter digitized video fluorescent microscopy. Anal Biochem. 1993 May 1;210(2):219–225. doi: 10.1006/abio.1993.1186. [DOI] [PubMed] [Google Scholar]
- Buanes T., Grotmol T., Veel T., Landsverk T., Ridderstråle Y., Raeder M. G. Importance of carbonic anhydrase for canalicular and ductular choleresis in the pig. Acta Physiol Scand. 1988 Aug;133(4):535–544. doi: 10.1111/j.1748-1716.1988.tb08438.x. [DOI] [PubMed] [Google Scholar]
- Farouk M., Vigna S. R., McVey D. C., Meyers W. C. Localization and characterization of secretin binding sites expressed by rat bile duct epithelium. Gastroenterology. 1992 Mar;102(3):963–968. doi: 10.1016/0016-5085(92)90183-y. [DOI] [PubMed] [Google Scholar]
- Fitz J. G., Basavappa S., McGill J., Melhus O., Cohn J. A. Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest. 1993 Jan;91(1):319–328. doi: 10.1172/JCI116188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. A., Bhathal P. S. Isolation and culture of intrahepatic bile ducts and its application in assessing putative inducers of biliary epithelial cell hyperplasia. Br J Exp Pathol. 1987 Aug;68(4):501–510. [PMC free article] [PubMed] [Google Scholar]
- Gores G. J., Kost L. J., LaRusso N. F. The isolated perfused rat liver: conceptual and practical considerations. Hepatology. 1986 May-Jun;6(3):511–517. doi: 10.1002/hep.1840060331. [DOI] [PubMed] [Google Scholar]
- Gores G. J., Nieminen A. L., Wray B. E., Herman B., Lemasters J. J. Intracellular pH during "chemical hypoxia" in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J Clin Invest. 1989 Feb;83(2):386–396. doi: 10.1172/JCI113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Argent B. E. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988 Oct;105(2):131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Grotmol T., Buanes T., Raeder M. G. Effect of arterial pH and PCO2 on biliary HCO3- secretion in the pig. Acta Physiol Scand. 1987 Oct;131(2):183–193. doi: 10.1111/j.1748-1716.1987.tb08225.x. [DOI] [PubMed] [Google Scholar]
- Ishii M., Vroman B., LaRusso N. F. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989 Nov;97(5):1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- Kato A., Gores G. J., LaRusso N. F. Secretin stimulates exocytosis in isolated bile duct epithelial cells by a cyclic AMP-mediated mechanism. J Biol Chem. 1992 Aug 5;267(22):15523–15529. [PubMed] [Google Scholar]
- Lenzen R., Alpini G., Tavoloni N. Secretin stimulates bile ductular secretory activity through the cAMP system. Am J Physiol. 1992 Oct;263(4 Pt 1):G527–G532. doi: 10.1152/ajpgi.1992.263.4.G527. [DOI] [PubMed] [Google Scholar]
- Nathanson M. H., Boyer J. L. Mechanisms and regulation of bile secretion. Hepatology. 1991 Sep;14(3):551–566. [PubMed] [Google Scholar]
- Oda M., Yousef I. M., Phillips M. J. Isolation of bile ducts from rat liver: technique and preliminary ultrastructural characterization. Exp Mol Pathol. 1975 Oct;23(2):214–219. doi: 10.1016/0014-4800(75)90019-2. [DOI] [PubMed] [Google Scholar]
- Raeder M. G. The origin of and subcellular mechanisms causing pancreatic bicarbonate secretion. Gastroenterology. 1992 Nov;103(5):1674–1684. [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Sternlieb I. Special article: functional implications of human portal and bile ductular ultrastructure. Gastroenterology. 1972 Aug;63(2):321–327. [PubMed] [Google Scholar]
- Strazzabosco M., Mennone A., Boyer J. L. Intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1991 May;87(5):1503–1512. doi: 10.1172/JCI115160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler H. O., Mancusi-Ungaro P. L. Role of bile ducts during secretin choleresis in dogs. Am J Physiol. 1966 May;210(5):1153–1159. doi: 10.1152/ajplegacy.1966.210.5.1153. [DOI] [PubMed] [Google Scholar]